Abstract
Introduction
With an increasing prevalence of citizens of older age and with overweight, the health issues related to knee osteoarthritis (OA) will intensify. Weight loss is considered a primary management strategy in patients with concomitant overweight and knee OA. However, there are no widely available and feasible methods to sustain weight loss in patients with overweight and knee OA. The present protocol describes a randomised controlled trial evaluating the efficacy and safety of the glucagon-like peptide-1 receptor agonist liraglutide in a 3 mg/day dosing in patients with overweight and knee OA.
Methods and analysis
150 volunteer adult patients with overweight or obesity and knee OA will participate in a randomised, double-blind, placebo-controlled, parallel-group and single-centre trial. The participants will partake in a run-in diet intervention phase (week −8 to 0) including a low calorie diet and dietetic counselling. At week 0, patients will be randomised to either liraglutide 3 mg/day or liraglutide placebo 3 mg/day for 52 weeks as an add-on to dietetic guidance on re-introducing regular foods and a focus on continued motivation to engage in a healthy lifestyle. The co-primary outcomes are changes in body weight and the Knee Injury and Osteoarthritis Outcome Score pain subscale from week 0 to week 52.
Ethics and dissemination
The trial has been approved by the regional ethics committee in the Capital Region of Denmark, the Danish Medicines Agency and the Danish Data Protection Agency. An external monitoring committee (The Good Clinical Practice Unit at Copenhagen University Hospitals) will oversee the trial. The results will be presented at international scientific meetings and through publications in peer-reviewed journals.
Trial registration numbers
2015-005163-16, NCT02905864, U1111-1171-4970
Based on protocol version
V.6; 30 January 2017, 15:30 hours
Keywords: overweight, osteoarthritis, knee, weight loss, liraglutide, dietary intervention
Strengths and limitations of this study.
This will be the first randomised controlled trial examining the efficacy and safety of daily liraglutide 3 mg/day in patients with overweight and knee osteoarthritis (OA).
Participants will be randomised to receive either liraglutide 3 mg/day or liraglutide placebo 3 mg/day for 52 weeks as an add-on to dietetic guidance.
The selected primary and secondary outcomes are aligned with outcome measures in rheumatology recommendations.
This trial has a strict focus on an adult population with clinical knee OA who can successfully go through an intensive dietary programme (the target being to lose at least 5% of their initial body weight).
The trial has implication for the large number of patients impacted by overweight and knee OA.
Introduction
Obesity is a serious medical condition with increasing incidence and prevalence worldwide. The achievement and maintenance of a healthy body weight is the main strategy for prevention and management of obesity-related diseases.1 2 The majority of subjects with a successful weight loss regain weight over the subsequent years and the maintenance of a clinically significant weight loss over time remains a challenge for healthcare professionals, patients and societies.1
Osteoarthritis (OA) is the most common type of arthritis, characterised by pain and physical disability.3 In addition, >10% of those aged >55 years have symptomatic OA, primarily involving the knees.4 Due to the pivotal role of the knee in basic mobility and locomotion, knee OA is associated with significant impairments and limitations to basic activities of daily living, such as walking and moving around, self-care and housekeeping activities, as well as participation in community life and recreational activities—all contributing to reduced quality of life and needs for assistance. Epidemiological data link obesity to the development of knee OA,5 obesity and knee OA share pathogenic phenotypes, and the development of one disease increases the risk of the other, potentially triggering the onset of a vicious cycle.6
Glucagon-like peptide 1 (GLP-1) is an incretin hormone that stimulates endogenous insulin secretion in a glucose-dependent manner. GLP-1 also lowers blood glucagon levels, reduces gastric emptying by decreasing gastric motility and increases satiety. These mechanisms have been exploited therapeutically in the treatment of type 2 diabetes for more than a decade, and recently the satiety promoting and body weight lowering effects of the GLP-1 analogue liraglutide prompted the US Food and Drug Administration and the European Medicines Agency to approve liraglutide in a once-daily dose of 3 mg for the treatment of patients with overweight or obesity. Published data, investigating the use of liraglutide 3 mg/day in a population of patients with obesity as well as dyslipidaemia and/or hypertension, revealed a long-term positive impact on both body weight and related health benefits.7
Scientific gap
The present trial has been designed to further investigate the potential of liraglutide 3 mg/day to safely induce and maintain long-term weight loss in patients with overweight or obesity and knee OA. Weight loss is strongly recommended as a management strategy of patients with concomitant overweight or obesity and knee OA, and improves both pain and function.8–11 However, no widely available and feasible means to sustain weight loss in patients with overweight or obesity and knee OA has been presented.
Hypotheses
A diet intervention combined with liraglutide 3 mg/day, for 52 weeks, is sufficient to induce and maintain weight loss and to improve knee OA-related pain symptoms. As such, the primary hypotheses to be tested are:
In patients with overweight or obesity and knee OA, a diet intervention combined with liraglutide 3 mg/day will induce and maintain a significantly greater weight loss compared with a diet intervention combined with an identically appearing liraglutide placebo treatment.
In patients with overweight or obesity and knee OA, a diet intervention combined with liraglutide 3 mg/day will induce and maintain a significantly greater reduction in knee OA pain compared with a diet intervention combined with an identically appearing liraglutide placebo treatment.
Objectives
The primary objectives are to establish the efficacy and safety of a diet intervention combined with liraglutide 3 mg/day, or placebo, in inducing and maintaining weight loss over 52 weeks and in improving symptomatic knee pain.
Methods and analysis
Trial design
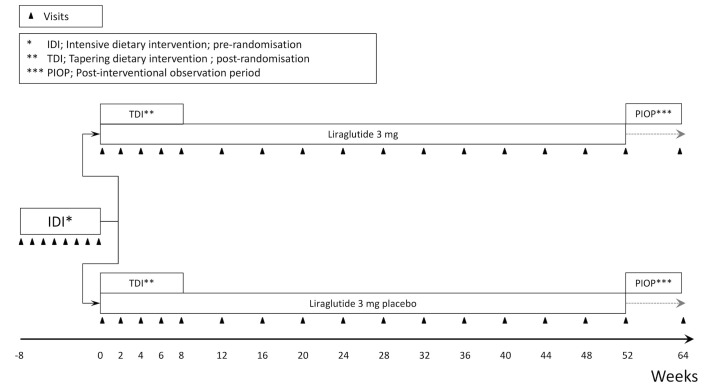
The trial is designed as a single-centre, randomised, placebo-controlled, participant, investigator and outcome assessor blinded, parallel-group trial. It contains three periods. Participants will initially be enrolled in an 8-week intensive dietary intervention (IDI) period. If successfully achieving a weight loss of minimum 5% during this period participants will continue with a tapering dietary intervention (TDI) for 8 weeks (week 0–8) and be randomised to receive either liraglutide 3 mg/day or identically appearing placebo throughout the 52-week main trial period (week 0–52). The trial will be completed by a 12-week postinterventional observation period (figure 1).
Figure 1.
Trial design.
Trial conduct
Participants will be recruited within 12 months from the OA outpatients' clinic at the Parker Institute at Copenhagen University Hospital, Bispebjerg and Frederiksberg. General practitioners and collaborating clinical hospital departments in the Capital Region will be informed about the possibility to refer patients to the project. In addition, the trial will be advertised in newspapers and on the Parker Institutes website (www.parkerinst.dk).
Potential study participants will initially partake in a motivational assessment including an interview in which the nature of the initial IDI is explained together with a description of the overall study, a thorough outline of the interventions and visits, and a session in which the investigator addresses any questions the potential participant may have. The study will not use any standardised scoring system to assess motivation, but during the interview the investigator will assess the individual’s motivation for weight loss through both the IDI period and the subsequent participation in the randomised part of the study.
Before enrolment takes place, potential participants will be provided with written and verbal information about the trial and the procedures involved, in accordance with local requirements. Potential participants will have the opportunity to ask questions and have ample time to consider their participation. Following the signature of the informed consent form participants will be enrolled in the 8-week IDI period.
The IDI period comprises a supervised dietary weight loss programme in which participants receive a hypocaloric formula diet containing 800–1000 kcal/day. The formula diet consists of ready-to-use meal bars and powders to mix with water to make shakes, soups or porridge. The weight loss programme consists of an 8-week period with full meal replacement according to a standard liquid energy intake protocol. To facilitate adherence to the programme, patients will be scheduled for weekly facility-based group sessions with six to eight patients led by a dietician. The recommendations for daily nutrient intake will be met during this period.
Participants achieving a weight loss ≥5% during the IDI will be randomised to daily receive either liraglutide 3 mg/day or an identical placebo throughout the subsequent 52-week main trial period.
The initial 8 weeks of the main trial period consist of an 8-week TDI period (week 0–8) focusing on a partial re-introduction of regular meals in combination with formula diet products. In this period, all participants (irrespective of randomisation) will be scheduled to meet for group sessions led by a dietician every 2 weeks. No dietary consultancies will be offered from the trial after week 8, but to prevent attrition patients will be offered one to two daily meal replacements with a formula diet from week 8 to 52 to be administered by themselves. Participants will be instructed to aim for an intake of 1200 kcal/day from week 0 to 8 and for an intake of 1500 kcal/day from week 8 and onwards.
For the main trial period (drug intervention period running from week 0 to 52), participants will be randomised at week 0 to one of the two experimental arms described below:
- Liraglutide, 3 mg/day
- Arm description: subjects will be up-titrated to liraglutide 3 mg once daily and stay on that dose for the remainder of the 52-week drug intervention period.
- Drug: liraglutide 3 mg once daily administered in a 6 mg/mL, 3 mL pen for subcutaneous injection.
- Dose escalation/titration scheme: initial dosage of 0.6 mg/day, escalated biweekly by 0.6–3 mg/day over a total of 8 weeks. Visits will be conducted at weeks 0, 2, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and 52.
- Liraglutide placebo, 3 mg/day
- Arm description: subjects will be up-titrated to liraglutide 3 mg placebo once daily and stay on that dose for the remainder of the 52-week drug intervention period.
- Drug: liraglutide 3 mg placebo once daily administered in a 6 mg/mL drug equivalent volumes, 3 mL pen for subcutaneous injection.
- Dose escalation scheme: initial dosage of a 0.6 mg drug equivalent volume per day, escalated biweekly by a 0.6 mg drug equivalent volume per day to a 3 mg drug equivalent volume per day over a total of 8 weeks. Visits will be conducted at weeks 0, 2, 4, 6, 8, 12, 16, 20, 24, 28, 32, 36, 40, 44, 48 and 52.
Research nurses with experience in trials involving self-administered injections will instruct participants in the use of pens, and the materials used to support the verbal instructions will be the publicly available materials produced by Novo Nordisk for liraglutide. Dose escalation will be based on safety as well as tolerability and if dose escalation is not feasible, then delayed increments are allowed. Subjects will be maintained at the highest tolerated dose level and the reduction of the achieved maintenance dose will lead to patient discontinuation.
The trial will end when the last patient has i) completed the last visit as well as the 12-week postinterventional observation period, ii) prematurely discontinued the intervention or iii) withdrawn from the trial, whichever comes last.
For all potential trial participants, the following will be recorded: number of individuals initially assessed for eligibility, number excluded before enrolment (including reasons for non-eligibility), number enrolled, number randomised and number withdrawn/dropped out during the trial (including reasons for withdrawal or exclusion). From allocation and onwards study personnel will assess the use of study medication to evaluate adherence.
Prior to the start of the study, the sponsor-investigator will ensure that the other investigators are familiar with the protocol, electronic case report forms (eCRFs) and other study documents and procedures. The sponsor-investigator will be visited by the monitor prior to trial commencement and thereafter on a regular basis. The monitor will check trial procedures, including safety assessments, drug handling, data recording and complete source data verification procedures.
Visits, assessments and procedures will take place as visualised in table 1.
Table 1.
Visit schedule
| Information | Screening | Enrolment | Preallocation | - | - | - | - | - | - | - | Allocation | Postallocation | - | - | - | - | - | - | - | - | - | - | - | - | - | End of trial | - | Off-treatment follow-up | |
| Visit name | −Ty | −Tz | −Tx | −T8 | -T7 | -T6 | -T5 | -T4 | -T3 | -T2 | -T1 | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 | T13 | T14 | T15 | Tx | Tz |
| Week no. | X | X | X | −8 | −7 | −6 | −5 | −4 | −3 | −2 | −1 | 0 | 2 | 4 | 6 | 8 | 12 | 16 | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 48 | 52 | X | 64 |
| Information and screening | |||||||||||||||||||||||||||||
| Motivation appraisal | X | ||||||||||||||||||||||||||||
| Exchange of information | X | ||||||||||||||||||||||||||||
| Eligibility screening and consent | X | ||||||||||||||||||||||||||||
| X-ray and physical examination | X | ||||||||||||||||||||||||||||
| Blood testing and urine stick | X | ||||||||||||||||||||||||||||
| Medical and medication history | X | ||||||||||||||||||||||||||||
| KOOS pain, WOMAC pain, C-SSRS and PHQ-9 | X | ||||||||||||||||||||||||||||
| Vital signs, height, body weight and ECG | X | ||||||||||||||||||||||||||||
| Intervention | |||||||||||||||||||||||||||||
| IDI | X | X | X | X | X | X | X | X | |||||||||||||||||||||
| TDI | X | X | X | X | X | ||||||||||||||||||||||||
| Lira 3 mg OR Lira 3 mg pbo | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Assessments | |||||||||||||||||||||||||||||
| Physical examination | X | X | X | X | |||||||||||||||||||||||||
| Dietician (group session) | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||||
| Titration visit (MD) | X | X | X | X | X | (X) | (X) | ||||||||||||||||||||||
| Medical history | X | ||||||||||||||||||||||||||||
| Update of medical history | X | X | X | ||||||||||||||||||||||||||
| Medication history | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| KOOS, WOMAC, SF-36, IWQoL-lite and O-O resp. crit. | X | X | X | X | |||||||||||||||||||||||||
| ICOAP | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| KOOS pain and WOMAC pain | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||||
| C-SSRS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||
| PHQ-9 and BES | X | X | X | X | X | ||||||||||||||||||||||||
| TRIM-Weight | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||||
| Adverse events report form | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Vital signs and waist and hip circumferences | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||||||||||
| Body weight | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| Blood testing, height and DXA | X | X | X | X | |||||||||||||||||||||||||
| X-ray | X | X | X | ||||||||||||||||||||||||||
| ECG | X | X | X | X | X | ||||||||||||||||||||||||
| Medicine hand-out | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||||||||||||
| Adherence assessment | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X |
BES, Binge Eating Scale; C-SSRS, Columbia Suicide Severity Rating Scale; ICOAP, Intermittent and Constant Osteoarthritis Pain; IDI, intensive dietary intervention; IWQoL-Lite, Impact of Weight on Quality of Life-Lite; KOOS, Knee Injury and Osteoarthritis Outcome Score; Lira, liraglutide; O-O resp. crit., OMERACT-OARSI responder criteria; pbo, placebo; PHQ-9, Patient Health Questionnaire-9; SF-36, SF-36, Short Form 36; TDI, tapering dietary intervention; TRIM-Weight, Treatment-Related Impact Measure-Weight; WOMAC, Western Ontario and McMaster Universities Arthritis Index.
Trial site
The trial will be conducted at the Parker Institute at Copenhagen University Hospital, Bispebjerg and Frederiksberg. The Parker Institute is a well-established research institute and clinical department with secretariat, data managers and Good Clinical Practice (GCP) educated healthcare professionals such as physicians, trained specialists in rheumatology and radiology, nurses and laboratory technicians. Moreover, access to other departments and specialities within the hospital is available on request if deemed necessary.
Trial population
To be enrolled in this trial, the following eligibility criteria, assessed at screening, must be met:
Inclusion criteria
Informed consent obtained.
Clinical diagnosis of knee OA (American College of Rheumatology criteria) with early to moderate radiographic changes (Kellgren-Lawrence [KL] grades 1, 2 or 3).
Age≥18 years and <75 years.
Body mass index (BMI) ≥27 kg/m2.
Stable body weight during the previous 3 months (<5 kg self-reported weight change).
Motivated for weight loss.
Achieved at least 5% weight loss during the initial 8-week IDI (assessed at allocation visit; T0).
Exclusion criteria
Ongoing participation, or participation within the last 3 months, in an organised weight loss programme (or within the last 3 months).
Current or history of treatment with medications that may cause significant weight gain for at least 3 months before this trial.
Current use or use within 3 months before this trial of GLP-1 receptor agonist, pramlintide, sibutramine, orlistat, zonisamide, topiramate or phentermine.
Type 1 diabetes.
Type 2 diabetes treated with glucose-lowering drugs other than metformin.
Alloplasty in target knee joint (most symptomatic knee at screening).
End-stage disease in target knee joint (KL grade 4).
Immuno-inflammatory disease.
Chronic widespread pain.
Pregnancy or insufficient anticonception therapy for female fertile patients.
Breast feeding.
Estimated glomerular filtration rate <60 mL/min/1.73 m2.
Alanine aminotransferase (ALT) or aspartate aminotransferase>3×above upper normal range.
Elective surgery scheduled during the trial duration period, except for minor surgical procedures.
Surgical procedures such as arthroscopy or injections into a knee within 3 months prior to enrolment.
Previous surgical treatment for obesity (excluding liposuction >1 year before trial entry).
Thyroid-stimulating hormone outside of the range of 0.4–6.0 mIU/L.
Obesity secondary to endocrinological or eating disorders, or to treatment with medicinal products that may cause weight gain.
Family or personal history of medullary thyroid carcinoma or multiple endocrine neoplasia type 2.
Inflammatory bowel disease.
Congestive heart failure, New York Heart Association class III–IV.
Diabetic gastroparesis.
History of or current diagnosis of pancreatitis (acute and/or chronic) or pancreatic cancer.
History of cancer with the exception of in situ malignancies of the skin or cervix uteri.
History of major depressive disorder, a Patient Health Questionnaire-9 (PHQ-9) score of >15 or a history of other severe psychiatric disorders or diagnosis of an eating disorder.
Subjects with a lifetime history of a suicide attempt or history of any suicidal behaviour within the past month before entry into the trial.
Inability to speak Danish fluently.
A mental state impeding compliance with the programme.
Use of opioids or similar strong analgesics.
Allergic reactions to the active ingredients of Saxenda, such as hypotension, palpitations, dyspnoea and oedema.
Data collection
Patients will take part in a series of examinations and tests throughout the study, as mentioned below.
Knee Injury and Osteoarthritis Outcome Score
The Knee Injury and Osteoarthritis Outcome Score (KOOS) is designed to assess health-related quality of life (HRQoL) in patients with knee injuries and knee OA.12 The KOOS consists of 42 items covering five domains, namely, pain, symptoms, activities of daily living, sports and recreation and knee-related QoL. A normalised score is calculated for each domain with 100 indicating no symptoms and functional impairment and zero indicating extreme symptoms and functional impairment.
Western Ontario and McMaster Universities Arthritis Index
Western Ontario and McMaster Universities Arthritis Index (WOMAC) is a disease-specific questionnaire designed to assess pain, stiffness and physical function in patients with hip and/or knee OA.13 It consists of 24 items divided into three subscales concerning pain, stiffness and physical function. Higher scores on the WOMAC indicate worse pain, stiffness and functional limitations.
Intermittent and Constant Osteoarthritis Pain Questionnaire
The Intermittent and Constant Osteoarthritis Pain (ICOAP) is a diagnosis-specific 11-item questionnaire designed to assess the pain experience within the last week among people suffering from knee and hip OA.14 The questionnaire is divided into two domains, a 5-item scale for constant pain and a 6-item scale for intermittent pain. Normalised scores, for the two subscales and for the total pain score, ranges from zero (no pain) to 100 (extreme pain).
Columbia Suicide Severity Rating Scale
The Columbia Suicide Severity Rating Scale (C-SSRS) is a detailed questionnaire assessing both suicidal behaviour and suicidal ideation. Four constructs are assessed.15 The first is the severity of ideation, rated on a 5-point ordinal scale. The second is the intensity of ideation subscale comprising five items each rated on a 5-point ordinal scale. The third is the behaviour subscale, which is rated on a nominal scale. And the fourth is the lethality subscale, which is rated on a 6-point ordinal scale, and if actual lethality is zero, potential lethality of attempts is rated on a 3-point ordinal scale. The Baseline-Screening version will be employed to assess inclusion/exclusion criteria and to provide a pretreatment assessment at baseline. On all subsequent visits, the Since Last Visit version will be used.
Patient Health Questionnaire
The PHQ-9 is a diagnostic tool for mental health disorders.16 The PHQ-9 is the depression subscale of the PHQ and contains nine questions related to depression disorder symptoms during the past 14 days. The answer categories are based on a 4-point response scale and the summed PHQ-9 score can range from zero to 27. A score of ≥15 is considered an indication of moderately severe or severe depression.
Binge Eating Scale
The Binge Eating Scale (BES) is a self-report instrument that assesses the behavioural and emotional/cognitive symptoms associated with binge eating.17 The BES comprises 16 items assessing key behavioural (eg, rapid eating, eating large amounts of food) and affective/cognitive symptoms (eg, guilt, feeling out of control or unable to stop eating) that precede or follow a binge. Each item contains three to four statements that are weighted response options, which reflect a range of severity for each characteristic. Participants are asked to select the statement that best describes their experience. The scale’s possible total scores range from zero to 46, with higher scores indicating more severe binge eating symptoms.
Outcome Measures in Rheumatoid Arthritis Clinical Trials/Osteoarthritis Research Society International (OMERACT-OARSI) responder criteria
The Outcome Measures in Rheumatoid Arthritis Clinical Trials/Osteoarthritis Research Society International responder criteria include a composite index based on pain, function and patient global.18 For the purpose of assessing this, we will employ three questions regarding knee pain, physical function and the patients’ global assessment of disease impact on their daily life. The answers to each of these questions are given on 100 mm visual analogue scales (VAS).
To assess pain, the patients are asked to indicate ‘the degree of knee pain in your daily life’ (VAS-pain). The anchors on the 100 mm VAS are 0=‘no pain’ and 100=‘worst imaginable pain’. To assess physical function, the patients are asked to indicate ‘the degree of physical impairment of your knee in your daily life’ (VAS-function). The anchors on the 100 mm VAS are 0=‘no impairment’ and 100=‘worst imaginable impairment’. To assess the patients’ global assessment of disease impact on their daily life, the patients are asked to indicate ‘the overall impact of your knee OA on your daily life’ (VAS-global). The anchors on the 100 mm VAS are 0=‘no impact’ and 100=‘worst imaginable impact’.
Categorisation as a responder requires an improvement in the above-mentioned pain or function VAS scores of at least 50% and an absolute change of 20 mm. Alternatively, a response can be achieved by meeting at least two of the following three criteria: (1) an improvement of 20% and an absolute change of 10 mm in VAS-pain, (2) an improvement of 20% and an absolute change of 10 mm in VAS-function or (3) an improvement of 20% and an absolute change of 10 mm in VAS-global.
Impact of Weight on Quality of Life-Lite
The Impact of Weight on Quality of Life-Lite (IWQoL-Lite) is a 31-item, self-report, obesity-specific measure of HRQoL that consists of a total score and scores on each of five scales: physical function, self-esteem, sexual life, public distress and work.19 Scores range from zero to 100, where 100 represent the best HRQoL and zero represents the worst.
Treatment-Related Impact Measure-Weight
The TRIM-Weight is an obesity treatment-specific patient-reported outcomes measure designed to assess the key impacts of prescription antiobesity medication and be applicable to the wide range of prescription medications currently available.20 The TRIM-Weight is based on 22 items within seven thematic domains related to a patient’s experience with a weight loss medication: i) satisfaction in terms of weight loss, ii) the burden of taking the medication, iii) satisfaction in terms of appetite-control, iv) the impact of weight stabilisation, mood swings or tiredness, v) convenience, vi) discomfort and vii) impact on social aspects, productiveness and relationships. All items are scored on anchored rating scales with five levels of response (1–5) in which higher scores indicate better quality of life.
Short Form 36
The Short Form 36 (SF-36) is a generic, short-form health status questionnaire composed of 36 questions within eight multi-item domains assessing physical function, social function, role-emotional, role-physical, bodily pain, general health, mental health and vitality.21 These can be combined into two summary scores (physical and mental health summary scores). For each summary score, the ordinal scores are transformed to a linear zero to 100 scale; zero indicating the least favourable health state and 100 indicating the best state of health.
Anthropometrics
Waist circumference will be measured mid-way between the lower rib margin and the iliac crest, while hip circumference will be measured at the point over the buttocks yielding the maximum circumference. Waist and hip circumferences will be measured to the nearest 1 cm. All anthropometric measurements will be taken in accordance with the WHO report on measuring obesity.
Height without shoes will be measured using a stadiometer and rounded to the nearest 1 cm.
Body weight will be measured to the nearest 0.1 kg with a decimal weighing scale (TANITA BW-800, Tanita Europe BV Hoogoorddreef 56e, 1101BE Amsterdam, The Netherlands), with participants fasting and wearing underwear or light clothing, only.
The BMI (kg/m2) will be calculated from body weight and height.
Blood samples
Standard biochemistry: C reactive protein (CRP), ALT, calcium, creatinine, potassium, sodium, low-density lipoprotein (LDL), high-density lipoproteins (HDL), triglycerides (TG) and total cholesterol (TC). Haematology: haemoglobin, leucocytes, differential cell count and thrombocytes. Glucose metabolism: haemoglobin A1c (HbA1c) and fasting plasma glucose (FPG).
Radiography
Radiographic examinations include standard clinical semi-flexed weight-bearing posterior-anterior radiographs of both knees. During the examination, patients will be facing the plate of the radiography equipment with the knees touching the cassette holder or a reclining table top. The radiography plate or cassette holder is placed so that the centre of the film will be at the level of the patient’s tibiofemoral joint line. The radiography beam is centred between the knees with a 10 degrees cranio-caudal angle and the body weight will be distributed equally between the two legs. The radiographs will be assessed by use of the KL grading system; a categorical grading scale of knee OA going from 0 to 4 by means of an evaluation of osteophytes, joint space narrowing, sclerosis and altered bone shapes.
Vitals signs
Blood pressure (BP), systolic and diastolic, will be measured with the patient in sitting position with the legs uncrossed and the back and arm supported. Patients resting pulse will be measured following a resting period of 5 min in a sitting position with the legs uncrossed and the back and arms supported. Patients will be instructed to avoid caffeine, smoking and physical activity within 30 min prior to both of these measurements.
Visit schedule
Outcomes
Patient characteristics
Height, age, gender and KL grading will be collected and reported as patient characteristics.
Co-primary outcomes
The co-primary outcomes are changes in body weight and the KOOS pain subscale from baseline (week 0) to the last visit in the main trial period (week 52).
Confirmatory secondary outcomes
The confirmatory secondary outcomes are changes in the KOOS symptom, ADL, sport and recreation and HRQoL subscales, the WOMAC pain, stiffness and function subscales, the total score and subscales in the ICOAP questionnaire, BMI, waist circumference or the waist/hip ratio from baseline (week 0) to the last visit in the main trial period (week 52). Moreover, the proportion of patients with ≥5% or ≥10% weight loss at the last visit in the main trial period (week 52) also constitutes confirmatory secondary outcomes.
Supportive secondary outcomes
The supportive secondary outcomes are changes in biomarkers (CRP, HbA1c, FPG, ALT, LDL, HDL, TG and TC), BP, resting heart rate, the impact of weight on quality of life (IWQoL-Lite), treatment-related impact on quality of life (TRIM-Weight) and the general health status (SF-36) from baseline (week 0) to the last visit in the main trial period (week 52). Changes in proportion of patients meeting the criteria for metabolic syndrome or prediabetes from week 0 to 52 as well as the proportion of patients classified as responders (the O-O responder criteria) at the last visit in the main trial period (week 52) also constitutes confirmatory secondary outcomes.
Safety outcomes
The safety outcomes include the incidence of adverse events (AEs), suicidal behaviour and/or ideation (C-SSRS), depression (PHQ-9), binge eating (BES) and measures outside reference limits for haemoglobin, thrombocytes, leucocytes, differential cell count, creatinine and electrolytes (±2 SD) and for ALT and CRP (+150%).
Data management
The collection, preservation and dissemination of the clinical data is specified in this clinical trial protocol and abide by the standard requirements for GCP-compliant data management in clinical trials.
The source data and documents, eCRF, protocol and amendments, drug accountability forms, correspondence, patient identification list, informed consent forms and other essential GCP documents will be retained for at least 10 years after the study is completed at the study site. Data entries are quality ensured by double data entry, classification of data type (ie, text and numbers) and/or range checks for data values.
All data collected during this project will be managed and quality certified by the Parker Institutes data management team, composed by the primary investigator, a data manager and a chief administrator. This team is responsible for ensuring data completeness and accuracy as well as source data verification. The latter will be performed by the monitor and the relevant investigators. The team is also responsible for ensuring operations of a secure database established for the collection of clinical data collected via the eCRF platform through a secure connection. All data obtained during the study will be documented in the individual eCRFs. Reasons for any missing data will be noted in the database, and logging and tracking of data changes will be documented.
Randomisation
Participants will be randomised to treatment daily with liraglutide 3 mg/day or liraglutide placebo 3 mg at week 0, that is, after the initial 8-week IDI period. Participants will be randomised in a 1:1 manner to receive either 3 mg/day liraglutide or identically appearing placebo; stratified randomisation will be based on gender (male vs female), age (<60 years vs ≥60 years) and obesity class (BMI <40 vs ≥40 kg/m2) status at trial enrolment (week −8). A computer-generated randomisation sequence will be produced using SAS PROC PLAN to generate the eight randomisation schedules before any participant is enrolled, allocating participants in permuted blocks of two to six to the daily liraglutide 3 mg/day or placebo group (1:1). The randomisation sequence is entered into the eCRF by a data manager.
Allocation concealment and blinding
The trial uses a computer-generated allocation process in which the patient identifier is coupled to one of the experimental arms when the physician clicks on the ‘randomisation button’, appearing at visit T0 in the eCRF system. On allocation to one of the two experimental arms the patient identifier is automatically coupled to specific pens, each of them labelled by a single and unique Dispensing Unit Number. The entire process is blinded for all investigators, clinical, academic and administrative trial personnel.
Unblinding will only take place in exceptional circumstances when knowledge of the actual treatment is essential for further management of the patient. If unblinding is deemed necessary, the investigator will activate a data solution, within the eCRF, build for this specific purpose and controlled by an independent data manager and the chief administrator. The actual allocation will not be disclosed to the patient and/or other study personnel including other site personnel, monitors, corporate sponsors or project office staff.
Sample size and power considerations
The co-primary outcomes are changes in body weight and KOOS pain from randomisation to the end of the trial, 52 weeks after randomisation. The sample size of 150 is designed to provide a reasonable power (>80%) to detect a 5 kg difference in body weight change between the groups, and an 8-units difference in the KOOS pain. All power and sample size analyses were conducted using ’SAS Power and Sample Size', V.3.1 (SAS Institute, Cary, North Carolina, USA):
Body weight: for a two-sample pooled t-test of a normal mean difference with a two-sided significance level of 0.05 (p<0.05), assuming a common SD of 10 kg (conservatively estimated based on our previous weight loss trial in this patient population22), a sample size of 75 per group, has a power of 92% to detect a mean difference of 5.5 kg in the group mean change in body weight.
KOOS pain: for a two-sample pooled t-test of a normal mean difference with a two-sided significance level of 0.05 (p<0.05), assuming a common SD of 15 KOOS points, a sample size of 75 patients per group has a power of 90% to detect a mean difference in the group mean changes of 8 KOOS points (corresponding to a moderate Cohen’s effect size of 0.5).
The combined power for the two end points is 83%.
Statistical methods
The prespecified efficacy analyses will be based on the data from the full-analysis set, that is, the intention-to-treat (ITT) population—including all participants who are randomised and assessed at baseline. In case of missing data at week 52, the observation from enrolment will be carried forward in case of missing data in the ITT population. The safety analysis set includes all patients who are randomly assigned to a trial group and have had exposure to a trial drug (ie, liraglutide 3 mg/day or placebo). This trial does not plan for any interim analyses.
The primary analysis will be the comparison between liraglutide and placebo using an analysis of covariance (ANCOVA) model with treatment, gender, age and obesity stratification as fixed effects and with adjustment for the level at baseline as a covariate. From this model, the observed differences in weight change and KOOS pain change between liraglutide treatment and placebo will be estimated together with the associated 95% CI and the p value corresponding to the test of the hypothesis of no difference between treatments (ie, the null hypothesis).
To establish the efficacy of liraglutide 3 mg/day compared with placebo in patients with overweight or obesity and knee OA, inducing and maintaining weight loss and pain relief over 52 weeks, end points are tested in a hierarchical manner in the order in which the end points are presented. Liraglutide 3 mg/day will be considered statistically significantly better than liraglutide placebo 3 mg/day placebo with respect to change in body weight if the null hypothesis is rejected (p<0.05). But, overall, liraglutide 3 mg/day will only be confirmed as statistically significantly better than placebo with respect to change in KOOS pain subscale, if it is also statistically significantly better with respect to change in body weight.
Categorical changes for dichotomous end points will be analysed with the use of logistic regression with the same fixed effects and covariates as the respective ANCOVA. Sensitivity analyses will be performed to assess the robustness of the primary analyses, including ‘per-protocol’ scenarios, repeated-measures linear mixed models and multiple imputation techniques.
Management
The project management team consists of the Primary Investigator, Dr Henrik Rindel Gudbergsen who is responsible for the execution of the project, the Sponsor-Investigator Professor Henning Bliddal who is responsible for the overall scientific planning of the project and Chief Administrator Claus Bomhoff who is responsible for administrative and financial tasks. The steering committee (SC), led by the Primary Investigator, is the principal management body with respect to the operational and scientific facets of this trial. Members of the SC are the investigators involved in the development of this protocol and include HG (chairman), HB (sponsor), MH, EEW, HB, RC, MB, FKKK, AA, MUR and LEK. The SC will ensure trial resources, schedule all trial-related activities and ensure execution of the trial.
The independent ethics committee (ICE) monitors any safety and/or ethical concerns arising during the project and to advise the SC. The members of the ICE are Lennart Jacobsson, Professor in Rheumatology, Department of Rheumatology, Sahlgrenska University Hospital, Gothenburg, Sweden and Karl Agner Kristensen MD, PhD, Specialist in Obstetrics and Gynaecology, Department of Obstetrics and Gynaecology, Lund University Hospital, Lund, Sweden.
Patient and public involvement
Via a formal review process, the authors retrieved input from an appointed knee OA patient advisor in a discussion focusing on the development of hypotheses, interventions and outcomes related to this study. The design of the study was not discussed with patients, whereas the burden of the study was assessed by all patients via an initial appraisal of their motivation to participate in the study and via a thorough description of the study in relation to the signing of the informed consent.
The institute’s patient board and the appointed knee OA advisor were also involved in proposing potential routes for communication regarding recruitment of patients, including websites and patient associations. Patients will be informed, via dialogue and a briefing document, that they may access results on an individual basis throughout the trial and that the study personnel will engage in presenting the overall results for each individual patient once the trial is complete. On trial completion, patients will also be invited to a meeting where the project results are presented in a manner that is understandable by laymen.
Ethics and dissemination
The investigator will monitor each participant for clinical and laboratory evidence of AEs on a routine basis throughout the trial. AEs, whether in response to a query, observed by site personnel or reported spontaneously by the participant, will be recorded. The investigator will assess and record any AE in detail, including the date of onset, description, severity, duration and outcome, relationship of the AE to trial drug and any action(s) taken.
The treatment and investigations in this trial are associated with minimal discomfort for the participants. The injection is practically pain-free but may leave a small haemorrhage, resolving spontaneously within a few days in the vast majority of patients. Less commonly, the patients may experience abdominal pain, insomnia, reflux, gastritis and dizziness. Uncommon AEs comprise dehydration, tachycardia, pancreatitis, cholecystitis, urticarial and malaise.
When collecting blood samples some participants may experience minor discomfort when the needle penetrates the skin, and rarely a small bleeding occurs. The planned radiographs will be identical and obtained at the same frequency as recommended in the current care model at the involved outpatient clinics.
The participants included in the planned clinical trial will not receive any financial compensation. Neither the sponsor-investigator nor any of the other members of the project group has financial interest in neither the conduct nor the results of the trial.
All participants will be covered by a patient-insurance, according to national requirements and common conduct, during the conduction of the clinical trials.
The successful planning and conduction of this trial may provide the basis for a significant improvement in the disease management of the many overweight citizens impacted by knee OA.
The number and timing of visits has been outlined to ensure observation of any safety issues as well as thorough management of medication handout and usage throughout the study.
Based on involvement of patients as well as the existing experience within the field of weight loss and knee OA management, the study design is considered to be acceptable for patients as well as feasible to implement. Nevertheless, this study will deliver comprehensive insights into the practicality and acceptability of the interventions studied in this specific context, and provide valuable information regarding the generalisability of the interventions in question.
At the end of the trial, one or more manuscripts will be prepared for publication in peer-reviewed journals. The manuscripts will be written in accordance with the Consolidated Standards of Reporting Trials statement. The manuscripts will include positive, negative as well as inconclusive results. In addition, the results from the trial will be presented as posters or oral presentations at national and/or international conferences.
Novo Nordisk and The Cambridge Weight Plan had no influence on the study design, nor will they have any influence on the analyses and interpretation of data or the writing of manuscripts/abstracts based on trial data. Novo Nordisk and The Cambridge Weight Plan will be given 4 weeks to review any manuscript/abstract or other means intended for publication or presentation of the data. All authors will qualify for authorship according to International Committee of Medical Journal Editors, 1997, and must have participated sufficiently in the work to take public responsibility for the content.
Supplementary Material
Acknowledgments
The authors would like to thank the Parker Institutes patient board, the institutes’ knee OA patient advisor and the study personnel involved in executing this trial.
Footnotes
Contributors: HG, EEW, MH, HB, RC, MPB, FKK, MH, AA, EMB and LEK have contributed by conceptualising, designing, writing, reviewing and approving the protocol for this trial. AO, MUR, CB, CD, BLH and KE have contributed by writing, reviewing and approving the protocol and are all part of the acquisition of data from the trial. Moreover, all authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Funding: This work was supported by Novo Nordisk A/S, both financially and through the delivery of active and placebo medicine, and by The Cambridge Weight Plan through the delivery of dietary supplements. The trial is an investigator-initiated study, initiated by the primary investigator Henrik Gudbergsen and the sponsor-investigator Henning Bliddal. In addition, this work was supported by a core grant from the Oak Foundation (OCAY-13-309) given to the Parker Institute, Copenhagen University Hospital, Bispebjerg and Frederiksberg.
Competing interests: HG has received speaker fees from Pfizer and MSD. MB has received speaker fees from Esaote, AbbVie and UCB. FKK has received lecture fees from, participated in advisory boards of and/or consulted for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD/Merck, Novo Nordisk, Sanofi and Zealand Pharma. AA is member of advisory boards/consultant for Acino, Switzerland, BioCare Copenhagen, DK, Gelesis, USA, Groupe Éthique et Santé, France, McCain Foods Limited, USA, Novo Nordisk, DK, Pfizer, USA, Saniona, DK, Weight Watchers, USA and Zaluvida, Switzerland. He is recipient of travel grants and honoraria as speaker for a wide range of Danish and international concerns. Commercial interests: co-owner and member of the Board of the consultancy company Dentacom Aps, Denmark (2005). Co-founder and co-owner of UCPH spin-outs Mobile Fitness A/S (2005), Flaxslim ApS (were also member of Board, 2015, and Personalized Weight Management Research Consortium ApS (Gluco-diet.dk, 2017). Co-inventor of a number of patents owned by UCPH, in accordance with Danish law. AA is not advocate or activist for specific diets, and is not strongly committed to any specific diet, for example, veganism, Atkins diet, gluten-free diet, high animal protein diet or dietary supplements. LEK has received fees for speaking and consultancy from Pfizer, AbbVie, Amgen, Sanofi, UCB, Celgene, BMS, Biogen, Novo Nordisk, MSD, Novartis, Eli Lilly and Janssen Pharmaceuticals.
Ethics approval: The trial is approved by the regional ethics committee in the Capital Region of Denmark; approval ID H-16019969.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: Once the trial has been completed data and information about the study may be accessed by contacting the corresponding author after obtaining and documenting legitimate approval from the Danish data authorities and to the extent possible according to Danish national law.
Correction notice: This article has been corrected since it was published. Author name has been changed from Filip K Krag Knop to Filip Krag Knop.
Patient consent for publication: Not required.
References
- 1. Hawkes C, Jewell J, Allen K. A food policy package for healthy diets and the prevention of obesity and diet-related non-communicable diseases: the NOURISHING framework. Obes Rev 2013;14:159–68. 10.1111/obr.12098 [DOI] [PubMed] [Google Scholar]
- 2. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes 2015;39:1188–96. 10.1038/ijo.2015.59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Felson DT, Lawrence RC, Dieppe PA, et al. Osteoarthritis: new insights. Part 1: the disease and its risk factors. Ann Intern Med 2000;133:635–46. 10.7326/0003-4819-133-8-200010170-00016 [DOI] [PubMed] [Google Scholar]
- 4. Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis 2001;60:91–7. 10.1136/ard.60.2.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coggon D, Reading I, Croft P, et al. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord 2001;25:622–7. 10.1038/sj.ijo.0801585 [DOI] [PubMed] [Google Scholar]
- 6. Bliddal H, Leeds AR, Christensen R. Osteoarthritis, obesity and weight loss: evidence, hypotheses and horizons - a scoping review. Obes Rev 2014;15:578–86. 10.1111/obr.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double-blind trial. Lancet 2017;389:1399–409. 10.1016/S0140-6736(17)30069-7 [DOI] [PubMed] [Google Scholar]
- 8. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 2012;64:465–74. 10.1002/acr.21596 [DOI] [PubMed] [Google Scholar]
- 9. Fernandes L, Hagen KB, Bijlsma JW, et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann Rheum Dis 2013;72:1125–35. 10.1136/annrheumdis-2012-202745 [DOI] [PubMed] [Google Scholar]
- 10. McAlindon TE, Bannuru RR, Sullivan MC, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage 2014;22:363–88. 10.1016/j.joca.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 11. Zhang W, Nuki G, Moskowitz RW, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage 2010;18:476–99. 10.1016/j.joca.2010.01.013 [DOI] [PubMed] [Google Scholar]
- 12. Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes 2003;1:64 10.1186/1477-7525-1-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 1988;15:1833–40. [PubMed] [Google Scholar]
- 14. Hawker GA, Davis AM, French MR, et al. Development and preliminary psychometric testing of a new OA pain measure--an OARSI/OMERACT initiative. Osteoarthritis Cartilage 2008;16:409–14. 10.1016/j.joca.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kessler S, Grammozis A, Günther KP, et al. [The intermittent and constant pain score (ICOAP) - a questionnaire to assess pain in patients with gonarthritis]. Z Orthop Unfall 2011;149:22–6. 10.1055/s-0030-1249967 [DOI] [PubMed] [Google Scholar]
- 16. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry 2011;168:1266–77. 10.1176/appi.ajp.2011.10111704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gormally J, Black S, Daston S, et al. The assessment of binge eating severity among obese persons. Addict Behav 1982;7:47–55. 10.1016/0306-4603(82)90024-7 [DOI] [PubMed] [Google Scholar]
- 19. Kolotkin RL, Crosby RD, Kosloski KD, et al. Development of a brief measure to assess quality of life in obesity. Obes Res 2001;9:102–11. 10.1038/oby.2001.13 [DOI] [PubMed] [Google Scholar]
- 20. Brod M, Hammer M, Kragh N, et al. Development and validation of the Treatment Related Impact Measure of Weight (TRIM-Weight). Health Qual Life Outcomes 2010;8:19 10.1186/1477-7525-8-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;30:473–83. [PubMed] [Google Scholar]
- 22. Christensen R, Henriksen M, Leeds AR, et al. Effect of weight maintenance on symptoms of knee osteoarthritis in obese patients: a twelve-month randomized controlled trial. Arthritis Care Res 2015;67:640–50. 10.1002/acr.22504 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.