Abstract
Objectives
Treating acute decompensated heartfailure (ADHF) for improving congestion with diuretics may cause worsening renal function (WRF), but the clinical efficacy of tolvaptan add-on therapy on reducing WRF in ADHF patients is inconsistent. This analysis is to evaluate the effects of tolvaptan add-on therapy on reducing WRF in ADHF patients.
Methods
Meta-analysis of randomised trials of tolvaptan add-on therapy on reducing WRF in ADHF patients. The MEDLINE, Embase and Cochrane Central Register of Controlled Trials databases were searched for relevant articles from their inception to 31 October, 2017. Two reviewers filtrated the documents on WRF, short-term all-cause mortality, body weight decreased, elevated sodium level for calculating pooled relatives risks, weighted mean difference and associated 95% CIs. We used fixed-effects or random-effects models according to I2 statistics.
Achievements
Seven random controlled trials with 937 patients were included for analysis. Compared with the control, tolvaptan add-on therapy did not improve incidence of worsening renal function (RR 0.78, 95% CI 0.48 to 1.26, p=0.31, I2=66%) and short-term all-cause mortality (RR 0.85, 95% CI 0.47 to 1.56, p=0.61, I2=0%). On subgroup analyses, there was a suggestion of possible effect modification by dose of tolvaptan, in which benefit was observed in low-dose (≤15 mg/day) group (RR 0.48, 95% CI 0.23 to 1.02, p=0.05, I2=54%), but not with high-dose (30 mg) group (RR 1.33, 95% CI 0.99 to 1.78, p=0.05, I2=0%). However, tolvaptan add-on therapy reduced body weight in 2 days (standardised mean difference −0.49, 95% CI −0.64 to −0.34, p<0.00001, I2=0%), increased sodium level (mean difference 1.56, 95% CI 0.04 to 3.07, p=0.04, I2=0%).
Conclusion
The result suggests that comparing with the standard diuretic therapy, tolvaptan add-on therapy did not reduce the incidence of WRF and short-term mortality, however, it can decrease body weight and increase the sodium level in patients who are with ADHF. Further researches are still required for confirmation.
Keywords: tolvaptan, worsening renal function, acute decompensated heart failure, meta-analysis
Strengths and limitations of this study.
Increased the worsening renal function of tolvaptan in patients with acute decompensated heart failure.
Tolvaptan was not reducing the incidence of worsening renal function or short-term all-cause mortality, however, it decreases the body weight while increases the sodium level.
Only seven randomised controlled studies were included, however, some studies have limitations.
Lack of unified standards for the dosage, the tolvaptan use duration and follow-up time, which may affect the clinical outcomes.
Only English language studies included.
Introduction
Congestion is the primary reason for patients hospitalisation with acute decompensated heart failure (ADHF). Despite in-patient use of diuretics and vasodilators targeting decongestion, congestion is persistent in many ADHF patients at hospital discharge and has been associated with increasing morbidity and mortality.1 Currently, various types of therapeutic agents are used for heart failure (HF) as the standard treatment which includes diuretics, angiotensin-receptor blockers, angiotensin-converting enzymes inhibitors and beta-blockers. These drugs are still playing an important role in the treatment of HF patients. Diuretics is the therapy cornerstone for the treatment of congestion, which is an important component of ADHF treatment for improving oxygenation and relieving the symptoms of oedema, despite the potential adverse effects related to renin angiotensin aldosterone system activation, electrolyte disturbances and worsening renal function.2
Arginine-vasopressin (AVP) controls the body water’s content and blood pressure by affecting water excretion rate through kidney.3 AVP is secreted from the posterior pituitary in response to elevation in plasma osmolality and the decreases in arterial pressure.4 AVP causes water retention through the V2 receptor to maintain the blood pressure. In patients with HF, contributing to such symptoms as oedema, dyspnoea and congestion,5 the level of AVP in increased. The fatal disadvantages of loop diuretic treatment for patients with ADHF are activating neurohumoral factors and worsening renal function (WRF).6 WRF was defined as an increase in serum creatinine of 0.3 mg/dL from baseline within 7 days from admission. Tolvaptan is an orally active, non-peptide, selective V2 receptor antagonist. Selective AVP V2 receptor antagonists induce hypotonic diuresis without significantly influencing the excretion of electrolytes.7 Tolvaptan has been mentioned in many studies. Tolvaptan benefits patients with symptomatic HF in reducing body weight, increasing urine volume and serum sodium, but without worsening renal function.8 9 Previous studies and meta-analysis have demonstrated that in ADHF patients, early administration of oral tolvaptan should be combined with standardise therapy, including conventional diuretics, improved heart failure signs and symptoms without serious events.10–13 The purpose of this study is to conduct a meta-analysis of randomised control trials (RCTs) focusing on the renal effects of tolvaptan in patients with ADHF in comparison with the effects of other traditional diuretic agents.
Methods
This meta-analysis was conducted in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement.14
Search procedure
We searched the MEDLINE, Embase and Cochrane Central Register of Controlled Trials databases from the date of their inception to 31 October, 2017, with no language restrictions. We used the combinations of the terms like, ‘Tolvaptan’, ‘vasopressin V2-receptor blocker’, ‘Acute Heart Failure’, ‘Acute Decompensated Heart Failure’ as the test words and as Medical Subject Headings (MESH) headings. The MEDLINE search strategy is available to view (see online supplementary appendix 1). All articles were available until 31 October, 2017. Relevant studies were identified from the reference lists of selected articles and review articles.
bmjopen-2018-025537supp001.pdf (34KB, pdf)
Study selection
Randomised controlled trials of tolvaptan add-on therapy comparing with traditional therapy or other diuresis agents in patients with evidence of ADHF were included with constraints on the time period till 31 October, 2017. The processes of selection, data extraction and quality assessment were independently executed by two reviewers. Disagreement was solved by reviewing the relevant studies to reach consensus.
Inclusion criteria
The inclusion criteria for the studies are as follows; it should, (1) be a randomised controlled trial, (2) include participants who are adult patients with ADHF and defined as patients had dyspnoea at rest requiring urgent hospital admission for evaluation and treatment, (3) compare tolvaptan add-on therapy with traditional diuretics agents and (4) include any relevant outcomes: all-cause mortality, WRF, sodium level, body weight reduction and fluid loss.
Exclusion criteria
The exclusion criteria are as follows: (1) observational study and (2) study on CHF or not reporting the desired outcome.
Data extraction
Data extraction from reports was processed in line with the protocol, by the reviewers; disagreements were resolved by negotiations. Attempts to contact all investigators were made to obtain raw data or to confirm details of the study design for all included trials. However, these attempts were not always successful as expected.
For each of the trials included in the review, the following characteristics were recorded: (1) First author’s surname, (2) Year of publication, (3) Country where the study was performed, (4) Study design and characteristics, (5) Total number of participants, (6) Inclusion and exclusion criteria, (7) Details about intervention arm, (8) Details about traditional/control arm, (9) Dose of tolvaptan, (10) Treatment duration, (11) Primary outcome evaluated, (12) Other outcome variables evaluated and (13) Quality indicators.
Quality and risk of bias of included trials
The quality of the included trials and the risk of bias were assessed by two independent reviewers using the components described by the Cochrane Collaboration,15 including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. Disagreements were resolved by negotiation.
Statistical analysis
All of the meta-analytic procedures were conducted by Review Manager, V.5.3. Two-tailed p values <0.05 were regarded as statistically significant. We used Q statistics, the related p values, and the I2 statistic to investigate the heterogeneity of each study. I2 statistic is a quantitative measure describing the percentage of total variations due to heterogeneity. The extracted I2 statistic value was used to assess the heterogeneity of each variable across the study. According to the Cochrane Handbook,16 heterogeneity of variables is indicating significant heterogeneity when the I2 range from 50% to 90%. Therefore, an I2 of <50% is considered acceptable. If the research results were not statistically different, the fixed effect model would be used for meta-analysis. If there is a statistical heterogeneity among the research results, the sources of heterogeneity will need further analysis. After excluding the obvious clinical heterogeneity, the random effects model was exploited in analysing the meta.
Patient and public involvement
No patients were directly involved in the development of the research question, selection of the outcome measures, design and implementation of the study or interpretation of the results.
Achievements
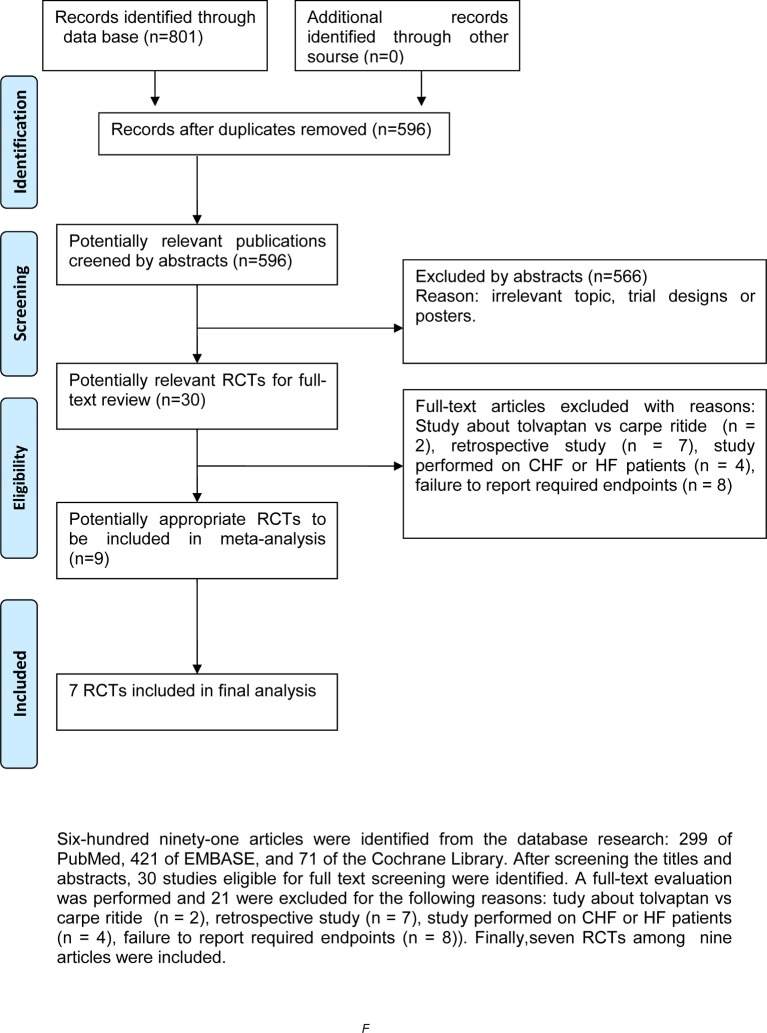
In total, 801 articles and documents were identified from the database research: 299 of PubMed, 421 of EMBASE and 71 of the Cochrane Library. By screening titles and abstracts, 566 apparently irrelevant articles were first excluded. Then, the detailed full texts of remainders were downloaded to assess. A full-text evaluation was performed and 21 of them were excluded for they are studies on: tolvaptan versus carperitide17 18(n=2), retrospective studies (n=7), study articles19–21 defined as one randomised controlled study.20 Finally, there are seven RCTs12 20 22–26 among nine articles included. The flow diagram of study selection is shown in figure 1.
Figure 1.
Flow diagram of study selection. CHF, congestive heart failure; HF, heart failure; RCTs, randomised control trials.
Study characteristics and quality
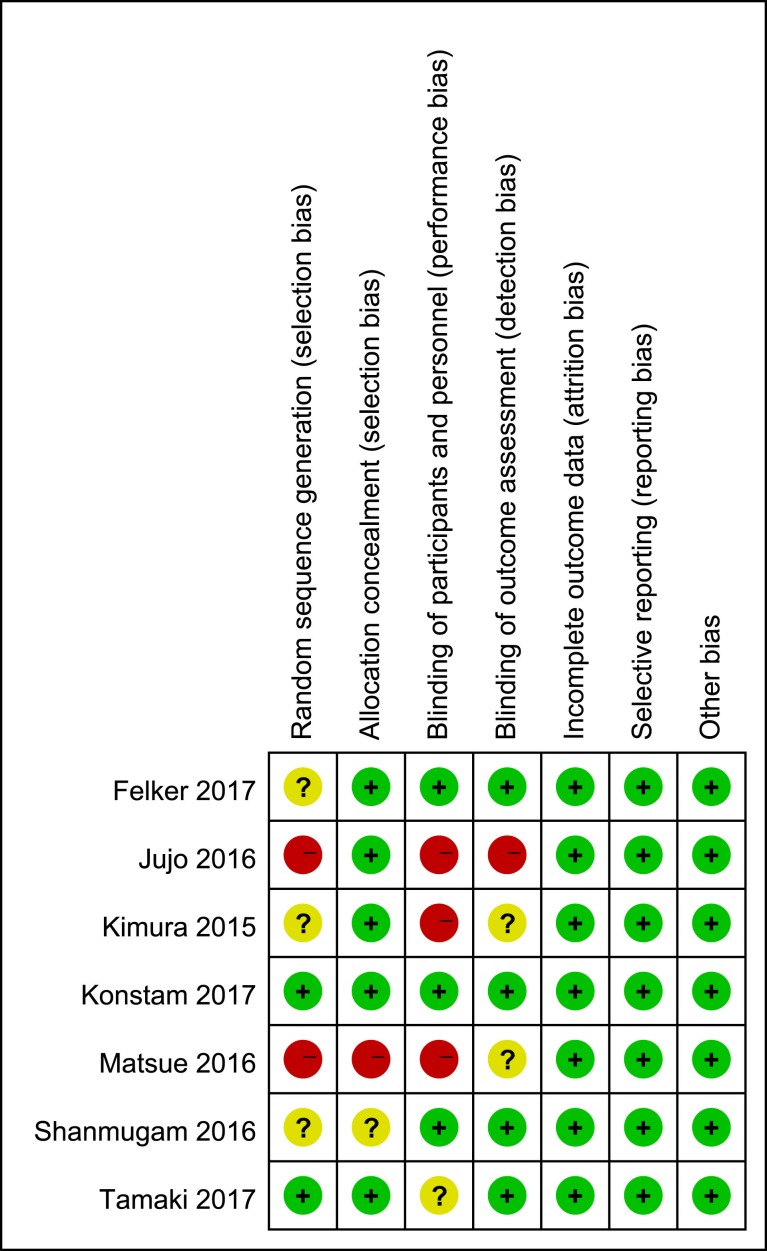
The study characteristics of the seven RCTs from USA, India and Japan from 2012 to 2017 with 937 patients involved are presented in table 1. The duration of observations ranged from 2 to 636 days. Most participants12 20 22–25 had ADHF (left ventricular ejection fraction [LVEF] <50%) of New York Heart Association class II to IV. One study focuses on the ADHF patients with Heart failure with preserved ejection fraction (HFpEF).26Three of the studies used carperitide.20 25 26 The risk of bias was evaluated with the Cochrane risk of bias tool.14 Most items for all included RCTs showed with low risk, however, the information in some studies is still insufficient, which made the evaluation even more difficult. Generally speaking, the RCTs included in our meta-analysis are of relatively high quality, except one study by Matsue et al,20 which shows a high risk of bias. The results are summarised in figure 2.
Table 1.
Baseline characteristics of the studies included in meta-analysis
| Study /year /reference |
Study location | Sample size | Intervention | LVEF,% | Age, years | Follow-up duration | Primary outcome | ||||
| Tolvaptan | Control | Tolvaptan | Control | Tolvaptan | Control | Tolvaptan | Control | ||||
| Jujo et al 201625 | Japan | 30 | 30 | Tolvaptan 7.5 mg/day + carperitide |
Furosemide + carperitide |
45(33,55)* | 46(37,60)* | 79±11 | 79±11 | 5 days | WRF, changes in urine volume, serum creatinine, BUN, BNP and catecholamines |
| Tamaki et al 201726 | Japan | 26 | 24 | Tolvaptan 7.5 or 15 mg/day + diuretic |
Diuretic | 60.7±10.0 | 59.7±7.5 | 79±7 | 75±10 | 48 hours | WRF, changes in serum creatinine, BUN, body weight, urine volume, secrum sodium and eGFR |
| Konstam et al 201722 | USA | 122 | 128 | Tolvaptan 30 mg/day + diuretic |
Placebo + diuretic |
35±16 | 33±17 | 70±11 | 67±13 | 7 days | WRF, changes in body weight, dyspnoea relief, eGFR and serum creatinine, 30 day mortality or re-hospitalisation |
| Felker et al 201723 | USA | 129 | 128 | Tolvaptan 30 mg/day + loop diuretic |
Placebo + loop diuretic |
34±17 | 32±17 | 66±13 | 63±16 | 48 hours | WRF, changes in body weight, serum sodium, dyspnoea relief and urine volume, worsening HF and 30 day mortality |
| Shanmugam et al 201624 | India | 25 | 26 | Tolvaptan 15 mg/day + diuretic |
Diuretic | 31.9±12.2 | 29.2±8.7 | 58.9±12.1 | 57±12 | 5 days | Changes in plasma sodium and dyspnoea relief, adverse effects |
| Matsue et al 201620 | Japan | 108 | 109 | Tolvaptan 15 mg/day + conventional therapy |
Conventional therapy | 45.4±18.1 | 46.8±16.4 | 72.99±8.9 | 72.95±10.24 | 636 days | WRF, changes in body weight, serum sodium, dyspnoea relief, BNP and urine volume, in-hospital death, adverse effects |
| Kimura et al 201612 | Japan | 26 | 26 | Tolvaptan 15 mg/day + furosemide 20 mg |
Furosemide | 47.54±16.75 | 56.73±11.52 | 80.54±12.15 | 86.15±4.95 | 7 days | WRF, changes in mean creatinine clearance and eGFR, adverse effects |
Data are given as the mean ±SD deviation
*Data presented as median with IQR.
BNP, B-type natriuretic peptide; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; WRF, worsening renal function.
Figure 2.
Risk of bias summary.
Effect of tolvaptan add-on therapy on WRF
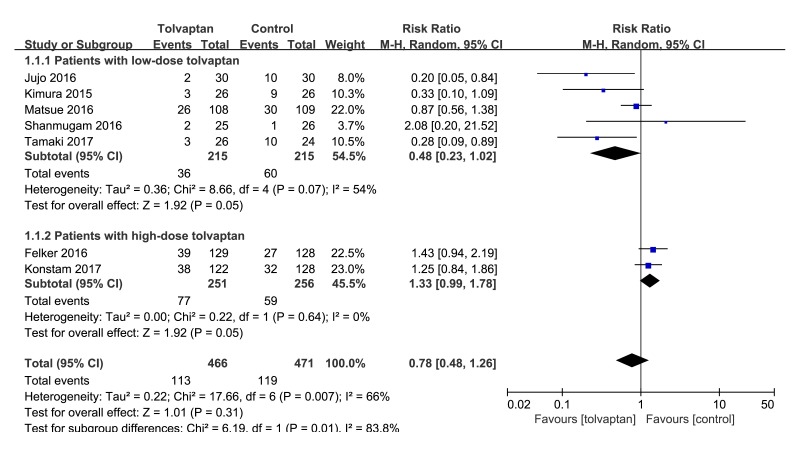
Seven studies12 20 22–26 have evaluated the effect of tolvaptan add-on therapy on WRF in patients with acute decompensated heart failure. Meta-analysis showed that I2=66%, p=0.007, the heterogeneity was high, so a random effect model was used. Meta-analysis (random effect model) showed that tolvaptan adding on loop diuretic comparing with controls or loop diuretic agents cannot significantly reduce the incidence of WRF (RR 0.78, 95% CI 0.48 to 1.26, p=0.31) in acute heart failure patients complicated with hyponatraemia or renal dysfunction. Shown in figure 3 is sub-analysis on differences in WRF between low (≤15 mg/day) and high (>15 mg/day) doses of tolvaptan. Low-dose group is in favour of add-on therapy compared with control (RR 0.48, 95% CI 0.23 to 1.02, p=0.05, I2=54%). High-dose group is not in favour of add-on therapy compared with control (RR 1.33, 95% CI 0.99 to 1.78, p=0.05, I2=0%). As shown in figure 3.
Figure 3.
Forest plot depicting the effect of tolvaptan on worsening renal function versus control.
Effects of tolvaptan add-on therapy on body weight
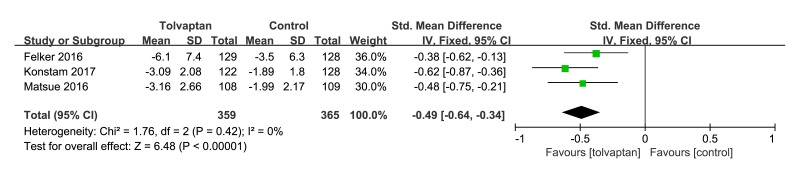
Mean body weight reflected the aquaretic effect of tolvaptan add-on therapy in ADHF patients. Three studies20 22 23 were included in the meta-analysis of the changings in body weight from baseline to 48 hours. There was a significant difference between the tolvaptan add-on therapy and control arms in favour of tolvaptan add-on therapy, which is an standardised mean difference (SMD −0.49, 95% CI −0.64 to −0.34, p<0.00001, I2=0%) in body weight changing. As shown in figure 4.
Figure 4.
Forest plot depicting the effect of tolvaptan on body weight reductions versus control.
Effects of tolvaptan add-on therapy on short-term mortality
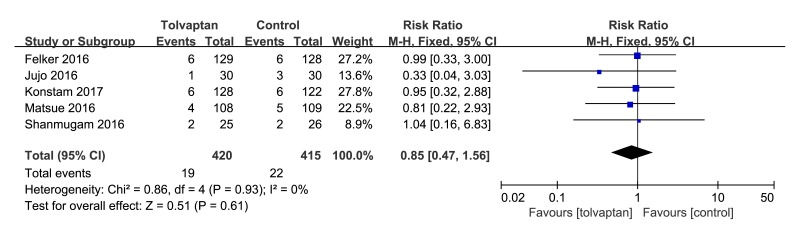
Five studies20 22–25 described the effects of tolvaptan add-on therapy on all-cause mortality. The pooled effects of tolvaptan add-on therapy on mortality that included in those five trials were not significantly different from control (RR 0.85, 95% CI 0.47 to 1.56, p=0.61, I2=0%). As shown in figure 5.
Figure 5.
Forest plot depicting the effect of tolvaptan on mortality versus control.
Effects of tolvaptan add-on therapy on serum sodium
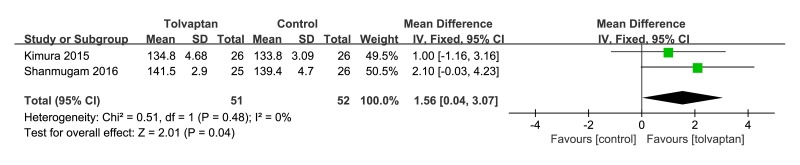
Although studies looked at changes of serum sodium at 5 days, there was a change in serum sodium in favour of tolvaptan add-on therapy (mean difference [MD] 1.56, 95% CI 0.04 to 3.07, p=0.04, I2=0%). As shown in figure 6.
Figure 6.
Forest plot depicting the effect of tolvaptan on serum sodium versus control.
Discussion
The main findings of this meta-analysis indicate that tolvaptan add-on therapy does not ameliorate the incidence of WRF or the short-term all-cause mortality in patients with ADHF. However, tolvaptan add-on therapy can reduce body weight, and increase the sodium level in patients with ADHF. A great majority of ADHF admissions are related to volume overload and congestion while loop diuretics decongestion remains the mainstay of current ADHF therapy. It was suggested that that WRF can be caused by immediate intravascular volume reduction induced by decongestion therapy using loop diuretics. WRF may through activation of the renin-angiotensin-aldosterone (RAA) and sympathetic nervous systems, and then leading to a decrease in renal perfusion and glomerular filtration pressure.27 Renal dysfunction is also a common comorbidity in ADHF patients, and it forebodes higher rates of mortality and hospitalisation in patients with ADHF to a great extent.28
There is an urgent need for an alternative approach to achieve adequate decongestion with minimum risk of WRF in ADHF patients.29 Tolvaptan has been alleviating congestion without reducing the renal blood flow or activation of the RAA and sympathetic nervous systems.5 The prognosis of HF patients30 can be greatly improved by the renal protective treatment. However, in this analysis, WRF has no statistical significance, the mean body weight has decreased and sodium concentration has increased.
Sub-analysis of studies with low dose, tolvaptan add-on therapy may decrease the rate of WRF. The results indicate that the use of tolvaptan add-on therapy in AHF may reduce WRF compared with the increasing loop diuretics. The improvement of kidney function may be attributed to the dose reduction of loop diuretics, which is facilitated through the aquaresis by tolvaptan. Consistent with that low-dose tolvaptan add-on therapy in HF patients with diuretic resistance and renal impairment increased urine volume without further renal impairment compared with patients who received an increased dose of furosemide.31The high-dose group consisted of US studies (placebo-controlled studies) may cause increasing the rate of WRF. In this analysis, although tolvaptan has no effect on WRF, while in the subgroup of low-dose tolvaptan group decreased the rate of WRF. The result indicates that high-dose (30 mg) tolvaptan in AHF may increase WRF compared with low-dose tolvaptan. The dose of tolvaptan may be related to the incidence of WRF. This result should be carefully interpreted, however, because of the limitation of the present data (p=0.05), so more well-designed randomised clinical trials are needed.
Aggressive fluid removal therapy is strongly recommended for symptom relieving and haemodynamic improvement in ADHF. Tolvaptan add-on therapy can significantly reduce body weight, however, it cannot ameliorate the incidence of WRF and short-term all-cause mortality. Tolvaptan may be like ultrafiltration acting as a decongestion method. Therefore, rapid and aggressive decongestion treatment may precede WRF to ameliorate congestion during hospitalisation, irrespective of the decongestion method. In the ultrafiltration versus intravenous diuretics for patients hospitalised for acute decompensated congestive heart failure (UNLOAD) trial, greater weight loss and a trend toward WRF by ultrafiltration compared with conventional diuretic therapy were associated with a reduced rate of re-hospitalisation for HF.32 The short-term of therapy may have been one factor for the failure in achieving long-term effects, although other short-term interventions can at times have long-term effects.
The present overall results are, in part, consistent with previous meta-analyses of tolvaptan in acute heart failure.13The current analysis exclude the trials comparing to tolvaptan and carperitide17 18 and include a placebo-controlled study from USA22 and a controlled study from Japan.26 Regarding the subgroup analysis of WRF in ADHF patients, low-dose tolvaptan may decrease the rate of WRF.
Limitations
There are a number of limitations in the meta-analysis. First, a total of seven random controlled studies were included, but most of the studies have their limitations. The inclusions of the study were more concentrated in the same region and country. Although the studies were randomised controlled trials, but the study of the distribution are hidden, the specific random method is not a completed description, there is no solid evidence to regulate the possibility of patient selection bias. Only two studies from the selected trials measured long-term mortality and four studies had the outcomes of short-term mortality. Second, there is no unified standard for the dosage, the tolvaptan use duration and follow-up time, which may affect the clinical outcomes. Third, differences in race, age and complication among studies also may result in slightly diverse response to therapy. Fourth, different control treatments may also lead to the inaccurate results. In addition, the sample size of some RCTs was too small and the adverse effects of tolvaptan such as dry mouth, dehydration were not reported in some study. Therefore, this meta-analysis also has certain enlightenment to the future randomised controlled trial: (1) Unified drug administration time and dosage and (2) The articles included in the study should come from different countries and regions in order to clarify the clinical effect of different countries and nationalities for an accurate conclusion.
Conclusion
We observed that tolvaptan add-on therapy does not ameliorate incidence of WRF, short-term all-cause mortality in patients with ADHF. However, tolvaptan add-on therapy can reduce body weight and elevate sodium level in patients with ADHF. Due to the limitations of the quality and quantity of the articles and documents, further researches for this conclusion are needed.
Supplementary Material
Footnotes
Contributors: GM is the guarantor. GM drafted the manuscript. All the authors contributed to the development of the selection criteria, the risk of bias assessment strategy and data extraction criteria, the search strategy and statistical expertise. All the authors read, provided feedback and approved the final manuscript. GM and XM conceived and designed the experiments. GM, XM and GW performed the experiments. GM and WT analysed the data. GM and XH contributed reagents/materials/analysis tools. GM wrote the paper.
Funding: This research was supported by Health Commission of Henan Province (grant number 2018020315).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: No additional data are available.
Patient consent for publication: Not required.
References
- 1. Ambrosy AP, Pang PS, Khan S, et al. Clinical course and predictive value of congestion during hospitalization in patients admitted for worsening signs and symptoms of heart failure with reduced ejection fraction: findings from the EVEREST trial. Eur Heart J 2013;34:835–43. 10.1093/eurheartj/ehs444 [DOI] [PubMed] [Google Scholar]
- 2. Hardin EA, Grodin JL. Diuretic strategies in acute decompensated heart failure. Curr Heart Fail Rep 2017;14:127–33. 10.1007/s11897-017-0319-y [DOI] [PubMed] [Google Scholar]
- 3. Ishikawa SE, Saito T, Saito T, et al. Pathophysiological role of aquaporin-2 in impaired water excretion. Prog Brain Res 2008;170:581–8. 10.1016/S0079-6123(08)00445-7 [DOI] [PubMed] [Google Scholar]
- 4. Lee CR, Watkins ML, Patterson JH, et al. Vasopressin: a new target for the treatment of heart failure. Am Heart J 2003;146:9–18. 10.1016/S0002-8703(02)94708-3 [DOI] [PubMed] [Google Scholar]
- 5. Costello-Boerrigter LC, Smith WB, Boerrigter G, et al. Vasopressin-2-receptor antagonism augments water excretion without changes in renal hemodynamics or sodium and potassium excretion in human heart failure. Am J Physiol Renal Physiol 2006;290:F273–F278. 10.1152/ajprenal.00195.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salah K, Kok WE, Eurlings LW, et al. Competing risk of cardiac status and renal function during hospitalization for acute decompensated heart failure. JACC Heart Fail 2015;3:751–61. 10.1016/j.jchf.2015.05.009 [DOI] [PubMed] [Google Scholar]
- 7. Izumi Y, Miura K, Iwao H. Therapeutic potential of vasopressin-receptor antagonists in heart failure. J Pharmacol Sci 2014;124:1–6. 10.1254/jphs.13R13CP [DOI] [PubMed] [Google Scholar]
- 8. Alskaf E, Tridente A, Al-Mohammad A. Tolvaptan for heart failure, systematic review and meta-analysis of trials. J Cardiovasc Pharmacol 2016;68:196–203. 10.1097/FJC.0000000000000405 [DOI] [PubMed] [Google Scholar]
- 9. Wu MY, Chen TT, Chen YC, et al. Effects and safety of oral tolvaptan in patients with congestive heart failure: A systematic review and network meta-analysis. PLoS One 2017;12:e0184380 10.1371/journal.pone.0184380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Konstam MA, Gheorghiade M, Burnett JC, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA 2007;297:1319–31. 10.1001/jama.297.12.1319 [DOI] [PubMed] [Google Scholar]
- 11. Gheorghiade M, Konstam MA, Burnett JC, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332–43. 10.1001/jama.297.12.1332 [DOI] [PubMed] [Google Scholar]
- 12. Kimura K, Momose T, Hasegawa T, et al. Early administration of tolvaptan preserves renal function in elderly patients with acute decompensated heart failure. J Cardiol 2016;67:399–405. 10.1016/j.jjcc.2015.09.020 [DOI] [PubMed] [Google Scholar]
- 13. Wang C, Xiong B, Cai L. Effects of Tolvaptan in patients with acute heart failure: a systematic review and meta-analysis. BMC Cardiovasc Disord 2017;17:164 10.1186/s12872-017-0598-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med 2009;151:W–94. 10.7326/0003-4819-151-4-200908180-00136 [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Deeks JJ, Higgins JPT, Altman DG. Chapter 9: analysing data and undertaking Meta-analyses : Higgins JPT, Green S, Cochrane handbook for systematic reviews of interventions. version 5.1.0 [updated March 2011]: The Cochrane Collaboration, 2011. [Google Scholar]
- 17. Suzuki S, Yoshihisa A, Yamaki T, et al. Acute heart failure volume control multicenter randomized (AVCMA) trial: comparison of tolvaptan and carperitide. J Clin Pharmacol 2013;53:1277–85. 10.1002/jcph.197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Suzuki S, Yoshihisa A, Yamaki T, et al. Long-term effects and prognosis in acute heart failure treated with tolvaptan: the AVCMA trial. Biomed Res Int 2014;2014:1–8. 10.1155/2014/704289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Matsue Y, Suzuki M, Nagahori W, et al. Clinical effectiveness of tolvaptan in patients with acute decompensated heart failure and renal failure: design and rationale of the AQUAMARINE study. Cardiovasc Drugs Ther 2014;28:73–7. 10.1007/s10557-013-6491-8 [DOI] [PubMed] [Google Scholar]
- 20. Matsue Y, Suzuki M, Torii S, et al. Clinical effectiveness of tolvaptan in patients with acute heart failure and renal dysfunction. J Card Fail 2016;22:423–32. 10.1016/j.cardfail.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 21. Matsue Y, Suzuki M, Torii S, et al. Prognostic impact of early treatment with tolvaptan in patients with acute heart failure and renal dysfunction. Int J Cardiol 2016;221:188–93. 10.1016/j.ijcard.2016.07.063 [DOI] [PubMed] [Google Scholar]
- 22. Konstam MA, Kiernan M, Chandler A, et al. Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J Am Coll Cardiol 2017;69:1409–19. 10.1016/j.jacc.2016.12.035 [DOI] [PubMed] [Google Scholar]
- 23. Felker GM, Mentz RJ, Cole RT, et al. Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J Am Coll Cardiol 2017;69:1399–406. 10.1016/j.jacc.2016.09.004 [DOI] [PubMed] [Google Scholar]
- 24. Shanmugam E, Doss CR, George M, et al. Effect of tolvaptan on acute heart failure with hyponatremia--a randomized, double blind, controlled clinical trial. Indian Heart J 2016;68(Suppl 1):S15–S21. 10.1016/j.ihj.2015.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jujo K, Saito K, Ishida I, et al. Randomized pilot trial comparing tolvaptan with furosemide on renal and neurohumoral effects in acute heart failure. ESC Heart Fail 2016;3:177–88. 10.1002/ehf2.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tamaki S, Sato Y, Yamada T, et al. Tolvaptan Reduces the Risk of Worsening Renal Function in Patients With Acute Decompensated Heart Failure and Preserved Left Ventricular Ejection Fraction Prospective Randomized Controlled Study. Circ J 2017;81:740–7. 10.1253/circj.CJ-16-1122 [DOI] [PubMed] [Google Scholar]
- 27. Ronco C, Cicoira M, McCullough PA. Cardiorenal syndrome type 1: pathophysiological crosstalk leading to combined heart and kidney dysfunction in the setting of acutely decompensated heart failure. J Am Coll Cardiol 2012;60:1031–42. 10.1016/j.jacc.2012.01.077 [DOI] [PubMed] [Google Scholar]
- 28. Metra M, Cotter G, Senger S, et al. Prognostic significance of creatinine increases during an acute heart failure admission in patients with and without residual congestion: a post Hoc Analysis of the PROTECT Data. Circ Heart Fail 2018;11:e004644 10.1161/CIRCHEARTFAILURE.117.004644 [DOI] [PubMed] [Google Scholar]
- 29. Goldsmith SR, Bart BA, Burnett J. Decongestive therapy and renal function in acute heart failure: time for a new approach? Circ Heart Fail 2014;7:531–5. 10.1161/CIRCHEARTFAILURE.113.000828 [DOI] [PubMed] [Google Scholar]
- 30. Damman K, Valente MA, Voors AA, et al. Renal impairment, worsening renal function, and outcome in patients with heart failure: an updated meta-analysis. Eur Heart J 2014;35:455–69. 10.1093/eurheartj/eht386 [DOI] [PubMed] [Google Scholar]
- 31. Inomata T, Ikeda Y, Kida K, et al. Effects of Additive Tolvaptan vs. Increased Furosemide on Heart Failure With Diuretic Resistance and Renal Impairment Results From the K-STAR Study. Circ J 2017;82:159–67. 10.1253/circj.CJ-17-0179 [DOI] [PubMed] [Google Scholar]
- 32. Costanzo MR, Guglin ME, Saltzberg MT, et al. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007;49:675–83. 10.1016/j.jacc.2006.07.073 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-025537supp001.pdf (34KB, pdf)