Abstract
Objective
To estimate the association between cumulative anticholinergic burden and falls and fractures in patients with overactive bladder (OAB).
Design
A retrospective claims-based study (2007–2015) of patients with OAB; outcomes from a subset were contrasted to a non-OAB comparison.
Setting
United States, commercially and Medicare-insured population.
Participants
154 432 adults with OAB and 86 966 adults without OAB, mean age of 56 years, and 67.9% women.
Main outcome measures
Cumulative anticholinergic burden, a unitless value representing exposure over time, was estimated over the 12 months pre-index (‘at baseline’) and every 6 months post index. Burden was categorised as no burden (0), low burden (1–89), medium burden (90–499) or high burden (500+). Unadjusted rates of falls or fractures were estimated, and the increased risk associated with anticholinergic burden (measured at the closest 6-month interval prior to a fall or fracture) was assessed using a Cox proportional hazards model and a marginal structural model.
Results
Median (IQR) baseline anticholinergic burden was 30 (0.0–314.0) and higher among older (≥65 years, 183 [3.0–713.0]) versus younger (<65 years, 13 [0.0–200.0]) adults. The unadjusted rate of falls or fractures over the period was 5.0 per 100 patient-years, ranging from 3.1 (95% CI 3.0–3.2) for those with no burden, to 7.4 (95% CI 7.1–7.6) for those with high burden at baseline. The adjusted risk of falls and fractures was greater with higher anticholinergic burden in the previous 6 months, with an HR of 1.2 (95% CI 1.2 to 1.3) for low burden versus no burden, to 1.4 (95% CI 1.3 to 1.4) for high versus no burden. Estimates from marginal structural models adjusting for time-varying covariates were lower but remained significantly higher with a higher anticholinergic burden. Rates of falls and fractures were approximately 40% higher among those with OAB (vs those without).
Conclusion
Higher levels of anticholinergic burden are associated with higher rates of falls and fractures, highlighting the importance of considering anticholinergic burden when treating patients with OAB.
Keywords: anticholinergic burden, overactive bladder, falls, fractures, observational study, marginal structural models
Strengths and limitations of this study.
This is the first study to demonstrate the association of higher cumulative anticholinergic burden with a higher risk of falls and fractures among those with overactive bladder (OAB).
This study contributes evidence to help inform the appropriate use of anticholinergic medications, both among younger and older patients with OAB who have a higher comorbidity burden.
The use of a validated generalisable dataset with a large sample size allowed for the calculation of precise estimates of risk and an understanding of the interplay of factors contributing to falls and fractures.
As with any retrospective study, the findings are limited by the use of claims data, and duration of follow-up available.
Introduction
Falls and fractures, which are major causes of morbidity and mortality among older adults,1 2 have numerous risk factors, both intrinsic and extrinsic.2–12 Interactions between one or more of these factors are also important contributors to fall risk.12–14 Overactive bladder (OAB) is a symptom complex including urinary urgency, with or without urinary incontinence and nocturia, symptoms that are intrinsic risk factors for falls or fractures.11–13 15–17 While many patients manage their OAB symptoms with lifestyle modifications alone,18 among those requiring pharmacotherapy most will initially be treated with anticholinergic medications called antimuscarinics.19 Cumulative or prolonged exposure to the broader class of anticholinergic medications, which are widely used to manage patients across a variety of health conditions, is also a risk factor for falls and fractures even among populations already at higher risk.3–7
To date, studies have infrequently evaluated the impact of OAB treatment17 20 and never the impact of anticholinergic burden on falls and fractures among those with OAB.21 22 Few randomised trials of antimuscarinic treatments report the occurrence of falls, and those that do, usually do not report significant differences between OAB treatments or placebo.23–25 One observational study examining OAB treatments and falls and fractures reported no difference in rates among over 100 000 patients initiating two different antimuscarinics over their first 90 days of treatment, though follow-up times were short.20 Another reported a slightly protective effect of OAB treatment on falls but did not measure fractures, nor the intensity of, duration of or adherence to OAB treatments.17 However, the impact of anticholinergic burden on falls and fractures risk in OAB would not be driven by antimuscarinic use only, but rather from the total of all prescribed anticholinergic medications; to the best of our knowledge, this has not yet been examined.
Understanding the magnitude of the association between anticholinergic burden and falls and fractures among those with OAB is important for several reasons. First, OAB is highly prevalent, particularly among older adults, affecting up to 18.5% of adults over 40 years of age.26 Second, the high frequency of major risk factors for falls and fractures (including older age, urinary symptoms11–13 and gait problems)27 among those with OAB contribute to an approximately 33% increased risk of falls compared with those without OAB,28 independent of any incremental risk possibly conveyed by anticholinergic burden. However, if antimuscarinics successfully manage the symptoms of OAB that are themselves risk factors for falls, it is conceivable that the impact of treating OAB with antimuscarinics could be a reduction in falls and fractures.17 Alternatively, anticholinergic burden could act as an effect modifier of the relationship between OAB symptoms and risk of falls or fractures, such that its impact would be more or less pronounced among different subgroups of patients with OAB. Finally, unlike certain fall risk factors such as older age or sex, anticholinergic burden is potentially modifiable, and at-risk patients with OAB could be managed by treatments that do not act by the anticholinergic pathway. Due to the multifactorial nature of falls and fractures risk,1 14 the application of rigorous statistical techniques is required to appropriately control for potential confounders while estimating the association between time-varying exposures such as anticholinergic burden and relevant outcomes.
The primary objective of this study was to estimate the association between anticholinergic burden and falls and fractures among individuals with OAB. A secondary objective was to compare rates of falls and fractures among those with OAB to an age-matched and sex-matched sample without OAB. These data will be important to help formulate treatment recommendations for patients with OAB at higher risk of falls and fractures.
Methods
Study design
This retrospective cohort study used the Truven MarketScan claims databases from the USA, which are large, nationally representative healthcare datasets of Commercial Claims and Encounters (commercial) and patients insured through Medicare Supplemental and Coordination of Benefits (medicare supplemental). These databases contain individually linked data for over 84 million people, allowing characterisation of patient populations, treatment patterns, clinical outcomes and healthcare resource use (including medication fills).29 These data have been widely validated for clinical, pharmacoepidemiological and pharmacoeconomic research.30–32
The study period was from January 2007 to December 2015. For the core analyses, the identification period for enrolment was from January 2008 to December 2014, to allow ≥1 year of pre-enrolment data per person for summarising baseline characteristics, determining whether an individual is an incident or prevalent case of OAB (see below) and establishing anticholinergic exposure. The final year of the study period was used to allow all cohort members the opportunity for ≥1 year of follow-up post-index date, defined according to the date of the first identified OAB-related code during the study period. Outcomes could occur at any time between the index date and censoring (eg, inpatient death, dis-enrolment in the insurance plan or the end of the study period).
For comparison with individuals without OAB, data from January 2008 to December 2009 were used to enrol patients into either a non-OAB cohort or an OAB cohort (‘OAB cohort 2’, representing a subset of the core OAB cohort). All patients were assigned an index date of 1 January 2010 to avoid immortal time bias in classifying subsequent outcomes to exposure groups.33 Data from January 2010 to December 2015 were used to observe the outcomes of interest.
Patient involvement
Patients and the public were not involved in this research.
Study sample
Study inclusion required that individuals be ≥18 years of age at the index date with medical and pharmaceutical coverage in the 12 months prior to the index date. Exclusion criteria were neurogenic bladder/neurogenic detrusor overactivity, pregnancy, malignant neoplasm, renal impairment, hepatic insufficiency, trauma or organ transplantation during the study period (online supplementary table 1). Study eligibility was determined based on the availability of insurance coverage rather than actual resource use, and no exclusion criteria related to the duration of post-index follow-up were imposed.
bmjopen-2018-026391supp001.pdf (778.7KB, pdf)
The OAB cohort included individuals with (1) validated International Classification of Diseases, Ninth Revision (ICD-9) codes in any position on ≥1 inpatient claims or ≥2 outpatient medical claims on separate dates, or (2) ≥2 OAB-specific medication claims (by National Drug Codes) during the identification period (online supplementary table 1). The date of the first relevant code during the identification period was the individual’s index date. Cohort members were classified as incident if, in the year prior to index, they had no OAB-specific claims. All other OAB cases were classified as prevalent.
For comparison with the non-OAB cohort, individuals in OAB cohort 2 were required to have 1 year of data availability pre-index and potential for data availability through at least 2010. The non-OAB cohort was randomly selected according to a 2:1 age and sex match to OAB cohort 2. Members of both cohorts were assigned an index date of 1 January 2010 (ie, the end of the identification period).
In terms of sample size, another recent MarketScan study identified over 1 million individuals with OAB over 5 years.34 Fractures, which occur more infrequently than falls, were observed among 5% of a separate OAB cohort over 5 years,15 which would correspond to 50 000 individuals from a cohort of 1 million. While little is known about anticholinergic burden and falls in OAB, in an Irish study in Parkinson’s disease, 24% of those with high anticholinergic burden had injurious falls versus 7% of those without anticholinergic burden.35 To detect a difference as great in OAB, at alpha=0.05 and power=0.8, 300 individuals per anticholinergic burden level would be required. Ultimately, of the eligible cohort of over 2 million persons with OAB, a 15% sample was randomly selected for computational feasibility.
Classifying exposure and outcomes
The exposure of interest was cumulative anticholinergic burden estimated by applying the score derived from a cross-sectional measure of anticholinergic exposure (the 2012 version of the Anticholinergic Cognitive Burden [ACB)] scale, a validated scale counting usage of 104 medications rated as contributing at least some anticholinergic burden)36 37 over time, as outlined in online supplementary figure 1.38 Briefly, a unitless value reflecting the intensity of anticholinergic exposure (by a medication’s defined daily dose),39 40 strength of anticholinergic activity (by drug-specific ACB score) and period of exposure is estimated, reflecting an individual’s cumulative standardised daily dose of all medications over time (online supplementary figure 1).41
Cumulative anticholinergic burden was calculated at baseline (over the 12-month pre-index period) and updated at 6-month intervals. At each time point, an individual’s anticholinergic burden was classified as no burden versus any anticholinergic burden, with burden characterised as low (1–89), medium (90–499) and high (500+), based on an initial review of histograms of score data. An example patient with low anticholinergic burden may have received two 90-day fills for 25 mg atenolol (ACB score=1) over a year (cumulative anticholinergic burden=60) versus a patient with high anticholinergic burden having received one 30-day fill for 100 mg carbamazepine (ACB score=1), two 30-day fills for solifenacin 10 mg (ACB score=3) and two 90-day fills for tolterodine 4 mg (ACB score=3) over a year (cumulative anticholinergic burden=912, additional example calculations provided in online supplementary figure 1).
The primary outcome was a composite of falls and fractures sufficiently severe to require inpatient or outpatient care, identified by validated ICD-9 and Healthcare Common Procedure Coding System/Current Procedural Terminology codes (online supplementary table 1). While these outcomes were initially considered individually (ie, as falls, vs fractures), due to consistency in the trends in results between the composite and individual outcomes (data not shown), the manuscript results focus on the composite outcome.
Statistical analysis
Baseline characteristics were summarised by means, SDs, medians and IQRs for continuous variables, and by number and percent for categorical variables. These included demographics, risk factors for falls and fractures, or high anticholinergic burden and other comorbidities. Comorbidities were considered by overall Elixhauser score42 and according to key comorbidities (see online supplementary table 1 for codes). Baseline characteristics were summarised overall and according to age (<65 vs >65 years) and sex.
Cumulative anticholinergic burden was summarised by the number and percent with no burden versus any burden at baseline and at 6-month intervals post index, mean (95% CI) scores at baseline and at 6-month intervals post index, and as the five most frequent anticholinergic medications from the ACB scale prescribed at least once (at the level of the medication and class), overall and by age.
The frequency of falls and fractures over the period was estimated according to baseline level of anticholinergic burden. The unadjusted rate (95% CI) per 100 person-years was estimated using negative binomial regression models, overall and by age, sex and timing (ie, an incident vs prevalent case) of OAB. Rates were compared according to baseline anticholinergic burden using rate ratios (RRs) with 95% CIs.
Time to first fall or fracture, according to time-varying levels of cumulative anticholinergic burden (measured at the closest 6-month interval prior to the fall or fracture) and adjusted for age, sex and other key covariates at baseline, was estimated using the Andersen-Gill formulation of the Cox proportional hazards model43 and was compared between cohorts at different levels of burden using HRs with 95% CIs. Potential covariates for adjustment were identified based on preliminary models, and covariates remaining significant were retained in the final model (see list of potential covariates, identified by literature review, in online supplementary table 1). While the inclusion of anticholinergic burden as a continuous variable was considered, it was ultimately included as a categorical variable due to the ease of interpretation from comparing estimates for categorical levels directly. To understand the impact of age, a subgroup analysis was performed among patients aged >65 years at index.
Changes in medications or comorbidities over the period may be related to both anticholinergic use and the occurrence of falls and fractures. To control for these time-varying covariates, as well as all other non-time-varying covariates included in the non-weighted Cox analysis, a marginal structural model was run.44 For its implementation, a multinomial logistic model estimating inverse-probability weights was first developed to predict anticholinergic burden (measured at the closest 6-month interval prior to the fall or fracture) based on age, sex and all covariates identified for inclusion. Any comorbidities included as covariates were set to time varying, with their indicator set to ‘absent’ unless a code for the comorbidity was found, after which all subsequent intervals for that individual had the indicator set to ‘present’. Then, the marginal structural model incorporating the inverse-probability weights was implemented to estimate the HR (95% CI) of falls and fractures associated with levels of anticholinergic burden among those with OAB, adjusting for age, sex and other key covariates at baseline. Further details on the marginal structural model, including estimation of stabilised weights and robust variances, are described by Robins et al.45
To compare falls and fractures among those with OAB with those among the non-OAB cohort, the increased risk according to the level of baseline anticholinergic burden and the OAB status was first estimated as unadjusted rates and RRs (95% CIs) and then using adjusted Cox and marginal structural models, as previously discussed. OAB status was handled as a fixed covariate in both the Cox and marginal structural models. To estimate the extent of the modification (by anticholinergic burden) of the association between OAB and falls and fractures, interaction terms were included in the model, and the effect of OAB for each level of anticholinergic burden was estimated.
All analyses were conducted in R V.3.4.0.
Results
Core analyses
The mean age of the OAB cohort (n=154 432) was 56 years, 75.9% were <65 years, 67.9% were women (table 1) and the median duration of follow-up post index was 2.5 years. The mean (SD) Elixhauser comorbidity score was 1.0 (3.9), and 3.6% had a fall or fracture in the preceding year. At baseline (measured over 12 months pre-index), 35.4% had no burden, 25.0% had low burden, 20.5% had moderate burden and 19.1% had high anticholinergic burden. Those with any baseline anticholinergic burden were slightly older on average and were more likely to be women. The median (IQR) baseline anticholinergic burden was 30 (0–314) and was substantially higher among those aged ≥65 (183 [3–713]) versus those aged <65 (13 [0–200]) years.
Table 1.
Demographic and clinical characteristics of the OAB cohort at baseline, Truven MarketScan databases 2007–2015
| Overall | By age | By baseline anticholinergic burden* | By sex | |||||||||||
| <65 | ≥65 | No burden | Some burden | Male | Female | |||||||||
| n=154 432 | n=117 271 | n=37 161 | n=54 602 | n=99 830 | n=49 597 | n=104 835 | ||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Age (years) | ||||||||||||||
| Mean (SD) | 55.7 | 15.2 | 49.4 | 11.0 | 75.7 | 7.5 | 51.4 | 14.6 | 58.1 | 15.0 | 56.2 | 14.0 | 55.5 | 15.8 |
| Median (IQR) | 56 | 46–64 | 52 | 43–58 | 75 | 69–81 | 52 | 42–60 | 58 | 49–68 | 57 | 48–64 | 55 | 46–64 |
| ≤45 | 36 039 | 23.3 | 36 039 | 30.7 | 0 | 0 | 17 300 | 31.7 | 18 739 | 18.8 | 9915 | 20 | 26 124 | 24.9 |
| 46–55 | 39 784 | 25.8 | 39 784 | 33.9 | 0 | 0 | 15 357 | 28.1 | 24 427 | 24.5 | 12 743 | 25.7 | 27 041 | 25.8 |
| 56–65 | 43 414 | 28.1 | 41 448 | 35.3 | 1966 | 5.3 | 14 610 | 26.8 | 28 804 | 28.9 | 16 160 | 32.6 | 27 254 | 26 |
| 66–75 | 17 649 | 11.4 | 0 | 0 | 17 649 | 47.5 | 4383 | 8 | 13 266 | 13.3 | 6115 | 12.3 | 11 534 | 11 |
| 76–85 | 13 099 | 8.5 | 0 | 0 | 13 099 | 35.2 | 2341 | 4.3 | 10 758 | 10.8 | 3765 | 7.6 | 9334 | 8.9 |
| 86+ | 4447 | 2.9 | 0 | 0 | 4447 | 12 | 611 | 1.1 | 3836 | 3.8 | 899 | 1.8 | 3548 | 3.4 |
| Female sex | 104 835 | 67.9 | 79 159 | 67.5 | 25 676 | 69.1 | 29 999 | 54.9 | 74 836 | 75.0 | 0 | 0 | 104 835 | 100.0 |
| Comorbidities† | ||||||||||||||
| Hypertension, uncomplicated | 55 900 | 36.2 | 35 332 | 30.1 | 20 568 | 55.3 | 14 401 | 26.4 | 41 499 | 41.6 | 19 895 | 40.1 | 36 005 | 34.3 |
| Diabetes mellitus and diabetic peripheral neuropathy | 21 490 | 13.9 | 13 424 | 11.4 | 8066 | 21.7 | 5540 | 10.1 | 15 950 | 16.0 | 8205 | 16.5 | 13 285 | 12.7 |
| Cerebrovascular disease and stroke | 8517 | 5.5 | 3180 | 2.7 | 5337 | 14.4 | 1599 | 2.9 | 6918 | 6.9 | 2905 | 5.9 | 5612 | 5.4 |
| Dizziness | 8398 | 5.4 | 5366 | 4.6 | 3032 | 8.2 | 1905 | 3.5 | 6493 | 6.5 | 2249 | 4.5 | 6149 | 5.9 |
| Osteoporosis | 6609 | 4.3 | 3162 | 2.7 | 3447 | 9.3 | 1626 | 3.0 | 4983 | 5.0 | 471 | 0.9 | 6138 | 5.9 |
| Arthritis | 6345 | 4.1 | 4370 | 3.7 | 1975 | 5.3 | 1295 | 2.4 | 5050 | 5.1 | 1097 | 2.2 | 5248 | 5.0 |
| Falls or fractures within the preceding year | 5542 | 3.6 | 3059 | 2.6 | 2483 | 6.7 | 1163 | 2.1 | 4379 | 4.4 | 1210 | 2.4 | 4332 | 4.1 |
| Lifestyle factors | ||||||||||||||
| Smoking | 13 548 | 8.8 | 8836 | 7.5 | 4712 | 12.7 | 2956 | 5.4 | 10 592 | 10.6 | 4426 | 8.9 | 9122 | 8.7 |
| Alcohol abuse | 768 | 0.5 | 658 | 0.6 | 110 | 0.3 | 188 | 0.3 | 580 | 0.6 | 374 | 0.8 | 394 | 0.4 |
| Medications | ||||||||||||||
| Opioids | 56 036 | 36.3 | 41 608 | 35.5 | 14 428 | 38.8 | 11 044 | 20.2 | 44 992 | 45.1 | 14 887 | 30.0 | 41 149 | 39.3 |
| Benzodiazepine use | 27 507 | 17.8 | 20 252 | 17.3 | 7255 | 19.5 | 2349 | 4.3 | 25 158 | 25.2 | 5882 | 11.9 | 21 625 | 20.6 |
| Chronic use of inhaled or oral corticosteroids | 5367 | 3.5 | 3306 | 2.8 | 2061 | 5.5 | 888 | 1.6 | 4479 | 4.5 | 1492 | 3.0 | 3875 | 3.7 |
| Risk factors for high anticholinergic burden | ||||||||||||||
| Depression, neurotic disorders or psychosis | 32 674 | 21.2 | 27 037 | 23.1 | 5637 | 15.2 | 7838 | 14.4 | 24 836 | 24.9 | 8135 | 16.4 | 24 539 | 23.4 |
| Chronic obstructive pulmonary disease | 10 016 | 6.5 | 5604 | 4.8 | 4412 | 11.9 | 1824 | 3.3 | 8192 | 8.2 | 3167 | 6.4 | 6849 | 6.5 |
| Parkinson’s disease/other neurological impairments | 5973 | 3.9 | 3401 | 2.9 | 2572 | 6.9 | 1059 | 1.9 | 4914 | 4.9 | 1847 | 3.7 | 4126 | 3.9 |
| Dementia | 1570 | 1.0 | 138 | 0.1 | 1432 | 3.9 | 216 | 0.4 | 1354 | 1.4 | 408 | 0.8 | 1162 | 1.1 |
| Intestinal motility disorders | 152 | 0.1 | 107 | 0.1 | 45 | 0.1 | 43 | 0.1 | 109 | 0.1 | 39 | 0.1 | 113 | 0.1 |
| Elixhauser score, mean (SD) | 1 | 3.9 | 1 | 3.3 | 3 | 5.0 | 1 | 3.0 | 1 | 4.3 | 1 | 3.9 | 1 | 3.9 |
| Timing of OAB | ||||||||||||||
| Incident case | 1 06 730 | 69.1 | 84 888 | 72.4 | 21 842 | 58.8 | 43 688 | 80.0 | 63 042 | 63.1 | 36 783 | 74.2 | 69 947 | 66.7 |
| Prevalent case | 47 702 | 30.9 | 32 383 | 27.6 | 15 319 | 41.2 | 10 914 | 20.0 | 36 788 | 36.9 | 12 814 | 25.8 | 34 888 | 33.3 |
| Anticholinergic burden | ||||||||||||||
| Mean (SD) | 266.7 | 486.5 | 213.8 | 443.9 | 433.8 | 570.3 | 0 | 0 | 412.6 | 553.2 | 154.2 | 365.3 | 320.0 | 526.1 |
| Median (IQR) | 30 | 0.0, 314.0 | 13 | 0.0, 200.0 | 183 | 3.0, 713.0 | 0 | 0.0, 0.0 | 180 | 36.0, 609.0 | 1 | 0.0, 120.0 | 60.0 | 0.0, 445.5 |
| No burden | 54 602 | 35.4 | 46 746 | 39.9 | 7856 | 21.1 | 54 602 | 100.0 | 0 | 0 | 24 603 | 49.6 | 29 999 | 28.6 |
| Low | 38 669 | 25.0 | 31 229 | 26.6 | 7440 | 20.0 | 0 | 0 | 38 669 | 38.7 | 11 504 | 23.2 | 27 165 | 25.9 |
| Medium | 31 719 | 20.5 | 22 006 | 18.8 | 9713 | 26.1 | 0 | 0 | 31 719 | 31.8 | 8460 | 17.1 | 23 259 | 22.2 |
| High | 29 442 | 19.1 | 17 290 | 14.7 | 12 152 | 32.7 | 0 | 0 | 29 442 | 29.5 | 5030 | 10.1 | 24 412 | 23.3 |
*Baseline anticholinergic burden assessed over the 12-month pre-index period.
†Only comorbidities identified among >2.5% are presented. The following were identified among <2.5% of the cohort: syncope, complicated hypertension, cognitive impairment, Alzheimer’s disease, musculoskeletal problems, hyperparathyroidism, decreased vision, chronic kidney disease, leg and foot amputation, hypotension, and palmonental reflex.
OAB, overactive bladder.
Cumulative anticholinergic burden over time
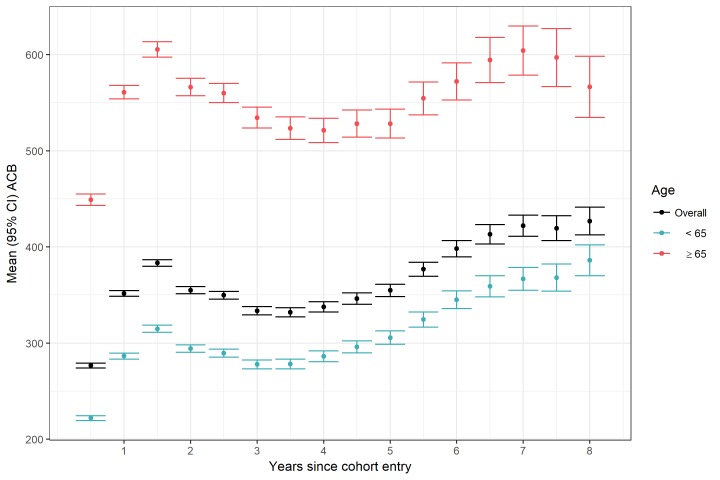
Despite 35% having no anticholinergic burden at baseline, over 80% of those with OAB (n=124 819) had at least some anticholinergic burden recorded during any of the 6-month intervals over the period. The five most frequent anticholinergic medications prescribed from the ACB scale, two of which were antimuscarinics for OAB, were codeine (n=27 639, 17.9%; ACB score=1), oxybutynin (n=27 554, 17.8%; ACB score=3), prednisone (n=27 131, 17.6%; ACB score=1), tolterodine (n=26 663, 17.3%; ACB score=3) and cyclobenzaprine (n=21 691, 14.0%; ACB score=2). The most frequent anticholinergic medication classes prescribed were genitourinary smooth muscle relaxants (n=44 497, 28.8%, which included other antimuscarinics, in addition to oxybutynin and tolterodine), antidepressants (n=22 638, 14.7%), beta-blockers (n=21 026, 13.6%), benzodiazepines (n=15 361, 9.9%) and adrenal medications (n=11 728, 7.6%). Mean (95% CI) levels of anticholinergic burden increased over the period and were higher among older versus younger cohort members (figure 1).
Figure 1.
Mean (95% CI) level of anticholinergic burden according to time since cohort entry and age. ACB, anticholinergic burden.
Rates of falls and fractures
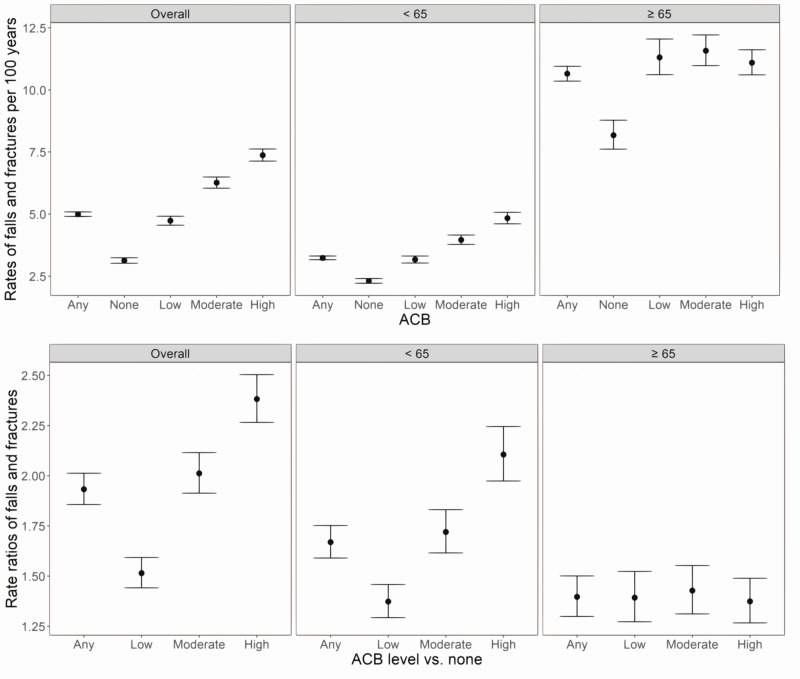
Among those with OAB, 15 287 (9.9%) experienced a fall (3.3%) or fracture (7.7%) over follow-up, including 3650 (2.4%) with no baseline anticholinergic burden, 3495 (2.3%) with low burden, 3802 (2.5%) with moderate burden and 4340 (2.8%) with high burden (measured over 12 months pre-index). The unadjusted rate of falls or fractures was 5.0 (95% CI 4.9 to 5.1) per 100 patient-years and ranged from 3.1 (95% CI 3.0 to 3.2) for no baseline anticholinergic burden to 7.4 (95% CI 7.1 to 7.6) for high burden (figure 2). For those aged ≥65 years, rates were high overall and higher among those with higher baseline anticholinergic burden; from 8.2 (95% CI 7.6 to 8.8) per 100 person-years for no burden to 11.1 (95% CI 10.6 to 11.6) for high burden. For those aged <65 years, rates were lower but also increased with increasing levels of baseline anticholinergic burden from 2.3 (95% CI 2.2 to 2.4) per 100 person-years for no burden to 4.8 (95% CI 4.6 to 5.1) for high burden. When comparing rates between those aged <65 vs >65 years at the same level of baseline anticholinergic burden, RRs demonstrated a 2.0-fold to 3.5-fold increased risk of falls and fractures among older adults with OAB compared with younger adults with OAB.
Figure 2.
Rates (top) and rate ratios (bottom), for falls and fractures* estimated over the study period in the overactive bladder cohort, according to baseline anticholinergic burden,** overall and according to age (<65 years vs >65 years); Truven MarketScan databases 2007–2015. *Point estimates (dots) and 95% CIs (lines) plotted. **Baseline anticholinergic burden assessed over the 12-month pre-index period. ACB, anticholinergic burden.
A 1.9-fold (95% CI 1.9 to 2.0) increased risk of falls and fractures was observed among those with some versus no baseline anticholinergic burden; RRs ranged from 1.5 (95% CI 1.4 to 1.6) for those with low versus no anticholinergic burden to 2.4 (95% CI 2.3 to 2.5) for those with high versus no anticholinergic burden (figure 2). The increased risk of falls and fractures associated with anticholinergic burden level was more pronounced among younger (<65 years; RR 1.7, 95% CI 1.6 to 1.8) versus older (≥65 years; RR 1.4, 95% CI 1.3 to 1.5) adults with OAB.
Adjusted rates of falls and fractures
A statistically significant association was observed between anticholinergic burden (measured at the closest 6-month interval prior to the fall or fracture) and falls and fractures in the Cox model adjusted for age, sex and key comorbidities; and the magnitude of the association increased with increasing levels of anticholinergic burden. All key covariates included in the final model are described in table 2. HRs (95% CI) for falls and fractures were 1.2 (95% CI 1.2 to 1.3) for low versus no anticholinergic burden, 1.3 (95% CI 1.2 to 1.4) for medium versus no burden and 1.4 (95% CI 1.3 to 1.4) for high versus no burden. Findings from the marginal structural model were consistent, although the magnitude of the association was slightly less: HRs were 1.2 (95% CI 1.1 to 1.2) for low versus no burden, 1.2 (95% CI 1.1 to 1.3) for medium versus no burden and 1.3 (95% CI 1.3 to 1.4) for high versus no burden. Among those aged ≥65 years, anticholinergic burden was significantly associated with falls and fractures in the Cox model, but the association was less than that for the overall OAB cohort: 1.1 (95% CI 1.0 to 1.2) for low burden versus no burden, 1.2 (95% CI 1.1 to 1.3) for medium burden versus no burden and 1.2 (95% CI 1.1 to 1.3) for high burden versus no burden (table 2). See online supplementary figure 2 for boxplots demonstrating the distribution of estimated weights by time and level of anticholinergic burden.
Table 2.
Adjusted (left) Cox model-estimated and (right) marginal structural model-estimated HRs (95% CI) for the association between falls and fractures and anticholinergic burden in the OAB cohort (n=154 432), including the subgroup aged >65 years (middle); Truven MarketScan databases 2007–2015
| Cox model* | Marginal structural model* | |||||
| Overall population | Subgroup aged >65 years | Overall population | ||||
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| By anticholinergic burden level versus no burden† | ||||||
| Low (1–89) | 1.2 (1.2 to 1.3) | <0.001 | 1.1 (1.0 to 1.2) | 0.006 | 1.2 (1.1 to 1.2) | <0.001 |
| Medium (90–499) | 1.3 (1.2 to 1.4) | <0.001 | 1.2 (1.1 to 1.3) | <0.001 | 1.2 (1.1 to 1.3) | <0.001 |
| High (500+) | 1.4 (1.3 to 1.4) | <0.001 | 1.2 (1.1 to 1.3) | <0.001 | 1.3 (1.3 to 1.4) | <0.001 |
| By age category versus ≤45 | ||||||
| 46–55 | 1.3 (1.2 to 1.3) | <0.001 | 1.7 (1.6 to 1.7)‡ | <0.001 | 1.2 (1.2 to 1.3) | <0.001 |
| 56–65 | 1.5 (1.4 to 1.6) | <0.001 | 1.5 (1.4 to 1.6) | <0.001 | ||
| 66–75 | 2.3 (2.2 to 2.4) | <0.001 | 2.3 (2.1 to 2.5) | <0.001 | ||
| 76–85 | 3.4 (3.2 to 3.6) | <0.001 | 3.5 (3.3 to 3.9) | <0.001 | ||
| 86+ | 5.0 (4.6 to 5.4) | <0.001 | 5.6 (5.0 to 6.3) | <0.001 | ||
| Sex | ||||||
| Women versus men | 1.5 (1.5 to 1.6) | <0.001 | 1.6 (1.5 to 1.7) | <0.001 | 1.5 (1.5 to 1.6) | <0.001 |
| Comorbidity categories at baseline | ||||||
| Cardiovascular diseases§ | 1.1 (1.1 to 1.1) | 0.018 | 1.2 (1.1 to 1.2) | <0.001 | 1.1 (1.0 to 1.1) | 0.043 |
| Neurological impairments | 1.5 (1.4 to 1.6) | <0.001 | 1.7 (1.5 to 1.8) | <0.001 | 1.5 (1.4 to 1.6) | <0.001 |
| Endocrine, nutritional and metabolic diseases | 1.1 (1.1 to 1.2) | <0.001 | 1.2 (1.1 to 1.4) | <0.001 | 1.2 (1.1 to 1.3) | <0.001 |
| Cardiovascular disease×neurological impairments | 1.1 (1.0 to 1.2) | 0.042 | 1.0 (0.9 to 1.1) | 0.945 | 1.1 (1.0 to 1.2) | 0.048 |
| Cardiovascular disease×endocrine, nutritional and metabolic diseases | 1.0 (1.0 to 1.1) | 0.750 | 0.9 (0.8 to 1.0) | 0.118 | 0.9 (0.8 to 1.0) | 0.219 |
| Neurological impairments×endocrine, nutritional and metabolic diseases | 1.1 (1.0 to 1.2) | 0.092 | 1.0 (0.9 to 1.1) | 0.786 | 1.0 (0.9 to 1.2) | 0.558 |
*The Cox models were implemented using function coxph from the R package survival V.2.41–3. The marginal structural model was implemented using function coxph from R package survival V.2.41–3, using the weight argument to apply time-varying weights and setting a cluster term for enrolment ID for robust variance estimation. Time-varying weights were calculated using function ipwtm from R package ipw V.1.0–11 and based on a multinomial logistic regression model (using a generalised logit link) with categorical time-varying anticholinergic burden as the outcome, where age, sex and time-varying comorbidity categories, as well as all two-way interactions between them, were included as predictor variables.
†Level of anticholinergic burden assessed using the closest 6-month measure prior to the fall or fracture.
‡Cardiovascular disease=cerebrovascular disease+stroke.
§For the subgroup analysis among those aged >65 years, age categories for comparison were 65 to <74 years, vs >75 vs <75 years.
OAB, overactive bladder.
Comparison with the non-OAB cohort
To understand the impact of OAB on the association between anticholinergic burden and falls and fractures, outcomes from 86 966 individuals without OAB and 43 483 individuals with OAB were analysed. Both cohorts were 71.0% women and had a mean age of 57.4 years. The mean (SD) Elixhauser comorbidity score was slightly lower in the non-OAB cohort (0.7 [2.9]) than in OAB cohort 2 (1.0 [3.6]), as was the percentage with a fall or fracture in the previous year (2.5% for the non-OAB cohort versus 3.3% for OAB cohort 2). The mean (SD) baseline anticholinergic burden (assessed over 12 months pre-index) for the OAB cohort two was substantially higher (347.6 [553.8]) than that for the non-OAB cohort (89.2 [243.3]), which was reflected in the difference in distribution according to anticholinergic burden level at baseline. In OAB cohort 2, 33.9% had no burden and 25.6% had high burden at baseline, compared with 59.2% with no burden and 4.7% with high burden at baseline in the non-OAB cohort.
The unadjusted rate (95% CI) of falls and fractures per 100 person-years was higher among those with OAB (4.8, 95% CI 4.7 to 5.0) versus those without (3.5, 95% CI 3.5 to 3.6). Rates in OAB cohort 2 ranged from 3.1 (95% CI 2.9 to 3.3) for those with no baseline burden to 6.9 (95% CI 6.6 to 7.3) for high baseline burden; and rates in the non-OAB cohort ranged from 2.7 (95% CI 2.6 to 2.8) for those with no burden to 8.1 (95% CI 7.4 to 8.8) among the small sample with high burden. Overall, those with OAB were at a 1.4-fold (95% CI 1.3 to 1.5) increased risk of falls and fractures compared with those without OAB. RRs ranged from 1.2 (95% CI 1.1 to 1.3) for those with no baseline burden to 0.9 (95% CI 0.8 to 0.9) among those at the highest level of burden (table 3).
Table 3.
Rates and RRs for falls and fractures estimated over the study period in OAB cohort 2 and in the non-OAB cohort, according to baseline anticholinergic burden; Truven MarketScan databases 2007–2015
| OAB cohort 2 | Non-OAB cohort | OAB versus non-OAB RRs | |
| n=43 483 | n=86 966 | n=130 449 | |
| Fall and fracture rates (95% CI) per 100 person-years | |||
| Overall, crude rate | 4.8 (4.7 to 5.0) | 3.5 (3.5, to 3.6) | 1.4 (1.3 to 1.5) |
| By baseline anticholinergic burden level* | |||
| No burden (0) | 3.1 (2.9 to 3.3) | 2.7 (2.6 to 2.8) | 1.2 (1.1 to 1.3) |
| Low (1–89) | 4.3 (4.0 to 4.6) | 3.8 (3.6 to 4.0) | 1.1 (1.0 to 1.2) |
| Medium (90–499) | 5.5 (5.2 to 5.8) | 5.1 (4.9 to 5.4) | 1.1 (1.0 to 1.2) |
| High (500+) | 6.9 (6.6 to 7.3) | 8.1 (7.4 to 8.8) | 0.9 (0.8 to 0.9) |
| RRs, by anticholinergic burden level | |||
| Any burden versus no burden | 1.8 (1.7 to 1.9) | 1.8 (1.7 to 1.9) | |
| Low burden versus no burden | 1.4 (1.3 to 1.5) | 1.4 (1.3 to 1.5) | |
| Medium burden versus no burden | 1.7 (1.6 to 1.9) | 1.9 (1.8 to 2.0) | |
| High burden versus no burden | 2.2 (2.0 to 2.4) | 3.0 (2.7 to 3.3) |
*Baseline anticholinergic burden assessed over the 12-vmonth pre-index period.
OAB, overactive bladder; RR, rate ratio.
Adjusted estimates from the Cox model confirmed the statistically significant association between OAB status and falls and fractures, which are modified by the level of anticholinergic burden (measured at the closest 6-month interval prior to the fall of fracture; see online supplementary table 2). Among those with OAB, the HR for low burden versus no anticholinergic burden was 1.3, that for medium burden versus no anticholinergic burden was 1.3 and that for high burden versus no anticholinergic burden was 1.4. Among those without OAB, the HR for low versus no anticholinergic burden was 1.4, that for medium versus no burden it was 1.4 and that for high versus no burden it was 1.7. Results from the marginal structural model were similar (online supplementary table 2), with boxplots demonstrating the distribution of estimated weights by time and level of anticholinergic burden in online supplementary figure 3.
Discussion
While anticholinergic exposure has been associated with higher rates of falls and fractures among those with other health conditions,3–7 until now, the impact of cumulative anticholinergic burden on the risk of falls and fractures among those with OAB has been unknown. This large cohort study demonstrated that, among those with OAB, higher cumulative anticholinergic burden is associated with a significantly higher rate of falls and fractures. After adjustment for age, sex and key comorbidities, the increased risk of falls and fractures was 23% among those with low burden, 30% among those with medium burden and 38% among those with high burden, compared with those without anticholinergic burden. Rates of falls and fractures were approximately 40% higher among those with OAB than in a non-OAB comparison group. These data suggest that both urinary symptoms and anticholinergic burden are important risk factors for falls and fractures. The magnitude of the dose–response-like association and temporal relationship and the biologic plausibility of the association46 lend credence to possible causality33 between increasing anticholinergic burden and falls and fractures in OAB.
The use of a validated generalisable dataset with a large sample size allowed for the calculation of precise estimates of risk and an understanding of the interplay of factors contributing to falls and fractures. While assessing cumulative exposure revealed a dose–response-like relationship with falls and fractures, validating that measure against cross-sectional assessments will be important. Varied statistical techniques were specified a priori, and results were consistent regardless of the approach selected. Finally, falls and fractures were assigned according to an individual’s OAB status prior to the follow-up period to avoid the potential for misclassification among those who developed OAB during that period.47
As with any retrospective study, the findings are limited by the data and duration of follow-up available. As the study used claims data, misclassification may have occurred if coding is driven by reimbursement-related factors. Additionally, adherence to anticholinergic medications could not be assessed using these data, only that a prescription claim was recorded. Given the sampling frame, findings may not be reflective of outcomes for individuals without or with other types of insurance. As those with intermittent coverage may have been included, both exposure and outcomes may be underestimated. Additionally, anticholinergic use may be underestimated as over-the-counter medications, or those not included in the ACB scale, would not have been captured. Further, many other scales for measuring anticholinergic burden exist, and each considers different medications. While we chose the ACB scale because of its relevance to the United States context and the comprehensive list of medications considered,36 37 the choice of anticholinergic burden scale could impact the results. Limitations to the ACB scale include that the scores assigned to various medications have not been validated against serum anticholinergic activity, and that it omits some medications with anticholinergic activity (eg, gabapentin) in its derivation, which is based on expert consensus and literature review. Finally, it is conceivable that those with higher anticholinergic burden would have more encounters with the medical system within which to detect falls or fractures. We did not adjust for this, however, as the health conditions underlying the increased healthcare resource use would also be on the causal pathway between anticholinergic exposure and falls and fractures.
Few other robust data on the impact of cumulative anticholinergic exposure on falls and fractures among those with OAB exist. A recent US claims-based study found that antimuscarinic-treated patients with OAB were at an almost 50% increased risk of falls and fractures compared with those without OAB, even without measuring overall anticholinergic burden, although those analyses did not account for other important risk factors.48 A borderline significant association was reported between antimuscarinic use and fractures among Taiwanese patients with OAB, although assessment of anticholinergic burden was based on a single dispensation only.49 That increased anticholinergic burden was associated with increased falls and fractures among those with OAB is consistent with findings from those with Parkinson’s disease,5 depression50 and among post-menopausal women.4 Exact estimates of increased risk are difficult to compare directly because most studies measured burden cross-sectionally and not cumulatively. Nonetheless, the available evidence suggests a consistent message of increased falls and fractures risk with increased anticholinergic exposure and that the amount of increased risk depends on the extent of anticholinergic burden, as well as the underlying disease. Future research may build off these findings by evaluating the impact of OAB-specific treatment on OAB symptoms that are risk factors for falls and fractures, while accurately accounting for background level of cumulative anticholinergic burden. This is important as successful management of OAB symptoms with antimuscarinics may, in itself, decrease the risk of falls and fractures.
Older adults had a 3.3-fold increased risk of falls and fractures compared with younger adults with OAB and a 2.0-fold to 3.5-fold increased risk at each level of anticholinergic burden. While we were surprised that the increased risk of falls and fractures associated with anticholinergic burden was less marked among older adults with OAB—a finding consistent in both the unadjusted and adjusted analyses—there are a few plausible explanations. One is that the baseline risk of falls or fractures is much higher among older adults. This suggests that older adults with OAB likely have many predisposing factors other than anticholinergic burden compared with younger patients with OAB for whom anticholinergic burden may be the main contributing risk factor. Regardless of the mechanism, these findings highlight the importance of medication review for falls risk among younger and older patients with OAB.51 52
In an administrative database study of patients with OAB, higher levels of anticholinergic burden are associated with a higher rate of falls and fractures, with those at the highest level at an almost 40% increased risk of falls and fractures compared with those without anticholinergic burden. These data will help inform on the appropriate use of anticholinergic medications among both younger and older patients with OAB with multifaceted comorbidity requiring anticholinergic exposure.53
Supplementary Material
Acknowledgments
We would like to thank Elizabeth Badillo for drafting, reviewing and editing this manuscript. Elizabeth Badillo is an employee of Broadstreet Health Economics & Outcomes Research, which received payment from Astellas.
The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Footnotes
Contributors: SMS and KG were responsible for the conception and design of the work. SMS, GL-O, BR and AD were responsible for data collection. SMS, GL-O, BR, KG, CS, DW, EV, NC and AD were responsible for data analysis and interpretation. SMS was responsible for drafting the article. SMS, KG, CS, DW, GL-O, BR, AD, EV and NC were responsible for critical revision of the article; were responsible for final approval of the version to be published.
Funding: The present study was initiated by Astellas Pharma Global Development, and funding for the conduct of this study was provided by Astellas Pharma Global Development. Publication of the study results was not contingent on permission from the sponsor.
Disclaimer: KG, CS and DW were employees of Astellas Pharma Global Development at the time of study completion. SMS, GL-O, BR and AD were employees of Broadstreet Health Economics & Outcomes Research, which received payment from Astellas to conduct the study. EV and NC received payment from Astellas for consultation.
Competing interests: All authors have completed the ICMJE form for Disclosure of Potential Conflicts of Interest (available on request from the corresponding author). SMS, GL-O, AD and BR declare a relevant conflict of interest for the work under consideration for publication as Broadstreet received funding from Astellas for the design and analysis of the data for this study; they declare no conflicts relative to financial activities outside of the submitted work, intellectual property or other relationships not covered. KC, CS and DW declare a relevant conflict of interest to the work under consideration for publication and for relevant financial activities outside of the submitted work as they are employees of Astellas Pharma, the funder of the study, at the time of study completion; they declare no conflicts relative to intellectual property or other relationships not covered. EV declares a relevant conflict of interest for the work under consideration for publication and for relevant financial activities outside of the submitted work as he received personal fees for consultancy for Astellas Pharma, the study funder; he declares no conflicts relative to intellectual property or other relationships not covered. NC declares a relevant conflict of interest for relevant financial activities outside of the submitted work as he received personal fees for consultancy for Astellas Pharma, the study funder; he declares no conflicts relative to the work under consideration for publication, intellectual property or other relationships not covered.
Ethics approval: Because the Truven MarketScan data are deidentified and are fully Health Insurance Portability and Accountability Act of 1996 compliant, and because this study did not involve the collection, use or transmittal of individually identifiable data, Institutional Review Board review or approval was not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data sharing statement: The authors confirm that all data required to replicate our findings are available for purchase by any researcher from Truven Marketscan via this link: https://marketscan.truvenhealth.com/marketscanportal/
References
- 1. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med 1988;319:1701–7. 10.1056/NEJM198812293192604 [DOI] [PubMed] [Google Scholar]
- 2. Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol 1991;46:M164–70. 10.1093/geronj/46.5.M164 [DOI] [PubMed] [Google Scholar]
- 3. Zia A, Kamaruzzaman S, Myint PK, et al. Anticholinergic burden is associated with recurrent and injurious falls in older individuals. Maturitas 2016;84:32–7. 10.1016/j.maturitas.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 4. Marcum ZA, Wirtz HS, Pettinger M, et al. Anticholinergic medication use and falls in postmenopausal women: findings from the women’s health initiative cohort study. BMC Geriatr 2016;16:76 10.1186/s12877-016-0251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Crispo JA, Willis AW, Thibault DP, et al. Associations between anticholinergic burden and adverse health outcomes in Parkinson disease. PLoS One 2016;11:e0150621 10.1371/journal.pone.0150621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aizenberg D, Sigler M, Weizman A, et al. Anticholinergic burden and the risk of falls among elderly psychiatric inpatients: a 4-year case-control study. Int Psychogeriatr 2002;14:307–10. 10.1017/S1041610202008505 [DOI] [PubMed] [Google Scholar]
- 7. Marcum ZA, Perera S, Thorpe JM, et al. Anticholinergic use and recurrent falls in community-dwelling older adults: findings from the health ABC study. Ann Pharmacother 2015;49:1214–21. 10.1177/1060028015596998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pariente A, Dartigues JF, Benichou J, et al. Benzodiazepines and injurious falls in community dwelling elders. Drugs Aging 2008;25:61–70. 10.2165/00002512-200825010-00007 [DOI] [PubMed] [Google Scholar]
- 9. O’Loughlin JL, Robitaille Y, Boivin JF, et al. Incidence of and risk factors for falls and injurious falls among the community-dwelling elderly. Am J Epidemiol 1993;137:342–54. 10.1093/oxfordjournals.aje.a116681 [DOI] [PubMed] [Google Scholar]
- 10. Muir SW, Gopaul K, Montero Odasso MM. The role of cognitive impairment in fall risk among older adults: a systematic review and meta-analysis. Age Ageing 2012;41:299–308. 10.1093/ageing/afs012 [DOI] [PubMed] [Google Scholar]
- 11. Takazawa K, Arisawa K. Relationship between the type of urinary incontinence and falls among frail elderly women in Japan. J Med Invest 2005;52(3-4):165–71. 10.2152/jmi.52.165 [DOI] [PubMed] [Google Scholar]
- 12. Nakagawa H, Niu K, Hozawa A, et al. Impact of nocturia on bone fracture and mortality in older individuals: a Japanese longitudinal cohort study. J Urol 2010;184:1413–8. 10.1016/j.juro.2010.05.093 [DOI] [PubMed] [Google Scholar]
- 13. Vaughan CP, Brown CJ, Goode PS, et al. The association of nocturia with incident falls in an elderly community-dwelling cohort. Int J Clin Pract 2010;64:577–83. 10.1111/j.1742-1241.2009.02326.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dionyssiotis Y. Analyzing the problem of falls among older people. Int J Gen Med 2012;5:805–13. 10.2147/IJGM.S32651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wagner TH, Hu TW, Bentkover J, et al. Health-related consequences of overactive bladder. Am J Manag Care 2002;8(19 Suppl):S598–607. [PubMed] [Google Scholar]
- 16. Coyne KS, Wein A, Nicholson S, et al. Comorbidities and personal burden of urgency urinary incontinence: a systematic review. Int J Clin Pract 2013;67:1015–33. 10.1111/ijcp.12164 [DOI] [PubMed] [Google Scholar]
- 17. Jayadevappa R, Chhatre S, Newman DK, et al. Association between overactive bladder treatment and falls among older adults. Neurourol Urodyn 2018;37:2688–94. 10.1002/nau.23719 [DOI] [PubMed] [Google Scholar]
- 18. Dumoulin C, Hay-Smith J. Pelvic floor muscle training versus no treatment for urinary incontinence in women. A Cochrane systematic review. Eur J Phys Rehabil Med 2008;44:47–63. [PubMed] [Google Scholar]
- 19. Gormley EA, Lightner DJ, Faraday M, et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol 2015;193:1572–80. 10.1016/j.juro.2015.01.087 [DOI] [PubMed] [Google Scholar]
- 20. Gomes T, Juurlink DN, Ho JM, Jmw H, et al. Risk of serious falls associated with oxybutynin and tolterodine: a population based study. J Urol 2011;186:1340–4. 10.1016/j.juro.2011.05.077 [DOI] [PubMed] [Google Scholar]
- 21. Szabo SM, Gooch KL, Walker DR, et al. The association between overactive bladder and falls and fractures: a systematic review. Adv Ther 2018;35:1831–41. 10.1007/s12325-018-0796-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. ISPOR European Meeting. The risk of falls and fractures among those with overactive bladder (OAB): a systematic review. Vienna, Austria, 2016. [Google Scholar]
- 23. Zinner NR, Mattiasson A, Stanton SL. Efficacy, safety, and tolerability of extended-release once-daily tolterodine treatment for overactive bladder in older versus younger patients. J Am Geriatr Soc 2002;50:799–807. 10.1046/j.1532-5415.2002.50203.x [DOI] [PubMed] [Google Scholar]
- 24. Dubeau CE, Kraus SR, Griebling TL, et al. Effect of fesoterodine in vulnerable elderly subjects with urgency incontinence: a double-blind, placebo controlled trial. J Urol 2014;191:395–404. 10.1016/j.juro.2013.08.027 [DOI] [PubMed] [Google Scholar]
- 25. Batista JE, Kölbl H, Herschorn S, et al. The efficacy and safety of mirabegron compared with solifenacin in overactive bladder patients dissatisfied with previous antimuscarinic treatment due to lack of efficacy: results of a noninferiority, randomized, phase IIIb trial. Ther Adv Urol 2015;7:167–79. 10.1177/1756287215589250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Onukwugha E, Zuckerman IH, McNally D, et al. The total economic burden of overactive bladder in the United States: a disease-specific approach. Am J Manag Care 2009;15(4 Suppl):S90–7. [PubMed] [Google Scholar]
- 27. Hausdorff JM. Gait variability: methods, modeling and meaning. J Neuroeng Rehabil 2005;2:19 10.1186/1743-0003-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Darkow T, Fontes CL, Williamson TE. Costs associated with the management of overactive bladder and related comorbidities. Pharmacotherapy 2005;25:511–9. 10.1592/phco.25.4.511.61033 [DOI] [PubMed] [Google Scholar]
- 29. IBM Watson Health.. MarketScan Databases. 2018. http://truvenhealth.com/markets/life-sciences/products/data-tools/marketscan-databases.
- 30. Wu JM, Matthews CA, Conover MM, et al. Lifetime risk of stress urinary incontinence or pelvic organ prolapse surgery. Obstet Gynecol 2014;123:1201–6. 10.1097/AOG.0000000000000286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marsico M, Mehta V, Chastek B, et al. Estimating the incidence and prevalence of juvenile-onset recurrent respiratory papillomatosis in publicly and privately insured claims databases in the United States. Sex Transm Dis 2014;41:300–5. 10.1097/OLQ.0000000000000115 [DOI] [PubMed] [Google Scholar]
- 32. Gauthier G, Guérin A, Zhdanava M, et al. Treatment patterns, healthcare resource utilization, and costs following first-line antidepressant treatment in major depressive disorder: a retrospective US claims database analysis. BMC Psychiatry 2017;17:222 10.1186/s12888-017-1385-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd edn Philadelphia: Wolters Kluwer Health/Lippincott Williams & Wilkins, 2008. [Google Scholar]
- 34. Durden E, Walker D, Gray S, et al. The direct and indirect costs associated with overactive bladder within a commercially-insured population in the United States. J Occup Environ Med 2018;60:847–52. 10.1097/JOM.0000000000001367 [DOI] [PubMed] [Google Scholar]
- 35. Richardson K, Bennett K, Maidment ID, et al. Use of medications with anticholinergic activity and self-reported injurious falls in older community-dwelling adults. J Am Geriatr Soc 2015;63:1561–9. 10.1111/jgs.13543 [DOI] [PubMed] [Google Scholar]
- 36. Boustani M, Campbell N, Munger S, et al. Impact of anticholinergics on the aging brain: a review and practical application. Aging health 2008;4:311–20. 10.2217/1745509X.4.3.311 [DOI] [Google Scholar]
- 37. Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr 2015;15:31 10.1186/s12877-015-0029-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. ISPOR 22nd Annual International Meeting. Assessment of anticholinergic burden scales and measures for estimating anticholinergic exposure in retrospective US database analyses. Baltimore, MD, 2018. [Google Scholar]
- 39. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD Index. 2017. http://www.whocc.no/atc_ddd_index/.
- 40. WHO Collaborating Centre for Drug Statistics Methodology. Definition and general considerations. https://www.whocc.no/ddd/definition_and_general_considera/.
- 41. Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015;175:401–7. 10.1001/jamainternmed.2014.7663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Elixhauser A, Steiner C, Harris DR, et al. Comorbidity measures for use with administrative data. Med Care 1998;36:8–27. 10.1097/00005650-199801000-00004 [DOI] [PubMed] [Google Scholar]
- 43. Vonesh E. Generalized linear and nonlinear models for correlated data: theory and applications using SAS. Cary, NC: SAS Institute Inc, 2012. [Google Scholar]
- 44. Hernán MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology 2000;11:561–70. 10.1097/00001648-200009000-00012 [DOI] [PubMed] [Google Scholar]
- 45. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. 10.1097/00001648-200009000-00011 [DOI] [PubMed] [Google Scholar]
- 46. Collamati A, Martone AM, Poscia A, et al. Anticholinergic drugs and negative outcomes in the older population: from biological plausibility to clinical evidence. Aging Clin Exp Res 2016;28:25–35. 10.1007/s40520-015-0359-7 [DOI] [PubMed] [Google Scholar]
- 47. Rothman KG. Modern Epidemiology. 2012.
- 48. Yehoshua A, Chancellor M, Vasavada S, et al. Health resource utilization and cost for patients with incontinent overactive bladder treated with anticholinergics. J Manag Care Spec Pharm 2016;22:406–13. 10.18553/jmcp.2016.22.4.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kao LT, Huang CY, Lin HC, et al. No increased risk of fracture in patients receiving antimuscarinics for overactive bladder syndrome: A retrospective cohort study. J Clin Pharmacol 2018;58:727–32. 10.1002/jcph.1067 [DOI] [PubMed] [Google Scholar]
- 50. Chatterjee S, Bali V, Carnahan RM, et al. Anticholinergic medication use and risk of fracture in elderly adults with depression. J Am Geriatr Soc 2016;64:1492–7. 10.1111/jgs.14182 [DOI] [PubMed] [Google Scholar]
- 51. Macdiarmid SA. Maximizing the treatment of overactive bladder in the elderly. Rev Urol 2008;10:6–13. [PMC free article] [PubMed] [Google Scholar]
- 52. Talasz H, Lechleitner M. Polypharmacy and incontinence. Z Gerontol Geriatr 2012;45:464–7. 10.1007/s00391-012-0358-7 [DOI] [PubMed] [Google Scholar]
- 53. Maman K, Aballea S, Nazir J, et al. Comparative efficacy and safety of medical treatments for the management of overactive bladder: a systematic literature review and mixed treatment comparison. Eur Urol 2014;65:755–65. 10.1016/j.eururo.2013.11.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2018-026391supp001.pdf (778.7KB, pdf)