Abstract
Purpose
We evaluated the repeatability of wide-field en face swept-source optical coherence tomography angiography (SS-OCTA) in healthy subjects.
Methods
Healthy subjects underwent two imaging sessions, on average 8 days apart, with a 100 kHz SS-OCTA instrument. The imaging protocol included a central 3 × 3 and 12 × 12 mm scans of the four quadrants resulting in more than a 70° wide-field OCTA of the posterior pole. Quantitative analysis was performed using the inbuilt Macular Density Algorithm Version v0.6.1 and AngioTool software. Consistency for the foveal avascular zone (FAZ), vessel density, and perfusion density of the superficial and deep capillary plexus slabs and the wide-field OCTA superficial slab, and the number of artefacts on the wide-field images were assessed.
Results
A total of 21 healthy volunteers (seven men and 14 women; mean age 32 years; range, 18–61; standard deviation, 10.28 years) were included in this analysis. Internal consistency was highest for FAZ area with an intraclass correlation (ICC) = 0.998 (95% confidence interval [CI], 0.997–0.999), a FAZ perimeter with an ICC = 0.995 (95% CI, 0.990–0.997), a FAZ circularity with an ICC= 0.976 (95% CI, 0.956–0.987), followed by the vessel density of the inner ring in the superficial slab with an ICC = 0.834 (95% CI, 0.691–0.911), and a vessel density of the inner ring in the deep slab with an ICC = 0.523 (95% CI, 0.113–0.744).
The reproducibility of the average vessels length of the wide-field OCTA cropped images was strong (ICC = 0.801; 95% CI, 0.624–0.895), followed by the reproducibility of total number of junctions (ICC = 0.795; 95% CI, 0.613–0.892) and the vessels percentage area (ICC = 0.662; 95% CI, 0.361–0.821).
Conclusions
The level of reproducibility for assessing the microvascular anatomy in wide-field OCTA is strong and can be used to quantify microvascular changes over time. Refinements in analysis strategies and a consensus of which parameters are most useful for quantitative assessment of wide-field OCTA images would be useful in the future.
Translational Relevance
These findings bridge the gap between basic imaging research and clinical use for quantitative wide-field OCTA.
Keywords: optical coherence tomography angiography, repeatability, retinal imaging
Introduction
Optical coherence tomography angiography (OCTA) is an imaging technique based on motion contrast and allows assessment of the microvascular anatomy of the retina without dye injection.1,2 Whereas the scanning range of OCTA was initially limited to the macula, technical advances, such as swept source OCT (SS-OCT), now allow scanning larger areas of the posterior pole. The PLEX Elite 9000 is a SS-OCT device containing a tunable laser with a central wavelength of 1060 nm and is able to scan areas of 12 × 12 mm, which can be extended to 70° to 80° by montage of scans in the four quadrants.3
Although wide-field SS-OCTA is assumed to be useful for wider and noninvasive evaluation of retinal vascular anatomy especially in the setting of retinal vascular disease, such as diabetic retinopathy or retinal vein occlusion, little research has been directed at investigating the repeatability of wide-field SS-OCTA.4 In this study, we examined the repeatability of wide-field SS-OCTA in healthy eyes.
Methods
This prospective observational study included 21 healthy volunteers of the outpatient clinic of the ophthalmology department of the University Hospital Bern, Switzerland between October 2017 and December 2017 (ClinicalTrials.gov registration No. NCT02811536). Approval for the collection and analysis of SS-OCTA images was obtained from the ethics committee at the University of Bern, Switzerland. The study was performed in accordance with International Conference on Harmonisation-Good Clinical Practice (ICH-GCP) guidelines, which correspond to the Health Insurance Portability and Accountability Act of 1996 (HIPAA) regulations. Written informed consent was obtained from all participants.
SS-OCTA images centered on the fovea with a scanning size of 3 × 3 mm and five additional 12 × 12 mm raster scans centered on the fovea, and temporal superior, temporal inferior, nasal superior, and nasal inferior quadrants were acquired using the PLEX Elite 9000 scanner (Carl Zeiss Meditec, Inc., Dublin, CA). The 3 × 3 mm raster scan consists of 300 A-scans per B-scan (with 10 μm spacing between adjacent scans), four B-scan repetitions per location and 300 B-scan positions in the raster. The 12 × 12 mm scanning pattern consists of 500 A-scans per B-scan (24 μm spacing between adjacent scans), 500 B-scans in the cube with two B-scan repetitions per location. The OCTA wide-field images consisted of five 12 × 12 mm scans and were automatically merged by the manufacturer's software covering all four quadrants and 70° to 80° of the posterior pole.
Evaluation of the Retinal Vasculature
Vessel and perfusion density as well as the foveal avascular zone (FAZ) areas for the central 3 × 3 mm scan were obtained by the software Macular Density Algorithm Version v0.6.1 (Carl Zeiss Meditec, Inc.) where perfusion density was defined as the total area of perfused vasculature per unit area in a region of measurement. The unitless results ranged from 0 (no perfusion) to 1 (fully perfused). Vessel density refers to the total length of perfused vasculature per unit area in a region of measurement in units of inverse millimeters.
Vessel density of the wide-field OCTA images was calculated with the AngioTool software for each imaging session (available in the public domain at https://ccrod.cancer.gov/confluence/display/ROB2/Downloads), which is a validated source for measuring vascular networks.5 The SS-OCTA wide-field images were cropped to include only imaged areas of the retina. Lacunarity describes the distribution of the sizes of gaps or lacunae surrounding the object within the image.6 AngioTool also provides a measure of number of vessel junctions, by computing the “branching index” (branch points/unit area) of the vascular network analyzed.5
Evaluation of Imaging Artefacts
Imaging artefacts were assessed by two graders (ME and MT). Motion artefacts were considered present when a white-line was detectable on the OCTA image with concomitant lateral displacement on B-scan images. Projection artefacts as well as motion artefacts,7,8 displacement artefacts (discontinuous blood vessels), shadowing (attenuation of the signal), vessel doubling (two copies of a blood vessel), and white line artefacts were assessed on sequential OCTA images.7
Statistical Analysis
Intraclass correlation coefficient (ICC) estimates and their 95% confident intervals (CIs) were calculated using SPSS statistical package version 21 (SPSS, Inc., Chicago, IL) based on a mean-rating (k = 2), absolute-agreement, 2-way mixed-effects model. Bland Altman plots were calculated with GraphPad Prism 5.0 software (GraphPad Software, Inc., San Diego, CA).
Results
Forty-two eyes of 21 volunteers (14 females and 7 males; average age, 32 years; range, 18–61 years; standard deviation [SD] ±10) were included. Mean interval between imaging sessions was 8 (9 ± 3) days.
Repeatability of Microvascular Anatomy in the Central 3 × 3 mm Scan
In the 3 × 3 mm scan average FAZ area was 0.2288 mm2 for the first and 0.2287 mm2 for the second scan. Internal consistency was highest for FAZ area with an ICC = 0.998 (95% CI, 0.997–0.999), followed by the FAZ Perimeter with an ICC = 0.995 (95% CI, 0.990–0.997) and FAZ Circularity with an ICC = 0.976 (95% CI, 0.956–0.987; Fig. 1). Quantification of the microvascular anatomy revealed the following information: The vessel density of the inner ring in the superficial layer showed an ICC = 0.834 (95% CI, 0.691–0.911), the perfusion density of the inner ring in the superficial layer an ICC = 0.269 (95% CI, −0.360–0.607), the inner ring deep layer vessel density an ICC = 0.523 (95% CI, 0.113–0.744), and the inner ring perfusion density of the deep layer an ICC = 0.532 (95% CI, 0.129–0.748; Fig. 2).
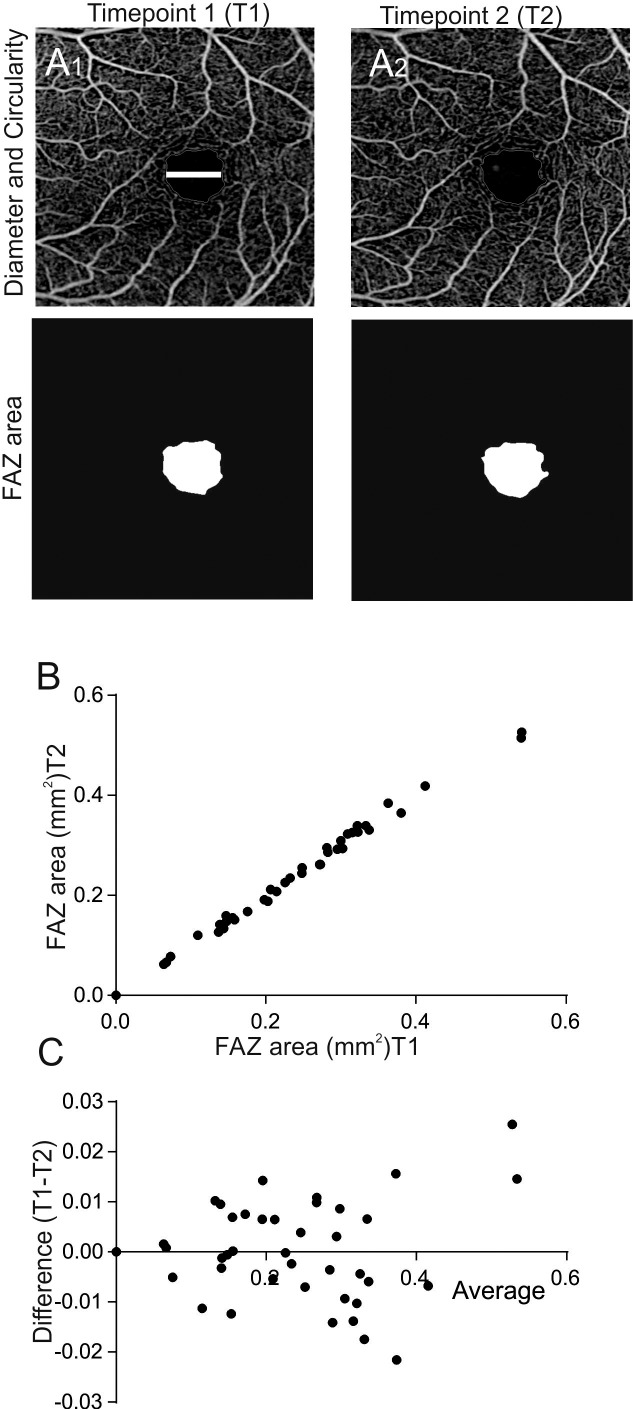
Figure 1.
Analysis of repeatability of FAZ parameters using OCTA. (A) Representative 3 × 3 mm OCTA scans showing the diameter and the circularity of the FAZ at time point 1 (A1, top) and time point 2 (A2, top). The white bar represents the FAZ diameter, the red line shows how the FAZ circularity was measured. Representative images of FAZ area at time points 1 and 2. (B) Correlation plot of FAZ area measured at two time points (n = 42, r = 0.997, P < 0.001). Four FAZ areas of 0 were calculated due to a software error (the imaging system was not able to detect the FAZ area). (C) Corresponding Bland-Altman plot shows the difference between T1 and T2 plotted against the average.
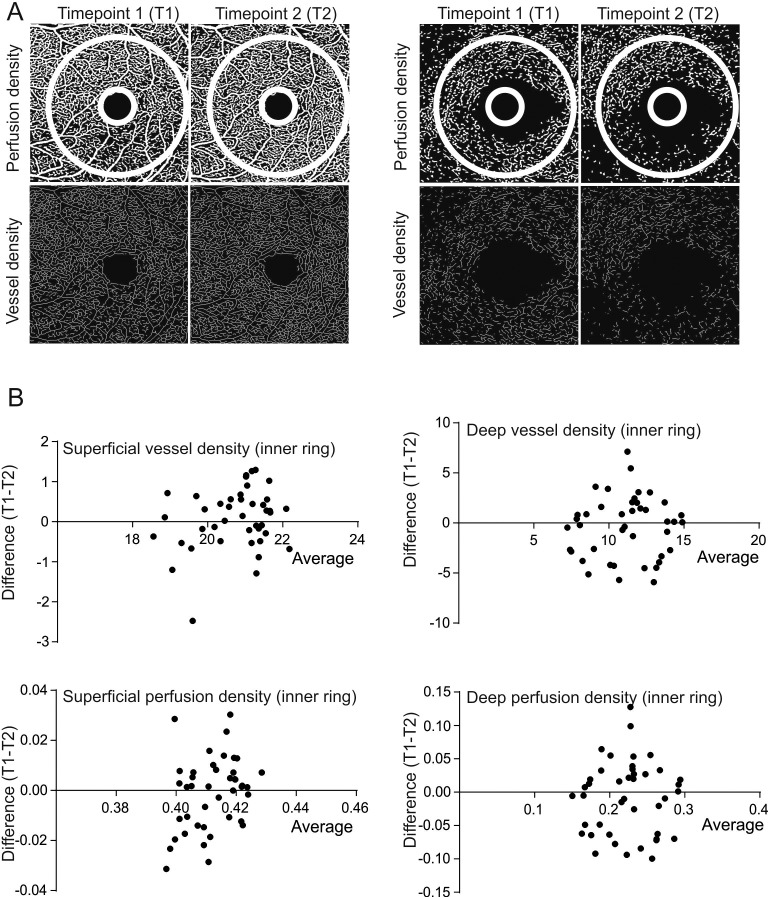
Figure 2.
Analysis of repeatability of vessel and perfusion density for the central 3 × 3 mm scan. (A) Representative images showing perfusion and vessel density analysis images for the superficial and deep retinal layers at two consecutive time points (T1 and T2). The white circle represents the area analyzed by the inbuilt OCTA device algorithm. (B) Bland-Altman plots show the level of agreement for the vessel density and perfusion density for the superficial and deep retinal layers.
Repeatability of Microvascular Anatomy in Wide-Field SS-OCTA
For wide-field SS-OCTA montage covering approximately 70° to 80° of the posterior pole, the vessels percentage area was 31.69% ± 1.588% for the first and 31.7% ± 1.895% for the second scan resulting in an ICC of 0.662 (95% CI, 0.361–0.821; Fig. 3). The following ICCs were determined: 0.854 (95% CI, 0.723–0.923) for the total number of end points, 0.845 (95% CI, 0.707–0.918) for total vessels length, 0.801 (95% CI, 0.624–0.895) for average vessels length, 0.795 (95% CI, 0.613–0.892) for total number of junctions, 0.677 (95% CI, 0.390–0.829) for mean lacunarity, and 0.662 (95% CI, 0.361–0.821) for vessel percentage area.
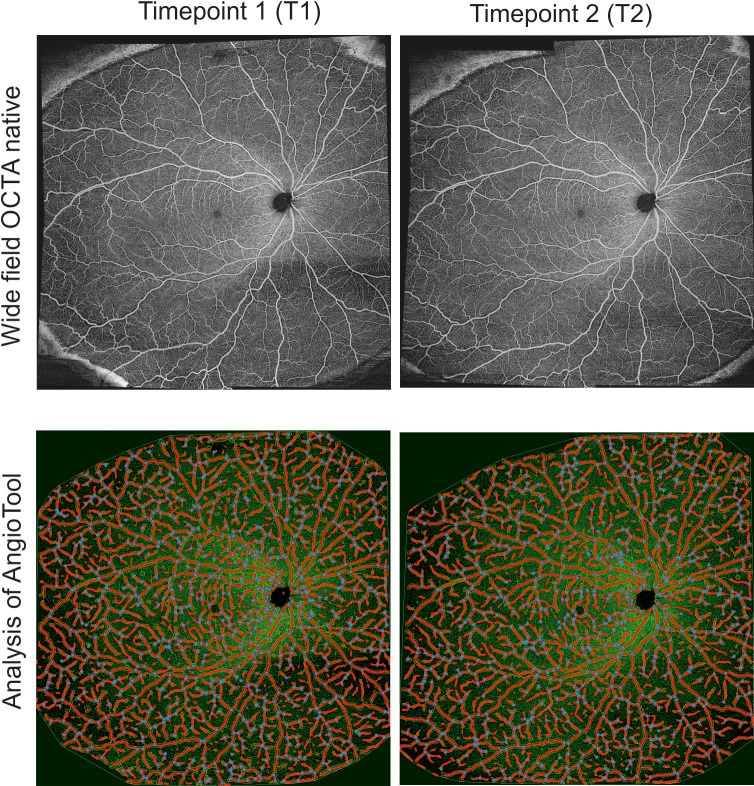
Figure 3.
Wide-field OCTA montage images of the superficial slab obtained with the PLEX Elite 9000 OCTA device. A representative wide-field OCTA image of the right eye is shown for two time points (top) and corresponding analysis using the AngioTool software (bottom). The blue dots represent junctions/branching points of the retinal vessels.
Analysis of Imaging Artefacts
Imaging artefacts as described by Spaide et al.7 were present in 100% of wide-field SS-OCTA slabs. The most prevalent imaging artefact was displacement artefact (present in 96.34%), followed by shadowing (present in 92.27%), white line artefacts (present in 63.41%), and vessel doubling (present in 35.37%). These values show the percentage of images where at least one artefact was present.
Internal consistency of artefact assessment was quite low with an ICC of 0.220 for displacement artefacts (95% CI, −0.474–0.558), 0.559 (95% CI, 0.166–0.767) for shadowing, 0.567 (95% CI, 0.181–0.771) for vessel doubling, and 0.620 (95% CI, 0.281–0.779) for white line artefacts (Fig. 4).
Figure 4.

Representative images show imaging artefacts using wide-field OCTA. (A) Small red circle (ROI) shows a displacement artefact. Enlargement of ROI represented by the large red circle shows discontinuous blood vessels. (B) Small red circle shows a shadowing artefact and corresponding enlargement of region of interest where an attenuation of the signal with loss of contrast can be seen. (C) White line artefact shown within red oval. Enlargement (insert) shows erroneously appearing lines (in this case a white horizontal line resembling a vessel; D) Vessel doubling artefact denoted by the red circle. Enlargement of this region (large red circle) shows that the same blood vessel appears twice in the image.
Discussion
Because of the recent advances in OCTA imaging with the capabilities of some devices to image retinal vasculature beyond the temporal arcades, it is important to establish reliability and repeatability for microvascular quantitative metrics. We examined the repeatability of quantitative parameters of wide-field SS-OCTA using the swept-source PLEX Elite 9000 scanner in healthy subjects.
So far, repeatability of different OCT-A devices has been focused mainly on the central 3 × 3 mm scan mode with the majority of studies focusing on FAZ area and diameter. The data of other studies are in keeping with our data, with excellent repeatability of the FAZ area using various OCTA devices. A report published in 2016 showed that ICC values for two consecutive scanning sessions for the superficial FAZ area were 0.95, superficial FAZ perimeter 0.93, deep FAZ area 0.84, and deep FAZ perimeter 0.77.9 Previous reports about the robustness of FAZ parameters and vessel density in 3 × 3 and 6 × 6 mm scans using the Angiovue OCT device found the FAZ parameters to be a robust outcome parameter and the vessel density to be more variable in particular in the 6 × 6 mm scans.10
For other metrics within the 3 × 3 mm scan area, such as perfusion density, the ICCs are generally lower, ranging from 0.3 to 0.9 across different studies, while the repeatability of the foveal vascular density found an excellent repeatability with ICCs ranging between 0.873 and 0.977. 9,11,12 This may be due to the fact that in vessel density (also called vessel skeleton density) the size of the vessel is negligible and all vessels are treated equally. This makes the parameter more sensitive to capillary dropout and flow void in capillaries, but therefore, also makes it more prone to noise and artefacts. The perfusion density in turn, evaluates the total area occupied by perfused vasculature per unit; therefore, larger vessels influence the measurement more than smaller vessels, which makes the parameter more robust, but also less sensitive for changes of the capillaries. Also, the trend that the deep vascular network yields lower repeatability values is in keeping with our findings.11
In our study, the repeatability of metrics of the vessel density of the superficial layer had an ICC of 0.834, whereas the vessel density of the deep layer showed a fair repeatability with an ICC of 0.523. This is likely due to the fact that the deep layers are more prone to projection artefacts.
The scan pattern used has been shown to affect repeatability of OCTA technology, which is in keeping with our data. Whereas the perfusion density of the superficial layer in the 3 × 3 mm was lowest with an ICC of 0.269 (95% CI, −0.360–0.607), the ICC in the wide-field SS-OCTA montage was 0.677 (95% CI, 0.390–0.829). As already pointed out in an earlier study, the reason for the lower repeatability score in the central 3 mm scan compared to the 6 mm or wide-field scan may be that the centration of the macula within the scan area has a greater impact on the 3 mm scan.11 Also the lower resolution in the 6 × 6 and 12 × 12 mm scans may contribute to this deviation, as well as the depiction of the peripapillary network, which is very dense. These differences highlight the critical need not only to use the same and consistent scan pattern if vessel length density (VLD) and perfusion density (PD) measurements are compared between different visits, but also the need for a uniform analysis platform within studies. Quantitative metrics of wide-field OCTA, such as the total number of end points as well as the total vessels length showed an excellent repeatability. As such, OCTA montage images may be useful for longitudinal studies investigating retinal vascular pathologies and may have advantages to other wide-field imaging modalities, such as ultra-wide-field fluorescein angiography.4
Our study has several limitations that must be considered. The overall cohort sizes were relatively small and quantification of anatomical features was assessed with two different analysis algorithms. The central 3 × 3 mm scan was analyzed by the customized software provided via the ARI-network platform, while the wide-field montage was analyzed by the AngioTool software and, as such, some of the variability may be explained by the algorithm used. Furthermore, we focused only on the superficial retinal vasculature for wide-field OCTA data, primarily because this layer provided the best contrast for retinal vessels and because the resolution of 24 μm on the 12 × 12 mm scans may make exact evaluation challenging and error prone. Although refinements in data processing and analysis will be necessary to render quantitative OCTA more user friendly, we were able to show that quantitative analysis of the retinal microvasculature can be performed with a high degree of repeatability.
Acknowledgments
Disclosure: M. Eastline, Zeiss (F); M.R. Munk, Zeiss (F), Lumithera (C), Bayer (C); S. Wolf, Allergan (C), Bayer (C), Novartis (C), Heidelberg Engineering (C), Zeiss (C), Chengdu Kanghong (C); K.B. Schaal, Zeiss (F); A. Ebneter, Zeiss (F), Bayer (C), Allergan (C), Novartis (C); M. Tian, Zeiss (F); H. Giannakaki-Zimmermann, Zeiss (F); M.S. Zinkernagel, Zeiss (F), Allergan (C), Bayer (F,C), Novartis (C,I), Heidelberg Engineering (F)
References
- 1.Jia Y, Bailey ST, Wilson DJ, et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology. 2014;121:1435–1444. doi: 10.1016/j.ophtha.2014.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Watanabe Y, Takahashi Y, Numazawa H. Graphics processing unit accelerated intensity-based optical coherence tomography angiography using differential frames with real-time motion correction. J Biomed Opt. 2014;19:021105. doi: 10.1117/1.JBO.19.2.021105. [DOI] [PubMed] [Google Scholar]
- 3.Schaal KB, Munk MR, Wyssmueller I, Berger LE, Zinkernagel MS, Wolf S. Vascular abnormalities in diabetic retinopathy assessed with swept-source optical coherence tomography angiography Widefield Imaging. Retina. 2019;39:79–87. doi: 10.1097/IAE.0000000000001938. [DOI] [PubMed] [Google Scholar]
- 4.Or C, Sabrosa AS, Sorour O, Arya M, Waheed N. Use of OCTA, FA, and ultra-widefield imaging in quantifying retinal ischemia: a review. Asia-Pacific J Ophthalmol. 2018;7:46–51. doi: 10.22608/APO.201812. [DOI] [PubMed] [Google Scholar]
- 5.Zudaire E, Gambardella L, Kurcz C, Vermeren S. A computational tool for quantitative analysis of vascular networks. PLoS One. 2011;6:e27385. doi: 10.1371/journal.pone.0027385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gould DJ, Vadakkan TJ, Poche RA, Dickinson ME. Multifractal and lacunarity analysis of microvascular morphology and remodeling. Microcirculation. 2011;18:136–151. doi: 10.1111/j.1549-8719.2010.00075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaide RF, Fujimoto JG, Waheed NK. Image artifacts in optical coherence tomography angiography. Retina. 2015;35:2163–2180. doi: 10.1097/IAE.0000000000000765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Vitis LA, Benatti L, Tomasso L, et al. Comparison of the performance of two different spectral-domain optical coherence tomography angiography devices in clinical practice. Ophthal Res. 2016;56:155–162. doi: 10.1159/000447094. [DOI] [PubMed] [Google Scholar]
- 9.Shahlaee A, Pefkianaki M, Hsu J, Ho AC. Measurement of foveal avascular zone dimensions and its reliability in healthy eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;161:50–55. doi: 10.1016/j.ajo.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 10.Chen FK, Menghini M, Hansen A, Mackey DA, Constable IJ, Sampson DM. Intrasession repeatability and interocular symmetry of foveal avascular zone and retinal vessel density in OCT angiography. Transl Vis Sci Technol. 2018;7:6. doi: 10.1167/tvst.7.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lei J, Durbin MK, Shi Y, et al. Repeatability and reproducibility of superficial macular retinal vessel density measurements using optical coherence tomography angiography en face images. JAMA Ophthalmol. 2017;135:1092–1098. doi: 10.1001/jamaophthalmol.2017.3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yanik Odabas O, Demirel S, Ozmert E, Batioglu F. Repeatability of automated vessel density and superficial and deep foveal avascular zone area measurements using optical coherence tomography angiography: diurnal findings. Retina. 2017;38:1238–1245. doi: 10.1097/IAE.0000000000001671. [DOI] [PubMed] [Google Scholar]