Abstract
Colorectal cancer found to be the most commonly occurring cancer worldwide which can be prevented by screening and its curable if diagnosed early. Lynch syndrome/HNPCC being an autosomal genetic disease and propensity in forming colorectal cancer is inherited wherein genomic instabilities and epigenetic changes are being the characteristic forms in hereditary cancers. It is very important to determine the polymorphism in several DNA repairing genes such as ATM, RAD51, XRCC2, XRCC3 and XRCC9 to study the risk exploring both the prognosis and the developing of colorectal cancer. The role of ATM gene has been studied which involves in the hereditary transfer of colorectal cancer associated with other related cancers such as stomach, lung and breast cancers. ATM found to be the mutation target and also a modifier gene with more risk of developing the disease by its polymorphism in variant of ATM D1853N. It was identified that ATM gene polymorphism did not drastically change HNPCC age of onset. ATM expression levels were studied and it has been concluded that the complete loss of ATM expression resulted in a propensity of worse survival and no better prognosis with increase in mortality rate. This ATM gene might be considered to be a predicted biomarker in colorectal cancer. (www.actabiomedica.it)
Keywords: ataxia-telangiectasia mutated, colorectal cancer, epigenetic change, lynch syndrome, polymorphism
Introduction
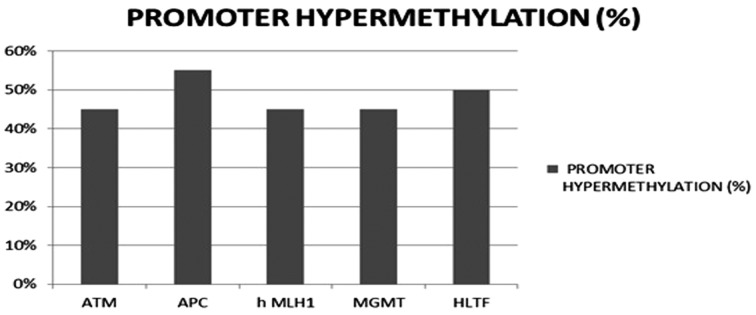
Colorectal cancer is found to be the fourth commonly occurring cancer worldwide (1, 2). It usually occurs from a pre-cancerous growth called a polyp in a very predictable way. It has been recognized that the ceaseless assembly of activation of oncogene and deactivation of tumor suppressor genes is primarily linked with the advancement of colorectal adenoma to cancer where the genes located on small arm of the chromosome 17 and large arm of the chromosome 18 which plays a significant part in the advancement of colon cancer (3). The primary alteration of genetic and epigenetic occurs that in succession promotes the colon cancer formation as they provide clonal expansion or clonal growth advantage to the cell. Thus, this cancer starts via a numerous step progression at both molecular level and morphological extent leading to the loss of genomic stability which is a primary molecular step in cancer emergence. Finally, hereditary cancer syndromes often tend to the germ line arrangement of key genetic flaws, which trigger the appearance of infrequent colon cancers. It is now widely accepted that the different types of genomic instability may result in distinct molecular pathways of carcinogenesis (33, 34). Three forms of genetic uncertainty in colorectal cancer have been reported (4). In 13% of colorectal cancer case studies, mismatch repair insufficiency tends to microsatellite instability. Around 40% colorectal cancer tumors are distinguished by epigenetic modifications mainly DNA methylation; an event known as CpG Islands Methylator Phenotype (CIMP). CIMP is more often detected in tumors with proximal location, microsatellite instability. About 15% of microsatellite instability is detected in colon cancer where 3% of these are linked to Lynch syndrome and the remaining 12% caused by sporadic, which has promoter hypermethylation of MLH1 gene occurring in the malignancy with the CpG island methylator phenotype but the part of this hypermethylation in previous stage of colorectal carcinogenesis, specifically in adenomas and non-cancer tissues of cancer patients, is not that well explained (5-7). In the last 47% of colorectal cancer, chromosomal uncertainty escort to gains and losses of large segments of chromosomes. This chromosomal instability leads to a pattern of gene alterations which culminates in tumor formation (8). All cancers are inherited of about 5-10% having the most in an autosomal dominant way with deficient penetrance (9). Ataxia-telangiectasia mutated gene was identified to change the phenotypic expression/pronouncement of HNPCC. This gene was linked with notably increased occurrenceof colon and other HNPCC-related cancers (10). Germ-line ATM mutations will not display the exact phenotype of the disease but are examined at more risk of developing epithelial tumors, such as breast and stomach cancers. The feasible involvement of ATM in a wide diversity of human solid cancers has also been recommended. Allelic loss studies have stipulated that the chromosome11q22.3 region, comprising the ATM locus, is often modified in many cancers, such as cancers of the breast, colon, lung, cervix and in neuroblastoma. ATM is considered to be a mutation target of microsatellite instability where abnormal transcripts are originated indirectly by intron mutations (11). Promoter hypermethylation were often recognized in more than 40% of colonic cancers and adenomas in APC, ATM, MGMT, HLTF, hMLH1 genes which was found to be more prone in older patients (12) (Fig. 1).
Figure 1.
Promoter hypermethylation recognized in colon cancers-following genes
Literature review
A study involving 167 Swiss individuals from around 20 HNPCC families was reported byP. Maillet et al. (2000), analyzed the genomic DNA from peripheral lymphocytes wherein 120 (72%) individuals were without symptoms and 47 (28%) was progressed with cancer. Among them 67 are MLH1 or MSH2 mutation carriers, the ATM1853N variant/mutant was found to be linked with a relatively increased emergence of colon and other HNPCC-related cancers, when compared with patients bearing the ATM1853D variant. In 2004, Wai K. Leung et al. analyzed the epigenetic transformations in fecal DNA of individuals with colorectal cancer of age ranging from 45-90 years as a molecular screening test. They examined the possibility of identifying promoter hyper-methylation of many tumor-type genes. The methyl-DNA located in colon tissues and stool was also identified by methyl-specific PCR. Deborah Thompson et al., in 2005 studied the likelihood of cancer in heterozygous ATM mutation carriers by usinglymphoblastoid cell lines took from blood samplesin 1160 relatives of 169 UK A-T patients comprising 247 obligate carriers obtained from the National Health Service Central Registry and were detected by genotyping; western blotting, PCR and statistical tests were carried out. The likelihood for all cancers other than breast cancer was found to be 2.05 (95% CI=1.09 to 3.84) in the female carriers and its 1.23 (95% CI=0.76 to 2.00) in the male carriers. An explicit risk increase was identified for breast cancer, although there was some confirmation of increased chances of colorectal cancer (RR=2.54, 95% CI=1.06 to 6.09) and also stomach cancer (RR=3.39, 95% CI=0.86 to 13.4). Alfa H.C. Bai et al. (2004), inspected the promoter hyper methylation in 10 tumor-type genes by using methyl specific PCR. Promoter hyper methylation was more often identified in 40% of colonic cancers and adenomas in APC, ATM, hMLH1, MGMT, HLTF, p14, p15, SOCS-1 and TIMP-3 genes. Methylation in ATM was found to be more prone in elder cancer patients with decreased protein expressions in colorectal adenomas. In 2012, Andrew D Beggs et al., analyzed and correlated the changed gene expression levels of ATM and Ku70 with chromosomal instability and with disease-free survival in 908 tumors from VICTOR clinical trial of stage 2 or 3 colorectal cancer using immunohistochemistry where the decreased ATM gene expression were found to have indigent disease free survival and the complete loss in ATM expression serves to be poor prediction in colorectal cancer and the expression of the Ku70 which has been downregulated was linked with chromosomal instability in colorectal cancer. Also Yosuke Ejima et al., in 2000analyzed the cancer cell lines acquired from solid malignancies for the ATM mutations which was procured from ATCC (Rockville, MD) included 10 colon adenocarcinoma by using PCR-SSCP analysis which resulted in 50 alterations of sequence in 16 cell lines. A high frequency of deletions in the 5 colon tumor cell lines of microsatellite uncertainty within the intronic mononucleotide tracts which has 62% of the sequence alterations whereas irregular splicing variants (497del22 or 1236del372) were linked with 2 intron deletions at splice acceptor locations preceded with ATM exon8 or exon12. The level of ATM protein, which was lower in the 3 cell lines, was observed here.
Genetic alteration
The most common genetic syndrome with elevated rates of colorectal cancer is the hereditary nonpolyposis colorectal cancer (HNPCC/Lynch syndrome) normally triggered by inherited mutations in one of five DNA mismatch repair (MMR) genes found in 3% of people with colon cancer (17). The mutation in DNA repair genes results in genomic instability in hereditary cancers and triggers the cancer development (18). Genomic instabilities that have been detected in colon cancer are microsatellite instability and as well as chromosome instability (19). HNPCC patients with tumors produce as adenomas and often modify to carcinoma. Ovarian, endometrial, urinary tract, gastric cancer and biliary tract are also characteristics of this syndrome and are identified more often in families with HNPCC which is suspected to account imprecisely 1% to 5% of all colorectal cancers and 0.5% to 1.4% of all endometrial cancers (20, 21). Ataxia-telangiectasia mutated gene (ATM gene) was found to be an interesting candidate as a remodeling gene in HNPCC where ATM 1853N variant having 8 times higher chance of forming a HNPCC-related cancer comparable with a MLH1 or MSH2 mutation carrier with the ATM 1853D variant in HNPCC patients. So this suggests that ATM 1853N plays a notable part in changing the penetrance of germ-line mutations (22). The sequence alteration resulted in intronic mononucleotide tract shortening preceding some of the ATM exons in colon tumor cell lines with microsatellite uncertainty followed by decreased depression in the level of ATM protein (18). From the studies of P. Maillet et al. (2000), it has been found that MLH1 and MSH2 mutation carriers with the ATM1853N variant had an 8 times higher chance of forming colorectal when comparable with MLH1 or MSH2 mutation carriers with the ATM1853D variant. This study suggests that the ATM D1853N polymorphism regulates MLH1 and MSH2 germ-line mutations. Six tumor-related genes was examined by Wai K. Leung et al. (2004), which includes APC, ATM, HLTF, MGMT and hMLH-1. Promoter hyper-methylation was also often detected in colorectal cancer such as ATM (45%), APC (55%), hMLH1 (45%), MGMT (45%) and HLTF (50%). (Fig. 3) To conclude, the samples had promoter hyper-methylation identified in one of the five tumor-related genes. According to Deborah Thompson et al. (2005), the carriers of mutations encoded a complete-length ATM protein had cancer risks alike to those of people carrying truncated mutations. These results in average risk of breast cancer in A-T heterozygotes and provide chance of more risk of other cancers. Alfa H.C. Bai et al. (2004), whoinspected the promoter hyper methylation in 10 tumor-type genes by methyl specific PCR concluded that there was no link between promoter hyper-methylation and other clinico-pathologic properties of cancer. Studies of Yosuke Ejima et al. (2000), indicated that ATM is a mutation target of microsatellite instability where aberrant transcripts were produced indirectly by intron mutations, which was found to be distinctand the exonic repeats were directly affected (Fig. 2).
Figure 3.
Promoter hypermethylation detection in genes (in %)
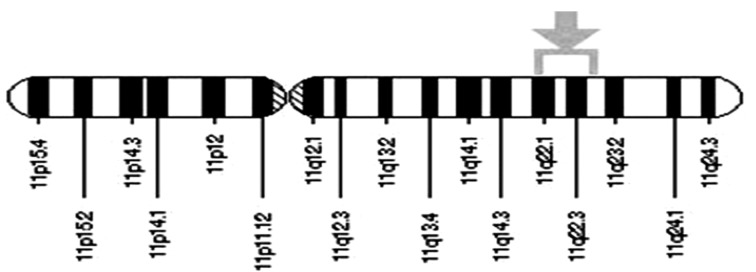
Figure 2.
The ATM gene is located on the long (q) arm of chromosome11 in-between positions 22 and 23. Cytogenetic Location: 11 q22-q23
Alterations of ATM are proposed to be susceptible in specific to lymphoproliferative syndromes (24). ATM allelic imbalance exhibited a high frequency in multiploid carcinomas in chromosome 11q22-23 when compared with diploid and aneuploidy carcinomas (25). The overall relative cancer risk was found to be higher for both gender carriers younger than 50 years of age, with minute proof of more risk for carriers aged around 50 years and also elder (14).
Other related cancers
In a study, ATM genotype showed link with lung cancer risk which suggests that polymorphisms of ATM plays a part in the progression of lung cancer (26). It has been initiated that ATM mutation heterozygotes had double fold more breast cancer chance comparable to the normal population. This likelihood was upgraded 5-fold in women with below age of 50 (27). ATM mutations of homozygous or heterozygous type cause ataxia and were recognized by progressive cerebellar ataxia, oculomotor apraxia, immunodeficiency, and increased chance of tumors (28). Epithelial cancers, lymphoid cancers primarily in childhood, breast cancer are seen normally in adults (29). There is an increased evidence for an complete chance of cancers other than breast cancer in ATM heterozygotes such as colorectal cancer and stomach cancer of clear higher mortality from cancer with statistically specific excess risks of colorectal and lung cancer deaths. There is a possible link between ATM and cancers of the gastrointestinal tract which was found to be statistically significant (30). It was found that the ATM variations may also modify the chance of neoplasia in breast and in digestive epithelia. ATM plays a very active role when exposed to tobacco smoke which induces expression of ATM in oesophageal cancer cell lines (31).
Conclusion
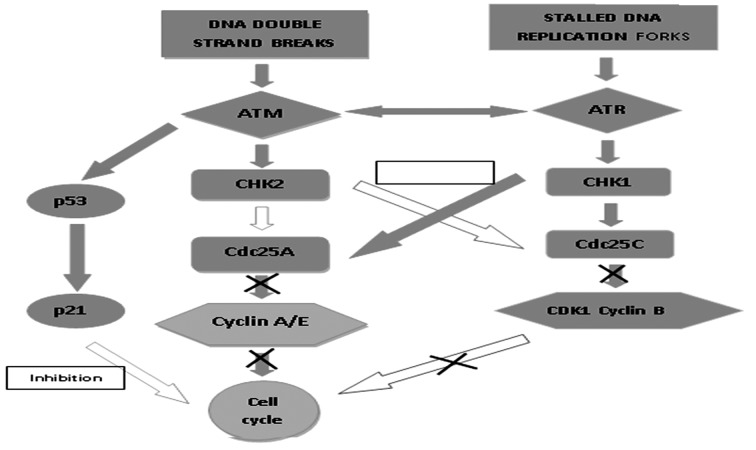
ATM is known to be the newer risk gene where the heterozygous carriers of ATM mutations are at higher chance of cancers and a major form of genetic instabilities. This study suggests that the variant of ATM D1853N polymorphism is linked with the more risk of developing a HNPCC-related cancer which remains to be highly significant (10) evidence of link between a single non-conservative point mutation in ATM and expression of phenotype in HNPCC (32) (Fig. 4). This study concludes that the occurrence of cancer in MLH1 or MSH2 mutation carriers is by the existence of a specific ATM polymorphism. The level of ATM protein expression in colon cancer cell lines was found to be three fold significantly different from that of breast cell lines (33). Decreased ATM expression is linked to indigent survival in patients with CRC (34). Loss of ATM expression resulted in a tendency towards the worse survival in colorectal cancer poorer prognosis in colorectal cancers associated with chromosomal instability (15). Link between the ATM genotypes and HNPCC age onset of survival analysis resulted that the ATM polymorphism did not transform HNPCC age of onset (35). As mismatch repair defects did not account for the majority of colorectal cancers, ATM was found to be attractive candidate gene for the inactivation and in the progress of chromosomal instability in tumor cells (36). The HNPCC condition in colorectal cancer varies from sporadic colorectal cancer in many ways where the moderate age onset is 45 years, it was found to be 63 years in the normal population. Thereby the mortality rate in ATM carriers was found to be high with the stomach and colorectal cancer of below 50 years of age (37, 38). There are many possible modifiers which includes loci encoded xenobiotic enzymes NAT1, GSTM1, NAT2, GSTT1 (7-9), MTHFR gene which results in changes in these loci further modulating the age onset, penetrance and position of cancer in MLH1 & MSH2 mutation carriers. Thus, this ATM gene might be considered as a prognostic biomarker in colorectal cancer; however the association between ATM gene and hereditary transfer of colorectal cancer still remains controversial.
Figure 4.
Genomic instability & DNA damage (ATM mutation)
Acknowledgments
The authors are thankful to Chettinad Academy of Research and Education (CARE), Chennai, India.
Funding Sources:
This study was financially supported by grants Chettinad Academy of Research and Education, Chennai, India.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Ashktorab H, Schaffer AA, Daremipouran M, Smoot DT, Lee E, Brim H. Distinct Genetic Alterations in Colorectal Cancer. PLOS One. 2010;5(1) doi: 10.1371/journal.pone.0008879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones PA, Laird PW. Cancer epigenetics comes of age. Nat Genet. 1999;21:163–7. doi: 10.1038/5947. [DOI] [PubMed] [Google Scholar]
- 4.Grabsch H, Dattani M, Barker L, et al. Expression of DNA Double-Strand Break Repair Proteins ATM and BRCA1 Predicts Survival in Colorectal Cancer. Clin Cancer Res. 2006;12:5. doi: 10.1158/1078-0432.CCR-05-2105. [DOI] [PubMed] [Google Scholar]
- 5.Sideris M, Papagrigoriadis S. Molecular biomarkers and classification models in the evaluation of the prognosis of colorectal cancer. Anticancer Res. 2014;34:2061–2068. [PubMed] [Google Scholar]
- 6.Aaltonen LA, Peltomäki P, Mecklin JP. Replication errors in benign and malignant tumors from hereditary nonpolyposis colorectal cancer patients. Cancer Res. 1994;54:1645. [PubMed] [Google Scholar]
- 7.Boland CR, Goel A. Microsatellite Instability in Colorectal Cancer. Gastroenterology. 2010;138(6):2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grady WM, Markowitz , Sanford MD. Genomic instability and colorectal cancer. Gastroenterology. 2000;16(1):62–67. doi: 10.1097/00001574-200001000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Rebecca N, Kevin S, Charis E. Highly penetrate hereditary cancer syndromes. Oncogene. 2004;23:6445–6470. doi: 10.1038/sj.onc.1207714. [DOI] [PubMed] [Google Scholar]
- 10.Maillet P, Chappuis PO, Vaudan G, et al. Polymorphism in the ATM gene modulates the penetrance of Hereditary Non-Polyposis Colorectal Cancer. Int J Cancer. 2000;88:928–931. doi: 10.1002/1097-0215(20001215)88:6<928::aid-ijc14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 11.Tamotsu S, Wataru H, Noriyuki U, et al. Frequent allelic imbalance at the ATM locus in DNA multiploid colorectal carcinomas. Oncogene. 2001;20:6095–6101. doi: 10.1038/sj.onc.1204731. [DOI] [PubMed] [Google Scholar]
- 12.Alfa HC, Tong BJ, To KF, et al. Promoter hypermethylation of tumor-related genes in the progression of colorectal neoplasia. Int J Cancer. 2004;112:846–853. doi: 10.1002/ijc.20485. [DOI] [PubMed] [Google Scholar]
- 13.Leung WK, To KF, Man EP, et al. Detection of Epigenetic Changes in Fecal DNA as a Molecular Screening Test for Colorectal Cancer: A Feasibility Study. Clin Chem. 2004;50:11. doi: 10.1373/clinchem.2004.039305. [DOI] [PubMed] [Google Scholar]
- 14.Deborah T, Silvia D, Jennifer K, et al. Cancer Risks and Mortality in Heterozygous ATM Mutation Carriers. J Natl Cancer Inst. 2005;97:11. doi: 10.1093/jnci/dji141. [DOI] [PubMed] [Google Scholar]
- 15.Beggs AD, Domingo E, McGregor M, et al. Loss of expression of the double strand break repair protein ATM is associated with worse prognosis in colorectal cancer and loss of Ku70 expression is associated with CIN. Oncotarget 3: 11. doi: 10.18632/oncotarget.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ejima Y, Yang L, Sasaki MS. Aberrant splicing of the ATM gene associated with shortening of the intronic mononucleotide tract in human colon tumor cell lines: A novel mutation target of microsatellite instability. Int J Cancer. 2000;86:262–268. doi: 10.1002/(sici)1097-0215(20000415)86:2<262::aid-ijc17>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 17.Muzny DM, Bainbridge MN, Chang K, et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–7. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability: an evolving hallmark of cancer. Nat Reviews Mol Cell Biol. 2000;11:220–228. doi: 10.1038/nrm2858. [DOI] [PubMed] [Google Scholar]
- 19.Grady WM, Carethers JM. Reviews in Basic and Clinical Gastroenterology Genomic and Epigenetic Instability in Colorectal Cancer Pathogenesis. Gastroenterology. 2008;1354:1079–1099. doi: 10.1053/j.gastro.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boland CR, Goel A. Microsatellite Instability in Colorectal Cancer. Gastroenterology. 2010;138(6):2073–2087. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor N, Mutch DG. Gynecologic Manifestations of Hereditary Nonpolyposis Colorectal Cancer. Oncology Journal. 2006 [PubMed] [Google Scholar]
- 22.Weischer M, Bojesen SE, Ellervik C, Tybjarg-Hansen A, Nordestgaard BG. CHEK2 genotyping for clinical assessment of breast cancer risk: meta-analyses of 26,000 patient cases and 27,000 controls. J Clin Oncol. 2008;26(4):542–548. doi: 10.1200/JCO.2007.12.5922. [DOI] [PubMed] [Google Scholar]
- 23.Rasio D, Negrini M, Maneti G, Dragani T, Croce CM. Loss of heterozygosity at chromosome 11q in lung adenocarcinoma: identification of three independent regions. Cancer Res. 1995;55:3988–3991. [PubMed] [Google Scholar]
- 24.Spector BD, Filipovich AM, Perry GS, Kersey KS. Ataxia-telangiectasia: a cellular and molecular link between cancer, neuropathology and immune deficiency. J Wiley Chichester. 1992:103–108. [Google Scholar]
- 25.Sugai T, Habano W, Uesugi N, et al. Frequent allelic imbalance at the ATM locus in DNA multiploid colorectal Carcinomas. Oncogene. 2001;20:6095–6101. doi: 10.1038/sj.onc.1204731. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Kim H, Lee KY, et al. Genetic polymorphisms of ataxia telangiectasia mutated affect lung cancer risk. Hum Mol Genet. 2006;15(7):1181–1186. doi: 10.1093/hmg/ddl033. [DOI] [PubMed] [Google Scholar]
- 27.Swift M, Morrell MD, Massey RB, Chase CL. Incidence of cancer in 161 families affected by Ataxia-telangiectasia. The N Engl J Med. 1991;325:26. doi: 10.1056/NEJM199112263252602. [DOI] [PubMed] [Google Scholar]
- 28.Chun HH, Gatti RA. Ataxia-telangiectasia, an evolving phenotype. DNA Repair. 2004;3(8-9):1187–1196. doi: 10.1016/j.dnarep.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Morrell D, Cromartie E, Swift M. Mortality and cancer incidence in 263 patients with ataxia-telangiectasia. J Natl Cancer Inst. 1986;77(1):89–92. [PubMed] [Google Scholar]
- 30.Inskip HM, Kinlen LJ, Taylor AM, Woods CG, Arlett CF. Risk of breast cancer and other cancers in heterozygotes for ataxia-telangiectasia. Br J Cancer. 1999;79:1304–7. doi: 10.1038/sj.bjc.6690209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang Y, Liang ZD, Wu TT, Cao L, Zhang H, Xu XC. Ataxia telangiectasia mutated expression is associated with tobacco smoke exposure in esophageal cancer tissues and benzo [a] pyrene diol epoxide in cell lines. Int J Cancer. 2007;120:91–5. doi: 10.1002/ijc.22121. [DOI] [PubMed] [Google Scholar]
- 32.Fitzgerald MG, Bean JM, Hegde SR. Heterozygous ATM mutations do not contribute to early onset of breast cancer. Nat Genet. 1997;15:307–310. doi: 10.1038/ng0397-307. [DOI] [PubMed] [Google Scholar]
- 33.Waha A, Sturne C, Kesseler A. Expression of the ATM gene is significantly reduced in sporadic breast Carcinomas. Int J Cancer. 1998;78:306–309. doi: 10.1002/(SICI)1097-0215(19981029)78:3<306::AID-IJC8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 34.Morio T, Ku KH. Artemis and ataxia-telangiectasia-mutated: signaling networks in DNA damage. Int J Biochem Cell Biol. 2008;4(40):598–603. doi: 10.1016/j.biocel.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Jones JS, Gu X, Lynch PM, Bigas MR, Amos CI, Frazier ML. ATM polymorphism and hereditary nonpolyposis colorectal cancer (HNPCC) age of onset (United States) doi: 10.1007/s10552-005-1540-7. [DOI] [PubMed] [Google Scholar]
- 36.Uhrhammer N, Bay J, Pernin D. Loss of heterozygosity at the ATM locus in colorectal carcinoma. 1999:655–663. doi: 10.3892/or.6.3.655. [DOI] [PubMed] [Google Scholar]
- 37.Lynch H, Boman B, Watson P, Lynch P, Bufo P, Mingazzini P. Gastroenterol. Clin N Am. 1998;17:679–711. [PubMed] [Google Scholar]
- 38.Lynch HT, Lynch JF, Attard TA. Diagnosis and management of hereditary colorectal cancer syndromes: Lynch syndrome as a model. CMAJ. 2009;181(5):273–280. doi: 10.1503/cmaj.071574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moisio AL, Sistonen P, Mecklin JP, Jarvinen H, Peltoma ki P. Genetic Polymorphisms in carcinogen metabolism and their modifying role in hereditary nonpolyposis colorectal cancer. Gastroenterology. 1998;115:1387–1394. doi: 10.1016/s0016-5085(98)70017-4. [DOI] [PubMed] [Google Scholar]