Abstract
The response to a bronchodilator is considered as crucial to diagnose COPD and to distinguish COPD from asthma. COPD is characterized by progressive airflow obstruction that is only partly reversible, whereas asthma is associated with airflow obstruction that is often reversible either spontaneously or with treatment. In spite of the partly reversible airflow obstruction, patients with COPD may show a significant bronchodilator response both in terms of an increase in forced expiratory volume in 1 second (FEV1) or in forced vital capacity (FVC) after an adequate dose of an inhaled bronchodilator. Changes in FEV1 or FVC characterize, respectively, flow or volume response after bronchodilator administration. This overview will deal with the reversibility testing characteristics and its clinical significance in COPD patients. (www.actabiomedica.it)
Keywords: COPD, airflow obstruction, bronchodilation
Introduction
The assessment of the bronchial response to drugs with bronchodilator activity plays an important role in the characterization of airflow obstruction in order to ascertain whether or not its reversibility can occur (1). From the clinical point of view, the response to a bronchodilator is considered as crucial to diagnose COPD and to distinguish COPD from bronchial asthma. COPD is characterized by progressive airflow obstruction that is only partly reversible (2), whereas asthma is associated with airflow obstruction that is often reversible either spontaneously or with treatment (3). It is of note that there is a group of individuals having characteristics of both COPD and asthma. Patients with asthma-COPD overlap syndrome (ACOS) include patients with COPD and eosinophilia, smoking asthmatics, long-standing asthmatics with airway remodeling and steroid-resistant asthmatics with neutrophilic inflammation (4). In spite of their clinical heterogeneity, patients with ACOS show a significant response to the bronchodilator (5).
Interestingly, in the general population, the bronchodilator response is not a nominal, all-or-nothing type, response, but has a continuous, Gaussian-type, distribution. This distribution was proved in the Eclipse study both in smoking subjects with or without bronchial obstruction and in healthy subjects (6). Given this normal distribution, some patients can experience a paradoxical response to β2 agonist, by showing a bronchoconstriction effect. Interestingly such a response, defined as a decrease in forced expiratory volume at 1st second (FEV1) and/or forced vital capacity (FVC) of 12% from baseline and 200 ml in absolute terms in the post-bronchodilator spirometry, was found in 5% of cases of a large smoking population with or without bronchial obstruction (7). The pathophysiological mechanisms underlying the paradoxical response to the bronchodilator are not known, even if the study by Bhatt et al (7) showed a higher prevalence of this response in the Black ethnic group and in patients with a higher rate of comorbidities.
This brief overview will deal with the reversibility testing characteristics and the clinical significance of the test in COPD patients.
The reversibility test
The bronchodilation test consists in a inhalation of a dose of a bronchodilator drug-acting after baseline spirometry, followed by a second forced expiratory maneuver to record any change in FEV1, FVC and in FEV1/FVC ratio, which is considered as the index of airflow obstruction. Changes in FEV1 or FVC characterize, respectively, flow or volume response after bronchodilator administration. The most commonly used drug for the test belongs to the class of short acting β2 agonist and is represented by Salbutamol at a dose of 400 mcg (1). However, this test has been also performed by using lower doses of Salbutamol (200 mcg) or other drug classes, such as antimuscarinics (i.e., Ipratropium bromide, 80 mg), alone or in combination with β2 agonists. The amount and type of drug used can influence the results. Notably, in subjects suffering from COPD it has been highlighted a better response when the β2 agonist is associated with the antimuscarinic (8). Thanks to the synergistic interaction between β2 agonists and antimuscarinic agents, when these drugs are given in combination, a more effective bronchial smooth muscle bronchodilation can occur (9). Therefore, a first important variable in the bronchial reversibility assessment is represented by drug class and dosage used during the test (Tab. 1).
Table 1.
Drugs used in the reversibility testing
| Drug class | Drug name | Dose | Pharmacology |
| β2 agonist | Salbutamol | 200 mcg 400 mcg |
The binding to the β2 receptor on smooth muscle cell membrane activates the adenylate cyclase enzyme leading to an increased cAMP synthesis. The following PKA (protein kinase A) activation brings to myosin light chains phosphorylation with transition in the inactive form and bronchodilation. The onset of action is within 15 minutes and lasts 3-4 hours. |
| Anti muscarinic | Ipratropium bromide | 80 mcg | This drug has antagonistic action on muscarinic M2 and M3 receptors. M3 receptor binding blocks phospholipase C action which normally activates the cascade of inositol triphosphate (IP3) and diacylglycerol (DAG); the first one is involved in calcium release from the sarcoplasmic reticulum, the second one in the opening of calcium channels with subsequent contraction of smooth muscles. The stimulation of these receptors on glandular epithelial cells surface increases mucus and secretions production. The onset of action is within 5 minutes and lasts 6 hours. |
Table 2.
Procedures relating a Bronchodilation test
| 1. Assess lung function at baseline |
| 2. Administer 400 mcg Salbutamol through a spacer |
| 3. Re-assess lung function after 15 minutes |
| 4. An increase in FEV1 and/or in FVC ≥12% and ≥200 mL constitutes a positive bronchodilator response |
Baseline condition may also influence the bronchodilator response. Post-bronchodilator percentage change is related to the baseline FEV1; low baseline FEV1 value is associated with more relevant percentage gain. For this reason, ATS/ERS guidelines added also an absolute increase in the amount of 200 ml, as a requirement for the positive test (8). Alternatively, to minimize this aspect, the choice of an absolute increase equal to 10% of predicted FEV1 has proved equally as effective (10). According to the ATS/ERS guidelines, bronchial reversibility test is considered as positive whether a 12% increase from baseline in FEV1 and/or FVC and 200 ml in absolute value in post-bronchodilator curve is reached (1) (11). Other criteria to consider the bronchodilator response as positive are a post-bronchodilator FEV1 percentage increase greater than 15% of baseline, an increase in post-bronchodilator FEV1 greater than 10% of the predicted value (10) or an absolute 400 ml increase in FEV1 (6). Evidence has shown how the ATS/ERS threshold tends to exceed normal variability values and placebo inhalation response (10) (12).
Measurements of lung volumes before and after bronchodilators add sensitivity when examining for bronchodilator responsiveness. Notably, in hyperinflated patients, the measurement of FVC before and after bronchodilator administration identifies a response that may not be uncovered by the FEV1 measurement (13). Bronchodilators reduce hyperinflation and FVC improvements after bronchodilator administration are related to the reduction in residual volume (RV), functional residual capacity (FRC) and total lung capacity (TLC), which also results in an increase in inspiratory capacity (IC), a parameter linked to the improvement in exercise tolerance and dyspnea perception (10).
Daily FEV1 and FVC variability in healthy subjects, regulated by sympathetic rather than parasympathetic nervous system prevalence on bronchial caliber, is approximately 150-180 ml in absolute terms (8% as a percentage) (1). It has been also estimated that in obstructive disease this variability is about 2-3 times higher than in normal subjects (14). It was also shown that the minimum clinically appreciable FEV1 change amounts to approximately 100-140 ml (15). In any case, the use of threshold values for distinction between responders and non-responders subjects involves a certain degree of arbitrariness, since the bronchial response to a bronchodilator is a variable which is continuous and normally distributed (8) (10).
COPD and bronchial reversibility
According to the GOLD statement, reversibility testing plays a key role in the diagnosis of COPD, that is made in case of FEV1/FVC post-bronchodilator <70%; again, according to the statement, the severity degree classification is evaluated by considering the post-bronchodilator FEV1 value (11). Importantly in COPD patients, the lack of bronchodilator response in the lung function laboratory does not preclude a clinical response to bronchodilator therapy (1).
Although it is essential for the diagnosis of COPD, the bronchodilator response does not seem to be a constant and reproducible characteristic in patients with COPD. In a cohort of patients subdivided into 3 groups (COPD, active smokers and non-smokers), the bronchial reversibility (only evaluated as a response in terms of FEV1) was studied on 4 occasions during a year (6). It has been shown that only 16% of patients considered to be reversible according to the ATS/ ERS criteria during the first visit kept this feature in all subsequent checks, while 66% of patients who were irreversible in the first control maintained that characteristic during the subsequent visits (6). Between permanently bronchodilator responders and non-responders patients no difference was found between the main clinical outcomes, such as mortality and severe exacerbations. However, in the same study, irreversible patients in at least 3 out of 4 occasions were characterized by a worst lung function, more severe emphysema revealed by low-dose CT and at greater risk of exacerbation. Another interesting finding of the study (6) concerned the FEV1 increase after bronchodilation, which was of comparable extent, when active smokers and COPD subjects were considered, but it was lower when non-smokers were considered.
In any case, given the intra-individual variability in reversibility tests, it is conceivable that a single test is not a reliable indicator nor any long-term response to inhaled bronchodilating therapy nor can be used as a parameter for distinguish patients into responders and non-responders (6). On the other hand, it has been shown as a negative response to bronchodilator test does not affect a long-term response to bronchodilator therapy in patients with COPD (16).
Clinical and functional features of COPD patients, who show a significant response in terms of flows with improvement in FEV1 (flow responders), of volume with increase in FVC (volume responders) or both of them are still under investigation (Figure 1). In a large population sample of patients with bronchial obstruction and severe parenchymal hyperinflation (TLC>133% predicted), only one third of the subjects improved significantly in terms of FEV1 after administration of salbutamol (13). However, in the same study, significant bronchodilator percentage response was up to three-quarters of the subjects if were also considered changes in terms of FVC (13). When the bronchodilator response in terms of FEV1 and FVC was considered in relation to the four GOLD classes distribution (17), it has been shown that patients belonging to the first GOLD classes (that is, patients with mild moderate COPD) tend to be more responsive in terms of flows, a feature that is lost in severe forms, where a volume response prevails.
Figure 1.
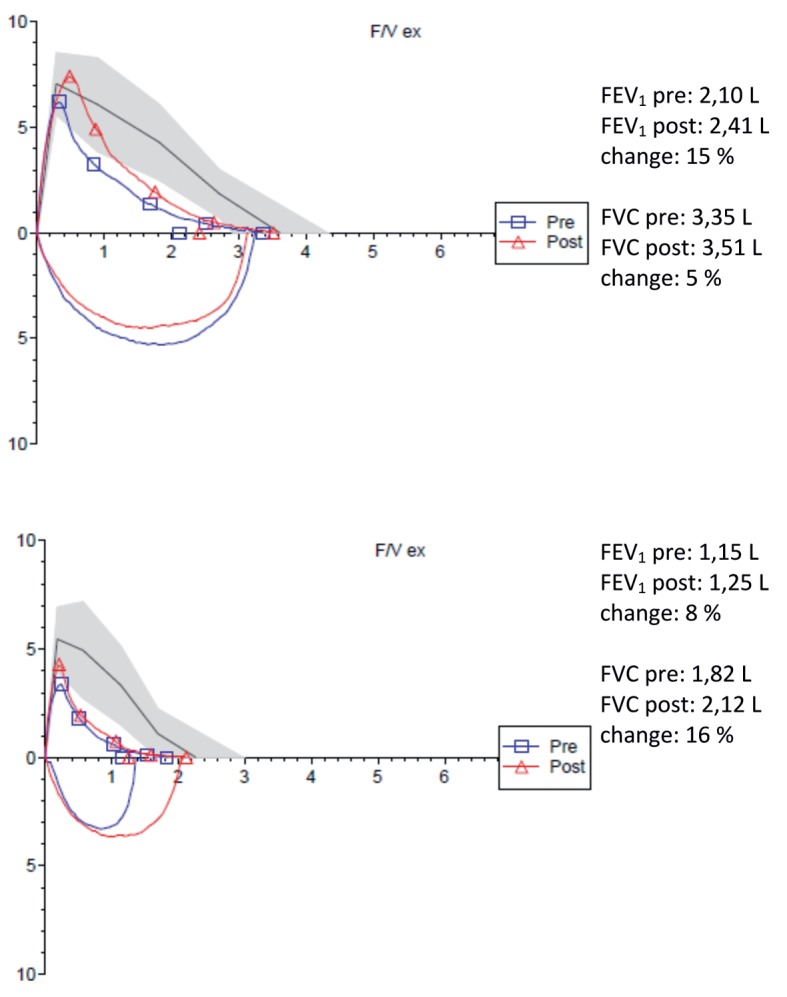
Flow volume curves in a flow responder patient (above) and in a volume responder patient (below)
In patients with COPD, the impulse oscillometry system (IOS) application has also brought to light a relationship between response to the bronchodilation test and small airways dysfunction. In a large cohort of COPD patients, the small airway dysfunction, expressed as an increase in peripheral airway resistance by IOS, was related to the bronchodilator responsiveness in terms of FVC, but not in terms of FEV1 (18). The small airways dysfunction was also associated with the worst airflow obstruction degree, with a major degree of hyperinflation and worse clinical conditions (18).
Taken together these studies (13, 18, 17) show that in COPD patients the volume responder pathophysiological trait is characterized by severe obstruction with marked hyperinflation, emphysema phenotype, small airway dysfunction and poorer health status.
Since FVC correlates with static volumes, such as VR, FRC and TLC, the response in terms of volumes can reduce patient’s hyperinflation, ensuring an improvement in inspiratory capacity and consequently a better exercise tolerance and a lower dyspnea perception (10). In such patients, characterized by long time constants, bronchodilator allows a better lung emptying reducing air trapping and the residual volume with displacement on the chest-lung curve to a more favorable level and consequent reduction in the work of breathing.
Conclusions
The reversibility testing is unavoidable to confirm the diagnosis of COPD in at risk patients, such as patients who smoke or have exposure to pollutants, patients who have symptoms, such as cough, sputum or dyspnea or patients who have a family history of chronic respiratory disease (2).
The bronchodilator response in COPD is a function of baseline FEV1 and increases in case of β2 agonist and an antimuscarinic agent are administered in association. Furthermore, this response in COPD, as in general population, is a normally distributed continuous variable and the categorization in responders and non-responders is based on arbitrary criteria. Indeed, this response cannot be used to phenotype patients, since it has been demonstrated that there is considerable intra-individual variability for that parameter. Also for this reason, a negative response does not preclude a long-term treatment with bronchodilators.
Any bronchodilator response can be evaluated in terms of a significant change in flows or volumes. Flow response prevails in COPD patients with mild to moderate degree of airflow obstruction, while volume response prevails in patients with severe one and/or with small airways dysfunction.
References
- 1.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function test. Eur Respir J. 2005;26:948–68. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 2.Celli BR, MacNee W. and the committee members. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–46. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 3.Bateman ED, Hurd SS, Barnes PJ, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31:143–78. doi: 10.1183/09031936.00138707. [DOI] [PubMed] [Google Scholar]
- 4.Putcha N, Wise RA. Asthma-Chronic Obstructive Pulmonary Disease Overlap Syndrome: Nothing New Under the Sun. Immunol Allergy Clin North Am. 2016;36:515–28. doi: 10.1016/j.iac.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nielsen M, Bårnes CB, Ulrik CS. Clinical characteristics of the asthma-COPD overlap syndrome-a systematic review. Int J Chron Obstruct Pulmon Dis. 2015 Jul 27;10:1443–54. doi: 10.2147/COPD.S85363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albert P, Agusti A, Edwards L, et al. Bronchodilator responsiveness as a phenotypic characteristic of established chronic obstructive pulmonary disease. Thorax. 2012;67:701–8. doi: 10.1136/thoraxjnl-2011-201458. [DOI] [PubMed] [Google Scholar]
- 7.Bhatt SP, Wells JM, Kim V, et al. Paradoxical Lung Function Response to Beta2-agonists: Radiologic Correlates and Clinical Implication. Lancet Respir Med. 2014;2:911–8. doi: 10.1016/S2213-2600(14)70185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calverley PMA, Burge PS, Spencer S, Anderson JA, Jones PW. Bronchodilator reversibility testing in chronic obstructive pulmonary disease. Thorax. 2003;58:659–64. doi: 10.1136/thorax.58.8.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panettieri RA. Bronchodilators, receptors and cross-talk: together is better? Postgrad Med. 2015;127:771–80. doi: 10.1080/00325481.2015.1080589. [DOI] [PubMed] [Google Scholar]
- 10.Tashkin DP, Celli B, Decramer M, et al. Bronchodilator responsiveness in patients with COPD. Eur Respir J. 2008 Apr;Apr(31):4–742. doi: 10.1183/09031936.00129607. [DOI] [PubMed] [Google Scholar]
- 11.GOLD (Global Initiative for Chronic Obstructive Lung Disease) 2016. http://goldcopd.org/
- 12.Tan WC, Vollmer WM, Lamprecht B, et al. Worldwide patterns of bronchodilator responsiveness: results from the Burden of Obstructive Lung Disease study. Thorax. 2012;67:718–26. doi: 10.1136/thoraxjnl-2011-201445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newton MF, O’Donnell DE, Forkert L. Response of lung volumes to inhaled salbutamol in a large population of patients with severe hyperinflation. CHEST. 2002;121:1042–50. doi: 10.1378/chest.121.4.1042. [DOI] [PubMed] [Google Scholar]
- 14.Van Noord A, Smeets J, Clément J, Van de Woestijne KP, Demedts M. Assessment of reversibility of airway obstruction. Am J Respir Crit Care Med. 1994;150:551–4. doi: 10.1164/ajrccm.150.2.8049845. [DOI] [PubMed] [Google Scholar]
- 15.Cazzola M, MacNee W, Martinez FJ, et al. Outcomes for COPD pharmacological trials: from lung function to biomarkers. Eur Respir J. 2008;31:416–68. doi: 10.1183/09031936.00099306. [DOI] [PubMed] [Google Scholar]
- 16.Mahler DA, Donohue JF, Barbee RA, et al. Efficacy of salmeterol xinafoate in the treatment of COPD. Chest. 1999;115:957–65. doi: 10.1378/chest.115.4.957. [DOI] [PubMed] [Google Scholar]
- 17.Schermer T, Heijdra Y, Zadel S, et al. Flow and volume responses after routine salbutamol reversibility testing in mild to very severe COPD. Respiratory Medicine. 2007;101:1355–62. doi: 10.1016/j.rmed.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 18.Pisi R, Aiello M, Zanini A, et al. Small airway dysfunction and flow and volume bronchodilator responsiveness in patients with chronic obstructive pulmonary disease. International Journal of COPD. 2015;10:1191–7. doi: 10.2147/COPD.S82509. [DOI] [PMC free article] [PubMed] [Google Scholar]


