Abstract
Juvenile fibromyalgia syndrome (JFMS) is a chronic condition characterized by symptoms of chronic diffuse musculoskeletal pain and multiple painful tender points on palpation. It is often accompanied by fatigue, disorders of sleep, chronic headaches, irritable bowel syndrome, and subjective soft tissue swelling. The complexity of the presenting clinical picture in JPFS has not been sufficiently defined in the literature. Similarities to adult fibromyalgia syndrome in JFMS are often difficult to compare, because many of the symptoms are “medically unexplained” and often overlap frequently with other medical conditions. However, a valid diagnosis of JFMS often decreases parents’ anxiety, reduces unnecessary further investigations, and provides a rational framework for a management plan. The diagnostic criteria proposed by Yunus and Masi in 1985 to define JFMS were never validated or critically analyzed. In most cases, the clinical diagnosis is based on the history, the physical examination that demonstrates general tenderness (muscle, joints, tendons), the absence of other pathological conditions that could explain pain and fatigue, and the normal basic laboratory tests. Research and clinical observations defined that JFMS may have a chronic course that impacts the functional status and the psychosocial development of children and adolescents. This paper briefly reviews the existing knowledge on JFMS focusing on the diagnosis, clinical and the epidemiological characteristics in children and adolescents for better understanding of this disorder. (www.actabiomedica.it)
Keywords: juvenile fibromyalgia syndrome, epidemiology, clinical characteristics, diagnosis
Introduction
Chronic pain (defined as persistent and recurrent pain) is a significant problem in the pediatric population, conservatively estimated to affect from 20% to 35% of children, especially adolescents, around the world (1). In general, pain can be categorized into 3 types that sometimes overlap: nociceptive or peripheral pain(related to damage of tissue by trauma or inflammation); neuropathic pain(associated with damage of peripheral or central nerves); and centralized pain ( has no identifiable nerve or tissue damage and is thought to result from persistent neuronal dysregulation, overactive ascending pain pathways, and a deficiency of descending inhibitory pain pathways) (2).
Fibromyalgia syndrome (FM) is an idiopathic condition, of a yet unidentified aetiology, characterized by chronic widespread musculoskeletal pain, fatigue and sleep disorders. The pain is not caused by local inflammation but may be due to abnormal function of pain receptors in the brain (3, 4)
Patients with FM commonly experience symptoms for several years prior to diagnosis. Repeated and often expensive laboratory investigations, frequent healthcare visits, and referral to a wide range of specialists contribute to considerable discomfort and cost to patients.
Recent guidelines are in agreement that the diagnosis remains clinical, and the purpose of a precise physical examination and limited laboratory investigations is to exclude other somatic diseases that have similar symptoms (3, 4). This has fostered a sense of insecurity in health care professionals leading to unnecessary investigations, excessive medicalization of patients (5, 6), and referral to numerous pediatric specialists (e.g., neurologists, rheumatologists, pain medicine) before the identification of the syndrome.
Although children and adolescents can meet the criteria of FM for adults (3), no general consensus has been achieved for the diagnosis and management of children and adolescents with chronic widespread pain (7).
This paper reviews briefly the existing knowledge regarding the diagnosis as well as the clinical and epidemiological characteristics of FM for better understanding of the juvenile fibromyalgia syndrome (JFMS).
Brief history of fibromyalgia
In 1592, in the book “Liber de rheumatismo”, Guillaume deBaillou described some of muscular pains similar to FM. This is probably the very first medical description of FM. An important step was made by William Balfour (8), a surgeon in Edinburgh who was the first, in 1815, to describe “A special pain, usually driven by an inflammatory action, involving fibrous and white tissues, belonging to muscles and joints, like tendons, aponevroses”. He called this widespread pain “fibrosistitis.”
In 1880, an U.S. psychologist named Beard wrote about a collection of symptoms consisting of fatigue, widespread pain, and psychological disturbances. He called it ‘neurasthenia’ and attributed the problems to the stress of modern life (9). For many years, lack of a unifying aetiology and a universal terminology hindered the understanding and recognition of FM.
In 1904, a pathologist, Ralph Stockman, reported evidence of inflammatory changes occurring in the fibrous, intramuscular septa of biopsies from afflicted patients (10). That finding led Gowers W. (11) to introduce the term “fibrositis” to describe the inflammation of fibrous tissue in his description of low back pain. In subsequent years, the terms fibrositis, fibromyositis, psychogenic, psychosomatic, or muscular rheumatism have all been used as descriptors for this syndrome.
The term “fibromyalgia” was first used by the Nobel Prize winner, Hench in 1976 (12). Smythe and Moldofsky (13), and Yunus et al. (14) formulated diagnostic criteria for FM, while Müller and Lautenschläger (1990) formulated diagnostic criteria for generalized tenomyopathy (15). The main manifestations were defined as pain in multiple parts of the body, in addition to constitutional and vegetative manifestations, and local hyperalgesia at muscle and tendon insertions (“tender points”).
Between the 1970s and the 90s, the recognition of the disease was an important step both for patients and physicians. The disease received a name, diagnostic criteria, and assessment tools.
In 1990 the American College of Rheumatology (ACR) first established criteria for the classification and diagnosis of the disease (16). New ACR classifications came into effect in 2010 (17), 2011(18) and 2016 (19) to validate the clinical diagnosis of FM.
Prevalence of fibromyalgia in children and adolescents
The prevalence of FM in adults is about 2% (95% CI, 1.4-2.7). It is higher among women (3.4%) than men (0.5%). The average age of diagnosis in adults is around 40-50 years, and 13-15 years for children and adolescents (3, 6, 20).
The reported prevalence of JFMS varies widely probably reflecting differences in ethnicity, socio-cultural background, psychological traits of the population and diverse methodologies that have been used in the published studies (21, 22).
The prevalence of JFMS reported in the literature in different countries is summarized in Table 1.
Table 1.
Prevalence of juvenile fibromyalgia syndrome in children and adolescents: review of the literature
| References | Diagnostic criteria | Cohort variables | Country/prevalence |
| Buskila et al. J Rheumatol 1993; 20(2): 368-70. | 1990 ACR criteria | 338 healthy school children, 179 boys and 159 girls, aged 9 to 15 yrs. | Israel Prevalence 6.2%. |
| Clark et al. J Rheumatol 1998; 25(10): 2009-14. | 1990 ACR criteria. | 548 children, 264 boys and 284 girls, aged 9-15. | Mexico Prevalence 1.2%. |
| Mikkelsson et al. Pain 1997; 73(1): 29-35 and J Rheumatol 1999; 26(3): 674-82. | Structured pain questionnaire to assess the prevalence and persistence of self-reported musculo-skeletal pain symptoms and disability caused by pain. | 1626 third and fifth grade schoolchildren | Finland Prevalence 1.3% at baseline. |
| Weir et al. J Clin Rheumatol 2006; 12(3): 124-8. | ICD-9 criteria (*) | 2595 incident cases of adult and juvenile FMS | U.S.A The estimated prevalence per age group was: 0.5 to1% for 0-4 yrs; 1 to 1.4% for 5-9 yrs; 2 to 2.6% for 10-14 yrs; and 3.5 to 6.2% for 15-19 yrs. |
| Fuda A et al. Egypt Rheumatol Rehabil 2014; 41: 135-138 | A questionnaire was completed by students. A clinical diagnosis of FM was established in only 25 cases. | 2000 students: 960 boys (48%) and 1040 girls (52%). Ages: 9-15 yrs, mean 11.9 yrs |
Egypt Prevalence 1.2%. |
Legend: JFMS: Juvenile fibromyalgia syndrome; FM: fibromyalgia; (*) International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to identify fibromyalgia cases (ICD code 729.1).
JFMS has a prevalence around 1-6%, more common in girls, and can be seen in children of all ages.
Pathophysiology
Current evidence indicates that FM is the result of the combination of a genetic predisposition and various extrinsic stressors that result in a process called central sensitization of the central nervous system (CNS) (23-26). Central sensitization is a complex phenomenon in which there is hyperexcitability of central nociceptive circuits brought on by activity dependent changes in synaptic transmission. This increased sensitization involves changes in receptors, neurotransmitters, ion channels and signalling pathways in the central nervous system to such an extent that even innocuous non-nociceptive stimuli are perceived as painful and the perception of noxious stimuli is exaggerated, prolonged and widespread.
Adolescents with FM were found to be more sensitive to pressure pain than their healthy peers; this suggests a tendency for sensitization of peripheral and/or central nociceptive information often reported in adults with FM (27). In addition, abnormalities associated with FM include an inability to sustain deep stage 4 sleep, neurotransmitter abnormalities (especially serotonin, dopamine, and norepinephrine), hypothalamic-pituitary-adrenal axis dysfunction, and peripheral sensitization.
Recent studies in patients with FM looked at functional activity in the brain. They have proposed that, in these patients, acute pain seems to activate somatosensory, insular and cingulate cortical areas, whereas chronic pain preferentially activates prefrontal and limbic cortical areas. In addition, anatomical changes such as regional decrease in grey matter density, volume or thickness in various regions of the brain, not limited to nociceptive areas, are described in patients with different chronic pain conditions. These anatomical and functional connectivity changes are likely to be secondary to chronic pain (28-31). In support of this view, treating chronic pain effectively can reverse these structural and functional abnormalities and restore normal brain function in humans (32).
Familial studies identified the possibility of genetic predisposition, with up to one-quarter of relatives of FM patients reporting chronic widespread pain (33). A genetic predisposition to fibromyalgia has been demonstrated linking certain genes to fibromyalgia, such as the SS genotype polymorphism in the promoter region of the serotonin transporter gene (5-HTT) (34), and the LL and LH genotype polymorphisms in the gene encoding the COMT (catechol-O-methyltransferase) enzyme (35).
Moreover, inflammatory processes may also play a significant role in the pathogenesis of FM (36).
There is a subset of people with FM who test positive for antinuclear antibody (ANA) and have constitutional symptoms that resemble those of patients with early lupus (37). Therefore, in individual cases, FM may be an early sign of an autoimmune disease (38).
Some studies have sought to demonstrate the occurrence of hormonal abnormalities in individuals with FM, but not specifically in young patients.
In summary, the pathophysiology of FM remains unclear, although some data indicate that a significant central sensitization component is at the root of the syndrome. It appears that the musculoskeletal system, neuroendocrine system, and central nervous system play a significant role in the pathogenesis of this disorder (39,40).
Clinical symptoms of fibromyalgia syndrome
Chronic widespread pain, the pivotal and most important symptom, is not localized to any specific body tissue and tends to move from site to site. Symptoms of fatigue, sleep disturbances, cognitive changes, mood disturbances and various somatic symptoms may occur to a greater or lesser extent.
Pain is described as being diffuse, deep, and continuous often with periods of exacerbation, and symptoms may be modulated by various factors including psychological stress, excessive physical activity, viral infections and fatigue. Therefore, the clinical presentation of FM can be quite diverse with some areas of the body more painful than others, fluctuations in intensity of pain and variable intensity of other associated symptoms. Patients also differ considerably in terms of severity of functional impairment (7, 25, 41).
Although JFMS has been less well studied than adult fibromyalgia, it is evident that the clinical features are basically similar, with minor differences (41), e.g., joint hypermobility is more common in JFMS (42), while psychological comorbidities are common but less severe; altered sleep pattern seems to be a common among patients with JFMS (21,43, 44).
Patients with JFMS may be particularly vulnerable to school absenteeism because of their unremitting widespread pain, disrupted sleep, and chronic fatigue (45).
Table 2 reviews the literature on the commonest clinical features in 277 patients with JFMS below or above 10 years of age.
Table 2.
Most common clinical findings in 277 patients below or above 10 years of age with juvenile fibromyalgia syndrome (From: Eraso RM et al. Clin Exp Rheumatol.2007;25:639-644; Gedalia et al. Clin Exp Rheumatol. 2000;18:415-419; Fuda A et al. Egypt Rheumatol Rehabil.2014:41:135–138; Siegel DM et al. Pediatrics. 1998;101:377-382, modified)
| Clinical feature | Eraso RM et al. Onset age 10 or under N: 46 (%) | Eraso RM et al. Onset > age 10 N:101 (%) | Gedalia A et al. N: 59 children (%) * | Fuda A et al. N: 15 children (%) ** | Siegel DM et al. N: 45 children (%) *** |
| Generalized aches & pain | (100) | (100) | (97) | (100) | (>90) |
| Headache | (78) | (80) | (76) | (52) | (71) |
| Sleep disturbances | (65) | (74) | (69) | (40) | (>90) |
| Morning muscle stiffness | (39) | (21) (§) | (29) | (56) | (53) |
| Fatigue / tiredness | (28) | (23) | (20) | (100) | (62) |
| Abdominal pain | (39) | (19) (§) | (17) | - | - |
| GI symptoms | (29) | - | |||
| Irritable bowel | (20) | - | |||
| Subjective joint swelling | (39) | (14) (§) | (24) | - | - |
| Joint hypermobility | (17) | (23) | (14) | - | - |
| Depression | (9) | (9) | (7) | (60) |
Legend = §: P value <age 10 vs, >age 10 - statistcally significant; GI: Gastrointestinal; * 41 F and 11 M diagnosed with primary JFMS. The mean age at onset was 13.1 years, and the mean age at diagnosis was 15.5 years; ** 1 M (18%) and 18 F (11%). Their ages ranged between 9 and 15 years, with a mean age of 11.9 years; *** 45 subjects (mean age, 13.3 years; 91% females), of whom 33 were available for telephone interview at a mean of 1.6 years from initial diagnosis (0.1 to 1.6 years).
Physical examination
Many musculoskeletal diseases seem capable of triggering the process of central sensitization with an associated increase in sleep disturbance, fatigue, widespread pain, and other symptoms common in FM (46-49). Therefore, physical examination (regardless of whether tender points are counted) remains the key in the evaluation of patients to assess the tenderness (allodynia and hyperalgesia) associated with FM as well as to aid in the differential diagnosis (50).
The clinical characteristics of FM among men are similar to those in women, except that men have fewer symptoms, pain sites and tender points, and less frequent fatigue and irritable bowel syndrome, (51).
The challenge of criteria for the diagnosis of fibromyalgia
There are no instrumental tests to confirm the diagnosis of JFMS, and thus differential diagnosis is by exclusion by means of an extensive clinical examination and patient’s history.
Several different classification schema have been proposed for adults. The most frequently used diagnostic criteria were published by the American College of Rheumatology (ACR) in 1990, with revised criteria proposed in 2010, 2011 and 2016 (16-19).
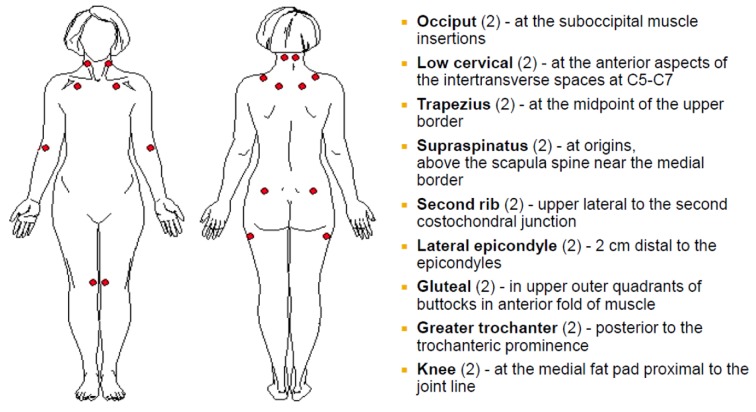
The first ACR criteria for FM in 1990 (16), based on 293 patients (mean age 44.7 years), emphasized chronic widespread musculo-skeletal pain (including pain in the axial skeleton), and the presence of pain on at least 11 of 18 specified tender point sites (pain is at the site of pressure, without radiation or referral) with digital palpation of 4 kg/cm2 (Figure 1).
Figure 1.
American College of Rheumatology 1990 criteria for the loci of tender point examination of fibromyalgia (Adapted from: Wolfe F et al. Arthritis Rheum 1990; 33: 160-172)
In practical terms, the pressure to assess tenderness with digital examination is the pressure needed to see the examiner’s own nail bed blanch. Tender points are located at soft tissue sites and reflect a reduction in pain threshold without any underlying tissue pathology. Although these criteria were widely accepted within the medical community, many primary care physicians were either not performing tender point examinations at all or performing them inaccurately (52).
The criteria proposed by Yunus for JFMS in 1985 (14) required fewer tender points for the diagnosis of FM in children than in adults (5 instead of 11). They also incorporate additional symptoms representing the broader spectrum of FM, as opposed to the ACR 1990 criteria that were based solely on the presence of pain and tenderness. These symptoms include anxiety, fatigue, poor sleep, headache, irritable bowel syndrome, subjective soft tissue swelling, numbness, pain modulation by physical activity, pain modulation by weather, and pain modulation by anxiety and distress; diagnosis of FM in a child depends on presentation of 3 of 10 symptoms (Table 3).
Table 3.
Yunus and Masi diagnostic criteria for juvenile primary fibromyalgia syndrome (Adapted from: Arthritis Rheum 1985; 28: 138-145)
Major Criteria
Minor Criteria Presence of three of the following features:
|
Debate considerations
Tender points have elicited considerable debate and their true value has been questioned because of many reasons. The criteria were originally designed to standardize patient classification in clinical trials rather than to diagnose FM in routine clinical practice and did not consider any other concomitant symptoms or severity of symptoms. They may be present in normal individuals and can increase with age. Their reliability is variable, ranging from good to poor (53, 54). Tender point examination remains a subjective test, open to individual interpretation and reflects an overall reduction in pain threshold, rather than a pathological process at the soft tissue site (55). Moreover, the association of pain report and tender point count is poorly correlated, suggesting that these measurements represent different parameters of pain experience in FM syndrome (56). There is a poor concurrent validity when tender points were examined digitally or by dolorimetry (57). The patient psychological state influences the measurement of tender points, suggesting an association with distress rather than an accurate indicator of pain (58).
In summary, although the tender point examination has been used in hundreds of studies and is recognized by the ACR for the diagnosis of FM, it had never been validated in the pediatric population. Furthermore, there is some evidence that application of the 4 kg cut off when assessing tenderness by dolorimetry is not applicable in children, and that a 3 kg cut off is more appropriate (58, 59).
Consequently, the 2010 ACR Diagnostic Criteria (17) eliminated the tender point examination and made diagnosis more difficult by requiring evaluation of symptoms and imposed a special burden on the examiner: the necessity to interview the patient in order to identify the extent and severity of the symptoms. The new criteria indicate two distinct but combined diagnostic pathways: a widespread pain index (WPI) and a symptom severity scale (SS). Furthermore, they have also included other domains, using a checklist of 41 associated symptoms, for exclusion of other conditions causing pain.
Thus, unlike the 1990 ACR criteria, the new 2010 criteria require exclusion of other conditions causing pain. Additionally, symptoms must have been present at a similar level for at least 3 months.
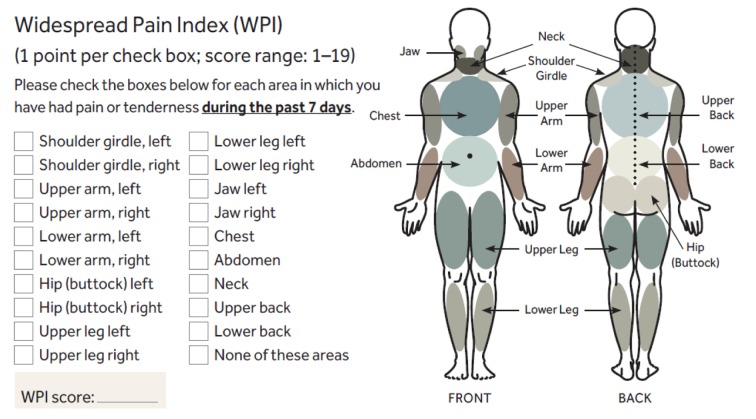
The WPI is calculated by summing up patient reports of pain in 19 separate regions of the body (Figure 2).
Figure 2.
Diagnostic and Severity Criteria for Fibromyalgia: Widespread Pain Index (WPI). (Adapted from: Wolfe F. et al. Arthritis Care Res (Hoboken) 2010; 62: 600-610).
The SS scale score is calculated by grading several symptoms (e.g., pain, fatigue, awaking unrefreshed) on a severity scale from 0 (“no problem”) to 3 (“severe, pervasive, continuous, life-disturbing problems”). Fatigue, waking unrefreshed, and cognitive symptoms are rated on the basis of the level of severity during the previous week. Additionally, in adults a list of 41 potential comorbid symptoms or signs is provided to represent an FM-related review of symptoms. The magnitude of their contributions to the patient’s behavior was categorically quantified from 0=none, 1=few (1-10), 2=many (11-30), to 3=very many (31-40).
A patient satisfies the proposed diagnostic criteria if the following 3 conditions are met:
The patient has a widespread pain index (WPI) of 7 or more and symptom severity score (SS) of 5 or more. Alternatively, a patient could meet criteria with a WPI of 3 to 6 and SS scale score of 9 or greater.
Symptoms have been present at a similar level for at least 3 months and must not be explained by another disease process.
The patient does not have a disorder that would otherwise explain the pain.
The use of WPI combined with SS enabled a 90.8% diagnostic accuracy (90.9% sensitivity and 85.9% specificity) when compared with the 1990 ACR criteria (17). Based on analysis of criteria studies and clinician and researchers’ comments, Wolfe et al. identified problematic areas, including: a) misclassification in asymmetric pain disorders, b) inconsistent and unclear instructions in the presence of other medical conditions, c) different clinician and self-report criteria, d) unclearly defined pain assessment regions. Therefore, they developed in 2016 a revision of the 2010/2011 fibromyalgia criteria (19). The revision makes the following changes:
1) Changes Criterion 1 to WPI ≥7 and SS score ≥5 or WPI 4-6 and SS score ≥9 (WPI minimum must be ≥4 instead of previous ≥3).
2) Adds a generalized pain criterion (Criterion 2) that is defined as pain in at least 4 of 5 regions (left upper, right upper, left lower, right lower, axial). In this definition, jaw, chest and abdominal pain are not evaluated as part of the generalized pain definition.
3) Standardizes and makes 2010 and 2011 criterion (Criterion 3) wording the same: “Symptoms have been generally present for at least 3 months.”
4) Removes the exclusion regarding disorders that could (sufficiently) explain the pain (Criterion 4) and adds the following text: “A diagnosis of fibromyalgia is valid irrespective of other diagnoses. A diagnosis of fibromyalgia does not exclude the presence of other clinically important illnesses.”
5) Adds the Fibromyalgia Symptom (FS) or polysymptomatic distress (PSD) scale as a full component of the fibromyalgia criteria.
6) Creates one set of criteria (2016) instead of having separate physician (2010) and patient (2011) criteria by replacing the physician estimate of somatic symptom burden with ascertainment of the presence of headaches, pain or cramps in lower abdomen, and depression during the previous 6 months.
Practical considerations
The 2016 revision combines physician and questionnaire criteria, minimizes misclassification of regional pain disorders, and eliminates the previously confusing recommendation regarding diagnostic exclusions. The physician-based criteria are valid for individual patient diagnosis. The self report version of the criteria is not valid for clinical diagnosis in individual patients but are valid for research studies.
The diagnosis of fibromyalgia in children and adolescents
The diagnosis of JFMS remains the subject of debate about which criteria to use: those of the ACR (2010) or those proposed by Yunus and Masi (14); the measurement of the force to be applied in the evaluation of tender points; the definition of headache and the most adequate assessment tools for the determination of anxiety and depression.
The 2010 criteria were validated in adolescent girls (aged 11-17 years) by Ting et al. (60). The authors suggested minor modifications to suit the developmental level of the patients, without compromising the integrity of the test.
They recommend extending the time frame of the WPI to “in the past 3 months” and to specify that the pain was persistent (“every day or almost every day”) to enhance the understanding of the nature of chronic or recurrent pain. For the somatic symptoms, several items rarely were endorsed by the youth (<10%), and some were not understood and/or were redundant. Therefore, they recommend removing these items (muscle pain, fatigue/tiredness, chest pain, fever, diarrhea, wheezing, Raynaud phenomenon, hives/welts, vomiting, oral ulcers, loss of/ change in taste, seizures, rash, sun sensitivity, hearing difficulties, hair loss, painful urination, and bladder spasms) from the list of somatic symptoms and to utilize only the most useful 22 remaining items.
They also emphasized that a standard detailed physical examination and history should be conducted as part of clinical practice to ensure other conditions (e.g. thyroid dysfunction, systemic lupus erythematosus, juvenile idiopathic arthritis, sleep disorders) are ruled out.
Practical considerations in children and adolescents
The use of the ACR 2010 diagnostic criteria, which do not require tender point examination, is recommended for clinical diagnosis, but should not preclude a thorough physical examination. However, these criteria do not precisely define how symptom severity is to be ascertained, leaving this to the clinician. Therefore, the concept of JFMS remains a work in progress with many current unanswered clinical and pathophysiologic issues. For example, the developmental changes which occur during childhood and adolescence make the measurement of paediatric pain particularly challenging (61), and the cognitive and metacognitive skills required for a child to give reliable self-reports of pain (such as the ability to rank-order objects, consider numerous options simultaneously, and retain and manipulate information) change significantly during childhood and adolescence (62).
Each symptom plays a variable role in the presentation of the individual patient and all contribute to a greater or lesser degree towards the overall effect of impaired quality of life and reduced functional activity. Therefore, such patients may be referred to numerous pediatric specialties (e.g., neurology, rheumatology, pain medicine) before symptoms of JFMS are finally identified.
Differential diagnosis of fibromyalgia in children and adolescents
Because the most prominent complaint of patients with FM is body pain, the differential diagnosis should consider a wide variety of other painful conditions. Similar to FM in adults, JFMS is often difficult to classify, because many of the symptoms are scientifically “medically unexplained” and often overlap with other medical conditions such as chronic fatigue syndrome (63), irritable bowel syndrome, and migraine headache.
Practically, the differential diagnosis of FM includes disorders that have symptoms of widespread pain and fatigue. These disorders include hypothyroidism, inflammatory and other myopathies, polymyalgia rheumatica, other rheumatic diseases, viral infections, and severe vitamin D deficiency (5-7, 41).
Laboratory testing
There are no specific tests to confirm the diagnosis, but many of the differential diagnoses diseases can be excluded by an extensive clinical examination and specific laboratory testing. Although not required to establish the diagnosis of FM, routine laboratory testing (if not already performed within the past 6-12 months) is frequently obtained. These tests include measurements: of erythrocyte sedimentation rate (ESR), C-reactive protein (CRP) levels, complete blood cell count, comprehensive metabolic panel, and thyroid function tests.
Depending on symptoms (e.g., duration of pain and acute vs. chronic), medical history, and physical examination findings, other tests may be indicated (e.g. antinuclear antibodies, rheumatoid factor, viral infections, vitamin D levels, imaging, electroencephalog-raphy, electrocardiography, genetic studies, biopsy) if there is a clinical suspicion for an alterna-tive cause of the pain (3-5, 64-66).
Psychological problems/disorders should be considered since they are even more common, e.g., depression (subtypes according to DSM-V), anxiety disorders (subtypes according to DSM-V), post-traumatic stress disorders and dissociative disorders with or without self-injurious behaviour. There is also the possi-bility of mental illness of parents, as seen in Munchhausen by proxy syndrome (64).
Debated is the possible role of hyperparathyroidism in FM, reported only in adults. A high frequency of hyperparathyroidism, in women with FM versus the general population, was reported by Costa et al. (65). By contrast, Ferrari and Russell found that the incidence of primary hyperparathyroidism in FM patients was not different than that seen in other patients with widespread or localized pain (66).
Ciregia et al. (67) investigated the presence of potential diagnostic and/or prognostic biomarkers in saliva which could be useful for the management of FM patients. Specifically, the salivary profile of FM patients was compared with those of healthy subjects, subjects suffering migraine (model of non-inflammatory chronic pain), and patients affected by rheumatoid arthritis (model of inflammatory chronic pain). Two-dimensional gel electrophoresis (2-DE) 2-DE serotransferrin and alpha-enolase were found differentially expressed in FM patients. The authors concluded that the identification of disease salivary biomarkers could be helpful in detecting FM clusters and targeted treatment.
Practical considerations
There are currently no instrumental tests or specific diagnostic markers for FM.and JFM. It is hoped that, in the near future, some tests such as salivary indicators can offer a clue for shedding light upon complex diseases like FM. The difficulty in diagnosing this condition, particularly in children and adolescents, can cause a great deal of frustration among patients and parents, when no definitive medical cause and/or explanation for the child’s symptoms is provided. Therefore, parents may look for multiple consultations in the conviction that a serious underlying disease is going to be missed.
Prognosis of fibromyalgia in children and adolescents
Initial studies indicated a positive long-term prognosis for JFMS (44, 68). By contrast, studies in subjects with JFMS recruited from hospital settings have shown a chronic and fluctuating course, with symptoms persisting in ~70% of young people (43, 69, 70).
One controlled study published in 2010 of patients with JFMS and matched healthy controls (mean age, 15 years) showed that about 50% of patients with JFM met the full ACR criteria for fibromyalgia at ~4 years follow-up (mean age, 19 years), and >70% had continuing symptoms of pain, fatigue or sleep difficulty (70).
Several authors report different prognoses between adults and youths with FM. (43, 45, 71). They suggested that the early detection of JFMS is an indication of a better prognosis (69), with significant gains in quality of life (73), and functionality for individuals who receive adequate treatment, whereas those with widespread pain that are not treated adequately have a greater chance of developing fibromyalgia (74). On the other hand, in a large prospective longitudinal study of JFMS patients, Kashikar-Zuck et al. (75) found that the majority of adolescent patients (~80%) with JFMS seen in a pediatric specialty care setting continued to report persistent pain and other FM symptoms as they transitioned into young adulthood (Table 4).
Table 4.
Long-term outcomes of juvenile fibromyalgia syndrome and review of the literature
| Reference | Results |
| Malleson PN et al. Idiopathic musculoskeletal pain syndromes in children. J Rheumatol 1991: 19: 1186-1189 | After a variable observation period between 1 and 48 months, 11 of 18 patients with so-called JFMS had persistent symptoms after an average of 11 months. |
| Buskila D et al. Fibromyalgia syndrome in children - an outcome study. J Rheumatol 1995; 11: 515-8. | A spontaneous remission of symptoms was observed in 13% of patients evaluated after a 30-month follow up. |
| Siegel DM et al. Fibromyalgia syndrome in children and adolescents: clinical features at presentation and status at follow-up. Pediatrics 1998; 101: 311-81. | In the present study, 46% of the patients improved, 43% remained unchanged, and in 11% symptoms became worse. There was no statistically significant difference between the younger and older patients. |
| Mikkelsson M. One year outcome of preadolescents with fibromyalgia. J Rheumatol 1999; 16: 614-81. | The JFMS persisted in only 16% of the patients evaluated at a one-year follow up. |
| Calvo I et al. Pediatric fibromyalgia patients: A follow-up study. Ann Rheum Dis. XIV European League Against Rheumatism Congress Abstracts, Glasgow, Scotland, 1999, p 353. | At 48-month follow-up, 15/11 (68.1%) had no longer fulfilled the FM criteria. |
| Gedalia A et al. Fibro-myalgia syndrome: experience in a pediatric rheu-matology clinic. Clin Exp Rheumatol 1000; 18: 415-419 | Conducted a retrospective study over a period of 4 years. At an average follow-up of 18 months (range 3-65 months), 60% of the children improved, 36% experienced no change and 4% experienced a worsening of pain symptoms. |
| Kashikar-Zuck S et al. Controlled follow-up study of physical and psycho-social functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology (Oxford) 1010; 49: 1104-1109 | Of 48 U.S. American children and adolescents diagnosed with JFMS, after an average of 3.1 years, 61.5% suffered from widespread musculoskeletal pain, and 60.4% fulfilled criteria for so-called JFMS. |
| Libby CJ et al. Protective and exacerbating factors in children and adolescents with fibromyalgia. Rehabil Psychol 1010; 55: 151-158 | Exacerbating factors for widespread musculoskeletal pain included the following: daily hassles, pain-related catastrophizing, lack of self-efficacy and lack of positive family support. |
| Kashikar-Zuck S et al. Long-Term Outcomes of Adolescents With Juvenile-Onset Fibromyalgia in Early Adulthood. Pediatrics 1014; 133: e591-600. | This prospective study demonstrated that pain and other symptoms persisted into adulthood for 80% of JFMS patients, with associated impairments in physical functioning and mood. At follow-up, one-half of the sample met full criteria for adult FM. |
In conclusion, most of youth with JFMS continue to experience symptoms into adulthood, which highlights the importance of early diagnosis and intervention. However, more research into the variability of outcomes within the JPFS group, with closer examination of risk and protective factors associated with future outcomes is essential to designing focused interventions.
General principles of treatment in children and adolescents
Goals of treatment should be pain re-lief, restoration of functioning, reduction of school absenteeism, dissolving so-cial isolation, strengthening self-aware-ness, mobilizing domestic resources and the development of strategies for cop-ing with pain. The inclusion of the family, the training of strategies in everyday life and the treatment of mental co-morbidi-ties are also important (64).
Evidence-based treatment guidelines in FM include those developed by the American Pain Society (APS) in 2005 (76, 77) and the European League Against Rheumatism (EULAR) in 2008 (78).
However, they were developed before FDA approved any medications for treating fibromyalgia and substantial heterogeneity exists between the recommendations. Furthermore, the studies evaluated during the development of these guidelines were not directly comparable as a result of variations in study design and short duration that limit their general applicability in clinical practice (79).
Little is known regarding treatment choices of youth diagnosed with JFMS as they move into young adulthood. The management of JFMS is centered on the issues of education, behavioral and cognitive change (cognitive-behavioral therapy (CBT)) with a strong emphasis on physical exercise), and a relatively minor role for pharmacological treatment with medications such as muscle relaxants, analgesics and tricyclic agents (80-84). Any patient being treated with a medication should be carefully evaluated for both efficacy as well as side effects, and medications should be discontinued unless there is evidence for definite benefit. More controlled studies are needed to investigate the effectiveness of these complementary methods to assist treatment providers in giving evidence-based treatment recommendations.
A recent Cochrane review has concluded that psychological treatments may improve pain control for children with a variety of pain conditions, including muscle pain, abdominal pain, headaches and FMS (85). Therefore, it appears that CBT should be offered as a preferred modality of non-pharmacological treatment for JFMS.
If disease development is assumed to be linked to the family background, hospitalization aimed to temporarily isolate the patient from his or her home environment should be taken into consideration (86).
Prompt recognition of JFMS may decrease problems for pediatric patients with chronic pain, while pediatric primary care providers’ lack of familiarity with JFMS can cause a delay in diagnosis and management (87, 88).
Conclusions
JFMS is characterized by persistent widespread musculoskeletal pain, sleep disturbances, fatigue, and the presence of multiple discrete tender points on physical examination. Its pathogenesis is not entirely understood, although it is currently believed to be the result of a central nervous system (CNS) malfunction that increases pain transmission and perception. To date, there is no “gold standard” for diagnosing FM because symptoms may be part of or overlap with other diseases or syndromes. Therefore, until a better clinical definition is achieved, all diagnostic criteria should be interpreted with caution and subject to modification. Recent guidelines agree that the diagnosis remains clinical, and the purpose of the physical examination and limited laboratory investigations is to rule out some other somatic disease that can sufficiently explain the symptoms.
Emerging evidence suggests that JFMS is a condition that frequently continues into adulthood with chronic physical and psychological symptoms, making it important to correctly identify and treat this condition in adolescence. A multidisciplinary approach, combining pharmacological, behavioral and exercise-based modalities is currently the standard of care for JFMS.
Conflict of interest:
None to declare
References
- 1.Stanford EA, Chambers CT, Biesanz JC, Chen E. The frequency, trajectories and predictors of adolescent recurrent pain: a population-based approach. Pain. 2008;138:11–21. doi: 10.1016/j.pain.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 2.Stanos S, Brodsky M, Argoff C, Clauw DJ, D’Arcy Y, Donevan S, Gebke KB, Jensen MP, Lewis Clark E, McCarberg B, Park PW, Turk DC, Watt S. Rethinking chronic pain in primary care setting. Postgraduate Med. 2016;128:502–513. doi: 10.1080/00325481.2016.1188319. [DOI] [PubMed] [Google Scholar]
- 3.Häuser W, Fitzcharles MA. Facts and myths pertaining to fibromyalgia. Dialogues Clin Neurosci. 2018;20:53–62. doi: 10.31887/DCNS.2018.20.1/whauser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rahman A, Underwood M, Carnes D. Fibromyalgia. BMJ. 2014 Feb 24;348:g1224. doi: 10.1136/bmj.g1224. doi: 10. 1136 /bmj.g1224. [DOI] [PubMed] [Google Scholar]
- 5.Arnold LM, Clauw DJ. Fibromyalgia syndrome: practical strategies for improving diagnosis and patient outcomes. Am J Med. 2010 Jun;123(6) S2 doi: 10.1016/j.amjmed.2010.04.001. doi: 10.1016/j.amjmed.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Kaufman EL, Tress J, Sherry DD. Trends in Medicalization of Children with Amplified Musculoskeletal Pain Syndrome. Pain Med. 2017;18:825–831. doi: 10.1093/pm/pnw188. [DOI] [PubMed] [Google Scholar]
- 7.Draheim N, Ebinger F, Schnobel-Muller E, Wolf B, Hauser W. Definition, diagnostics and therapy of chronic widespread pain and the (so-called) fibromyalgia syndrome in children and adolescents: updated guidelines 2017. Schmerz. 2017;31:296–307. doi: 10.1007/s00482-017-0208-z. [DOI] [PubMed] [Google Scholar]
- 8.Balfour W. Observations on the pathology and cure of rheumatism. Edinburgh Medical and Surgical Journal. 1815;11:168–187. [PMC free article] [PubMed] [Google Scholar]
- 9.Beard GM. New York: William Wood and Co; 1880. A Practical Treatise on Nervous Exhaustion (Neurasthenia) [Google Scholar]
- 10.Stockman R. The causes, pathology, and treatment of chronic rheumatism. Edinb Med J. 1904;15:107–16. doi: 10.1136/bmj.1.2252.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gowers W. Lumbago: Its Lessons and Analogues. Br Med J. 1904;1:117–121. doi: 10.1136/bmj.1.2246.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hench PK. Non articular rheumatism, 22nd rheumatism review: review of the American and English literature for the years 1973 and 1974. Arthritis Rheum. 1976;(19 suppl):1081–1089. [PubMed] [Google Scholar]
- 13.Smythe HA, Moldofsky H. Two contributions to understanding of the “fibrositis” syndrome. Bull Rheum Dis. 1977-1978;28:928–931. [PubMed] [Google Scholar]
- 14.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome. A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985;28:138–145. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]
- 15.Müller W, Lautenschläger J. Generalized tendomyopathy. I: Clinical aspects, follow-up and differential diagnosis. Z Rheumatol. 1990;49:11–21. [PubMed] [Google Scholar]
- 16.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe F, Clauw D, Fitzcharles MA, Goldenberg D, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology Preliminary Diagnostic. Criteria for Fibromyalgia and Measurement.of Symptom Severity. Arthritis Care Res. 2010;62:600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38:1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 19.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthr Rheum. 2016;46:319–329. doi: 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, Gabriel S, Hirsch R, Hochberg MC, Hunder GG, Jordan JM, Katz JN, Kremers HM, Wolfe F. National Arthritis Data Workgroup. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eraso RM, Bradford NJ, Fontenot CN, Espinoza LR, Gedalia A. Fibromyalgia syndrome in young children: onset at age 10 years and younger. Exp Rheumatol. 2007;25:639–644. [PubMed] [Google Scholar]
- 22.Clark P, Burgos-Vargas R, Medina-Palma C, Lavielle P, Marina FF. Prevalence of fibromyalgia in children: a clinical study of Mexican children. J Rheumatol. 1998;25:2009–2014. [PubMed] [Google Scholar]
- 23.Russel IJ, Larson AA. Neurophysiopathogenesis of fibromyalgia syndrome: a unified hypothesis. Rheum Dis Clin North Am. 2009;35:421–435. doi: 10.1016/j.rdc.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 24.Smith HS, Harris R, Clauw D. Fibromyalgia: an afferent processing disorder leading to a complex pain generalized syndrome. Pain Physician. 2011;14:E217–E245. [PubMed] [Google Scholar]
- 25.Yunus MB. Central sensitivity syndromes: a new paradigm and group nosology for fibromyalgia and overlapping conditions, and the related issue of disease versus illness. Semin Arthritis Rheum. 2008;37:339–352. doi: 10.1016/j.semarthrit.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Clauw DJ. Fibromyalgia and related conditions. Mayo Clin Proc. 2015;90:680–692. doi: 10.1016/j.mayocp.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Phillips K, Clauw DJ. Central pain mechanisms in chronic pain states-maybe it is all in their head. Best Pract Res Clin Rheumatol. 2011;25:141–154. doi: 10.1016/j.berh.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.King CD, Jastrowski Mano KE, Barnett KA, Pfeiffer M, Ting TV, Kashikar-Zuck S. Pressure Pain Threshold and Anxiety in Adolescent Females With and Without Juvenile Fibromyalgia: A Pilot Study. Clin J Pain. 2017;33:620–626. doi: 10.1097/AJP.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nebel MB, Gracely RH. Neuroimaging of fibromyalgia. Rheum Dis Clin North Am. 2009;35:313–327. doi: 10.1016/j.rdc.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 30.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 31.Apkarian AV. The brain in chronic pain: clinical implications. Pain Manag. 2011;1:577–586. doi: 10.2217/pmt.11.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seminowicz DA, Wideman TH, Naso L, Hatami-Khoroushahi Z, Fallatah S, Ware MA, Jarzem P, Bushnell MC, Shir Y, Ouellet JA, Stone LS. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31:7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korszun A, Young EA, Engleberg NC, Brucksch CB, Greden JF, Crofford LA. Use of actigraphy for monitoring sleep and activity levels in patients with fibromyalgia and depression. J Psychosom Res. 2002;52:439–443. doi: 10.1016/s0022-3999(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 34.Cohen H, Buskila D, Neumann L, Ebstein RP. Confirmation of an association between fibromyalgia and serotonin transporter promoter region (5-HTTLPR) polymorphism, and relationship to anxiety-related personality traits. Arthritis Rheum. 2002;46:845–847. doi: 10.1002/art.10103. [DOI] [PubMed] [Google Scholar]
- 35.Gürsoy S, Erdal E, Herken H, Madenci E, Alaşehirli B, Erdal N. Significance of catechol-O-methyltransferase gene polymorphism in fibromyalgia syndrome. Rheumatol Int. 2003;23:104–107. doi: 10.1007/s00296-002-0260-5. [DOI] [PubMed] [Google Scholar]
- 36.Metyas SK, Solyman JS, Arkfeld DG. Inflammatory fibromyalgia: is it real? Curr Rheumatol Rev. 2015;11:15–17. doi: 10.2174/1573397111666150522095004. [DOI] [PubMed] [Google Scholar]
- 37.Smart PA, Waylonis GW, Hackshaw KV. Immunologic profile of patients with fibromyalgia. Am J Phys Med Rehabil. 1997;76:231–234. doi: 10.1097/00002060-199705000-00014. [DOI] [PubMed] [Google Scholar]
- 38.Kötter I, Neuscheler D, Günaydin I, Wernet D, Klein R. Is there a predisposition for the development of autoimmune diseases in patients with fibromyalgia? Retrospective analysis with long term follow-up. Rheumatol Int. 2007;27:1031–1039. doi: 10.1007/s00296-007-0413-7. [DOI] [PubMed] [Google Scholar]
- 39.Nampiaparampil DE, Shmerling RH. A review of fibromyalgia. Am J Manag Care. 2004;10:794–800. [PubMed] [Google Scholar]
- 40.Bradley LA. Pathophysiologic mechanisms of fibromyalgia and its related disorders. J Clin Psych. 2008;69(suppl 2):6–13. [PubMed] [Google Scholar]
- 41.Kashikar-Zuck S, King C, Ting TV, Arnold LM. Juvenile Fibromyalgia: Different from the Adult Chronic Pain Syndrome? Curr Rheumatol Rep. 2016 Apr;18(4):19. doi: 10.1007/s11926-016-0569-9. doi: 10.1007/s11926-016-0569-9. [DOI] [PubMed] [Google Scholar]
- 42.Gedalia A, Press J, Klein M, Buskila D. Joint hypermobility and fibromyalgia in school children. Ann Rheum Dis. 1993;52:494–296. doi: 10.1136/ard.52.7.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Siegel DM, Janeway D, Baum J. Fibromyalgia syndrome in children and adolescents: Clinical features at presentation and status at follow-up. Pediatrics. 1998;101:377–382. doi: 10.1542/peds.101.3.377. [DOI] [PubMed] [Google Scholar]
- 44.Mikkelsson M. One year outcome of preadolescents with fibromyalgia. J Rheumatol. 1999;26:674–682. [PubMed] [Google Scholar]
- 45.Kashikar-Zuck S, Johnston M, Ting TV, Graham BT, Lynch-Jordan AM, Verkamp E, Passo M, Schikler KN, Hashkes PJ, Spalding S, Banez G, Richards MM, Powers SW, Arnold LM, Lovell D. Relationship between school absenteeism and depressive symptoms among adolescents with juvenile fibromyalgia. J Pediatr Psychol. 2010;35:996–1004. doi: 10.1093/jpepsy/jsq020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wolfe F, Michaud K. Severe rheumatoid arthritis (RA), worse outcomes, comorbid illness, and sociodemographic disadvantage characterize RA patients with fibromyalgia. J Rheumatol. 2004;31:695–700. [PubMed] [Google Scholar]
- 47.Clauw DJ, Katz P. The overlap between fibromyalgia and inflammatory rheumatic disease: when and why does it occur? J Clin Rheumatol. 1995;1:335–1342. doi: 10.1097/00124743-199512000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain. 2000;4:229–238. doi: 10.1053/eujp.2000.0175. [DOI] [PubMed] [Google Scholar]
- 49.McLean SA, Williams DA, Clauw DJ. Fibromyalgia after motor vehicle collision: evidence and implications. Traffic Inj Prev. 2005;6:97–104. doi: 10.1080/15389580580590931545. [DOI] [PubMed] [Google Scholar]
- 50.Rehm SE, Koroschetz J, Gockel U, Brosz M, Freynhagen R, Tölle TR, Baron R. A cross-sectional survey of 3035 patients with fibromyalgia: subgroups of patients with typical comorbidities and sensory symptom profiles. Rheumatology (Oxford) 2010;49:1146–1152. doi: 10.1093/rheumatology/keq066. [DOI] [PubMed] [Google Scholar]
- 51.Yunus MB, Inanici F, Aldag JC, Mangold RF. Fibromyalgia in men: comparison of clinical features with women. J Rheumatol. 2000;27:485–490. [PubMed] [Google Scholar]
- 52.Fitzcharles MA, Boulos P. Inaccuracy in the diagnosis of fibromyalgia syndrome: analysis of referrals. Rheumatology (Oxford) 2003;42:263–267. doi: 10.1093/rheumatology/keg075. [DOI] [PubMed] [Google Scholar]
- 53.Okifuji A, Turk DC, Sinclair JD, Starz TW, Marcus DA. A standardized manual tender point survey. I. Development and determination of a threshold point for the identification of positive tender points in fibromyalgia syndrome. J Rheumatol. 1997;24:377–383. [PubMed] [Google Scholar]
- 54.Bidari A, Ghavidel-Parsa B, Ghalehbaghi B. Reliability of ACR criteria over time to differentiate classic fibromyalgia from nonspecific widespread pain syndrome: a 6-month prospective cohort study. Mod Rheumatol. 2009;19:663–669. doi: 10.1007/s10165-009-0222-9. [DOI] [PubMed] [Google Scholar]
- 55.Granges G, Littlejohn G. Pressure pain threshold in pain-free subjects, in patients with chronic regional pain syndromes, and in patients with fibromyalgia syndrome. Arthritis Rheum. 1993;36:642–646. doi: 10.1002/art.1780360510. [DOI] [PubMed] [Google Scholar]
- 56.Jacobs JW, Rasker JJ, van der Heide A, Boersma JW, de Blécourt AC, Griep EN, van Rijswijk MH, Bijlsma JW. Lack of correlation between the mean tender point score and self-reported pain in fibromyalgia. Arthritis Care Res. 1996;9:105–111. doi: 10.1002/1529-0131(199604)9:2<105::aid-anr1790090206>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 57.Cott A, Parkinson W, Bell MJ, Adachi J, Bédard M, Cividino A, Bensen W. Interrater reliability of the tender point criterion for fibromyalgia. J Rheumatol. 1992;19:1955–1959. [PubMed] [Google Scholar]
- 58.Petzke F, Gracely RH, Park KM, Ambrose K, Clauw DJ. What do tender points measure? Influence of distress on 4 measures of tenderness. J Rheumatol. 2003;30:567–574. [PubMed] [Google Scholar]
- 59.Harth M, Nielson WR. The fibromyalgia tender points: use them or lose them? A brief review of the controversy. J Rheumatol. 2007;34:914–922. [PubMed] [Google Scholar]
- 60.Ting TV, Barnett K, Lynch-Jordan A, Whitacre C, Henrickson M, Kashikar-Zuck S. 2010 American College of Rheumatology adult fibromyalgia criteria for use in an adolescent female population with juvenile fibromyalgia. J Pediatr. 2016;169:181–187. doi: 10.1016/j.jpeds.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Howard RF, Liossi C. Pain assessment in children. Arch Dis Child. 2014;99:1123–1124. doi: 10.1136/archdischild-2014-306432. [DOI] [PubMed] [Google Scholar]
- 62.Chan JY, von Baeyer CL. Cognitive developmental influences on the ability of preschool-aged children to self-report their pain intensity. Pain. 2016;157:997–1001. doi: 10.1097/j.pain.0000000000000476. [DOI] [PubMed] [Google Scholar]
- 63.Itoh Y, Shigemori T, Igarashi T, et al. Fibromyalgia and chronic fatigue syndrome in children. Pediatr Int. 2012;54:266–271. doi: 10.1111/j.1442-200X.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 64.Zernikow B, Gerhold K, Bürk G, Häuser W, Hinze CH, Hospach T, Illhardt A, Mönkemöller K, Richter M, Schnöbel-Müller E, Häfner R. Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen Fachgesellschaften. Definition, diagnosis and therapy of chronic widespread pain and so-called fibromyalgia syndrome in children and adolescents. Systematic literature review and guideline. Schmerz. 2012;26:318–330. doi: 10.1007/s00482-012-1168-y. [DOI] [PubMed] [Google Scholar]
- 65.Costa JM, Ranzolin A, da Costa Neto CA, Marques CD, Duarte AL. High frequency of asymptomatic hyperparathyroidism in patients with fibromyalgia: random association or misdiagnosis? Rev Bras Rheumatol Engl Ed. 2016;56:391–397. doi: 10.1016/j.rbre.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 66.Ferrari R, Russell AS. Prevalence of primary hyperparathyroidism in a referred sample of fibromyalgia patients. Clin Rheumatol. 2015;34:1279–1283. doi: 10.1007/s10067-014-2735-7. [DOI] [PubMed] [Google Scholar]
- 67.Ciregia F, Giacomelli C, Giusti L, Boldrini C, Piga I, Pepe P, Consensi A, Gori S, Lucacchini A, Mazzoni MR, Bazzichi L. Putative salivary biomarkers useful to differentiate patients with fibromyalgia. J Proteomics. 2018 Apr 11 doi: 10.1016/j.jprot.2018.04.012. pii: S1874-3919(18)30172-6. doi: 10.1016/j.jprot. 2018.04.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 68.Buskila D, Neumann L, Hershman E, Gedalia A, Press J, Sukenik S. Fibromyalgia syndrome in children--an outcome study. J Rheumatol. 1995;22:525–528. [PubMed] [Google Scholar]
- 69.Gedalia A, García CO, Molina JF, Bradford NJ, Espinoza LR. Fibromyalgia syndrome in pediatric patients. Clin Exp Rheumatol. 2000;18:415–419. [PubMed] [Google Scholar]
- 70.Kashikar-Zuck S, Parkins IS, Ting TV, Verkamp E, Lynch-Jordan A, Passo M, Graham TB. Controlled follow-up study of physical and psychosocial functioning of adolescents with juvenile primary fibromyalgia syndrome. Rheumatology (Oxford) 2010;49:2204–2209. doi: 10.1093/rheumatology/keq254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hassett AL, Hilliard PE, Goesling J, Clauw DJ, Harte SE, Brummett CM. Reports of chronic pain in childhood and adolescence among patients at a tertiary care pain clinic. J Pain. 2013;14:1390–7.27. doi: 10.1016/j.jpain.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 72.Kashikar-Zuck S, Vaught MH, Goldschneider KR, Graham TB, Miller JC. Depression, coping, and functional disability in juvenile primary fibromyalgia syndrome. J Pain. 2002;3:412–419. doi: 10.1054/jpai.2002.126786. [DOI] [PubMed] [Google Scholar]
- 73.Alfvén G. Recurrent pain, stress, tender points and fibromyalgia in childhood: an exploratory descriptive clinical study. Acta Paediatr. 2012;101:283–291. doi: 10.1111/j.1651-2227.2011.02491.x. [DOI] [PubMed] [Google Scholar]
- 74.Black WR, Kashikar-Zuck S. Exercise interventions for juvenile fibromyalgia: current state and recent advancements. Pain Manag. 2017;7:143–148. doi: 10.2217/pmt-2016-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kashikar-Zuck S, Cunningham N, Sil S, Bromberg MH, Lynch-Jordan AM, Strotman D, Peugh J, Noll J, Ting TV, Powers SW, Lovell DJ, Arnold LM. Long-term outcomes of adolescents with juvenile-onset fibromyalgia in early adulthood. Pediatrics. 2014;133:e592–600. doi: 10.1542/peds.2013-2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Burckhardt C, Goldenberg D, Crofford L, Gerwin R, Gowans S, Kackson K. USA: American Pain Society, Glenview, Ill; 2005. Guideline for the management of fibromyalgia syndrome. Pain in adults and children. In: APS Clinical Practice Guideline Series no. 4. [Google Scholar]
- 77.American Pain Society. Glenview: American Pain Society; 2005. Guideline for the management of fibromyalgia syndrome pain in adults and children. [Google Scholar]
- 78.Carville SF, Arendt-Nielsen L, Bliddal H, Blotman F, Branco JC, Buskila D, Da Silva JA, Danneskiold-Samsøe B, Dincer F, Henriksson C, Henriksson KG, Kosek E, Longley K, McCarthy GM, Perrot S, Puszczewicz M, Sarzi-Puttini P, Silman A, Späth M, Choy EH. EULAR. EULAR evidence-based recommendations for the management of fibromyalgia syndrome. Ann Rheum Dis. 2008;67:536–541. doi: 10.1136/ard.2007.071522. [DOI] [PubMed] [Google Scholar]
- 79.Navarro RP. Contemporary management strategies for fibromyalgia. Am J Manag Care. 2009;15(7 suppl):S197–218. [PubMed] [Google Scholar]
- 80.Gmuca S, Sherry DD. Fibromyalgia: Treating Pain in the Juvenile Patient. Paediatr Drugs. 2017;19:325–338. doi: 10.1007/s40272-017-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Black WR, Kashikar-Zuck S. Exercise interventions for juvenile fibromyalgia: current state and recent advancements. Pain Manag. 2017;7:143–148. doi: 10.2217/pmt-2016-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tran ST, Guite JW, Pantaleao A, Pfeiffer M, Myer GD, Sil S, Thomas SM, Ting TV, Williams SE, Edelheit B, Ounpuu S, Rodriguez-MacClintic J, Zemel L, Zempsky W, Kashikar-Zuck S. Preliminary Outcomes of a Cross-Site Cognitive-Behavioral and Neuromuscular Integrative Training Intervention for Juvenile Fibromyalgia. Arthritis Care Res (Hoboken) 2017;69:413–420. doi: 10.1002/acr.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buskila D, Ablin JN. Treating juvenile fibromyalgia: cognitive-behavioral therapy, exercise and pharmacotherapy. Pain Manag. 2013;3:323–324. doi: 10.2217/pmt.13.37. [DOI] [PubMed] [Google Scholar]
- 84.Kashikar-Zuck S, Flowers SR, Strotman D, Sil S, Ting TV, Schikler KN. Physical activity monitoring in adolescents with juvenile fibromyalgia: findings from a clinical trial of cognitive-behavioral therapy. Arthritis Care Res (Hoboken) 2013;65:398–405. doi: 10.1002/acr.21849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Eccleston C, Palermo TM, Williams AC, Lewandowski A, Morley S. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database Syst Rev. 2009 Apr 15;(2):CD003968. doi: 10.1002/14651858.CD003968.pub2. doi: 10.1002/14651858. [DOI] [PubMed] [Google Scholar]
- 86.Yokota S, Kikuchi M, Miyamae T. Juvenile fibromyalgia: Guidance for management. Pediatr Int. 2013;55:403–409. doi: 10.1111/ped.12155. [DOI] [PubMed] [Google Scholar]
- 87.McLeod JD. Juvenile fibromyalgia syndrome and improved recognition by pediatric primary care providers. J Pediatr Health Care. 2014;28:e9–18. doi: 10.1016/j.pedhc.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 88.Buskila D, Ablin J. Pediatric fibromyalgia. Reumatismo. 2012;64:230–237. doi: 10.4081/reumatismo.2012.230. [DOI] [PubMed] [Google Scholar]