Abstract
Aim: Basal Cell Carcinoma (BCC) alone accounts for 80% of cases of non-melanoma skin cancer (NMSC), which characteristically develops on sun-exposed skin. Indeed the most common site of BCC is the head and neck region (80%). The purpose of this study to review the experience of our center with BCC in the head and neck region to report the sites of occurrence and treatment. Materials and method: We retrospectively reviewed 77 patients with BCC of the head and neck, who revived surgical treatment within our plastic surgery division. Basic demographic data, cancer site and size, surgical treatment and histological data were collected. The mean follow-up period was 12 months. Results: The study population included 37 males and 40 females, with a mean age of 74.12 years. The nasal unit was the main site of BCC (31.82%), followed by the periorbital (13.64%) and cervical (12.5%) units. Primary closure was the main surgical procedure performed (72.5%), followed by local flap (26.1%) and full-thickness skin grafts (1.4%). The safety resection margin ranged from 4.5 to 9 mm, with a 98.7% complete removal rate. Neither recurrence nor any newly-developed lesions were reported during follow-up in any patient. Discussion: Our work reflects the shift in the incidence of BCC, which now seems to be more frequent in females. Furthermore, our data strengthens the association between UVR exposure and BCC, confirms its predilection to occur on the nasal unit and validates surgical excision as the gold standard treatment for skin cancer. (www.actabiomedica.it)
Keywords: non-melanoma skin cancer, basal cell carcinoma, head and neck cancer, operative surgical procedure, epidemiology
Introduction
Non-melanoma skin cancer (NMSC) is the most common cancer in Caucasian populations worldwide (1). NMSC refers to all cutaneous cancers that do not develop from melanocytes, and is often incorrectly used to refer to basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), which together account for more than 95% of cases of NMSC (2). Each year 2-3 million patients are diagnosed with NMSC worldwide, with average yearly increases of 3-8% since 1960 (2-6). Since 80% of cases of NMSC occur in individuals > 60 years-old, its incidence will soon be equal to that of all other cancers taken together (8-10). BCC alone accounts for 80% of cases of NMSC (1). The incidence of BCC is rising by 10% per year and it is increasingly diagnosed in younger individuals (<40 years) (7). Death due to BCC is extremely rare as the incidence of metastasis is estimated to be 0.0028-0.55%; however, BCC can result in severe morbidity since the lesions tend to be located on the skin of the head and neck (11). Moreover, NMSC places a considerable economic burden on healthcare systems: the total annual cost of skin cancer care in the United States increased from $3.6 billion in 2002-2006 to $8.1 billion in 2007-2011 (12). Indeed NMSC is essentially an age-related disease and as populations grow older, its incidence and related costs will rise (2).
BCC develops from either the bulge region of the hair follicle, which is rich in keratinocyte stem cells, or from stem/progenitor cells in the basal cell layer of the epidermis (13). BCC grows slowly over a period of months to years, has a de novo onset with no visible pre-malignant phase, and may present as the nodular, superficial, sclerodermiform, pigmented or ulcus rodens subtypes (13). Metastasis are extremely rare; however, the tumor may be highly invasive and locally destructive. Since UV radiation (UVR) is one of the most important risk factors for skin cancer, which characteristically develops on sun-exposed skin, the most common site of BCC is the head and neck region (80%) followed by the trunk (15%) (13). BCC is usually associated with intermittent sun exposure and episodes of sunburn during childhood (13-17). Treatment varies depending on the clinical features, histological type, pattern of growth, size, location and comorbidities. Surgical excision with a 0.5 cm safety margin is the gold standard treatment. Wider excision margins are recommended for infiltrative, recurrent or multicentric-superficial BCC, and Mohs surgery has become established for these subtypes of BCC or when aesthetically relevant areas are involved (1,8). Thereconstructive options are direct suture, skin grafts and local flaps, and vary from case to case. Radiation therapy and topical medicines are valuable alternatives treatment for patients who are not eligible for surgery (1).
The purpose of this study was to review the experience of our center over BCC if head and neck district to report the sites of occurrence and treatment.
Materials and methods
For this study, we retrospectively reviewed 77 patients with BCC of the head and neck, who revived surgical treatment within the Cutaneous, Mininvasive, Regenerative and Plastic Surgery Unit of Parma University-Hospital, Italy, between January 2014 and February 2016. For all patients, basic demographic data (sex, age), cancer site and size, method of surgical treatment and histological data were collected. The sites of occurrence of BCC were classified based on the principal facial aesthetic regions: scalp, frontal, supraorbital, periorbital, temporal, zygomatyc, infraorbital, nasal, auricular, mandibular, perioral, mental and cervical (19, 20). The nasal, periorbital and labial regions were subdivided into aesthetic units. The nasal region included the dorsum, tip and alar lobe, while the periorbital region was subdivided into the upper and lower eyelid; and the perioral region, the upper and lower lip.
Patients provided signed informed consent prior to surgery and were educated about surgical and cosmetic risks. Depending on the size and site of BCC, patients underwent surgical excision by primary closure, local flap or skin graft. All patients were followed-up as outpatients on a weekly basis in the first month, and then at three, six and 12 months postoperatively. Patients were instructed to return to their normal level of activity 2 weeks after surgery. The minimum follow-up period was 3 months; early and late complications were recorded.
Results
At our plastic and reconstructive surgery department, 77 patients underwent surgical treatment for BCC of the head and neck region between January 2014 and February 2016. This retrospectively-assessed population included 37 males and 40 females, with a mean age of 74.12 years (range, 35 to 98 years). The age at diagnosis was younger for males than females (71.7 vs. 76.32 years). The age-frequency distribution peaked in the seventh decade. Eleven patients (14.29%) presented with two BCC tumors, seven (63.6%) of whom were female and four (36.4%) were male.
The average size of tissue excised was 1.8 × 1.17 cm (width × length; range, 7-0.6 cm and 4.5-0.3 cm, respectively) with a mean area of 2.77 cm2 (range, 31.5-0.18 cm2). The average size of the BCC tumors was 0.9×0.72 cm (width × length; range, 2.5-0.3 and 2.3-0.2 cm, respectively) with a mean area of 0.83 cm2 (range, 6.2-0.06 cm2). Nodular BCC accounted for 65.9% (58) of cases, while the superficial, sclerodermiform, and pigmented subtypes accounted for 12 (13.6%), 14 (15.9%), and 4 (4.5%) of cases. At the time of surgical excision, 64 (72.7%) of all tumors were ulcerated.
The sites of occurrence of BCC by facial aesthetic regions and units are summarized in Table 1. The nasal unit was the main site of BCC (31.82%), followed by the periorbital (13.64%) and cervical (12.5%) units. The alar lobes were the most common location within the nasal unit (50%), followed by the nasal dorsum (35.7%) and nasal tip (14.3%). There was no lateral predilection for BCC site: 32 (36.4%) tumors were on the right side, 31 (35.2%) on the left side and the side could not be classified for 25 cases (28.4%).
Table 1.
Anatomical sites of basal cell carcinoma in the head and neck region classified by aesthetic regions and units.
| Region Unit | Total | Left-sided | Right-sided | Not Classified |
| Scalp (n, %) | 4, 4.54% | |||
| Frontal (n, %) | 4, 4.54% | |||
| Supraorbital (n, %) | 0 | 1, 1.14% | ||
| Periorbital (n, %) | 12, 13.64% | |||
| Upper eyelid (n, %) | 0 | 0 | ||
| Lower eyelid (n, %) | 7, 7.95% | 5, 5.68% | ||
| Temporal (n, %) | 8, 9.09% | 5, 5.68% | 3, 3.41% | |
| Zygomatic (n, %) | 5, 5.68% | 2, 2.27% | 3, 3.41% | |
| Infraorbital (n, %) | 5, 5.68% | 4, 4.54% | 1, 1.14% | |
| Nasal (n, %) | 28, 31.82% | |||
| Alar lobe (n, %) | 14, 15.9% | 7, 7.95% | 7, 7.95% | |
| Dorsum (n, %) | 0, 11.38% | |||
| Tip (n, %) | 4, 4.54% | |||
| Auricular (n, %) | 5, 5.68% | 2, 2.27% | 3, 3.41% | |
| Mandibular (n, %) | 3, 3.41% | 1, 1.14% | 2, 2.27% | |
| Perioral (n, %) | 1, 1.14% | |||
| Upper lip (n, %) | 1, 1.14% | |||
| Lower lip (n, %) | 0 | |||
| Mental (n, %) | 1, 1.14% | |||
| Neck (n, %) | 11, 12.5% | 5, 5.68% | 6, 6.82% |
Figure 1.
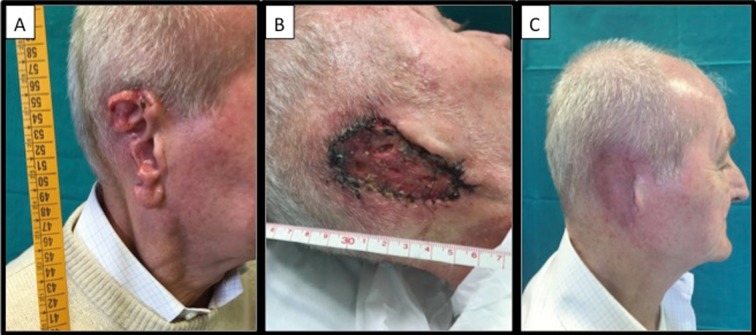
Images of a 73 year-old male patient who presented with ulcerated basal cell cancer of the right ear lobe. The patient had previously received incomplete surgery at another institution and requested complete auricular amputation at our unit. The patient refused any reconstructive surgery; therefore, we covered the resulting defect with a full-thickness skin graft harvested from the subclavicular area of the right chest. Images are shown before surgery (A), seven days after surgery (B), and three months after surgery (C)
Figure 2.
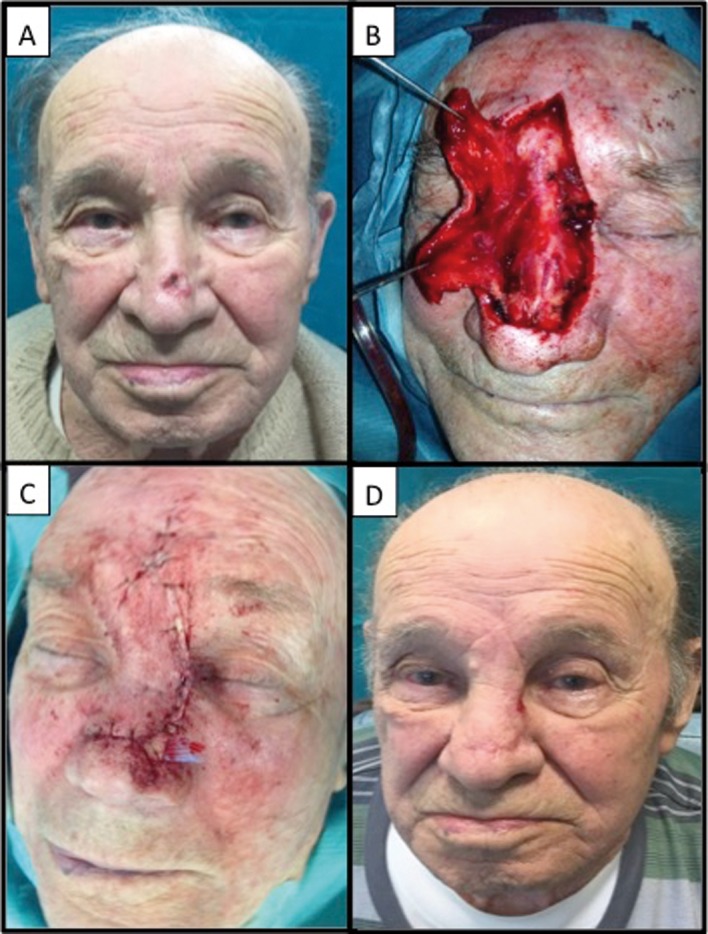
Images of an 82 year-old male patient who presented with nodular ulcerated basal cell cancer of the nasal dorsum. A dorsal nasal rotational flap was performed as the patient had adequate laxity of the glabbellar region. Laminar drainage was positioned in order to prevent hematoma formation and was removed on postoperative day 1. Images are shown before surgery (A), once the lesion had been excised and the flap elevated (B), immediately after the end of surgery (C), and three weeks after surgery (D)
Primary closure was the main surgical procedure performed (72.5%), followed by local flap (26.1%) and full-thickness skin grafts (1.4%). Partial wound dehiscence occurred in five (6.5%) patients, all of whom were receiving either oral anticoagulant therapy or aspirin that led to the formation of localized hematoma. Local cellulitis occurred in three (3.4%) cases, and was conservatively resolved by administration of topical antibiotics. In two (2.6%) patients, the scar developed into keloids, and one (1.3%) patient underwent revision surgery, as primary excision was incomplete. Neither recurrence nor any newly-developed lesions were reported during follow-up in any patient.
Discussion
The incidence of NMSC has dramatically increased worldwide over the last 30 years, mainly due to aging populations, as well as social and medical changes (2-6, 21, 22). BCC accounts for over 80% of cases of NMSC, and is the most frequently diagnosed cancer in Caucasian populations worldwide (23). Although death due to BCC is extremely rare, BCC is responsible for a significant economic burden and can lead to significant morbidity since most cases of BCC originate in key aesthetic and functional areas (24). UVR has been classified as a Class 1 ‘definite’ human carcinogen by the International Agency for Research on Cancer (IARC); therefore, the carcinogenic role of solar radiation is well established (25). The head and neck are commonly exposed to sunlight during an individual’s entire life, indeed numerous epidemiological studies have reported that approximately 80% of cases of BCC arise in the head and neck region (4, 18-26). In this cohort, the nasal region was the most commonly affected area of the head and neck; the nose is the most projected portion of the face and hence is more exposed to solar radiation. Moreover the fact that BCC developed on the lower eyelids, but not the upper eyelids, strengthens the evidence for UVR as an initiator of skin cancer. Similar findings have been reported in the literature. Choi et al.observed the highest occurrence of BCC on the nose (48.7%), followed by the orbital region; Kim et al.andJung and Kim also noted the nose was the most common site for BCC (47.3% and 38.4%) (27-29).
BCC is traditionally considered to occur more commonly in older patients, usually males, due to their more extensive exposure to sunlight (2,4,17). However, recent studies have reported a higher incidence of BCC among females than males (27, 30-34). In this work, we observed a male to female ratio of 0.925:1. These changes may be due to the extended life expectancy of women (35). Furthermore, de Vrjes et al. also suggested that the ‘typical’ patient with BCC in northwestern Europe is more often female as young females, and even females with higher education, report stronger sun-seeking behavior than their male counterparts (36). Surveys of the general Italian population also revealed what has been defined as the sunscreen paradox, which is described as a feeling of excessive protection when using sunscreen that disproportionately extends the sun exposure time and increases sun-seeking behaviors (37,38).
A study by Butler et al. observed skin cancers occurred more frequently on the left side of the body, which is supported by previous research that showed an increase in photo-damage and precancerous lesions on the left-side of the face due to exposure to sunlight through windows when driving and working (39-41). However, we observed a slightly higher incidence of BCC on the right side of the head and neck than the left side.
The primary aim of surgical treatment is complete removal of the lesion, while achieving the best aesthetic result as a main secondary goal. A 0.3 to 1 cm safety margin usually ensures a complete removal rate of 95%; unfortunately, radical surgical techniques may result in poor cosmetic outcomes depending on the site of BCC (17). In this cohort, the safety resection margin ranged from 4.5 to 9 mm, with a 98.7% complete removal rate, indicting the commonly-accepted surgical margin was adequate to ensure peripheral clearance in our experience. In this cohort, 72.5% of cases received primary closure while 26.1% required local flap surgery and 1.4% received a full-thickness skin graft. The high rate of direct closure may be explained by increased early diagnosis. As previously reported, BCC mostly occurs on the upper portion of the body and has a slow rate of growth, thus early diagnosis is usually relatively achievable. Indeed early diagnosis is mandatory in order to achieve the optimal surgical outcome, since smaller tumors generally require less invasive surgical procedures. Moreover, none of the patients in this cohort required topical medical therapy thanks to the high rate of early diagnosis.
Patients presenting with an NMSC are at a high risk of subsequent NMSC within the first year (42). Frankel et al.reported that 52% of patients developed subsequent NMSC within five years of therapy for the first skin cancer (43). Nevertheless we did not observe either recurrence or any newly-onset lesions within the first year of follow-up in this cohort.
This was a single center analysis of a small number of patients and therefore has several limitations. This data may not represent the most common sites of occurrence of BCC nor provide a general consensus for the treatment of BCC; multi-center studies of larger populations may help to precisely define the characteristics and optimal treatment for BCC. However, our work reflects the previously described shift in the incidence of BCC, which now seems to be more frequent in females. Furthermore, our data strengthens the association between UVR exposure and BCC and validates surgical excision as the gold standard treatment for skin cancer. Finally, this analysis highlights the key role of early diagnosis in achieving the optimal aesthetic outcome and - within the head and neck region - confirms the predilection of BCC to occur on the nasal unit.
Conflict of interest:
None to declare
References
- 1.Berking C, Hauschild A, Kölbl , et al. Basal cell carcinoma-treatments for the commonest skin cancer. Dtsch Arztebl Int. 2014;111(22):389–95. doi: 10.3238/arztebl.2014.0389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trakatelli M, Ulrich C, Del Marmol V, et al. Epidemiology of nonmelanoma skin cancer (NMSC) in Europe: accurate and comparable data are needed for effective public health monitoring and interventions. Br J Dermatol. 2007;156(s3):1–7. doi: 10.1111/j.1365-2133.2007.07861.x. [DOI] [PubMed] [Google Scholar]
- 3.Rogers HW, Weinstock MA, Harris AR, et al. Incidence estimate of non-melanoma skin cancer in the United States, 2006. Arch Dermatol. 2010;146:283–287. doi: 10.1001/archdermatol.2010.19. [DOI] [PubMed] [Google Scholar]
- 4.Diepgen TL, Mahler V. The epidemiology of skin cancer. Br J Dermatol. 2002;146(s61):1–6. doi: 10.1046/j.1365-2133.146.s61.2.x. [DOI] [PubMed] [Google Scholar]
- 5.Fransen M, Karahalios A, Sharma N, et al. Non-melanoma skin cancer in Australia. Med J Aust. 2012;197(10):565–8. doi: 10.5694/mja12.10654. [DOI] [PubMed] [Google Scholar]
- 6.Surdu S, Fitzgerald EF, Bloom MS, et al. Occupational exposure to ultraviolet radiation and risk of non-melanoma skin cancer in a multinational European study. PLoS ONE. 2013;8(4):e62359. doi: 10.1371/journal.pone.0062359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christenson LJ, Borrowman TA, Vachon CM, et al. Incidence of basal cell and squamous cell carcinomas in a population younger than 40 years. JAMA. 2005;294:681–90. doi: 10.1001/jama.294.6.681. [DOI] [PubMed] [Google Scholar]
- 8.Bakos RM, Kriz M, Mühlstädt M, Kunte C, Ruzicka T, Berking C. Risk factors for early-onset basal cell carcinoma in a German institution. Eur J Dermatol. 2011;21:705–9. doi: 10.1684/ejd.2011.1436. [DOI] [PubMed] [Google Scholar]
- 9.Caldeira S, Zehbe I, Accardi R, et al. The E6 and E7 proteins of the cutaneous human papillomavirus type 38 display transforming properties. J Virol. 2003;77(3):2195–2206. doi: 10.1128/JVI.77.3.2195-2206.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burnworth B, Arendt S, Muffler S, et al. The multi-step process of human skin carcinogenesis: a role for p53, cyclin D1, hTERT, p16, and TSP-1. Eur J Cell Biol. 2007;86(11):763–780. doi: 10.1016/j.ejcb.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.McCusker M, Basset-Seguin N, Dummer R, et al. Metastatic basal cell carcinoma: Prognosis dependent on anatomic site and spread of disease. Eur J Cancer. 2014;50:774–83. doi: 10.1016/j.ejca.2013.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Guy GP, Jr, Machlin SR, Ekwueme DU, Yabroff KR. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183–187. doi: 10.1016/j.amepre.2014.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahmed AH, Soyer HP, Saunders N, et al. Non-melanoma skin cancers. Drug Discov Today Dis Mech. 2008;5(1):e55–e62. [Google Scholar]
- 14.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem and Photobiol. 2001;63(1):8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 15.Trakatelli M, Barkitzi K, Apap C, et al. Skin cancer risk in outdoor workers: a European multicenter case-control study. J Eur Acad Dermatol Venereol. 2016;30(S3):5–11. doi: 10.1111/jdv.13603. [DOI] [PubMed] [Google Scholar]
- 16.John SM, Trakatelli M, Ulrich C. Non-melanoma skin cancer by solar UV: the neglected occupational threat. J Eur Acad Dermatol Venereol. 2016;30(S3):3–4. doi: 10.1111/jdv.13602. [DOI] [PubMed] [Google Scholar]
- 17.Madan V, Lear JT, Szeimies RM. Non-melanoma skin cancer. Lancet. 2010;375:673–85. doi: 10.1016/S0140-6736(09)61196-X. [DOI] [PubMed] [Google Scholar]
- 18.Hauschild A, Breuninger H, Kaufmann R, et al. Brief S2k guidelines - Basal cell carcinoma of the skin. J Dtsch Dermatol Ges. 2013;11(3):10–5. 11–6. doi: 10.1111/ddg.12015_3. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez-Ulloa M. Restoration of the face covering by means of selected skin in regional aesthetic units. Br J Plast Surg. 1956;9:212–21. doi: 10.1016/s0007-1226(56)80036-2. [DOI] [PubMed] [Google Scholar]
- 20.Baker SR. 2nd edn. Philadelphia: Elsevier Inc; 2007. Flap classification and design. In: Local Flaps in Facial Reconstruction; pp. 71–106. [Google Scholar]
- 21.Hong H, Ji JH, Choi EH. A clinical observation of cutaneous malignant tumors and premalignant lesions in gangwon province over 10 years (1999-2008) Korean J Dermatol. 2012;50:95–100. [Google Scholar]
- 22.Armstrong BK, Kricker A. Skin cancer. Dermatol Clin. 1995;13:583–94. [PubMed] [Google Scholar]
- 23.Lucas R, McMichael T, Smith W, Armstrong B. Geneva: World Health Organization; 2006. Solar Ultraviolet Radiation: Global Burden of Disease from Solar Ultraviolet Radiation. Environmental Burden of Disease Series, No. 13. [Google Scholar]
- 24.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. 2012;166(5):1069–1080. doi: 10.1111/j.1365-2133.2012.10830.x. [DOI] [PubMed] [Google Scholar]
- 25.IARR. France: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, International Agency for Research on Cancer, World Health Organization, Lyon; 1992. Solar and ultraviolet radiation; p. 316. [Google Scholar]
- 26.Fartasch M, Diepgen TL, Schmitt J, Drexler H. The relationship between occupational sun exposure and non-melanoma skin cancer. Dtsch Ärztebl Int. 2012;109(43):715–20. doi: 10.3238/arztebl.2012.0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JH, Kim YJ, Kim H, et al. Distribution of Basal cell carcinoma and squamous cell carcinoma by facial esthetic unit. Arch Plast Surg. 2013;40(4):387–391. doi: 10.5999/aps.2013.40.4.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim YP, Chun IK, Lee HH. A 10 year period (1968-1977) of clinical observation of cutaneous malignant tumors. Korean J Dermatol. 1978;16:19–29. [Google Scholar]
- 29.Jung YH, Kim SS. A clinical study in malignant skin tumors. J Korean Soc Plast Reconstr Surg. 1982;9:377–88. [Google Scholar]
- 30.Lee YJ, Seo SJ, Kim MN, et al. A 10 year period (1984-1993) of clinical observation of cutaneous malignant tumors. Korean J Dermatol. 1995;33:679–85. [Google Scholar]
- 31.Seo JJ, Won YH, Kim SJ, et al. A clinical observation of cutaneous malignant tumors over 10 years (1987-1996, Chonnam Province ) Korean J Dermatol. 1998;36:812–9. [Google Scholar]
- 32.Shin JH, Cho S, Whang KK, et al. An epidemiologic analysis of cutaneous malignant tumors over 15 years (1984-1998) Korean J Dermatol. 1999;37:1743–51. [Google Scholar]
- 33.Yoon TJ, Kim HS, Oh CW, et al. A clinical investigation of cutaneous malignant tumors in western Gyeongnam Province. Korean J Dermatol. 1997;35:956–62. [Google Scholar]
- 34.Jeong KB, Kim HC, Shin DH, et al. A clinical observation of cutaneous premalignant and malignant tumors. Korean J Dermatol. 2002;40:924–31. [Google Scholar]
- 35.Cho KH, Lee YS. A clinical observation of cutaneous malignant tumors. Korean J Dermatol. 1984;22:394–403. [Google Scholar]
- 36.de Vries E, Louwman M, Bastiaens M, et al. Rapid and continuous increases in incidence rates of basal cell carcinoma in the southeast Netherlands since 1973. J Invest Dermatol. 2004;123:634–8. doi: 10.1111/j.0022-202X.2004.23306.x. [DOI] [PubMed] [Google Scholar]
- 37.Autier P, Dore JF, Negrier S, et al. Sunscreen use and duration of sun exposure: a double-blind, randomized trial. J Natl Cancer Inst. 1999;91:1304–1309. doi: 10.1093/jnci/91.15.1304. [DOI] [PubMed] [Google Scholar]
- 38.Suppa M, Neri L, Bianchi L, et al. The first skin cancer screening day at the Italian parliament: a Euromelanoma initiative. Int J Dermatol. 2015;54(1):42–49. doi: 10.1111/ijd.12677. [DOI] [PubMed] [Google Scholar]
- 39.Butler ST, Fosko SW. Increased prevalence of left-sided skin cancers. J Am Acad Dermatol. 2010;63(6):1006–1010. doi: 10.1016/j.jaad.2009.11.032. [DOI] [PubMed] [Google Scholar]
- 40.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 41.Topczewska J, Postovit LM, Margaryan NV, et al. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nat Med. 2006;12:925–32. doi: 10.1038/nm1448. [DOI] [PubMed] [Google Scholar]
- 42.Schreiber MM, Moon TE, Fox SH, et al. The risk of developing subsequent nonmelanoma skin cancers. J Am Acad Dermatol. 1990;23:1114–8. doi: 10.1016/0190-9622(90)70343-g. [DOI] [PubMed] [Google Scholar]
- 43.Frankel DH, Hanusa BH, Zitelli JA. New primary nonmelanoma skin cancer in patients with a history of squamous cell carcinoma of the skin. Implications and recommendations for follow-up. J Am Acad Dermatol. 1992;26:720–6. doi: 10.1016/0190-9622(92)70100-t. [DOI] [PubMed] [Google Scholar]