Abstract
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) is a severe reaction to drugs. Incidence of DRESS in children is not well known and the mortality rate seems to be lower than 10%. Anticonvulsants are the main drugs involved both in adults and in children. The treatment of choice is intravenous immunoglobulins and corticosteroids used in synergy. Today there are not controlled clinical trials regarding DRESS treatment in children. Anyway, the prompt withdrawn of the offending drug is of paramount importance for a better prognosis. DRESS sequels may occur, consequently, follow-up visits are required at least until the first year after the reaction. (www.actabiomedica.it)
Keywords: children, drug reaction with eosinophilia and systemic symptoms, severe cutaneous adverse reactionst
Epidemiology
Drug reaction with eosinophilia and systemic symptoms (DRESS) is a serious and potentially fatal adverse reaction to therapeutic medications. Over the last 80 years, the nomenclature of this disease has been changing from drug-induced pseudolymphoma, anticonvulsant hypersensitivity syndrome, drug induced hypersensitivity syndrome (DIHS), drug induced de-layed multiorgan hypersensitivity syndrome to DRESS. DRESS is classified among severe cutaneous adverse reactions (SCARs) and in 1966 Bocquet et al. (1) identified it as a distinct clinical syndrome. Moreover, the meaning of “R” in DRESS acronym has been changed from Rash to Reaction due to the heterogeneity of skin eruptions (2). Initially, DRESS was thought to affect only adults, later it was diagnosed also in children (3).
The incidence of DRESS due to antiepileptics is in the range of 1:1000 to 1:10.000 in general population (4) and of 0.4:1000 (5) in hospital settings. In younger children the incidence of DRESS seems to be lower than in adults, although the real incidence is not known (6,7). Anyway, DRESS is more frequent than other severe immediate drug-induced reactions such as anaphylaxis (8), or exercise-induced anaphylaxis (9) but less common than food-induced anaphylaxis (10,11). The overall mortality rate is of 10% with a lower percentage in children than in adults (12-13).
Pathogenesis
DRESS is the result of a complex interplay of genetic factors [ethnic predisposition in people with certain human leucocyte antigen (HLA) alleles], immunological response, abnormalities in metabolic pathways (such as a deficiency or abnormality in epoxide hydroxylase, an enzyme that detoxifies the metabolites of aromatic amine anticonvulsants) and associated reactivations of herpes virus family members (HHV-6 and HHV-7, EBV and CMV) (14). In this context, African Americans are most likely to develop DRESS syndrome after initiation of aromatic anticonvulsants drugs whereas the Han Chinese are most likely to develop DRESS after allopurinol intake (15).
In fact, it has been found that DRESS syndrome is associated with certain human leukocyte antigens (HLAs), such as, HLA A*31:01 (aromatic anticonvulsant-induced DRESS); HLA A* 24:02 (lamotrigine-induced DRESS); HLA B*51:01, HLA B*15:13 and CYP2C9*3 (phenytoin-induced DRESS); HLA-B*57:01 and DRB1*01:01 and HLAB*35:05 (abacavir-induced DRESS) and HLA-B*58:01 (allopurinol-induced DRESS); HLA C*04:01 (nevirapine-induced DRESS) (16-19).
Apart from HLA, cytochrome P4502C9 marker has been reported to be involved in phenytoin induced SCARs (20-21).
Moreover, being a slow acetylator of drugs is thought to be a risk factor for DRESS syndrome (22).
Drugs may act as foreign antigens, binding to HLA/peptide/TCR complex and inducing hypersensitivity reactions. DRESS is a delayed type reaction according to Gell and Coombs classification (23).
There are four hypotheses regarding drug presentation mechanisms that have been suggested to explain how small drug molecules might interplay with HLA and TCR in drug hypersensitivity: (1) the hapten theory, (2) the pharmacological interaction with immune receptors (p-i) concept (i.e. carbamazepine directly interacts with HAL B*15:02) (3) the altered peptide repertoire model (i.e. abacavir binds to the F-pocket of HLA B*57:01), and (4) the altered TCR repertoire model (i.e. sulfamethoxazole directly interacts with TCR).
In delayed type reactions such as DRESS syndrome, drug antigens may activate specific T lymphocytes or natural killer cells with production of various cytokines/chemokines (i.e. TNF-α, IFN-γ, IL-2, IL-4, IL-5, TARC/CCL17, IL-6, IL-15, and IL-13) (16).
Furthermore, viruses have also been proposed to be involved in HLA/drug/TCR interactions and play an important role in drug hypersensitivity reactions, representing a source of exogenous peptides for drug presentation (24).
So far, the role of viruses in the pathogenesis of DRESS is unclear: a) Viral reactivation may be provoked by a cytokine storm secondary to an immune response against the drug (25); b) DRESS is a consequence of a strong immune response against an early viral reactivation (26). CD4+ and CD8+ drug-specific T cells proliferate after encountering the drug, but also anti-viral specific T cell can be cross-activated by drugs. In conclusion, the most common hypothesis is that the immunologic response to drugs induces a boost viral reactivation, consequently T lymphocytes and monocytes/macrophages release viruses that represents as an early marker of stimulation of these cells, rather than the triggering event in the pathogenesis of DRESS (27). In particular, toxic drug metabolites accumulation provoke an immunosuppression of B cells with hypogammaglobulinemia and subsequent viral re-activation (28). For example, in Asia and Europe the detection of HHV-6 copies in DRESS cases has been commonly reported with a frequency of 70-80%, making this data as an available diagnostic test (29, 30).
Clinical manifestations
The time onset of DRESS symptoms ranges from 2-6 weeks after initiation of treatment (2), anyway latency periods up to 105 days have been described (31).
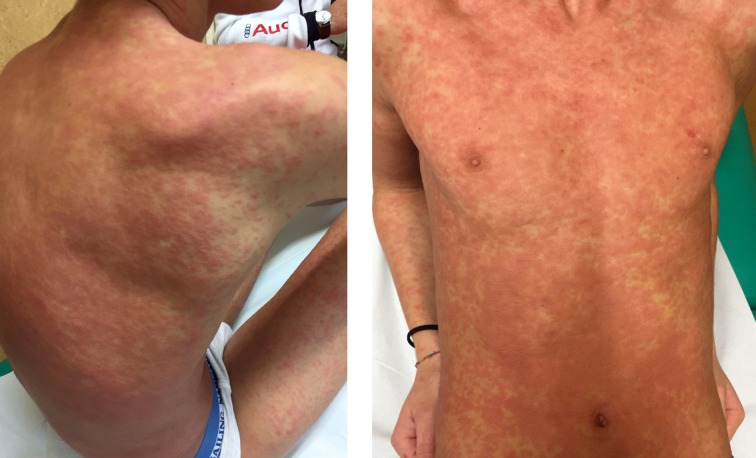
Retrospective studies have found that the average age of occurrence of DRESS syndrome is 9 years of age in children (7,13). The most common clinical feature is fever, which is usually high grade ranging from 38-40°C. The second most common feature is macular erythema. This kind of rash later evolves in more violaceous and papular lesions with or without pruritus (Figure 1), and over time, the eruption becomes potentially exfoliative. Consequently, although a maculopapular rash is the most common initial cutaneous manifestation other eruptions may be described, including targetoid, urticarial, pustular, blistering, lichenoid, exfoliative, and eczematous lesions. The skin eruption typically begins on the face associated to facial oedema and then involves the upper trunk progressively spreading at lower extremities. The skin can be involved from less than 50% of body surface to diffuse erythroderma, making consistent the cutaneous distribution of the eruption. Moreover, mucosal involvement has been frequently (>50%) described (i.e. conjunctivitis, oral mucositis and/or genital lesions) in DRESS (7).
Figure 1.
Acute Rash in DRESS syndrome
The eruption can persist for months after the offending drug has been discontinued. Lymphadenopathy is the third most common presentation, which is seen in 70-75% of patients (32).
Haematological abnormalities, such as leucocytosis, eosinophilia, atypical lymphocytosis, thrombocytopenia and agranulocytosis commonly occur in DRESS.
Eosinophilia is typically reported in DRESS studies from both Asia and Europe with percentages ranging from 48 to 95% of patients (2, 7, 33).
Among visceral organ, liver (i.e. hepatitis) is involved in 50-80% of patients, followed by kidney (i.e. nephritis with haematuria or acute renal failure) in 11-28% of patients. Unfortunately, in some patients, hepatic injury can progress to widespread hepatic necrosis and fulminant liver failure (29, 34, 35) and it represents the leading cause of mortality in these patients (36).
Lungs (i.e. pneumonitis) are involved in 2.6-5% patients, but also muscle (myositis), gastrointestinal (i.e. colitis) heart (i.e. myocarditis), pancreas (i.e. pancreatitis), brain (i.e. encephalitis), thyroid (i.e. thyroiditis) and conjunctiva (i.e. conjunctivitis) involvements have been described (31, 37). In table 1 are reported the clinical features of DRESS syndrome (29, 35, 36, 38-44). Clinical manifestations were similar between children and adults, with the exception of pulmonary involvement (excluding asthma), which was more frequent in adults, and gastrointestinal involvement, which was more frequent in children (42).
Table 1.
Most common clinical features of DRESS syndrome and percentages of organ involvement
| Fever (>38°C) | 86.5% | (38) |
| Acute Rash | 85% | (38) |
| Facial Swelling with periorbital involvement | 27% | (38) |
| Lymphadenopathy | 70% | (38) |
| Eosinophilia | 60-80% | (29, 30, 38) |
| Liver: Hepatomegaly and/or increase liver enzymes (AST/ALT) and/or hyperbilirubinemia; elevated Alkaline phosphatase (30) |
51-84% | (35, 36, 40-41) |
| Kidney: Elevation in creatinine Decrease in glomerular filtration rate (GFR) Proteinuria Haematuria *Allopurinol is most commonly implicated with renal involvement (36) |
11-57% | (35, 40-41) |
| Lungs: Interstitial pneumonitis Pneumonia Pleural effusion Acute respiratory distress Syndrome (ARDS) *Minocycline, Allopurinol, Abacavir are most commonly implicated with lung involvement (26, 37) |
2.6-5% | (29, 36) |
| Non specific Gastrointestinal Symptoms: Colitis Diarrhoea with or without electrolyte abnormalities |
8% | (35, 42) |
| Heart: Late onset Myocarditis (Troponin and CKMB elevated) | 4-27% | (43,44) |
| Tachycardia, arrhythmias, chest pain, non specific ECG changes, gross ST segment, elevation or depression, decrease in LV ejection fraction * Ampicillin is most commonly implicated with heart involvement |
Drugs Involved
More than 40 medications have been described as triggers of DRESS and among various drugs, aromatic antiepileptics are reported to be the most common cause followed by antibiotics. Moreover, aromatic anticonvulsants show cross-reactivity in 40-80% of cases while non aromatic anticonvulsants are well tolerated as alternative choice in case of reactions to aromatics. Anyway, data about DRESS in children are scarce and mostly come from case reports. In the study of Misirlioglu et al (45), antibiotics were the most common (50%) medication in the aetiology; 87.5% of the suspected antibiotics were beta-lactams, and 12.5% were macrolides. Antiepileptics were second (37.5%, n. 6) most common class of drugs in the aetiology. In Table 2 we reported the drugs most frequently involved in DRESS syndrome in children in the last ten years. Studies where children were included but not clearly specified in terms of age and culprit drugs, were excluded.
Table 2.
Most frequently reported drugs causing DRESS syndrome in children
| Single case or less than 10 children (mean age 7,6 years) (46-114) |
|
| 32 children (mean age 8,9 y) (13) |
|
| 33 children (mean age 5,8 y) (115) |
|
| 29 children (mean age 11 y) (116) |
|
| 11 children (mean age 6,6 y) (117) |
|
| 16 children (mean age 8,2 y) (45) |
|
Diagnosis
Due to the variability of its presentation, DRESS is known as “the great mimicker” making difficult the diagnosis (118). In particular, DRESS symptoms resemble those of cutaneous and systemic infectious diseases and can appear up to 3 months after the initial culprit drug exposure. The allergy work-up should start with a detailed record of clinical history by focusing on the chronology of drug assumptions and physical examination. Laboratory testing is fundamental, it should include liver, and kidney functions, search for viral infections, complete blood count and coagulation testing.
There are no clear and specific histopathological patterns in skin biopsy that are characteristic of DRESS Syndrome. Maculopapular exanthema (MPE) may be the initial presentation of SCARs including DRESS (119-120). When comparing DRESS with MPE, skin biopsies showed differences in terms of inflammatory infiltrate, atypical lymphocytes, keratinocyte damage, dermal involvement and leukocytoclastic vasculitis, these characteristics being more frequently observed in DRESS cases than in MPE cases (86, 121). Few necrotic keratinocytes were associated with non-severe DRESS cases, otherwise high amount of necrotic keratinocytes with confluent necrotic areas were associated with severe DRESS, respectively. Anyway, the role of skin or lymph node biopsies remains controversial (119).
Eosinophilia is a diagnostic criterion for DRESS. In physiologic conditions, eosinophils are not present in skin, liver, lungs or other internal organs otherwise in DRESS, eosinophils are typically increased in blood, in skin and in involved organs. Eosinophils infiltrate organs in response to cytokines and chemokines including eotaxin-1, TARC, IL-5 and granule release representing key factors of tissue damage (122).
The discovery of biomarkers of drug hypersensitivity could be useful for the diagnosis of DRESS syndrome. In DRESS cases, serum TARC levels have been reported to be significantly higher than those in patients with Steven-Johnson Syndrome (SJS)/Toxic epidermal necrolysis (TEN) and MPE during the acute phase and to be correlated with skin eruptions (122). For this reason, TARC could be considered a potential biomarker for the early phase and disease activity of DRESS syndrome.
Re-challenging with the offending drug has been considered the gold standard to diagnose drug eruptions, but in suspected DRESS cases, it should not be used because of the life-threatening nature of this syndrome (2, 123). Patch tests can be useful to prove a drug-specific immune response in DRESS syndrome (124). On the contrary, patch tests to different allergens such as foods have a low diagnostic accuracy (125). In vivo patch tests represent a low-risk method for reproducing delayed hypersensitivity by re-exposing patients to low amount of suspected offending drugs. Anyway, the sensitivity and specificity of patch tests are different according to the drug tested.
The lymphocyte transformation/activation test (LTT/LAT) measures the proliferation of T cells to a drug (126, 127). Unfortunately, it is not standardized for many medications and it is difficult to perform. Furthermore, it usually yields a negative result early in the course of the syndrome, and lacks sensitivity. A positive LTT/LAT is useful to confirm the diagnosis due to very low false positive results (only 2%), however a negative test cannot exclude the diagnosis (128). All these factors prevent widespread use of this test.
For the diagnosis of DRESS syndrome different criteria can be used such as Bocquet’s criteria (1), The European Registry of Severe Cutaneous Adverse Reactions to Drugs and Collection of Biological Samples (RegiSCAR) criteria (7) and the Japanese group of Severe Cutaneous Adverse Reactions to Drugs (SCAR-J) criteria (Table 3). The RegiSCAR is most often used to diagnose DRESS (129), it is based on seven independent parameters and three of them are required for the diagnosis of DRESS. According to RegiSCAR, the diagnosis of DRESS can be definite (score >5), probable (score 4-5), possible (score 2-3) and no (score <2) DRESS syndrome.
Table 3.
Three proposed diagnostic criteria of DRESS syndrome
| Bocquet et al. (1) | RegiSCAR (7) | J-SCAR (129) | |
| Requirement for diagnosis | ≥3 criteria | ≥3 criteria of the following asterisk marks | all 7 criteria = typical without 2 asterisk marks = atypical |
| History | - hospitalization - reaction suspected to be drug related |
- symptoms persisting at least 2 weeks after drug discontinuation | |
| Fever | - fever ≥38°C* | - fever ≥38°C | |
| Cutaneous finding | - acute drug eruption | - acute rash | - macular rash developing 3 weeks after starting offending drug |
| Hematologic abnormalities |
- eosinophilia >1.5×109/L or atypical lymphocytosis | one of the following hematologic abnormalities - eosinophilia over laboratory limits - lymphocyte count over and under normal limits - thrombocytopenia under laboratory limits |
one of the following hematologic abnormalities - leucocytosis (>11×109 /L) - atypical lymphocytes (>5%) - eosinophilia (>1.5×109 /L) |
| Other organ involvements | - lymphadenopathy ≥2 cm in diameter - hepatitis with liver transaminases ≥2 times of the normal values - interstitial nephritis - interstitial pneumonitis - carditis |
- lymphadenopathy involving ≥2 sites* - at least 1 internal organ involvement* |
- lymphadenopathy* - liver abnormalities (ALT >100 U/L) |
| Viral reactivation | - HHV-6 reactivation* |
Differential Diagnosis
DRESS should be differentiated from viral exanthemas especially EBV infectious mononucleosis, staphylococcal and streptococcal shock syndrome, meningococcemia, non infectious drug eruptions (e.g. SJS/TEN), autoimmune diseases (e.g. hypereosinophilic syndrome, Kawasaki disease, Stills diseases), urticaria vasculitis (130), neoplastic diseases (e.g. leukemia cutis, pseudolymphoma, mycosis fungoides), serum sickness like reaction, and atopic eritrodermia. In the last, for example, nephritis and hepatitis are lacking, being caused by bacterial infections (131).
Depending on organs involved, the differential diagnosis also includes viral hepatitis (liver), parasitic infection (gastrointestinal tract) and bacterial, viral and fungal pathogens (lung) (36).
Treatment
So far, there have been no prospective clinical trials on treatment of DRESS syndrome. Current recommendations are based on case reports and expert opinion (132). The first and most important step in treatment of DRESS is withdrawal of the causative drug, because a better prognosis is associated with an earlier discontinuation of the drug.
In mild forms, treatment is mainly supportive and symptomatic, consisting of topical steroids, systemic anti-H1 antihistamines and emollients for rash and itching. In patients with exfoliative dermatitis a prompt and appropriate fluid, electrolyte and nutritional support is of primary importance. In moderate cases without visceral involvement, corticosteroids are usually adequate (133).
In case of organ involvement, such as liver (transaminases >5 times upper limit of normal), kidney, lungs or heart, the expert opinion of French Society of Dermatology recommended to administer corticosteroid (prednisone, 1 mg/kg/day per os). Several aspects (i.e. optimal dose, route of administration, duration of treatment, and rapidity of dose tapering) of steroid treatment have not been compared in controlled trials (119). Tapering should take three to six months of time because rapid taper can be associated with relapse of DRESS (119, 134, 135). Systemic steroid therapy is advised to treat cases of moder-ate to severe disease taking into account the dramatic improvement in symptoms and frequent relapses of DRESS associated with quick prednisone taper. For all these reasons, intravenous pulses of methylprednisolone (1 g/d) are recommended especially in patients worsening despite adequate doses of oral corticosteroids (52).
Proposed mechanism by which corticosteroids benefit the patient is inhibition of IL-5, which attracts eosinophils, which are responsible for visceral organ damage by accumulation in DRESS syndrome (35). For the same reason, some authors proposed the use of mepolizumab (anti IL-5) in the treatment of DRESS (136).
Today, cyclosporine may be considered a second-line therapy for patients with severe organ involvement who do not respond to systemic corticosteroids and for patients in whom corticosteroids are contraindicated (137). Intravenous immunoglobulins (IVIG) have been reported to be useful in a few patients with DRESS and detrimental in others (138). Periodical controls (both clinical and laboratory parameters) are necessary to check progression of the skin eruption and/or development of clinical fatal life-threatening signs, which include hemophagocytosis with bone marrow failure, encephalitis, severe hepatitis, renal failure, and respiratory failure requiring treatment with steroids generally administered at a dose of 2 g/kg over 5 days with IVIG. The largest series of paediatric patients have been described by Marcu N et al. (62) who reported 7 patients with severe DRESS in whom treatment with IVIG (1-2 gr/kg) in addition to systemic corticosteroids was successful. One possible explanation is that IVIG preparations contain antiviral neutralizing antibodies that help clear the viral infection/reactivation found to be fundamental in the pathophysiology of DRESS. Anyway, IVIG should not be administered in the absence of steroids.
Due to the fact that there is a major viral reactivation along with presence of life-threatening signs, it has been proposed to administer anti-viral medications (e.g. ganciclovir) in combination with steroids with or without IVIG but the efficacy is unclear (139).
In severe and corticosteroid-resistant cases, more potent immunosuppressant medications includ-ing cyclosporine, azathioprine, rituximab, infliximab and mycophenolate have been used, sometimes alongside adjunctive treatment with IVIG and plasmapheresis (42, 66, 140, 141). N-acetyl cysteine (NAC), which acts as detoxifying drug, can also be used in DRESS.
Finally, the treatment of DRESS syndrome should be started immediately after diagnosis, even if the result of viral markers are still ongoing. Further studies with appropriate designs (i.e. randomized controlled trials) are needed to establish a standard of care in DRESS. Such studies should also assess the potential application of anti-viral drugs or probiotics for treating infections (142, 143, 144).
Prognosis
After withdrawal of the causative drug, most of the patients need some weeks to completely recover. The prevalence of sequelae is unknown. Long-term sequelae may be renal failure, chronic anaemia, autoimmune diseases (autoimmune thyroid disease, diabetes melli-tus type I, systemic lupus erythematous (SLE), systemic scle-rosis, adrenal insufficiency and autoimmune haemolytic anaemia). For example, thyroiditis has been reported in the 12.5% of children with a previous DRESS (7).
Moreover, recurrence of DRESS with unrelated drugs can be observed in 25% of cases, whereas very little or no flares are reported in patients after SJS/TEN (145, 146).
Those manifestations can occur months to years following the initial episode and awareness of association with a drug administration is crucial to promptly recognise and treat a possible DRESS. Fol-low-up visits at 2, 3, 4, 5, 6, 12 months and then once a year are recommended (146, 148).
Conflict of interest:
None to declare
References
- 1.Bocquet H, Bogot M, Roujeau JC. Drug-induced pseudolymphoma and drug hypersensitivity syndrome (Drug Rash with Eosinophilia and Systemic Symptoms: DRESS) Semin Cutan Med Surg. 1996;15:250–57. doi: 10.1016/s1085-5629(96)80038-1. [DOI] [PubMed] [Google Scholar]
- 2.Choudhary S, McLeod M, Torchia D, Romanelli P. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome. J Clin Aesthet Dermatol. 2013;6:31–7. [PMC free article] [PubMed] [Google Scholar]
- 3.Kani Y, Shiohara T. The variable clinical picture of drug-induced hypersensitivity syndrome/drug rash with eosinophilia and systemic symptoms in relation to the eliciting drug. Immunol Allergy Clin North Am. 2009;29:481–501. doi: 10.1016/j.iac.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 4.Mockenhaupt M. Epidemiology of cutaneous adverse drug reactions. Allergol Select. 2017;4(1):96–108. doi: 10.5414/ALX01508E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiszenson-Abala F, Auzerie V, Mahe E, et al. A 6-month prospective survey of cutaneous drug reactions in a hospital setting. Br J Dermatol. 2003;149:1018–22. doi: 10.1111/j.1365-2133.2003.05584.x. [DOI] [PubMed] [Google Scholar]
- 6.Chang DKM, Shear NH. Cutaneous reaction to antiepileptics. Semin Neurol. 1992;12:329–337. doi: 10.1055/s-2008-1041189. [DOI] [PubMed] [Google Scholar]
- 7.Kardaun SH, Sekula P, Valeyrie-Allanore L, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): an original multisystem adverse drug reaction. Results from the prospective RegiSCAR study. Br J Dermatol. 2013;169:1071–80. doi: 10.1111/bjd.12501. [DOI] [PubMed] [Google Scholar]
- 8.Caimmi S, Caimmi D, Bernardini R, et al. Perioperative anaphylaxis: epidemiology. Int J Immunopathol Pharmacol. 2011;24(3):S21–6. doi: 10.1177/03946320110240s304. [DOI] [PubMed] [Google Scholar]
- 9.Povesi Dascola C, Caffarelli C. Exercise-induced anaphylaxis: A clinical view. Ital J Pediatr. 2012;38:43. doi: 10.1186/1824-7288-38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vetander M, Protudjer JLP, Lilja G, et al. Anaphylaxis to foods in a population of adolescents: incidence, characteristics and associated risks. Clin Exp Allergy. 2016;46:1575–87. doi: 10.1111/cea.12842. [DOI] [PubMed] [Google Scholar]
- 11.Caffarelli C, Ricò S, Rinaldi L, Povesi Dascola C, Terzi C, Bernasconi S. Blood pressure monitoring in children undergoing food challenge: association with anaphylaxis. Ann Allergy Asthma Immunol. 2012;108:285–6. doi: 10.1016/j.anai.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Carroll M, Yueng-Yue K, Esterly N, Drolet BA. Drug-Induced hypersensitivity syndrome in pediatric patients. Pediatrics. 2001;108:485–92. doi: 10.1542/peds.108.2.485. [DOI] [PubMed] [Google Scholar]
- 13.Newell B, Moinfar M, Mancini A, Nopper A. Retrospective analysis of 32 pediatric patients with anticonvulsant hypersensitivity syndrome (ACHSS) Pediatr Dermatol. 2009;26:536–46. doi: 10.1111/j.1525-1470.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- 14.Descamps V. Human herpesvirus 6 involvement in paediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:858–59. doi: 10.1111/bjd.13592. [DOI] [PubMed] [Google Scholar]
- 15.Chiu M, Hu M, Ng M, et al. Association between HLA-B58:01 allele and severe cutaneous adverse reactions with allopurinol in Han Chinese in Hong Kong. Br J Dermatol. 2012;167:44–9. doi: 10.1111/j.1365-2133.2012.10894.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen CB, Abe R, Pan RY, et al. An updated review of the molecular mechanisms in drug hypersensitivity. J Immunol Res. 2018;13:6431694. doi: 10.1155/2018/6431694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hung SI, Chung WH, Jee SH, et al. Genetic susceptibility to carbamazepine-induced cutaneous adverse drug reactions. Pharmacogenet Genomics. 2006;16:297–306. doi: 10.1097/01.fpc.0000199500.46842.4a. [DOI] [PubMed] [Google Scholar]
- 18.Chung WH, Hung SI, Hong HS, et al. Medical genetics: a marker for Stevens-Johnson syndrome. Nature. 2004;428:486. doi: 10.1038/428486a. [DOI] [PubMed] [Google Scholar]
- 19.Mallal S, Phillips E, Carosi G, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–79. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 20.Tassaneeyakul W, Prabmeechai N, Sukasem C, et al. Associations between HLA class I and cytochrome P450 2C9 genetic polymorphisms and phenytoin-related severe cutaneous adverse reactions in a Thai population. Pharmacogenet Genomics. 2016;26:225–34. doi: 10.1097/FPC.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 21.Suvichapanich S, Jittikoon J, Wichukchinda N, et al. Association analysis of CYP2C9*3 and phenytoin-induced severe cutaneous adverse reactions (SCARs) in Thai epilepsy children. J Hum Genet. 2015;60:413–17. doi: 10.1038/jhg.2015.47. [DOI] [PubMed] [Google Scholar]
- 22.Rieder MJ, Shear NH, Kanee A, Tang BK, Spielberg SP. Prominence of slow acetylator phenotype among patients with sulfonamide hypersensitivity reactions. Clin Pharmacol Ther. 1991;49:13–7. doi: 10.1038/clpt.1991.3. [DOI] [PubMed] [Google Scholar]
- 23.Pichler WJ. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139:683–93. doi: 10.7326/0003-4819-139-8-200310210-00012. [DOI] [PubMed] [Google Scholar]
- 24.White KD, Chung WH, Hung SI, Mallal S, Phillips EJ. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: the role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136:219–34. doi: 10.1016/j.jaci.2015.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shiohara T, Inaoka M, Kano Y. Drug-induced hypersensitivity syndrome (DIHS): a reaction induced by a complex interplay among herpesviruses and antiviral and antidrug immune responses. Allergol Int. 2006;55:1–8. doi: 10.2332/allergolint.55.1. [DOI] [PubMed] [Google Scholar]
- 26.Picard D, Janela B, Descamps V, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): a multiorgan antiviral T cell response. Sci Transl Med. 2010;2:46–62. doi: 10.1126/scitranslmed.3001116. [DOI] [PubMed] [Google Scholar]
- 27.Roujeau J-C, Dupin N. Virus Reactivation in Drug Reaction with Eosinophilia and Systemic Symptoms (Dress) Results from a strong drug-specific immune response. J Allergy Clin Immunol Pract. 2017;5:811–12. doi: 10.1016/j.jaip.2016.11.027. [DOI] [PubMed] [Google Scholar]
- 28.Yazicioglu M, Elmas R, Turgut B, Genchallac T. The association between DRESS and the diminished numbers of peripheral B lymphocytes and natural killer cells: Peripheral B lymphocytes and NK cells in DRESS. Pediatr Allergy Immunol. 2012;23:289–96. doi: 10.1111/j.1399-3038.2012.01268.x. [DOI] [PubMed] [Google Scholar]
- 29.Cacoub P, Musette P, Descamps V, et al. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–97. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 30.Shiohara T, Kano Y. A complex interaction between drug allergy and viral infection. Clin Rev Allergy Immunol. 2007;33:124–33. doi: 10.1007/s12016-007-8010-9. [DOI] [PubMed] [Google Scholar]
- 31.Um SJ, Lee SK, Kim YH, et al. Clinical features of drug induced hypersensitivity syndrome in 38 patients. J Investig Allergol Clin Immunol. 2010;20:556–62. [PubMed] [Google Scholar]
- 32.Peyriere H, Dereure O, Breton H, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2006;155:422–28. doi: 10.1111/j.1365-2133.2006.07284.x. [DOI] [PubMed] [Google Scholar]
- 33.Wongkitisophon P, Chanprapaph K, Rattanakaemakorn P, Vachiramon V. Sixyear retrospective review of drug reaction with eosinophilia and systemic symptoms. Acta Derm Venereol. 2012;92:200e5. doi: 10.2340/00015555-1222. [DOI] [PubMed] [Google Scholar]
- 34.Lee JY, Lee SY, Hahm JE, Ha JW, Kim CW, Kim SS. Clinical features of drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome: a study of 25 patients in Korea. Int J Dermatol. 2017;56:944–51. doi: 10.1111/ijd.13667. [DOI] [PubMed] [Google Scholar]
- 35.Chen YC, Chiu HC, Chu CY. Drug reaction with eosinophilia and systemic symptoms: a retrospective study of 60 cases. Arch Dermatol. 2010;146:1373–9. doi: 10.1001/archdermatol.2010.198. [DOI] [PubMed] [Google Scholar]
- 36.Velasco M, McDermott J. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome and hepatitis induced by phenytoin: DRESS syndrome and hepatitis induced by phenytoin. Int J Dermatol. 2014;53:490–93. doi: 10.1111/j.1365-4632.2012.05547.x. [DOI] [PubMed] [Google Scholar]
- 37.Behera SK, Das S, Xavier AS, Selvarajan S. DRESS syndrome: a detailed insight. Hosp Pract (1995) 2018;46:152–62. doi: 10.1080/21548331.2018.1451205. [DOI] [PubMed] [Google Scholar]
- 38.Wang L, Mei XL. Drug Reaction with Eosinophilia and Systemic Symptoms: Retrospective analysis of 104 cases over one decade. Chin Med J (Engl) 2017;130:943–9. doi: 10.4103/0366-6999.204104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hiransuthikul A, Rattananupong T, Klaewsongkram J, Rerknimitr P, Pongprutthipan M, Ruxrungtham K. Drug-induced hypersensitivity syndrome/drug reaction with eosinophilia and systemic symptoms (DIHS/DRESS): 11 years retrospective study in Thailand. Allergol Int. 2016;65:432–38. doi: 10.1016/j.alit.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Wei CH, R. Chung-Yee Hui R, Chang CJ, et al. Identifying prognostic factors for drug rash with eosinophilia and systemic symptoms (DRESS) Eur J Dermatol. 2011;21:930–7. doi: 10.1684/ejd.2011.1550. [DOI] [PubMed] [Google Scholar]
- 41.Roujeau JC, Stern RS. Severe adverse cutaneous reactions to drugs. N Engl J Med. 1994;331:1272–85. doi: 10.1056/NEJM199411103311906. [DOI] [PubMed] [Google Scholar]
- 42.Williams KW, Ware J, Abiodun A, Holland-Thomas NC, Khoury P, Klion AD. Hypereosinophilia in children and adults: a retrospective comparison. J Allergy Clin Immunol Pract. 2016;4:941–7. doi: 10.1016/j.jaip.2016.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bourgeois GP, Cafardi JA, Groysman V, Hughey LC. A review of DRESS-associated myocarditis. J Am Acad Dermatol. 2012;66:e229–36. doi: 10.1016/j.jaad.2010.11.057. [DOI] [PubMed] [Google Scholar]
- 44.Thongsri T, Chularojanamontri L, Pichler WJ. Cardiac involvement in DRESS syndrome. Asian Pacific J Allergy Immunol. 2017;35:3–10. doi: 10.12932/AP0847. [DOI] [PubMed] [Google Scholar]
- 45.Misirlioglu DE, Guvenir H, Bahceci S. Severe cutaneous adverse drug reactions in pediatric patients: a multicenter study. J Allergy Clin Immunol Pract. 2017;5:757–63. doi: 10.1016/j.jaip.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 46.Belver MT, Michavila A, Bobolea I, Feito M, Bellón T, Quirce S. Severe delayed skin reactions related to drugs in the paediatric age group: A review of the subject by way of three cases (Stevens-Johnson syndrome, toxic epidermal necrolysis and DRESS) Allergol Immunopathol (Madr) 2016;44:83–95. doi: 10.1016/j.aller.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 47.Silva-Feistner M, Ortiz E, Rojas-Lechuga MJ, Muñoz D. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome in pediatrics. Clinical case. Rev Chil Pediatr. 2017;88:158–63. doi: 10.1016/j.rchipe.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 48.Tonekaboni SH, Jafari N, Chavoshzadeh Z, Shamsian BS, Rezaei N. DRESS Syndrome Presents as Leukoencephalopathy. Turk J Pediatr. 2015;57:541–4. [PubMed] [Google Scholar]
- 49.Walsh S, Diaz-Cano S, Higgins E, et al. Drug reaction with eosinophilia and systemic symptoms: is cutaneous phenotype a prognostic marker for outcome? A review of clinicopathological features of 27 cases. Br J Dermatol. 2013;168:391–401. doi: 10.1111/bjd.12081. [DOI] [PubMed] [Google Scholar]
- 50.Polivka L, Diana JS, Soria A, et al. Probable DRESS syndrome induced by IL-1 inhibitors. Orphanet J Rare Dis. 2017;12:87. doi: 10.1186/s13023-017-0645-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castellazzi ML, Esposito S, Claut LE, Daccò V, Colombo C. Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome in two young children: the importance of an early diagnosis. Ital J Pediatr. 2018;44:93. doi: 10.1186/s13052-018-0535-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koca T, Akcam M. Ibuprofen induced DRESS syndrome in a child. Indian Pediatr. 2016;53:745. doi: 10.1007/s13312-016-0924-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kocaoglu C, Cilasun C, Solak ES, Kurtipek GS, Arslan S. Successful Treatment of Antiepileptic Drug-Induced DRESS Syndrome with Pulse Methylprednisolone. Case Rep Pediatr. 2013;2013:928910. doi: 10.1155/2013/928910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Terlemez S, Demir F, Bulut Y, et al. DRESS syndrome developed related to acetylsalicylic acid use. Pediatr Allergy Immunol. 2016;27:227–30. doi: 10.1111/pai.12484. [DOI] [PubMed] [Google Scholar]
- 55.Smith RJ, Boos MD, McMahon P. Probable griseofulvin-induced drug reaction with eosinophilia and systemic symptoms in a child. Pediatr Dermatol. 2016;33:e290–1. doi: 10.1111/pde.12935. [DOI] [PubMed] [Google Scholar]
- 56.Fong CY, Hashim N, Gan CS, Chow TK, Tay CG. Sulthiame-induced drug reaction with eosinophiliavand systemic symptoms (DRESS) syndrome. Eur J Paediatr Neurol. 2016;20:957–61. doi: 10.1016/j.ejpn.2016.07.023. [DOI] [PubMed] [Google Scholar]
- 57.Karakayalı B, Yazar AS, Çakir D, et al. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome associated with cefotaxime and clindamycin use in a 6 year-old boy: a case report. Pan Afr Med J. 2017;28:218. doi: 10.11604/pamj.2017.28.218.10828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chow ML, Kim D, Kamath S, Peng D, Luu M. Use of antiviral medications in drug reaction with eosinophilia and systemic symptoms (DRESS): A case of infantile DRESS. Pediatr Dermatol. 2018;35:e114–e116. doi: 10.1111/pde.13408. [DOI] [PubMed] [Google Scholar]
- 59.Dursun A, Bayram AK, Tekerek NÜ, Akyıldız BN, Per H. A case of DRESS syndrome associated with carbamazepine treatment. Turk Pediatri Ars. 2018;53:48–50. doi: 10.5152/TurkPediatriArs.2017.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bayram AK, Canpolat M, Çınar SL. Drug reaction with eosinophilia and systemic symptoms syndrome induced by levetiracetam in a pediatric patient. J Emerg Med. 2016;50:e61–6. doi: 10.1016/j.jemermed.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Pereira-Ospina RDP, Bejarano-Quintero AM, Suescún-Vargas JM, Pinzón-Salamanca JY. Drug reaction with eosinophilia and systemic symptoms due to carbamazepine. Pediatric case. Arch Argent Pediatr. 2018;116:e433–e436. doi: 10.5546/aap.2018.e433. [DOI] [PubMed] [Google Scholar]
- 62.Anil H, Harmanci K, Tekin RT, Kocak A. Presence of a single nucleotide polymorphism (RS3758581) in a boy with DRESS syndrome. Cent Eur J Immunol. 2017;42:409–11. doi: 10.5114/ceji.2017.72821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Marcus N, Smuel K, Almog M, et al. Successful Intravenous Immunoglobulin Treatment in Pediatric Severe DRESS Syndrome. J Allergy Clin Immunol Pract. 2018;6:1238–42. doi: 10.1016/j.jaip.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 64.Song SM, Cho MS, Oh SH, et al. Liver transplantation in a child with acute liver failure resulting from drug rash with eosinophilia and systemic symptoms syndrome. Korean J Pediatr. 2013;56:224–6. doi: 10.3345/kjp.2013.56.5.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lin YC, Sheu JN, Chung WH, et al. Vancomycin-Induced Stevens-Johnson Syndrome in a boy under 2 years old: an early diagnosis by Granulysin Rapid Test. Front Pediatr. 2018;6:26. doi: 10.3389/fped.2018.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim H, Chadwick L, Alzaidi Y, Picker J, Poduri A, Manzi S. HLA-A*31:01 and Oxcarbazepine-Induced DRESS in a patient with seizures and complete DCX deletion. Pediatrics. 2018;141:S434–S438. doi: 10.1542/peds.2017-1361. [DOI] [PubMed] [Google Scholar]
- 67.Chua GT, Rosa Duque JS, Chong PCY, Lee PPW, Lau YL, Ho MHK. Paediatric case series of drug reaction with eosinophilia and systemic symptoms (DRESS): 12-year experience at a single referral centre in Hong Kong and the first reported use of infliximab. Eur Ann Allergy Clin Immunol. 2018;50:273–76. doi: 10.23822/EurAnnACI.1764-1489.47. [DOI] [PubMed] [Google Scholar]
- 68.Goswami JN, Vaidya PC, Saini AG, De D, Radotra BD, Singhi PD. Drug reaction with eosinophilia and systemic symptoms in a child on multiple antiepileptics. Turk J Pediatr. 2017;59:197–99. doi: 10.24953/turkjped.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Vignesh P, Kishore J, Kumar A, et al. A young child with eosinophilia, rash, and multisystem illness: Drug Rash, Eosinophilia, and Systemic Symptoms Syndrome after receipt of fluoxetine. Pediatr Dermatol. 2017;34:e120–e125. doi: 10.1111/pde.13131. [DOI] [PubMed] [Google Scholar]
- 70.Mattoussi N, Ben Mansour A, Essadam L, Guedri R, Fitouri Z, Ben Becher S. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) Syndrome in children: a case report. J Investig Allergol Clin Immunol. 2017;27:144–6. doi: 10.18176/jiaci.0140. [DOI] [PubMed] [Google Scholar]
- 71.Vinson AE, Dufort EM, Willis MD, Eberson CP, Harwell JI. Drug rash, eosinophilia, and systemic symptoms syndrome: Two pediatric cases demonstrating the range of severity in presentation-A case of vancomycin-induced drug hypersensitivity mimicking toxic shock syndrome and a milder case induced by minocycline. Pediatr Crit Care Med. 2010;11:e38–43. doi: 10.1097/PCC.0b013e3181c5911a. [DOI] [PubMed] [Google Scholar]
- 72.Irga N, Kosiak W, Jaworski R, Zielinski J, Adamkiewicz-Drozynska E. Pediatrician! Do you know the symptoms of DRESS Syndrome? Pediatr Emerg Care. 2013;29:504–7. doi: 10.1097/PEC.0b013e31828a3854. [DOI] [PubMed] [Google Scholar]
- 73.Silva-Feistner M, Ortiz E, Rojas-Lechuga MJ, Muñoz D. DRESS syndrome in paediatrics: clinical case. Rev Chil Pediatr. 2017;88:158–63. doi: 10.1016/j.rchipe.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Coughlin CC, Jen MV, Boos MD. Drug Hypersensitivity Syndrome with prolonged course complicated by parvovirus infection. Pediatr Dermatol. 2016;33:e364–e365. doi: 10.1111/pde.13007. [DOI] [PubMed] [Google Scholar]
- 75.Kang H, Min TK, Yang HJ, Pyun BY. Cefotaxime-induced drug rash with eosinophilia and systemic symptoms syndrome in a 7-year-old boy. Ann Allergy Asthma Immunol. 2016;117:202–4. doi: 10.1016/j.anai.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 76.Lan J, Lahoti A, Lew DB. A severe case of minocycline-induced DRESS resulting in liver transplantation and autoimmune sequelae. Ann Allergy Asthma Immunol. 2016;116:367–8. doi: 10.1016/j.anai.2015.12.010. [DOI] [PubMed] [Google Scholar]
- 77.Rueda-Valencia Mde L, Infante S, Campos M, Beléndez C, Saavedra Lozano J. Trimethoprim-sulfamethoxazole induced DRESS syndrome in a 4-year-old child. Ann Allergy Asthma Immunol. 2016;116:366–7. doi: 10.1016/j.anai.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 78.Vasanthan T, Rajaguru G, Venkatesh C, Narayanan P, Gulati R, Toi PC. DRESS Syndrome with peripheral neuropathy due to reactivation of cytomegalovirus in a child. J Glob Infect Dis. 2015;7:89–90. doi: 10.4103/0974-777X.157249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dilek N, Özkol HU, Akbaş A, et al. Cutaneous drug reactions in children: a multicentric study. Postepy Dermatol Alergol. 2014;31:368–71. doi: 10.5114/pdia.2014.43881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Teng P, Tan B. Carbamazepine-induced DRESS syndrome in a child: Rapid response to pulsed corticosteroids. Dermatol Online J. 2013;19:18170. [PubMed] [Google Scholar]
- 81.Dewan AK, Quinonez RA. Allopurinol-induced DRESS syndrome in an adolescent patient. Pediatr Dermatol. 2010;27:270–3. doi: 10.1111/j.1525-1470.2009.00983.x. [DOI] [PubMed] [Google Scholar]
- 82.Tempark T, Satapornpong P, Rerknimitr P, et al. Dapsone-induced severe cutaneous adverse drug reactions are strongly linked with HLA-B*13:01 allele in the Thai population. Pharmacogenet Genomics. 2017;27:429–37. doi: 10.1097/FPC.0000000000000306. [DOI] [PubMed] [Google Scholar]
- 83.Rioualen S, Dufau J, Flatres C, Lavenant P, Misery L, Roué JM. DRESS complicated by hemophagocytic lymphohistiocytosis in an infant treated for congenital toxoplasmosis. Ann Dermatol Venereol. 2017;144:784–7. doi: 10.1016/j.annder.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 84.Lavenant P, Roue JM, Huet F, Abasq C, Misery L, Rioualen S. DRESS syndrome and agranulocytosis, a rare combination. Arch Pediatr. 2017;24:752–6. doi: 10.1016/j.arcped.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 85.Besli GE, Yildirim S, Yilmaz K, Yuksel E. Drug Reaction With Eosinophilia and Systemic Symptoms Syndrome or hematologic malignancy?: a case report of a 4-year-old boy. Pediatr Emerg Care. 2017;33:494–6. doi: 10.1097/PEC.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 86.Erdem SB, Nacaroglu HT, Bag O, Karkiner CS, Korkmaz HA, Can D. DRESS syndrome associated with type 2 diabetes in a child. Cent Eur J Immunol. 2015;40:493–6. doi: 10.5114/ceji.2015.54606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Avancini J, Maragno L, Santi CG, Criado PR. Drug reaction with eosinophilia and systemic symptoms/drug induced hypersensitivity syndrome: clinical features of 27 patients. Clin Exp Dermatol. 2015;40:851–9. doi: 10.1111/ced.12682. [DOI] [PubMed] [Google Scholar]
- 88.González Díaz C, González Hermosa A, García-Lirio E, Martínez-Aranguren R, Gamboa Setien P. Dress induced by piperacillin-tazobactam in a child. J Allergy Clin Immunol Pract. 2015;3:615–7. doi: 10.1016/j.jaip.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 89.Shimabukuro K, Gibbon F, Kerstetter J, Tinsley C, Ashwal S. DRESS associated with perampanel administration in a child with drug-resistant epilepsy. Neurology. 2014;83:2188. doi: 10.1212/WNL.0000000000001052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lomairi NE, Abourazzak S, Chaouki S, Atmani S, Hida M. Drug Reaction with Eosinophilia and Systemic Symptom (DRESS) induced by carbamazepine: a case report and literature review. Pan Afr Med J. 2014;18:9. doi: 10.11604/pamj.2014.18.9.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suthar R, Sankhyan N, Shree H, Singhi P. Reversible Vegetative State in a Child Due to Drug Reaction with Eosinophilia and Systemic Symptoms. Indian J Pediatr. 2017;84:249–50. doi: 10.1007/s12098-016-2265-1. [DOI] [PubMed] [Google Scholar]
- 92.Correa-de-Castro B, Paniago AM, Takita LC, Murback ND, Hans-Filho G. Drug reaction with eosinophilia and systemic symptoms: a clinicopathological study of six cases at a teaching hospital in midwestern Brazil. Int J Dermatol. 2016;55:328–34. doi: 10.1111/ijd.12899. [DOI] [PubMed] [Google Scholar]
- 93.Ho CH, Uzunyan MY. Myocarditis in drug rash with eosinophilia and systemic symptoms. Cardiol Young. 2015;25:1210–3. doi: 10.1017/S1047951114001735. [DOI] [PubMed] [Google Scholar]
- 94.Nanishi E, Hoshina T, Ohga S, Nishio H, Hara T. Drug reaction with eosinophilia and systemic symptoms during primary Epstein Barr virus infection. J Microbiol Immunol Infect. 2015;48:109–12. doi: 10.1016/j.jmii.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 95.Ramírez A, Abril JC, Cano J. DRESS syndrome due to antibiotic therapy of osteoarticular infections in children: two case reports. Rev Esp Cir Ortop Traumatol. 2015;59:360–4. doi: 10.1016/j.recot.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Cheng J, Rawal S, Roberts A, Guttman OR. Drug reaction with eosinophilia and systemic symptoms syndrome associated with antituberculosis medications. Pediatr Infect Dis J. 2013;32:1388–90. doi: 10.1097/INF.0b013e3182a09f20. [DOI] [PubMed] [Google Scholar]
- 97.Yusuf IH, Sahare P, Hildebrand GD. DRESS syndrome in a child treated for toxoplasma retinochoroiditis. J AAPO. 2013;17:521–3. doi: 10.1016/j.jaapos.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 98.Shahbaz S, Sivamani RK, Konia T, Burrall B. A case of Drug Rash with Eosinophilia and Systemic Symptoms (DRESS) related to rufinamide. Dermatol Online J. 2013;19:4. [PubMed] [Google Scholar]
- 99.Naveen KN, Ravindra MS, Pai VV, Rai V, Athanikar SB, Girish M. Lamotrigine induced DRESS syndrome. Indian J Pharmacol. 2012;44:798–800. doi: 10.4103/0253-7613.103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zurina Z, Elizawaty O, Thevarajah S, Norlijah O. Dapsone syndrome-first Malaysian paediatric case report. Med J Malaysia. 2012;67:105–7. [PubMed] [Google Scholar]
- 101.Deka NM, Dass R, Das BK, Hoque R. Phenytoin Induced DRESS Syndrome. Indian J Pediatr. 2013;80:266. doi: 10.1007/s12098-012-0751-7. [DOI] [PubMed] [Google Scholar]
- 102.Buyuktiryaki AB, Bezirganoglu H, Sahiner UM, et al. Patch testing is an effective method for the diagnosis of carbamazepine-induced drug reaction, eosinophilia and systemic symptoms (DRESS) syndrome in an 8 year-old girl. Austral J Dermatol. 2012;53:274–7. doi: 10.1111/j.1440-0960.2012.00887.x. [DOI] [PubMed] [Google Scholar]
- 103.Bauer KA, Brimhall AK, Chang TT. Drug reaction with eosinophilia and systemic symptoms (DRESS) associated with azithromycin in acute Epstein-Barr virus infection. Pediatr Dermatol. 2011;28:741–3. doi: 10.1111/j.1525-1470.2011.01558.x. [DOI] [PubMed] [Google Scholar]
- 104.Orbak Z, Sepetcigil O, Karakelleoğlu C, Gülses S. Penicillin V-induced drug rash with eosinophilia and systemic symptoms. West Indian Med J. 2010;59:722–5. [PubMed] [Google Scholar]
- 105.Silva R, Botelho C, Cadinha S, Lisboa C, Azevedo I, Cernadas JR. Possible DRESS syndrome in a child with borreliosis. Allergol Immunopathol (Madr) 2012;40:129–31. doi: 10.1016/j.aller.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 106.Hubiche T, Milpied B, Cazeau C, Taïeb A, Léauté-Labrèze C. Association of immunologically confirmed delayed drug reaction and human herpesvirus 6 viremia in a pediatric case of drug-induced hypersensitivity syndrome. Dermatology. 2011;222:140–1. doi: 10.1159/000324506. [DOI] [PubMed] [Google Scholar]
- 107.Piñana E, Lei SH, Merino R, et al. DRESS-syndrome on sulfasalazine and naproxen treatment for juvenile idiopathic arthritis and reactivation of human herpevirus 6 in an 11-year old Caucasian boy. J Clin Pharm Ther. 2010;35:365–70. doi: 10.1111/j.1365-2710.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- 108.Rosenbaum J, Alex G, Roberts H, Orchard D. Drug rash with eosinophilia and systemic symptoms secondary to sulfasalazine. J Paediatr Child Health. 2010;46:193–6. doi: 10.1111/j.1440-1754.2009.01660.x. [DOI] [PubMed] [Google Scholar]
- 109.Scagni P, Morello M, Ramus MV, Agostini M, Pagliero R. Drug-induced hypersensivity syndrome associated with Epstein-Barr virus infection: a pediatric case report. Pediatr Dermatol. 2009;26:229–31. doi: 10.1111/j.1525-1470.2009.00891.x. [DOI] [PubMed] [Google Scholar]
- 110.Armin S, Chavoshzadeh Z, Mohkam M, Rezaei N. Antiepileptic hypersensitivity and DRESS syndrome due to phenytoin in two pediatric cases. Turk J Pediatr. 2009;51:76–7. [PubMed] [Google Scholar]
- 111.Kawakami T, Fujita A, Takeuchi S, Muto S, Soma Y. Drug-induced hypersensitivity syndrome: drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome induced by aspirin treatment of Kawasaki disease. J Am Acad Dermatol. 2009;60:146–9. doi: 10.1016/j.jaad.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 112.Seth D, Kamat D, Montejo J. DRESS Syndrome: A Practical Approach for Primary Care Practitioners. Clin Pediatr (Phila) 2008;47:947–52. doi: 10.1177/0009922808320703. [DOI] [PubMed] [Google Scholar]
- 113.Crowell CS, Melin-Aldana H, Tan TQ. Fever, rash, and hepatic dysfunction in a 3-year-old child: a case report. Clin Pediatr (Phila) 2008;47:517–20. doi: 10.1177/0009922807312084. [DOI] [PubMed] [Google Scholar]
- 114.Garnier A, El Marabet el H, Kwon T. Acute renal failure in a 3-year-old child as part of the drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome following hepatitis A. Pediatr Nephrol. 2008;23:667–9. doi: 10.1007/s00467-007-0676-y. [DOI] [PubMed] [Google Scholar]
- 115.Manuyakorn W, Siripool K, Kamchaisatian W, et al. Phenobarbital-induced severe cutaneous adverse drug reactions are associated with CYP2C19*2 in Thai children. Pediatr Allergy Immunol. 2013;24:299–303. doi: 10.1111/pai.12058. [DOI] [PubMed] [Google Scholar]
- 116.Ahluwalia J, Abuabara K, Perman MJ, Yan AC. HHV6 involvement in pediatric drug hypersensitivity syndrome. Br J Dermatol. 2015;172:1090–5. doi: 10.1111/bjd.13512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Asidharanpillai S, Sabitha S, Riyaz N, et al. Drug Reaction with Eosinophilia and Systemic Symptoms in Children: A Prospective Study. Pediatr Dermatol. 2016;33:e162–5. doi: 10.1111/pde.12803. [DOI] [PubMed] [Google Scholar]
- 118.Fleming P, Marik PE. The DRESS syndrome: the great clinical mimicker. Pharmacotherapy. 2011;31:332. doi: 10.1592/phco.31.3.332. [DOI] [PubMed] [Google Scholar]
- 119.Husain Z, Reddy BY, Schwartz RA. DRESS syndrome: Part II. Management therapeutics. J Am Acad Dermatol. 2013;68:709–e1-9. doi: 10.1016/j.jaad.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 120.Gonçalo MM, Cardoso JC, Gouveia MP, et al. Histopathology of the exanthema in DRESS is not specific but may indicate severity of systemic involvement. Am J Dermatopathol. 2016;38:23–33. doi: 10.1097/DAD.0000000000000439. [DOI] [PubMed] [Google Scholar]
- 121.Skowron F, Bensaid B, Balme B, et al. Drug reaction with eosinophilia and systemic symptoms (DRESS): clinicopathological study of 45 cases. J Eur Acad Dermatol Venereol. 2015;29:2199–205. doi: 10.1111/jdv.13212. [DOI] [PubMed] [Google Scholar]
- 122.Ogawa K, Morito H, Hasegawa A, et al. Identification of thymus and activation-regulated chemokine (TARC/CCL17) as a potential marker for early indication of disease and prediction of disease activity in drug-induced hypersensitivity syndrome (DIHS)/drug rash with eosinophilia and systemic symptoms (DRESS) J Dermatol Science. 2013;69:38–43. doi: 10.1016/j.jdermsci.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 123.Caffarelli C, Franceschini F, Caimmi D, et al. SIAIP position paper: provocation challenge to antibiotics and non-steroidal anti-inflammatory drugs in children. Ital J Pediatr. 2018;44:147. doi: 10.1186/s13052-018-0589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barbaud A, Collet E, Milpied B, et al. A multicentre study to determine the value and safety of drug patch tests for the three main classes of severe cutaneous adverse drug reactions. Br J Dermatol. 2013;168:555–62. doi: 10.1111/bjd.12125. [DOI] [PubMed] [Google Scholar]
- 125.Caglayan Sozmen S, Povesi Dascola C, Gioia E, Mastrorilli C, Rizzuti L, Caffarelli C. Diagnostic accuracy of patch test in children with food allergy. Pediatr Allergy Immunol. 2015;26:416–22. doi: 10.1111/pai.12377. [DOI] [PubMed] [Google Scholar]
- 126.Pichler WJ, Tilch J. The lymphocyte transformation test in the diagnosis of drug hypersensitivity. Allergy. 2004;59:809–20. doi: 10.1111/j.1398-9995.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 127.Kano Y, Hirahara K, Mitsuyama Y, Takahashi R, Shiohara T. Utility of the lymphocyte transformation test in the diagnosis of drug sensitivity: dependence on its timing and the type of drug eruption. Allergy. 2007;62:1439–44. doi: 10.1111/j.1398-9995.2007.01553.x. [DOI] [PubMed] [Google Scholar]
- 128.Jurado-Palomo J, Cabañas R, Prior N, et al. Use of the lymphocyte transformation test in the diagnosis of DRESS syndrome induced by ceftriaxone and piperacillin-tazobactam: two case reports. J Investig Allergol Clin Immunol. 2010;20:433. [PubMed] [Google Scholar]
- 129.Shiohara T, Iijima M, Ikezawa Z, Hashimoto K. The diagnosis of a DRESS syndrome has been sufficiently established on the basis of typical clinical features and viral reactivations. Br J Dermatol. 2007;156:1083–84. doi: 10.1111/j.1365-2133.2007.07807.x. [DOI] [PubMed] [Google Scholar]
- 130.Caffarelli C, Cuomo B, Cardinale F, et al. Aetiological factors associated with chronic urticaria in children: a systematic review. Acta Derm Venereol. 2013;93:268–72. doi: 10.2340/00015555-1511. [DOI] [PubMed] [Google Scholar]
- 131.Galli E, Neri I, Ricci G, et al. Consensus Conference on Clinical Management of pediatric Atopic Dermatitis. Ital J Pediatr. 2016;42:26. doi: 10.1186/s13052-016-0229-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Garcia-Doval I, LeCleach L, Bocquet H, Otero XL, Roujeau JC. Toxic epidermal necrolysis and Stevens-Johnson syndrome: does early withdrawal of causative drugs decrease the risk of death? Arch Dermatol. 2000;136:323–7. doi: 10.1001/archderm.136.3.323. [DOI] [PubMed] [Google Scholar]
- 133.Descamps V, Ranger-Rogez S. DRESS syndrome. Joint Bone Spine. 2014;81:15–21. doi: 10.1016/j.jbspin.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 134.Natkunarajah J, Goolamali S, Craythorne E, et al. Ten cases of drug reaction with eosinophilia and systemic symptoms (DRESS) treated with pulsed intravenous methylprednisolone. Eur J Dermatol. 2011;21:385–91. doi: 10.1684/ejd.2011.1300. [DOI] [PubMed] [Google Scholar]
- 135.Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353–6. doi: 10.1159/000069956. [DOI] [PubMed] [Google Scholar]
- 136.Ange N, Alley S, Fernando SL, Coyle L, Yun J. Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) syndrome successfully treated with mepolizumab. J Allergy Clin Immunol Pract. 2018;6:1059–60. doi: 10.1016/j.jaip.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 137.Kirchhof MG, Wong A, Dutz JP. Cyclosporine Treatment of Drug-Induced Hypersensitivity Syndrome. JAMA Dermatol. 2016;152:1254–7. doi: 10.1001/jamadermatol.2016.2220. [DOI] [PubMed] [Google Scholar]
- 138.Joly P, Janela B, Tetart F, Rogez S, et al. Poor benefit/risk balance of intravenous immunoglobulins in DRESS. Arch Derm. 2012;148:543–4. doi: 10.1001/archderm.148.4.dlt120002-c. [DOI] [PubMed] [Google Scholar]
- 139.Moling O, Tappeiner L, Piccin A, et al. Treatment of DIHS/DRESS syndrome with combined N-acetylcysteine, prednisone and valganciclovir--a hypothesis. Med Sci Monit. 2012;18:CS57–62. doi: 10.12659/MSM.883198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Alexander T, Iglesia E, Park Y, et al. Severe DRESS syndrome managed with therapeutic plasma exchange. Pediatrics. 2013;131:e945–e949. doi: 10.1542/peds.2012-2117. [DOI] [PubMed] [Google Scholar]
- 141.Zuliani E, Zwahlen H, Gilliet F, Marone C. Vancomycin-induced hypersensitivity reaction with acute renal failure: resolution following cyclosporine treatment. Clin Nephrol. 2005;64:155. doi: 10.5414/cnp64155. [DOI] [PubMed] [Google Scholar]
- 142.De Clercq E. Selective anti-herpesvirus agents. Antivir Chem Chemother. 2013;23:93–101. doi: 10.3851/IMP2533. [DOI] [PubMed] [Google Scholar]
- 143.Caffarelli C, Bernasconi S. Preventing necrotising enterocolitis with probiotics. Lancet. 2007;369:1578–80. doi: 10.1016/S0140-6736(07)60721-1. [DOI] [PubMed] [Google Scholar]
- 144.Caffarelli C, Cardinale F, Povesi-Dascola C, Dodi I, Mastrorilli V, Ricci G. Use of probiotics in pediatric infectious diseases. Expert Rev Anti Infect Ther. 2015;13:1517–35. doi: 10.1586/14787210.2015.1096775. [DOI] [PubMed] [Google Scholar]
- 145.Picard D, Vellar M, Janela B, Roussel A, Joly P, Musette P. Recurrence of drug-induced reactions in DRESS patients. J Eur Acad Dermatol Venereol. 2015;29:801–4. doi: 10.1111/jdv.12419. [DOI] [PubMed] [Google Scholar]
- 146.Aota N, Shiohara T. Viral connection between drug rashes and autoimmune diseases: how autoimmune responses are generated after resolution of drug rashes. Autoimmun Rev. 2009;8:488–94. doi: 10.1016/j.autrev.2009.02.029. [DOI] [PubMed] [Google Scholar]
- 147.Ushigome Y, Kano Y, Ishida T, Hirahara K, Shiohara T. Short- and long-term outcomes of 34 patients with drug-induced hypersensitivity syndrome in a single institution. J Am Acad Dermatol. 2013;68:721–8. doi: 10.1016/j.jaad.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 148.Duong TA, Duong TA, Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390:1996–2011. doi: 10.1016/S0140-6736(16)30378-6. [DOI] [PubMed] [Google Scholar]