Abstract
The most frequent pancreatic cancer is pancreatic adenocarcinoma. It has high and early locally and distant invasiveness; this is the reason why it often shows little sign or symptoms in early stage and poor prognosis after the diagnosis, frequently in advanced stage. Although it is possible to detect this tumor in early stage because of its neoplastic precursor (PanINs). Epidemiological data shows that pancreatic cancer is not very common but obvious it is one of the most neoplastic death-cause in the world. The trend of incidence is quite increasing through years, proportionally to the increase of risk factors. About risk factors, it is not easy to detect in all the cases but it is known the role of some of that: there are hereditary risk factors, such as genetic pattern like HBOC, HNPCC, FAP, PJS, FAMMM, HP and CF and environmental ones (modifiable) such as smoke, alcohol consumption, chronic pancreatitis, obesity and diabetes mellitus. This narrative review aims to analyze the epidemiological data of pancreatic cancer and associated risk factors. (www.actabiomedica.it)
Keywords: pancreatic cancer, pancreatic adenocarcinoma, epidemiology, risk factors
Introduction
All the pancreatic epithelial tumors are classified by WHO as benign (we will not consider these tumors in this review) and malignant ones; they are split out considering some macroscopic and microscopic features: on a macroscopic level they are solid, cystic or intraductal, while on a microscopic level they are ductal, acinar or endocrine tumors.
The most frequent pancreatic malignant tumor is the solid one represented by ductal cells and it is called Pancreatic Ductal Adenocarcinoma with his variants (1).
In this review we’re only considering pancreatic adenocarcinoma referring to it as “pancreatic cancer”.
About its behavior, pancreatic cancer has high invasiveness and the tendency to infiltrate peri-pancreatic tissues, in addiction to that, it has high trend to show nodal metastasis (peri-pancreatic, gastric, mesenteric, omental and peri-portal nodes) and hepatic, bone or pulmonary metastasis.
Rarely pancreatic cancer manifests specific signs or symptoms and because of its poorness of symptoms, it is often diagnosed in an advanced stage; frequently, when patients have symptoms like asthenia, jaundice, abdominal pain and weight loss, they already have a local advanced pancreatic neoplasia.
On a biological level pancreatic cancer has a clear multistep carcinogenesis; just as colo-rectal cancer presents the adenoma-carcinoma sequence, pancreatic cancer shows a similar one starting from Pancreatic Intraductal Neoplasia (PanINs IA, IB, II and III) and ending with an invasive neoplastic lesion. PanINs’ progression is supported by a succession of a lot of gene mutations (2).
This specific multistep carcinogenesis suggests that if we will diagnose an early stage lesion, by means of PanINs’ detection, we could stop the progression mentioned above, treating early lesions. This is the safer way to change the natural history of pancreatic cancer. Unfortunately, early-stage pancreatic cancer is usually clinically silent, highlighting the need for improved methods of early detection of precursor.
All the above-mentioned aspects show that pancreatic cancer is one of the most big killing neoplasia loaded by a poor prognosis after diagnosis; in this context, the key to solve this problem is the primary prevention knowing the epidemiology and the risk factors associated.
In this review, the purpose is to define the epidemiology of pancreatic cancer by means of descriptive data coming from the literature and identify risk factors associated with this cancer.
Epidemiology
In 1999 Parkin D et al. had compared cancer registries to obtain the incidences and mortality data bank to obtain information on cancer death in 23 world areas. The result was that pancreatic cancer was responsible for 168,000 deaths per year and it was the 9th most common cause of death from cancer in both sexes combined and about incidence it was the 13th. Unfortunately the mortality to incidence ratio was 98% because of the poor prognosis, worse in developed countries than in developing ones (3).
To investigate the most recent worldwide epidemiology, we are basing our research on the GLOBOCAN estimates. The Global Cancer Observatory (GCO) is an interactive web-based platform presenting global cancer statistics to inform cancer control and cancer research.
Incidence and mortality rates were estimated using GLOBOCAN by country, using the most recently available data collected by the IARC or available in routine reports from the registries themselves.
GLOBOCAN 2012 estimates demonstrate different results of incidence and mortality, both lower than 350,000 (338,000 were new cases and 331,000 were the deaths); but already 6 years ago pancreatic cancer was the 7th leading cause of cancer death in both sexes and it was more frequent in developed countries (4).
GLOBOCAN 2018 estimates show that, worldwide, pancreatic cancer is the 14th neoplasia sorting by new cases per year. There is little difference between male and female incidence rate. Because of its poor prognosis, with almost as many deaths (n = 432,000) as cases (n = 459,000), pancreatic cancer is the 7th leading cause of cancer death in both males and females (5).
The trend of incidence of pancreatic cancer through years is increasing as reported by the recent Cancer Statistics Review (CSR) (6).
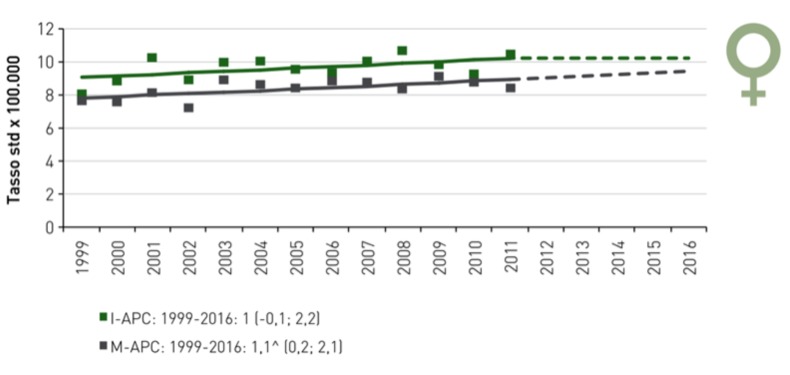
Several authors, agree with that result, such as in Taiwan Tseng CM et al., analyzing Taiwan National Cancer Registry and the National Cause of Death Registry, support the evidence we mentioned above and they formulate that presumably the incidence and mortality will continue to rise in Taiwan (7). In Germany, Quante AS et al. have noted a constant increase in the incidence of pancreatic cancer, which will surpass colorectal and breast cancer to rank as the second most common cause of cancer-related deaths by 2030 (8); this trend is the same we can observe in US, where current projections suggest that pancreatic cancer will become the second-highest cause of cancer death (9). Finally we show the epidemiological data in Italy: in 2017 there was over 13,000 new cases and in 2015 over 11,000 deaths; in 2016 the incidence was 22/100,000 new cases per year and it is raising in both sexes, even if more in male population (10).
Figure.
Pancreatic cancer is mostly frequent in elderly people, the risk of developing pancreatic cancer goes up as people age: about 80% are at least 60 years old and 71 is the average age at the time of diagnosis (11). A population-based epidemiological sudy (12) conclude that the secular trends in the incidence of pancreatic cancer match trends in the prevalence in known risk factors for pancreatic adenocarcinoma such as smoking, overweight and obesity, and diabetes.
Risk factors
Despite other gastrointestinal tumors, evidences of risk factors for development of pancreatic cancer are poor and they don’t explain the whole pancreatic cancer world: we can identify risks factor only in 40% of cases. We have genetics factors (10%) and environmental (modifiable) factors (13).
Curiously, it is difficult to understand the effect-cause relationship of some risk factors, such as diabetes mellitus: in literature some authors tried to clarify the question (14).
About unmodifiable (hereditary) risk factors, we can remember:
HBOC (Hereditary breast and ovarian cancer syndrome): the BRCA1 and BRCA2 mutations can cause early-onset malignant tumors, most of all breast and ovarian cancer, and including pancreatic cancer, especially if in BRCA2 mutation. This pathway can explain 17-19% of hereditary pancreatic cancer (15).
HNPCC (Hereditary Non Polyposic Colorectal Cancer or Lynch syndrome): people who have Lynch syndrome, because of the microsatellite instability (MSH2, MSH6, MLH1, PMS2 and EPCAM genes), are predisposed to early-onset colorectal cancer without polyposic lesions and other-site neoplasia, including pancreatic cancer (RR=8.6) (16).
FAP (Familial Adenomatous polyposis): this syndrome, caused by a mutation in the APC gene, is characterized of early-onset polyps in gastrointestinal tract that can be develop in malignant neoplasia. If FAP is involved in an increased risk of pancreatic cancer is uncertain because it may reflects a misdiagnosed of ampulla carcinomas (17).
PJS (Peutz-Jeghers Syndrome): The STK11/LKB1 genes mutation characterize an hamartomatous polyposis syndrome and this condition can determine gastrointestinal neoplasia and other tumors like pancreatic cancer (RR=132) (18).
FAMMM (Familial Athypical Multiple Mole Melanoma syndrome): this syndrome is characterized by malignant melanoma in one or more first-degree or second-degree relatives. In 38% of cases this pathology is caused by a p16INK4a gene mutation, dysregulates the normal cellular cycle. These people have an higher relative risk for pancreatic cancer (13 to 22-fold increased risk of developing pancreatic cancer) (19).
HP (Hereditary Pancreatitis): In 80% of cases of hereditary pancreatitis it is possible to identify a PRSS1 gene mutation: this is a condition characterized by recurrent acute pancreatitis starting in childhood, that can be evolve in a praecox pancreatic failure (20); the pathogenetic mechanisms, involved in pancreatic cancer onset, are triggered by the pancreatic chronic inflammation (21). Some authors found an high relative risk (RR= 69) for pancreatic cancer for patients with HP compared to the general population (22).
CF (Cystic Fibrosis): this pathology, caused by CFTR gene mutation, has the same pathogenetic mechanisms explained in HP, because recurrent acute pancreatitis can be involved in pancreatic cancer onset (23).
Figure.
More interesting is the acknowledgment about environmental risk factors:
Tobacco use: smoking is the main demonstrated environmental risk factor for the development of pancreatic cancer; the pathogenetic mechanisms include genes mutations inducement (KRAS, p53) and, on the other hand, a chronic inflammation and these two factors can induce cytokines and growth factors output, providing the right pathway to cellular transformation. Tobacco smoking habits in considered responsible of 20-35% of pancreatic cancer cases (24).
Alcohol consumption: the evidence of this association is limited to heavy alcohol assumption: more than three drinks consumption per day has a relative risk from 1.22 to 1.36 of developing pancreatic cancer, with a dose-response relationship. Alcohol and its metabolites make a pro-carcinogenetic pathway through chronic inflammation (considering alcohol consumption is responsible of 60-90% of chronic pancreatitis) and cellular gene instability (25).
Chronic pancreatitis: The morphological and functional modification in chronic pancreatitis are the same we can found in pancreatic cancer; this similarity is represented also on a molecular level. The chronic inflammation causes the production of TNFα, IL-6, IL-8, PDGF, TGFβ and other cytokines that can induce cellular proliferation and reduce immune-surveillance (26). The main damage on DNA is caused by ROS production, promoting the progression to cellular transformation (27). The risk of pancreatic cancer is significantly elevated in subjects with chronic pancreatitis and appears to be independent from sex, country, and type of pancreatitis (28). In a 2010 meta-analysis, Raimondi S. et al. identify the association between chronic pancreatitis and pancreatic cancer with a 13.3 relative risk (22).
Obesity: Some studies had demonstrated a relative risk increase of 1.12 for each increase in 5 kg/m2 in the BMI (29). In obese people the pathogenesis is characterized by adiposopathy, a chronic adipose disease in which macrophages product pro-inflammatory cytokines and there is a dysregulation of hormonal level: in particular we can find high level of leptin and low level of adiponectin (30). It is interesting to observe that there is a temporal relationship between BMI and the neoplasia: obesity since childhood have a higher risk relative for pancreatic cancer development (31). On a dietetic level, high consumption of red meat and fatty diet may have a role in the pathogenesis (32).
Diabetes mellitus: About 80% of people with pancreatic cancer have also glucose intolerance or diabetes. The association between these two diseases is clear but it is important to define the relationship. The majority of patients with pancreatic cancer has diabetes in close up the diagnosis of the tumor validating the hypothesis which support that this diabetes is a consequence of the neoplasia (33). But there is a relevant association also with diabetes mellitus type 2 with OR=1.8 (34); the pathogenetic mechanisms sustain this relationship are the hyperinsulinemia, often detected in DMT2, and high level of IGF1: that modification can induce pancreatic glandular proliferation and specific cellular interaction (35). The cellular interaction is between Pancreatic Stellate Cells (PaSCs) and Tumor-associated Macrophages (TAMs): while their dysregulation, because of hyperinsulinemia and hyperglycemia, can induce fibrosis, cellular proliferation and apoptosis inhibition, they are the actors of desmoplastic reaction and hyperplasia frequently detected in pancreatic neoplastic tissues (36).
There are some others factors pointed to have a role in pancreatic carcinogenesis, but there isn’t enough evidence in literature, e.g. biliary obstructive diseases. Some studies underline the role of cholecystectomy: the pivot of this association is the CCK levels, often high in cholecystectomized patients, CCK is responsible for pancreatic glandular hyperplasia (RR=1.23) (37). Some other studies support the hypothesis according to which the risk factor isn’t the surgery but the history of gallstones (RR=1.70) (38). We have to consider that risk factors associated with gallstones, like obesity, diabetes, alcohol consumption and fatty diet are the same involved in the pathogenesis of pancreatic cancer; for this reason some studies clear the role of biliary obstructive diseases (39).
Figure.
Figure.
Conclusions
Pancreatic cancer is one of the most oncological big killer; in few years it will become a non-marginal healthy problem, because of its increasing trend of incidence and poor prognosis after diagnosis. In several cases it is difficult to identify certain risk factors and the hereditary ones is clear just in small portion; the large majority of pancreatic cancer results from environmental factors: smoke, alcohol consumption, chronic pancreatitis, obesity and diabetes mellitus.
References
- 1.Bosman FT, Carneiro F, Hurban RH, Theise ND WHO Classification of Tumours of the Digestive System. WHO Classification of Tumors. 2010;3 [Google Scholar]
- 2.Hruban, Ralph H, Wilentz, Robb E, Kern, Scott E. Genetic progression in the pancreatic ducts. The American journal of pathology. 2000;156(6):1821. doi: 10.1016/S0002-9440(10)65054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parkin D. Max, Pisani Paola, Ferlay J. Global cancer statistics. CA: a cancer journal for clinicians. 1999;49(1):33–64. doi: 10.3322/canjclin.49.1.33. [DOI] [PubMed] [Google Scholar]
- 4.Torre Lindsey A, et al. Global cancer statistics, 2012. CA: a cancer journal for clinicians. 2015;65(2):87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 5.Bray Freddie, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians. 2018 doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 6.Howlader N, Noone A-M, Krapcho M, et al. Bethesda: National Cancer Institute; 2017. SEER Cancer Statistics Review, 1975–2014. [Google Scholar]
- 7.Tseng Chao-Ming, et al. Incidence and mortality of pancreatic cancer on a rapid rise in Taiwan, 1999–2012. Cancer epidemiology. 2017;49:75–84. doi: 10.1016/j.canep.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 8.Quante Anne S, et al. Projections of cancer incidence and cancer-related deaths in Germany by 2020 and 2030. Cancer medicine. 2016;5.9:2649–2656. doi: 10.1002/cam4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahib Lola, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer research. 2014 doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 10.AIOM; AIRTUM. I Numeri del Cancro in Italia 2018 [Google Scholar]
- 11.Gold E. B, Goldin S. B. Epidemiology of and risk factors for pancreatic cancer. Surgical oncology clinics of North America. 1998;7.1:67–91. [PubMed] [Google Scholar]
- 12.Gordon-Dseagu Vanessa L, et al. Pancreatic cancer incidence trends: evidence from the Surveillance, Epidemiology and End Results (SEER) population-based data. International journal of epidemiology. 2017;47.2:427–439. doi: 10.1093/ije/dyx232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Becker Andrew E, et al. Pancreatic ductal adenocarcinoma: risk factors, screening, and early detection. World journal of gastroenterology: WJG. 2014;20.32:11182. doi: 10.3748/wjg.v20.i32.11182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Magruder J, Trent Elahi, Dariush, Andersen, Dana K. Diabetes and pancreatic cancer: chicken or egg? Pancreas. 2011;40.3:339–351. doi: 10.1097/MPA.0b013e318209e05d. [DOI] [PubMed] [Google Scholar]
- 15.Moran Anthony, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Familial cancer. 2012;11.2:235–242. doi: 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 16.Kastrinos Fay, et al. Risk of pancreatic cancer in families with Lynch syndrome. Jama. 2009;302.16:1790–1795. doi: 10.1001/jama.2009.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giardiello F. M, et al. Increased risk of thyroid and pancreatic carcinoma in familial adenomatous polyposis. Gut. 1993;34.10:1394–1396. doi: 10.1136/gut.34.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giardiello Francis M, et al. Very high risk of cancer in familial Peutz–Jeghers syndrome. Gastroenterology. 2000;119.6:1447–1453. doi: 10.1053/gast.2000.20228. [DOI] [PubMed] [Google Scholar]
- 19.De Snoo, Femke A, et al. Increased risk of cancer other than melanoma in CDKN2A founder mutation (p16-Leiden)-positive melanoma families. Clinical Cancer Research. 2008;14.21:7151–7157. doi: 10.1158/1078-0432.CCR-08-0403. [DOI] [PubMed] [Google Scholar]
- 20.Larusch Jessica, Whitcomb, David C. Genetics of pancreatitis. Current opinion in gastroenterology. 2011;27.5:467. doi: 10.1097/MOG.0b013e328349e2f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Chanjuan, Hruban Ralph H, Klein, Alison P. Familial pancreatic cancer. Archives of pathology & laboratory medicine. 2009;133.3:365–374. doi: 10.5858/133.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raimondi Sara, et al. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best practice & research Clinical gastroenterology. 2010;24.3:349–358. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Maisonneuve Patrick, Marshall B. C, Lowenfels A. B. Risk of pancreatic cancer in patients with cystic fibrosis. Gut. 2007;56.9:1327–1328. doi: 10.1136/gut.2007.125278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iodice Simona, et al. Tobacco and the risk of pancreatic cancer: a review and meta-analysis. Langenbeck’s archives of surgery. 2008;393.4:535–545. doi: 10.1007/s00423-007-0266-2. [DOI] [PubMed] [Google Scholar]
- 25.Tramacere Irene, et al. Alcohol drinking and pancreatic cancer risk: a meta - analysis of the dose - risk relation. International journal of cancer. 2010;126.6:1474–1486. doi: 10.1002/ijc.24936. [DOI] [PubMed] [Google Scholar]
- 26.Gukovsky Ilya, et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144.6:1199–1209.e4. doi: 10.1053/j.gastro.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cavestro Giulia Martina, et al. The race from chronic pancreatitis to pancreatic cancer. Jop. 2003;4.5:165–8. [PubMed] [Google Scholar]
- 28.Lowenfels Albert B, et al. Pancreatitis and the risk of pancreatic cancer. New England Journal of Medicine. 1993;328.20:1433–1437. doi: 10.1056/NEJM199305203282001. [DOI] [PubMed] [Google Scholar]
- 29.Larsson Susanna C, Orsini Nicola, Wolk Alicja. Body mass index and pancreatic cancer risk: a meta - analysis of prospective studies. International journal of cancer. 2007;120.9:1993–1998. doi: 10.1002/ijc.22535. [DOI] [PubMed] [Google Scholar]
- 30.Stolzenberg-Solomon Rachael Z, et al. Circulating leptin and risk of pancreatic cancer: a pooled analysis from 3 cohorts. American journal of epidemiology. 2015;182.3:187–197. doi: 10.1093/aje/kwv041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stocks Tanja, et al. Blood glucose and risk of incident and fatal cancer in the metabolic syndrome and cancer project (me-can): analysis of six prospective cohorts. PLoS medicine. 2009;6.12:e1000201. doi: 10.1371/journal.pmed.1000201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsson S. C, Wolk A. Red and processed meat consumption and risk of pancreatic cancer: meta-analysis of prospective studies. British journal of cancer. 2012;106.3:603. doi: 10.1038/bjc.2011.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gullo Lucio, et al. Diabetes and the risk of pancreatic cancer. New England Journal of Medicine. 1994;331.2:81–84. doi: 10.1056/NEJM199407143310203. [DOI] [PubMed] [Google Scholar]
- 34.Huxley R, et al. Type-II diabetes and pancreatic cancer: a meta-analysis of 36 studies. British journal of cancer. 2005;92.11:2076. doi: 10.1038/sj.bjc.6602619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang Jiayue, et al. Insulin promotes proliferation and fibrosing responses in activated pancreatic stellate cells. American Journal of Physiology-Gastrointestinal and Liver Physiology. 2016;311.4:G675–G687. doi: 10.1152/ajpgi.00251.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Apte Minoti V, et al. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144.6:1210–1219. doi: 10.1053/j.gastro.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Genlai, et al. Cholecystectomy and risk of pancreatic cancer: a meta-analysis of observational studies. Cancer Causes & Control. 2012;23.1:59–67. doi: 10.1007/s10552-011-9856-y. [DOI] [PubMed] [Google Scholar]
- 38.Fan Yonggang, et al. Increased risk of pancreatic cancer related to gallstones and cholecystectomy: a systematic review and meta-analysis. Pancreas. 2016;45.4:503–509. doi: 10.1097/MPA.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 39.Schernhammer E. S, et al. Gallstones, cholecystectomy, and the risk for developing pancreatic cancer. British journal of cancer. 2002;86.7:1081. doi: 10.1038/sj.bjc.6600193. [DOI] [PMC free article] [PubMed] [Google Scholar]