Abstract
Background: Contrast enhanced Computed Tomography (CCT) is the most used imaging test to investigate acute abdominal clinical conditions, because of its high sensitivity and specificity. It is mandatory to make a correct and prompt diagnosis when life threatening abdominal diseases as mesenteric ischemia are suspected. Contrast medium administration was linked to acute renal failure, therefore radiologist often prefer to perform CCT without contrast in patients needing to undergo the exam with increased serum creatinine. The aim of the review was to focus on the incidence of contrast induced nephropathy in patients presenting non-traumatic acute abdominal clinical conditions, who underwent CCT with intravenous contrast agent administration in emergency setting.Materials and Methods: The systematic review protocol was guided by the Preferred Reporting Items for Systematic Reviews and Meta-analyses Protocol (PRISMA-P). Quality of the evidence will be evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology. Results: The strongest currently available evidence on the incidence of post-contrast acute kidney injury (AKI) following intravenous contrast agent administration consists in a meta-analysis of observational studies. Data extracted from meta-analyses demonstrate that, compared with non-contrast CT, CCT was not significantly associated with AKI. Moreover, the risk of AKI (RR=0.79; 95% confidence interval [CI]: 0.62, 1.02; P=.07), death (RR=0.95; 95% CI: 0.55, 1.67; P=.87), and dialysis (RR=0.88; 95% CI: 0.23, 3.43; P=.85) is similar, compared with the risk of AKI in the non-contrast medium group. Furthermore, intravenous low-osmolality iodinated contrast material is a nephrotoxic risk factor, but not in patients with a stable SCr level less than 1.5 mg/dL, therefore many factors other than contrast material could affect PC-AKI rates. Discussion and conclusions: The benefits of diagnostic information gained from contrast enhanced TC in assessing AA are fundamental in some clinical scenarios. The risk of contrast induced nephropathy (CIN) is negligible in patients with normal renal function but the incidence appears to rise to as high as 25% in patients with pre-existing renal impairment or in the presence of risk factors such as diabetes, advanced age, vascular disease and use of certain concurrent medications. The incidence of CIN/AKI after intravenous contrast administration is very low in general population. Radiologists and referring physicians should be familiar with the risk factors for renal disease, CIN and preventing measures. (www.actabiomedica.it)
Keywords: contrast induced nephropathy, acute abdomen, Emergency Department, acute kidney injury, prevention strategy
Background
In clinical practice, physical examination of the patient, plasmatic dosage of C-Reactive Protein and White Blood Cells count alone are not always sufficient to discriminate the grade of urgency of abdominal diseases in patients admitted for acute abdominal pain in emergency surgery department (1).
Contrast enhanced Computed Tomography (CCT) is the most used imaging test to investigate acute abdomen (AA) because of its high sensitivity and specificity (1).
Contrast medium (CM) administration was linked to acute renal failure, above all in patients undergoing primary angioplasty or coronary procedures with higher dose of CM than in patients who received CCT with intravascular contrast medium in ED (2-4).
In literature, several studies showed that CM administration can lead to contrast-induced nephropathy (CIN) especially in “high risk” patients including the elderly and patients with chronic renal impairment, diabetes, congestive heart failure and anemia (2-8).
The American College of Radiology (ACR) stated that CIN is a real, albeit rare, entity and recommends more restricted use of intravenous contrast material among “high-risk” patients (9).
The “fear” of CIN makes radiologists reluctantto submit a patient to CCT with intravenous contrast administration if serum creatinine is minimally increased even in emergency situations, when making diagnosis is the priority to promptly manage the patient presenting with AA, and CCT is mandatory, especially when clinical findings and laboratory results are inconclusive.
Post-contrast induced Acute Kidney Injury (PC-AKI) is a general term used to describe a sudden deterioration in renal function that occurs within 48 hours after intra-venous administration of iodinated contrast agent (9).
PC-AKI is a correlative diagnosis and may occur regardless of whether CM was the cause of the deterioration (9).
CIN is a specific term used to describe a sudden deterioration in renal function that is caused by the intra-venous administration of iodinated CM; therefore, CIN is a subgroup PC-AKI (9).
CIN is considered the development of AKI after the administration of radiographic CM in the absence of other identifiable causes and is widely accepted as the third most common cause of hospital-acquired AKI; it occurs above all in patients undergoing primary angioplasty or coronary procedures which require intra-arterial high dose of CM than in patients who underwent to CCT with intravenous CM in emergency surgery department (2, 3, 9).
The incidence of CIN in the general population ranges from 0.6% to 2.3%, but, when focusing on specific high-risk patients, the incidence can increase to more than 40% (5-7, 9).
The precise pathophysiological mechanism of CIN is not entirely understood. The leading theories hypothesize that it results from hypoxic injury to the renal tubules induced by renal vasoconstriction or by direct cytotoxic effects of CM (9); alternatively, some experts have argued that PC-AKI is caused by coexisting risk factors and is only coincidentally related to CM, especially when administered intravenously (10, 11).
The osmolality and dose of CM are key factors determining its renal tolerability (12, 13).
Besides, CIN is reported to be a self-limited phenomenon: serum creatinine typically increases over 1 to 3 days, peaks at 4 to 5 days, and returns to baseline in 7 to 14 days. More severe CIN may be associated with oliguria and a delayed peak in serum creatinine and a slower return to steady state, which may remain above baseline values. In a small subset of patients, temporary or permanent dialysis may be required (14).
High risk patients are considered those with preexisting renal dysfunction, acute or chronic renal failure, diabetes mellitus, congestive heart failure because of poor renal perfusion from atherosclerosis, chronic hypertension, or diminished cardiac output (7).
Risk factors for CIN (table 1) are classified in:
Table 1.
Risk factors for contrast induced nephropathy
| Risk Factors for CIN non modifiable | Risk factors for CIN modifiable |
|
|
- Patient related risk factors, divided into major (pre-existing renal disease and diabetes), and minor (advanced age, female gender, hypertension and nephrotoxic drugs).
- Contrast related risk factors: related to concentration of CM, volume administered, repeated contrast administration within 24-48 hours (7-8).
In this “potentially” at risk patients, usually radiologists prefer to perform a CT without contrast, often useless to make diagnosis or eliminate a suspected surgical abdominal disease.
Acute abdominal conditions may be particularly challenging in the elderly, as they have a diminished sensorium, which allows the pathology to advance to an emergency state before developing symptoms. The most frequent disorders that occurs in elderly patients are: mesenteric ischemia, intestinal perforation by colon rectal cancer, diverticulitis and cholecystitis (15).
CT with intravenous contrast provides anatomical details and has high diagnostic specificity (15).
A clear imaging is mandatory to guide emergency surgery in differential diagnosis with the aim to plan the definitive management of the patient, choosing the best surgical approach.
ACR established that CCT has superior diagnostic performance compared to un-enhanced CT and that failure to diagnose an important clinical entity carries its own risk (9).
Preventive measures such as pre-hydration can significantly decrease the risk of CIN in low and high-risk patients.
In emergency setting, the timing of diagnosis is fundamental to obtain the best outcomes, decreasing morbidity and mortality rates. In patients presenting AA, CCT is mandatory to differentiate between surgical and non-surgical conditions.
The fear of CI-AKI is one of the most frequent reason why CM is withheld from patients undergoing computed tomography and thus frequently compromises the diagnostic information gained from CT and delays treatment.
We decided to perform a systematic literature review about contrast-induced nephropathy aimed to quantify the “real” risk of developing CIN and/or AKI for patients presenting with non-traumatic AA after CCT with intravenous contrast administration and to understand if the risk of CIN can be considered a strong contraindication to perform CCT and to report available strategies, recommendations and guidelines to decrease this risk.
Materials and methods
The systematic review protocol was guided by the Preferred Reporting Items for Systematic Reviews and Meta-analyses Protocol (PRISMA-P) (20). The methodological approach included the development of selection criteria, definition of search strategies, assessment of study quality, and extraction of relevant data. Quality of the evidence will be evaluated using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology (21).
Literature search strategy
A literature search was performed on the following online databases: MEDLINE (through PubMed), EMBASE and Cochrane Libraries using the combination of the following keywords and/or MeSH terms: “contrast induced nephropathy” OR “contrast enhanced tomography” OR “acute kidney injury” OR “contrast agent” AND “non traumatic abdominal pain” OR “acute abdomen” OR “emergency surgery” and “”emergency department”.
In addition, the reference lists from the eligible studies and relevant systematic review articles were cross-checked to identify additional records. The literature search was performed on March 2018 and was restricted to articles published since 2000. Only studies were written in English and met the selection criteria were reviewed.
Study selection
The title and abstract from all references were screened independently and blindly by two reviewers using the pre-defined inclusion and exclusion criteria. Full-text copies of relevant reports were then obtained and reviewed independently by two reviewers for final inclusion decision. Two independent reviewers extracted data from included studies using a standardized data extraction form. Disagreements were resolved by consensus and by consultation with a third independent reviewer, when needed.
Study inclusion criteria
The study selection criteria were defined before initiating data collection for proper identification of studies eligible for the analysis. All studies in which the primary objective was to describe the role of contrast enhanced tomography in the management of AA, the incidence of CIN and related implication in emergency department in the diagnostic pathway of AA in patients aged ≥18 years, were retrieved and analyzed.
Types of study
Observational and prospective studies, meta-analyses, randomized controlled trial and epidemiological studies were considered eligible for inclusion in this systematic review. Conference abstracts, letters, retrospective studies, case reports and commentaries were not considered.
Exclusion criteria
The search was limited to studies published in English, analyzing data from a population of study aged ≥18 years.
Data extraction
Data extracted from the included studies were processed for qualitative and possibly quantitative analyses.
Quality of evidence
The Grading of Recommendations Assessment Development and Evaluation (GRADE) system (21-22) was used to enable consistent judgment of the “body of evidence” and was included in the systematic review. GRADE specifies four categories: high, moderate, low, and very low. In the context of a systematic review, the quality of evidence reflects the confidence that the estimates of the effect are correct.
Results
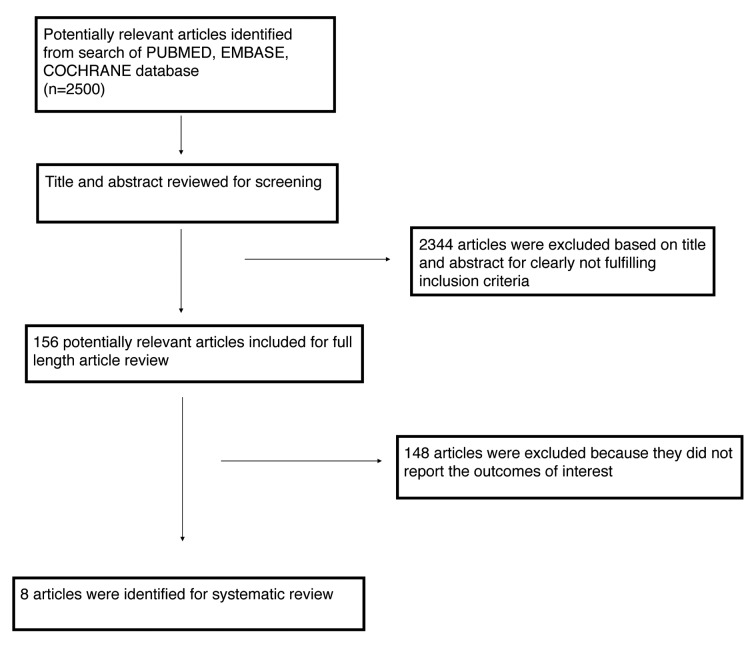
Out of the 2500 articles initially identified, 8 articles (table 2) met the inclusion criteria and were selected for the analysis.
Table 2.
Studies included in the systematic review
| References | Type of study | Numb. of patients | Setting | Area of CCT | Incidence AKI in CCT group (%) | Incidence AKI in non-CCT group (%) |
| Mc Donald 2014 (12) | Retrospective matched | 21346 | multiple | any | 4,8 | 5,1 |
| Davenport 2013 (63) | Retrospective matched | 20242 | inpatient | any | 8,3 | 8,6 |
| Hinson 2017 (40) | Retrospective matched | 12700 | ED | any | 10,6 | 10,2 |
| Sonhaye 2015 (41) | Prospective observational | 1292 | ED | any | 3,4 | 1,8 |
| Haveman 2006 (38) | Retrospective | 340 | ICU | Abdo/pelvis | 2,2 | NR |
| Heller 2016 | Retrospective | 7863 | ED | any | 8,6 | 9,6 |
| Kidoh 2013 (39) | Retrospective | 470 | multiple | abdo-pelvis | 9,1 | 8,3 |
| Tremblay 2005 (37) | Retrospective | 95 | ED/trauma | any | 3,6 | 15,4 |
ED=emergency department; ICU=intensive care unit; NR=non-reported; AKI= acute kidney injury; CCT=contrast enhanced to¬mography
The flow chart of study identification and inclusion/exclusion process is shown in Figure 1.
Figure 1.
Search strategy according to PRISMA
Limitations
This systematic review will address both CIN and PC-AKI because in literature the 2 terms we can’t separate CIN from PC-AKI even if these terms are not interchangeable.
• CIN/PC-AKI: definition, incidence, risk factors.
CIN is broadly defined as an absolute (≥0.5 mg/dl) or relative (≥25%) increase in serum creatinine compared with baseline after exposure to intravascular CM when alternative explanations for renal impairment have been excluded. (23).
The CIN Consensus Panel recommended using a relative increase in serum creatinine to define CIN given that this definition is independent of baseline renal function (24).
CIN typically develops within 24-72 hours post-exposure to contrast medium, with renal function returning to baseline level in two weeks. The overall incidence of CIN in the general population is <2%. In high-risk patients, including the elderly population and patients with chronic renal impairment, diabetes, congestive heart failure and anemia, the incidence of CIN is much higher (≥20%) (25).
The serum creatinine level returns within 1 to 3 weeks to baseline or a new baseline on serial follow up and contrast induced nephropathy is believed to resolve within 3 weeks. Sometimes it progresses to severe renal failure (serum creatinine >=3.0mg/dL) needing for dialysis and death (24-25).
The pathophysiological mechanisms of CIN is still unclear; iodine contrast in animal model has toxic effect for renal epithelial and endothelial cells and can increase renal tubular viscosity in vitro resulting in tubular obstruction and elevated interstitial pressure (24-25-26).
The term AKI was introduced to define abrupt damage to the kidneys whether permanent leading to acute then chronic renal failure, or temporary leading to short-term compromise in renal function. This damage is manifested by abnormal fluid balance, acid-base disturbances, and electrolyte imbalances (25). Acute renal failure is associated with high mortality rate (40-90%) (9-25).
PC-AKI is commonly defined as a rise in blood urea nitrogen, serum creatinine, or a decline in estimated glomerular filtration rate (eGFR) occurring in a narrow time window - typically 24-72 hours - after administration of iodinated CM (9).
The Acute Kidney Injury Network (AKIN) defines AKI if at least one out of of three conditions is met: (a) an absolute increase in serum creatinine levels by ≥0.3 mg/dL from baseline, (b) a relative increase in serum creatinine by ≥50% from baseline, or (c) a urine output reduced to ≤0.5 mL/kg/hour for at least 6 hours (24-26).
Urine output is not routinely measured in non-critically ill patients, consequently the first two of the 3 criteria listed above have been used to define AKI in recent studies on PC-AKI with intravenous CM (9, 10, 25).
The RIFLE (risk, injury, failure, loss, and end-stage renal disease) is a classification system which defines different stages of AKI based on changes in serum creatinine or eGFR and urine output.
It was developed by the Acute Dialysis Quality Initiative group in 2004 and introduced as measures for grading the level of kidney damage, the outcome of this damage, the interventions necessary for each class, and thus the mortality associated with this damage upon the administration of nephrotoxic drugs or contrast medium and classify patients (27, 28).
AKI reported incidence rates vary greatly depending on the patient population, the nature of the radiologic procedure, and definition of AKI. It can range from 0-11% (27).
PC-AKI is generally estimated to occur in approximately 5-15% of patients after contrast injection. A decreased baseline renal function has consistently been found to be a strong predictor of PC-AKI. Some comorbidities such as diabetes, proteinuria, hypertension, and dehydration, and nephrotoxic co-medications further increase the risk of AKI following angiographic procedures (3-5).
In clinical practice, there are different types of contrast agents with different severity of their side effects, used for varying diagnostic studies, the oldest of which is the ionic contrast agent that is also known as the first generation of CM. It is a hyper-osmolar agent that produces good images but causes more renal damage. Since the 1990s, low-osmolar CM (2 to 3 times plasma osmolality) have been the standard of care for intravascular injection. The second generation of the CM is a non-ionic agent that has lower osmolality in comparison to the first generation. The newest agent is a non-ionic iso-osmolar contrast agent; it is isotonic to plasma and it has an even lower osmolality than the second generation, thus associated with less incidence of CIN. Iodixanol is the only iso-osmolar CM available for intravascular injection. in patients with intra-arterial administration and renal insufficiency, iodixanol is associated with a reduced risk of CIN compared with iohexol (low osmolar contrast agent), whereas no significant difference between iodixanol and other low osmolar CM could be found (30, 31).
The risk of post-PC-AKI has been shown to be significantly higher with high-osmolar CM compared to low-osmolar CM, but the iso-osmolar CM iodixanol has had conflicting results in further reducing risk even in vulnerable patients (12, 13, 31-33). In diabetic population, iodixanol is not associated with a significant reduction of CIN risk. Iodixanol is associated with a reduced risk of CIN compared with iohexol, whereas no significant difference between iodixanol and other low osmolar CM could be found (31).
Several studies have found evidence of a dose-dependent risk increasing with CM volume administered during the procedure (3, 4, 32).
In their meta-analysis of controlled studies of intravenous CM, McDonald et al. (32) report an overall AKI rate of 6.5% in the non-contrast CT group averaged over varying definitions of AKI. Davenport et al. found AKI incidence rates in the non-contrast group of 8.6% and 12.4% based on the AKIN and more traditional CIN criteria, respectively (33). Using an absolute increase of serum creatinine 0.5 mg/dL from baseline to define AKI, McDonald et al. (13) found AKI rates in the non-contrast control group of 4, 9, and 13 % for patients with a baseline creatinine of <1.5, 1.5-2, and ≥2 mg/dL, respectively. These background rates of AKI need to be considered when assessing whether CM exposure increases the frequency of AKI.
The incidence of AKI is substantially higher following coronary angiographic procedures than following contrast-enhanced CT because the patient population undergoing coronary procedures typically has more advanced vascular disease and a higher rate of hemodynamic compromise and is thus more predisposed for AKI than the average population undergoing contrast-enhanced CT (12, 32, 33). Furthermore, in CCT, the contrast agent dose administered is lower than in angiographic procedures (3, 4, 12, 13, 32, 33).
The site of CM injection (intra-arterial versus intravenous) may also have a direct influence possibly due to a higher initial concentration of CM in the renal vessels, since it has been demonstrated for aortography that the risk of AKI is greater if the CM is injected immediately proximal to the renal arteries (3, 4, 23).
Furthermore, coronary angiography leads a variety of iatrogenic risk factors for AKI, which may increase the risk of PC-AKI and are unrelated to the CM itself. It is well known that cholesterol emboli and microemboli from scraping of aortic plaque occur in a high percentage of patients during invasive angiographic procedures. Iatrogenic (micro-) embolization of renal parenchyma may contribute to AKI following angiographic procedures. Other potential complications of coronary angiography including arrhythmias, hemorrhage, myocardial infarction, or aortic dissection can all lead to hypotension or reduced cardiac output and thus contribute to post-procedural AKI which may be falsely attributed to the CM (23).
In the recent literature, CIN is reported to be a self-limited phenomenon but concern remains that intravenous iodinated contrast material exposure can lead to irreversible nephrotoxicity. Although self-limiting in most cases, PC-AKI carries a risk of more permanent renal insufficiency, dialysis, and death. Levy and colleagues retrospectively compared the outcomes of 174 patients who developed AKI after CM administration for various procedures with matched controls who received CM but did not develop AKI (34). This study found a significantly increased risk of in-hospital mortality (34% versus 7%) for those patients who developed post-AKI. However, it has been pointed out that AKI in most of these patients was probably not due to contrast material but to other comorbid and iatrogenic risk factors (23, 35).
Most available evidence on the outcome of PC-AKI relates to intra-arterial CM administration for cardiac catheterization or other angiographic procedures. In a retrospective study of patients with preexisting renal insufficiency undergoing percutaneous coronary interventions, Gruberg and colleagues found a significantly increased risk of in-hospital mortality (15% versus 5%) for those patients who had a ≥25% increase in serum creatinine within 48 hours following coronary procedures compared to those who did not (35).
The incidence of AKI requiring dialysis after percutaneous coronary interventions is <1% in most published cohorts (13, 32, 33).
An adverse prognostic value of PC-AKI has also been demonstrated for longer-term mortality after percutaneous coronary interventions. In summary, the literature has consistently demonstrated that patients who develop postcontrast AKI after catheterization procedures have a significantly higher risk of death during the hospital stay (34, 35).
Unlike intra-arterial CM for cardiac catheterization or other angiographic procedures, the risk of adverse outcome from post-contrast administration is lower for intravenously administered CM. In an analysis of 6 prospective studies including >1,000 total patients undergoing contrast-enhanced CT with an overall PC-AKI rate of 5.1%, there was no case of dialysis or death resulting from PC-AKI (36).
• Is intravenous contrast administration for CCT safe in emergency?
Tremblay et al (37) carried out a retrospective analysis of data from 95 trauma patients to assess if the benefits outweigh the risks of intravenous contrast in trauma patients who present with an elevated serum creatinine. The incidence of AKI after CCT with intravenous contrast administration reported was 3.6% versus 15.4% in the control group. This result suggested that the benefits outweigh the risks for proceeding with iv contrast in trauma patients with an elevated creatinine.
Haveman et al (38) in a previous retrospective analysis investigated the incidence of AKI in ICU patients and concluded that CT with modern contrast was associated with a very low incidence of nephropathy in predominantly non-diabetic surgical ICU patients and that intravenous contrast should only rarely be withheld in these patients.
Furthermore, Kidoh et al (39) demonstrated that there were no significant differences in the incidence of AKI between the low-contrast dose and unenhanced CT protocols (9.1% vs 8.3%, P=0.77) in patients with renal insufficiency.
Sonhaye et al (40) confirmed these data with a prospective review of 620 patients admitted to the emergency room undergone CCT using intravenous contrast and 672 patients who received CT without intravenous contrast. Among the patients who received intravenous contrast, 3% developed CIN during their admission. At discharge, no patient had continued renal impairment. The multivariate analysis of all patients who had serial creatinine levels (including those who did not receive any contrast load) shows no increased risk for acute kidney injury associated intravenous contrast (odds ratio=0.619, p value=0.886); only diabetes remains an independent risk factor of acute kidney injury (odds ratio=6.26, p value=0.031).
The fear of causing or exacerbating renal damage should not be a reason for with-holding contrast studies.
• The importance of CT Scan in emergency surgery
Acute abdominal pain is a common condition of admission in ED. The term “acute abdomen” defines a clinical syndrome characterized by the sudden onset of severe abdominal pain requiring emergency medical or surgical treatment.
In an analysis of more than 10,000 patients presenting with acute abdominal pain the etiology could not be determined in one-third of these cases; of those patients in whom a diagnosis was made, 28% had appendicitis, 9.7% acute cholecystitis, 4.1% small bowel obstruction, 4% acute gynecological disease, 2.9% acute pancreatitis, 2.9% acute renal colic, 2.5% perforated peptic ulcer, and 1.5% acute diverticulitis (42).
Various potentially life-threatening diseases can cause acute abdominal pain; thus a rapid and accurate diagnosis is essential to reduce morbidity and mortality.
Physical and laboratory examinations are often non-specific, and the clinical presentation of many entities overlaps. Therefore, diagnostic and efficient imaging evaluations are indispensable.
Computed tomography (CT) has gained widespread acceptance as the first-line imaging modality in patients presenting with acute abdominal pain (1, 43).
Although clinical data, physical examination and laboratory tests guide the clinician in diagnosis, they are not sufficient to reach definitive diagnosis especially if pain spreads throughout the abdomen rather than involving a specific region or abdominal quadrant, particularly among the elderly, obese and immunocompromised patients.
The best diagnostic imaging test in these clinical scenarios is contrast enhanced multidetector CT (42-44).
In a cohort study comparing ultrasound and CT in 1021 consecutive patients, CT was significantly more sensitive than ultrasound (89% vs 70%, p<0.001), although the approach achieving the highest sensitivity was a diagnostic strategy combining an initial ultrasound scan, followed by CT, only when ultrasound examination yielded negative or inconclusive findings (1).
To compare the effect of an initial early CT examination versus standard practice on the length of hospital stay, diagnostic accuracy, and mortality of adults presenting with AA, Sala et al. conducted a randomized controlled trial involving 205 adults presenting with acute abdominal pain; the study showed that early abdominal CT in patients with acute abdominal pain improves diagnostic certainty, even if it does not reduce the length of hospital stay and 6 month mortality (45).
Tsushima et al. prospectively analyzed data about 125 adult patients presenting with acute abdominal pain to determine the value of CT on the diagnosis and treatment plan of these patients; authors concluded that CCT frequently changed the initial clinical diagnoses, increasing the diagnostic yield (46).
Catena et al focused on the diagnostic impact of CT scans in abdominal trauma and in non-traumatic acute abdomen. The aim was to guide emergency surgeons and physicians in the choice of the best radiological exam taking in account sensitivity and specificity of CCT for the different diseases underlying acute abdominal pain (47).
Moreover, in emergency setting time is the essence for survival and to obtain decreasing in morbidity and mortality rates related to the AA.
CCT with intravenous contrast administration in patients presenting with non-traumatic AA has the advantage to give information about the presence and feature of fluids or abscesses in the abdominal cavity, about the cause of small or large bowel obstruction, it allows to detect the site of a gastrointestinal perforation and to check vascular changes in the small or large bowel wall.
CCT is mandatory to make diagnosis in some life-threatening gastrointestinal emergencies as in case of acute mesenteric ischemia, gastrointestinal perforation, obstruction, diverticulitis and to early manage the patient with the best operative or non-operative strategy.
• How to prevent CIN
CIN is not as frequent as it was believed in the past few years in general population, but it occurs in high risk patients (23). This suggests using all precautions that may prevent contrast media-nephrotoxicity in all patients, especially in high-risk patients.
All the available studies about CIN prevention strategies suggest to identify patients at risk for developing CIN before administering CM. Methods to identify patients at risk include use of patient questionnaire, a review of the patient’s complete medical history looking for comorbidities as hypertension, renal disease, dyslipidemia, hyperuricemia, diabetes, heart failure, myeloma, treatment with nephrotoxic drugs, and measurement of serum creatinine before CM administration (table 1).
The absence of risk factors for renal disease effectively eliminates the likelihood of a patient having renal impairment (48).
Renal function is usually assessed with a serum creatinine (sCR), which is used in either the Cockcroft-Gault or modification of diet on renal disease formula to estimate glomerular filtration rate (eGFR). The risk of CIN increases as the estimated glomerular filtration rate falls, particularly below 60 ml/min (48-50).
In all patients admitted with AA, suspected to have an acute surgical pathology, it is suggested to:
- discontinue all potentially nephrotoxic drugs (aminoglycosides, vancomycin, amphotericin B, dipyridamole, metformin, and nonsteroidal anti-inflammatory drugs). Special attention should be paid to the use of metformin, because of its prevalent renal excretion and its tendency to cause a severe lactic acidosis. Thus, this medication should be discontinued 12 hours before the administration of contrast agent and not be resumed until at least 36 hours after the procedure, or even longer if the serum creatinine has not returned to baseline (50).
- Provide as soon as possible an adequate hydration of the patient. Iso-tonic fluids were significantly less risky than half iso-tonic fluids for developing CIN (51).
In high-risk patients it may be useful to implement the i.v. infusion of 0.9% saline solution at a rate of about 3 mL/kg/hour, 1 hour before and for the 6 hours after the procedure, for procedure scheduled the same day. At least 300-500 mL of IV hydration should be administrated before contrast is given (10).
The European Renal Best Practice (55) recommends volume expansion with either isotonic sodium chloride or sodium bicarbonate solutions, rather than no volume expansion, in patients at increased risk for CIN.
All potentially surgical patients should be hydrated by IV fluids since admission.
Other agents are used to prevent CIN but they should not be considered as a substitute for hydration; the most used are N-acetylcysteine (NAC), beta blockers such as nebivolol, adenosine antagonists, simvastatin, furosemide, dopamine and dopamine agonists, recombinant human erythropoietin (55-59).
Most of these agents has been studied in patients undergone intra-arterial CM administration.
There is no evidence of outcomes regarding the application of short-term prophylaxis protocols for contrast-induced nephropathy (CIN), that may be most feasibly convenient, in emergency settings.
The European Renal Best Practice does “not recommend using prophylactic intermittent hemodialysis or hemofiltration for the purpose of prevention of CIN (55).
In preventing CIN, the radiologist has a major role, in choosing to administrate the least nephrotoxic iodinated agent as iodixanol (iso-osmolar CM) and iopamidol (low-osmolar CM) at the lowest dosage possible that would produce good imaging and enable diagnosis of the underlying cause of AA (58).
High doses of contrast agents are required in coronary angiography and percutaneous coronary interventions. For these procedures some formulas have been suggested to calculate the dosage that is least dangerous for renal function: (a) Cigarroa’s formula: 5 mL of contrast per kg b.w./serum creatinine (mg/dL) with maximum dose acceptable of 300 mL for diagnostic coronary arteriography; (b) Laskey’s formula: volume of contrast to eGFR ratio with a cut-off point of the ratio at 3.7 for percutaneous coronary intervention (58).
Discussion
CIN and CCT with intravenous contrast administration: dogma or reality?
The strongest currently available evidence on the incidence of PC-AKI following intravenous CM administration consists in a meta-analysis of observational studies.
Twenty-eight studies involving 107,335 participants were included in the final analysis.
Included articles specifically compared rates of renal insufficiency, need for renal replacement therapy, or mortality in patients who received intravenous contrast versus those who received no contrast.
Meta-analysis demonstrated that, compared with non-contrast CT, contrast-enhanced CT was not significantly associated with either AKI (odds ratio [OR] 0.94; 95% confidence interval [CI] 0.83 to 1.07), need for renal replacement therapy (OR 0.83; 95% CI 0.59 to 1.16), or all-cause mortality (OR 1.0; 95% CI 0.73 to 1.36).
Therefore, given similar frequencies of AKI in patients receiving non-contrast CT, other patient- and illness-level factors, rather than the use of contrast material, likely contribute to the development of AKI.
Before that study, another meta-analysis of controlled studies demonstrated a similar incidence of AKI, dialysis, and death between patients who received CM and control group.
This meta-analysis was performed by Mc Donald et al. (32) to examine the incidence of AKI and other outcomes in patients exposed to intravenous CM compared with patients who underwent an imaging examination without contrast medium or were otherwise unexposed (control group).
Thirteen non-randomized studies were included for meta-analysis with a total of 25,950. In the group that received contrast medium (contrast medium group), risk of AKI (RR=0.79; 95% confidence interval [CI]: 0.62, 1.02; P=.07), death (RR=0.95; 95% CI: 0.55, 1.67; P=.87), and dialysis (RR=0.88; 95% CI: 0.23, 3.43; P=.85) was similar, compared with the risk of AKI in the non-contrast medium group. This pattern was observed regardless of i.v. contrast medium type, diagnostic criteria for AKI, or whether patients had diabetes mellitus or renal insufficiency.
It is important to remind that all controlled studies included in the meta-analysis had a nonrandomized study design, which inevitably makes them vulnerable to selection bias, since patients perceived to be at risk for AKI are more likely to receive non-contrast CT examinations.
In the following studies, statistical methods of propensity score adjustment were used to neutralize differences in AKI risk factors between the contrast-enhanced and the non-contrast CT group and thus neutralize the effects of selection bias. McDonald et al. (13) found that after propensity matching there was no significant difference in AKI incidence between contrast-enhanced and non-contrast group. Subgroup analysis was performed for patients with a baseline serum creatinine of <1.5, 1.5-2, and ≥2 mg/dL, and no significant difference between exposed and nonexposed patients was found in either group.
Mc Donald and al. (64) examined the association of intravenous iodinated contrast material administration with the subsequent development of PC-AKI, emergent dialysis, and short-term mortality using a propensity score-adjusted analysis of a cohort of intensive care unit (ICU) patients who underwent CT examination and confirmed that intravenous contrast material administration was not associated with an increased risk of AKI, emergent dialysis, and short-term mortality in ICU patients with pre-CT eGFR >45. An increased risk of dialysis was observed in patients with pre-CT eGFR ≤45.
Hinson and al. performed a single-center retrospective cohort analysis with the aim to determine whether intravenous contrast administration for computed tomography was independently associated with increased risk for AKI and adverse clinical outcomes. They reported that contrast administration was not associated with increased incidence of AKI and was not associated with increased incidence of chronic kidney disease, dialysis, or renal transplant at 6 months. They demonstrated also that clinicians were less likely to prescribe contrast to patients with decreased renal function and more likely to prescribe intravenous fluids if contrast was administered (65).
Analyzing all data available about PC-AKI after intravenous administration of CM, evidence strongly suggests that the risk caused by CM is much smaller than previously thought based on noncontrolled studies. For patients with a baseline creatinine of <1.5 mg/dL and an eGFR of ≥45 mL/min/1.73 m2 the risk of PC-AKI is likely to be nonexistent.
In emergency setting the first aim is making diagnosis.
Acute abdominal pain is one of the most common conditions that calls for prompt diagnosis and early treatment. Having the correct diagnosis is the essential premise to set up the best management for the patient. In patients who are acutely ill, delays in imaging may adversely affect patient care.
The “golden hour” concept can be applied also to the evaluation of AA patients: rapid intervention improves the outcomes. The relationship between timing and mortality is well known in literature and as CCT in evaluation of trauma patients is mandatory both in hemodynamically stable patients and in unstable patients after adequate resuscitative maneuvers to assess and treat all the lesions.
CCT with intravenous administration of CM is the most used imaging technique to investigate life threatening causes underlying AA and it is mandatory when intestinal ischemia, small or large bowel obstruction, diverticulitis or peritonitis are suspected diseases.
The risk of CIN is negligible in patients with normal renal function but the incidence appears to rise to as high as 25% in patients with pre-existing renal impairment or in the presence of risk factors such as diabetes, advanced age, vascular disease and use of certain concurrent medications (66, 67).
Patients admitted for non-traumatic AA in emergency surgery department are often elderly, dehydrated and present with hypotension, acute or chronic nephropathy or acute pathologies that independently affect the risk for developing CIN; this are widespread conditions which cannot limit diagnostic CT in emergency.
At admission, before beginning clinical evaluation, laboratory and imaging exams, the patient with AA should:
- be hydrated as early as possible;
- stop medications as anticoagulants and nephrotoxic drugs.
Delaying inpatient CT scans worsens patient outcomes and increases morbidity and mortality rates.
Explorative laparoscopy or laparotomy are not the best diagnostic tools in case of high-risk patients.
The radiologist should not refuse to perform CCT if it is necessary for diagnosis, even in patients at high risk for CIN.
Preventing measures to decrease the risk of CIN and improve outcomes should be applied.
The incidence reported of AKI in patients undergoing contrast IV administration for CCT is not high as thought before.
Conclusions
The benefits of diagnostic information gained from contrast enhanced TC in assessing AA are fundamental in some clinical scenarios. Radiologists and referring physicians should be familiar with the risk factors for renal disease and CIN. The incidence of CIN/AKI after intravenous contrast administration is very low in general population.
Since volume expansion is the only proven preventive strategy, in emergency setting it is advised to start volume expansion as early as possible before contrast medium administration.
References
- 1.Gans S, L, Pols M, A, Stoker J, Boermeester M. A: Guideline for the Diagnostic Pathway in Patients with Acute Abdominal Pain. Dig Surg. 2015;32:23–31. doi: 10.1159/000371583. doi: 10.1159/000371583. [DOI] [PubMed] [Google Scholar]
- 2.Marenzi G, Lauri G, Assanelli E, et al. Contrast-induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–5. doi: 10.1016/j.jacc.2004.07.043. [DOI] [PubMed] [Google Scholar]
- 3.Maioli M, Toso A, Gallopin M, et al. Preprocedural score for risk of contrast-induced nephropathy in elective coronary angiography and intervention. J Cardiovasc Med (Hagerstown) 2010;11:444–9. doi: 10.2459/JCM.0b013e328335227c. doi: 10.2459/JCM.0b013e328335227c [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: development and initial validation. J Am Coll Cardiol. 2004;44:1393–9. doi: 10.1016/j.jacc.2004.06.068. doi: 10.1016/j.jacc.2004.06.068 [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Moore A, Dickerson E, Dillman JR, Vummidi D, Kershaw DB, Khalatbari S, Davenport MS. Incidence of nonconfounded post-computed tomography acute kidney injury in hospitalized patients with stable renal function receiving intravenous iodinated contrast material. Curr Probl Diagn Radiol. 2014 Sep-Oct;43(5):237–41. doi: 10.1067/j.cpradiol.2014.05.001. doi: 10.1067/j.cpradiol.2014.05.001. Epub 2014 Jun 6. PubMed PMID: 24909428. [DOI] [PubMed] [Google Scholar]
- 6.Huang MK, Hsu TF, Chiu YH, Chiang SC, Kao WF, Yen DH, Huang MS. Risk factors for acute kidney injury in the elderly undergoing contrast-enhanced computed tomography in the emergency department. J Chin Med Assoc. 2013 May;76(5):271–6. doi: 10.1016/j.jcma.2013.01.007. doi: 10.1016/j.jcma.2013.01.007. Epub 2013 Mar 21. PubMed PMID: 23683260. [DOI] [PubMed] [Google Scholar]
- 7.Feldkamp T, Kribben A. Contrast media induced nephropathy: definition, incidence, outcome, pathophysiology, risk factors and prevention. Minerva Med. 2008;99(2):177–96. [PubMed] [Google Scholar]
- 8.Parfrey PS1, Griffiths SM, Barrett BJ, Paul MD, Genge M, Withers J, Farid N, McManamon PJ. Contrast material-induced renal failure in patients with diabetes mellitus, renal insufficiency, or both. A prospective controlled study. N Engl J Med. 1989 Jan 19;320(3):143–9. doi: 10.1056/NEJM198901193200303. [DOI] [PubMed] [Google Scholar]
- 9. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf .
- 10.Owen, Richard J, et al. Canadian Association of Radiologists Consensus Guidelines for the Prevention of Contrast-Induced Nephropathy: Update 2012. Canadian Association of Radiologists Journal. 65(2):96–105. doi: 10.1016/j.carj.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Eng J, Wilson RF, Subramaniam RM, Zhang A, Suarez-Cuervo C, Turban S, et al. Comparative Effect of Contrast Media Type on the Incidence of Contrast-Induced Nephropathy: A Systematic Review and Meta-analysis. Ann Intern Med. 164:417–424. doi: 10.7326/M15-1402. doi: 10.7326/M15-1402. [DOI] [PubMed] [Google Scholar]
- 12.McDonald RJ, McDonald JS, Carter RE, Hartman RP, Katzberg RW, Kallmes DF, Williamson EE. Intravenous contrast material exposure is not an independent risk factor for dialysis or mortality. Radiology. 2014 Dec;273(3):714–25. doi: 10.1148/radiol.14132418. doi: 10.1148/radiol.14132418. Epub 2014 Sep 9. [DOI] [PubMed] [Google Scholar]
- 13.McDonald JS1, McDonald RJ, Carter RE, Katzberg RW, Kallmes DF, Williamson EE. Risk of intravenous contrast material-mediated acute kidney injury: a propensity score-matched study stratified by baseline-estimated glomerular filtration rate. Radiology. 2014 Apr;271(1):65–73. doi: 10.1148/radiol.13130775. doi: 10.1148/radiol.13130775. Epub 2014 Jan 16. [DOI] [PubMed] [Google Scholar]
- 14.Rear R, Bell RM, Hausenloy DJ. Contrast-induced nephropathy following angiography and cardiac interventions. Heart. 2016;102(8):638–648. doi: 10.1136/heartjnl-2014-306962. doi: 10.1136/heartjnl-2014-306962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reginelli A1, Russo A2, Pinto A3, Stanzione F4, Martiniello C5, Cappabianca S6, Brunese L7, Squillaci E8. The role of computed tomography in the preoperative assessment of gastrointestinal causes of acute abdomen in elderly patients. Int J Surg. 2014;12(Suppl 2):S181–S186. doi: 10.1016/j.ijsu.2014.08.345. doi: 10.1016/j.ijsu.2014.08.345. Epub 2014 Aug 23. [DOI] [PubMed] [Google Scholar]
- 16.Marc C. Heinrich, Lothar Häberle, Volker Müller, Werner Bautz, Michael Uder. Nephrotoxicity of Iso-osmolar Iodixanol Compared with Nonionic Low-osmolar Contrast Media: Meta-analysis of Randomized Controlled Trials Radiology. 2009;250(1):68–86. doi: 10.1148/radiol.2501080833. [DOI] [PubMed] [Google Scholar]
- 17.McCullough P, A, Brown J. R: Effects of Intra-Arterial and Intravenous Iso-Osmolar Contrast Medium (Iodixanol) on the Risk of Contrast-Induced Acute Kidney Injury: A Meta-Analysis. Cardiorenal Med. 2011;1:220–234. doi: 10.1159/000332384. doi: 10.1159/000332384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta RK, Bang TJ. Prevention of Contrast-Induced Nephropathy (CIN) in Interventional Radiology Practice. Semin Intervent Radiol. 2010;27(4):348–59. doi: 10.1055/s-0030-1267860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deek H1, Newton P2, Sheerin N3, Noureddine S4, Davidson PM5. Contrast media induced nephropathy: a literature review of the available evidence and recommendations for practice. Aust Crit Care. 2014 Nov;27(4):166–71. doi: 10.1016/j.aucc.2013.12.002. doi: 10.1016/j.aucc.2013.12.002. Epub 2014 Jan 23. [DOI] [PubMed] [Google Scholar]
- 20.Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. PRISMA-P Group. Syst Rev. 2015 Jan 1;4(1) doi: 10.1186/2046-4053-4-1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brozek JL, Akl EA;, Jaeschke R;, Lang DM;, Bossuyt P;, Glasziou P;, Helfand M;, Ueffing E;, Alonso-Coello P;, Meerpohl J;, Phillips B;, Horvath AR;, Bousquet J;, Guyatt GH;, Schünemann HJ. “Grading quality of evidence and strength of recommendations in clinical practice guidelines: part 2 of 3. The GRADE approach to grading quality of evidence about diagnostic tests and strategies”. Allergy. 2009;64(8):1109–16. doi: 10.1111/j.1398-9995.2009.02083.x. doi: 10.1111/j.1398-9995.2009.02083.x. PMID 19489757. [DOI] [PubMed] [Google Scholar]
- 22.Aguayo-Albasini JL1, Flores-Pastor B2, Soria-Aledo V1. GRADE system: classification of quality of evidence and strength of recommendation. Cir Esp. 2014 Feb;92(2):82–8. doi: 10.1016/j.ciresp.2013.08.002. doi: 10.1016/j.ciresp.2013.08.002. Epub 2013 Dec 20. [DOI] [PubMed] [Google Scholar]
- 23.Meinel FG, De Cecco CN, Schoepf UJ, Katzberg R. Contrast-induced acute kidney injury: definition, epidemiology, and outcome. Biomed Res Int. 2014;2014:859328. doi: 10.1155/2014/859328. doi: 10.1155/2014/859328. Epub 2014 Mar 10. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCullough PA, Stacul F, Becker CR, Adam A, Lameire N, Tumlin JA, Davidson CJ. CIN Consensus Working Panel. Contrast-Induced Nephropathy (CIN) Consensus Working Panel: executive summary. Rev Cardiovasc Med. 2006 Fall;7(4):177–97. [PubMed] [Google Scholar]
- 25.McCullough PA. Contrast-induced acute kidney injury. J Am Coll Cardiol. 2008 Apr 15;51(15):1419–28. doi: 10.1016/j.jacc.2007.12.035. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
- 26.Davenport MS, Cohan RH, Khalatbari S, Ellis JH. The challenges in assessing contrast-induced nephropathy: where are we now? AJR Am J Roentgenol. 2014 Apr;202(4):784–9. doi: 10.2214/AJR.13.11369. doi: 10.2214/AJR.13.11369. Review. [DOI] [PubMed] [Google Scholar]
- 27.Cruz DN1, Bagshaw SM, Ronco C, Ricci Z. Acute kidney injury: classification and staging. Contrib Nephrol. 2010;164:24–32. doi: 10.1159/000313717. doi: 10.1159/000313717. Epub 2010 Apr 20. [DOI] [PubMed] [Google Scholar]
- 28.Kim MH, Koh SO, Kim EJ, Cho JS, Na S-W. Incidence and outcome of contrast-associated acute kidney injury assessed with Risk, Injury, Failure, Loss, and End-stage kidney disease (RIFLE) criteria in critically ill patients of medical and surgical intensive care units: a retrospective study. BMC Anesthsiology. 2015;15:23. doi: 10.1186/s12871-015-0008-x. doi: 10.1186/s12871-015-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ülger F, Pehlivanlar Küçük M, Küçük AO, İlkaya NK, Murat N, Bilgiç B, Abanoz H. Evaluation of acute kidney injury (AKI) with RIFLE, AKIN, CK, and KDIGO in critically ill trauma patients. Eur J Trauma Emerg Surg. 2018 Aug;44(4):597–605. doi: 10.1007/s00068-017-0820-8. doi: 10.1007/s00068-017-0820-8. Epub 2017 Jul 17. [DOI] [PubMed] [Google Scholar]
- 30.Heinrich MC1, Häberle L, Müller V, Bautz W, Uder M. Nephrotoxicity of iso-osmolar iodixanol compared with nonionic low-osmolar contrast media: meta-analysis of randomized controlled trials. Radiology. 2009 Jan;250(1):68–86. doi: 10.1148/radiol.2501080833. doi: 10.1148/radiol.2501080833. [DOI] [PubMed] [Google Scholar]
- 31.Han XF1,2, Zhang XX2, Liu KM2, Tan H3, Zhang Q. Contrast-induced nephropathy in patients with diabetes mellitus between iso- and low-osmolar contrast media: A meta-analysis of full-text prospective, randomized controlled trials. PLoS One. 2018 Mar 20;13(3):e0194330. doi: 10.1371/journal.pone.0194330. doi: 10.1371/journal.pone.0194330. eCollection 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McDonald JS1, McDonald RJ, Comin J, Williamson EE, Katzberg RW, Murad MH, Kallmes DF. Frequency of acute kidney injury following intravenous contrast medium administration: a systematic review and meta-analysis. Radiology. 2013 Apr;267(1):119–28. doi: 10.1148/radiol.12121460. doi: 10.1148/radiol.12121460. Epub 2013 Jan 14. [DOI] [PubMed] [Google Scholar]
- 33.Davenport MS, Khalatbari S, Cohan RH, Dillman JR, Myles JD, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material: risk stratification by using estimated glomerular filtration rate. Radiology. 2013 Sep;268(3):719–28. doi: 10.1148/radiol.13122276. doi: 10.1148/radiol.13122276. Epub 2013 Apr 11. [DOI] [PubMed] [Google Scholar]
- 34.Levy EM, Viscoli CM, Horwitz RI. The effect of acute renal failure on mortality. A cohort analysis. JAMA. 1996 May 15;275(19):1489–94. [PubMed] [Google Scholar]
- 35.Gruberg L, Mintz GS, Mehran R, Gangas G, Lansky AJ, Kent KM, Pichard AD, Satler LF, Leon MB. The prognostic implications of further renal function deterioration within 48 h of interventional coronary procedures in patients with pre-existent chronic renal insufficiency. J Am Coll Cardiol. 2000 Nov 1;36(5):1542–8. doi: 10.1016/s0735-1097(00)00917-7. [DOI] [PubMed] [Google Scholar]
- 36.Katzberg RW, Lamba R. Contrast-induced nephropathy after intravenous administration: fact or fiction? Radiologic Clinics of North America. 2009;47(5):789–800. doi: 10.1016/j.rcl.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Tremblay LN, Tien H, Hamilton P, Brenneman FD, Rizoli SB, Sharkey PW, Chu P, Rozycki GS. Risk and benefit of intravenous contrast in trauma patients with an elevated serum creatinine. J Trauma. 2005 Nov;59(5):1162–6. doi: 10.1097/01.ta.0000194694.71607.0c. discussion 1166-7. [DOI] [PubMed] [Google Scholar]
- 38.Haveman JW, Gansevoort RT, Bongaerts AHH, et al. Intensive Care Med. 2006;32:1199. doi: 10.1007/s00134-006-0198-2. https://doi.org/10.1007/s00134-006-0198-2 . [DOI] [PubMed] [Google Scholar]
- 39.Kidoh M1, Nakaura T, Awai K, Matsunaga Y, Tanoue K, Harada K, Uemura S, Yamashita Y. Low-contrast dose protection protocol for diagnostic computed tomography in patients at high-risk for contrast-induced nephropathy. J Comput Assist Tomogr. 2013 Mar-Apr;37(2):289–96. doi: 10.1097/RCT.0b013e318279bd20. doi: 10.1097/RCT.0b013e318279bd20. [DOI] [PubMed] [Google Scholar]
- 40.Sonhaye L, Kolou B, Tchaou M, et al. Intravenous Contrast Medium Administration for Computed Tomography Scan in Emergency: A Possible Cause of Contrast-Induced Nephropathy. Radiol Res Pract. 2015;2015:805786. doi: 10.1155/2015/805786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heller M, Krieger P, Finefrock D, Nguyen T, Akhtar S. Contrast CT Scans in the Emergency Department Do Not Increase Risk of Adverse Renal Outcomes. West J Emerg Med. 2016;17(4):404–8. doi: 10.5811/westjem.2016.4.28994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah S. An update on common gastrointestinal emergencies. Emerg Med Clin North Am. 2013 Aug;31(3):775–93. doi: 10.1016/j.emc.2013.05.002. doi: 10.1016/j.emc.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Radwan RW, Tang AM, Beasley WD. Computed tomography as a first-line investigation for elderly patients admitted to a surgical assessment unit. Ann R Coll Surg Engl. 2018 Apr;100(4):285–289. doi: 10.1308/rcsann.2017.0231. doi: 10.1308/rcsann.2017.0231. Epub 2018 Jan 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rosen MP, Sands DZ, Longmaid HE, 3rd, Reynolds KF, Wagner M, Raptopoulos V. Impact of abdominal CT on the management of patients presenting to the emergency department with acute abdominal pain. AJR Am J Roentgenol. 2000 May;174(5):1391–6. doi: 10.2214/ajr.174.5.1741391. [DOI] [PubMed] [Google Scholar]
- 45.Sala E1, Watson CJ, Beadsmoore C, Groot-Wassink T, Fanshawe TR, Smith JC, Bradley A, Palmer CR, Shaw A, Dixon AK. A randomized, controlled trial of routine early abdominal computed tomography in patients presenting with non-specific acute abdominal pain. Clin Radiol. 2007 Oct;62(10):961–9. doi: 10.1016/j.crad.2007.01.030. Epub 2007 Jul 2. [DOI] [PubMed] [Google Scholar]
- 46.Tsushima Y1, Yamada S, Aoki J, Motojima T, Endo K. Effect of contrast-enhanced computed tomography on diagnosis and management of acute abdomen in adults. Clin Radiol. 2002 Jun;57(6):507–13. doi: 10.1053/crad.2001.0925. [DOI] [PubMed] [Google Scholar]
- 47.Catena F, et al., editors. Ct scan in abdominal emergency surgery. Hot topics in Acute Care Surgery and Trauma. https://doi.org/10,1007/978-3-319-48347-4_1 . [Google Scholar]
- 48.Cochran S.T. Determination of serum creatinine level prior to administration of radiographic contrast media. JAMA. 1997;277:517–518. doi: 10.1001/jama.1997.03540310015008. [DOI] [PubMed] [Google Scholar]
- 49.Solomon R, et al. How to prevent contrast-induced nephropathy and manage risk patients: Practical recommendations. Kidney International. 69:S51–S53. doi: 10.1038/sj.ki.5000375. [DOI] [PubMed] [Google Scholar]
- 50.Thomson K. Safe use of radiographic contrast media. Australian Prescriber. 2010;33(1):19–22. [Google Scholar]
- 51.Mueller C. Prevention of contrast-induced nephropathy with volume supplementation. Kidney International. 2006;69:S16–S19. doi: 10.1038/sj.ki.5000369. doi: 10.1038/sj.ki.5000369. [DOI] [PubMed] [Google Scholar]
- 52.Balemans CEA, Reichert LJM, van Schelven BIH, van Den Brand JAJG, Wetzels JFM. Epidemiology of contrast material-induced nephropathy in the era of hydration. Radiology. 2012;263(3):706–713. doi: 10.1148/radiol.12111667. doi: 10.1148/radiol.12111667/-/DC1.] [DOI] [PubMed] [Google Scholar]
- 53.Merten GJ, BurgeInss WP, Gray LV, Holleman JH, Roush TS, Kowalchuk GJ, Bersin RM, Van Moore A, Simonton CA, Rittase RA, III, Norton HJ, Kennedy TP. Prevention of contrast-induced nephropathy with sodium bicarbonate: a randomized controlled trial. The Journal of the American Medical Association. 2004;291(19):2328–2334. doi: 10.1001/jama.291.19.2328. doi: 10.1001/jama 291.19.2328. [DOI] [PubMed] [Google Scholar]
- 54.Masuda M, Yamada T, Mine T, Morita T, Tamaki S, Tsukamoto Y, Okuda K, Iwasaki Y, Hori M, Fukunami M. Comparison of usefulness of sodium bicarbonate versus sodium chloride to prevent contrast-induced nephropathy in patients undergoing an emergent coronary procedure. The American Journal of Cardiology. 2007;100(5):781–786. doi: 10.1016/j.amjcard.2007.03.098. doi: 10.1016/j.amjcard.2007.03.098. [DOI] [PubMed] [Google Scholar]
- 55.Fliser D, Laville M, Covic A, Fouque D, Vanholder R, Juillard L, van Biesen WA. European Renal Best Practice (ERBP) position statement on the Kidney Disease Improving Global Outcomes (KDIGO) Clinical Practice Guidelines on Acute Kidney Injury: part 1: definitions, conservative management and contrast-induced nephropathy. Nephrology Dialysis Transplantation. 2012;27(12):4263–4272. doi: 10.1093/ndt/gfs375. doi: 10.1093/ndt/gfs375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Andreucci M, Faga T, Pisani A, Sabbatini M, Russo D, Michael A. Prevention of contrast-induced nephropathy through a knowledge of its pathogenesis and risk factors. Scientific World Journal. 2014;2014:823169. doi: 10.1155/2014/823169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Owen RJ, Hiremath S, Myers A, Fraser-Hill M, Barrett BJ. Canadian Association of Radiologists consensus guidelines for the prevention of contrast-induced nephropathy: update 2012. Can Assoc Radiol J. 2014 May;65(2):96–105. doi: 10.1016/j.carj.2012.11.002. doi: 10.1016/j.carj.2012.11.002. Epub 2014 Feb 20. [DOI] [PubMed] [Google Scholar]
- 58.Stacul F, van der Molen AJ, Reimer P, Webb JA, Thomsen HS, Morcos SK, Almén T, Aspelin P, Bellin MF, Clement O, Heinz-Peer G. Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). Contrast induced nephropathy: updated ESUR Contrast Media Safety Committee guidelines. Eur Radiol. 2011 Dec;21(12):2527–41. doi: 10.1007/s00330-011-2225-0. doi: 10.1007/s00330-011-2225-0. Epub 2011 Aug 25. [DOI] [PubMed] [Google Scholar]
- 59.Cheungpasitporn W, Thongprayoon C, Brabec BA, Edmonds PJ, O’Corragain OA, Erickson SB. Oral Hydration for Prevention of Contrast-Induced Acute Kidney Injury in Elective Radiological Procedures: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. North American Journal of Medical Sciences. 2014;6(12):618–624. doi: 10.4103/1947-2714.147977. doi: 10.4103/1947-2714.147977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Marenzi G, Ferrari C, Marana I, Assanelli E, De Metrio M, Teruzzi G, Veglia F, Fabbiocchi F, Montorsi P, Bartorelli AL. Prevention of contrast nephropathy by furosemide with matched hydration: the MYTHOS (Induced Diuresis With Matched Hydration Compared to Standard Hydration for Contrast Induced Nephropathy Prevention) trial. JACC Cardiovasc Interv. 2012 Jan;5(1):90–7. doi: 10.1016/j.jcin.2011.08.017. doi: 10.1016/j.jcin.2011.08.017. [DOI] [PubMed] [Google Scholar]
- 61.Kama A1, Yılmaz S, Yaka E, Dervişoğlu E, Doğan NÖ, Erimşah E, Pekdemir M. Comparison of short-term infusion regimens of N-acetylcysteine plus intravenous fluids, sodium bicarbonate plus intravenous fluids, and intravenous fluids alone for prevention of contrast-induced nephropathy in the emergency department. Acad Emerg Med. 2014 Jun;21(6):615–22. doi: 10.1111/acem.12400. doi: 10.1111/acem.12400. [DOI] [PubMed] [Google Scholar]
- 62.Aycock RD, Westafer LM, Boxen JL, Majlesi N, Schoenfeld EM, Bannuru RR. Acute Kidney Injury After Computed Tomography: A Meta-analysis. Ann Emerg Med. 2018 Jan;71(1):44–53.e4. doi: 10.1016/j.annemergmed.2017.06.041. doi: 10.1016/j.annemergmed.2017.06.041. Epub 2017 Aug 12. Review. [DOI] [PubMed] [Google Scholar]
- 63.Davenport MS, Khalatbari S, Dillman JR, Cohan RH, Caoili EM, Ellis JH. Contrast material-induced nephrotoxicity and intravenous low-osmolality iodinated contrast material. Radiology. 2013;267(1):94–105. doi: 10.1148/radiol.12121394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McDonald JS, McDonald RJ, Williamson EE, Kallmes DF, Kashani K. Post-contrast acute kidney injury in intensive care unit patients: a propensity score-adjusted study. Intensive Care Med. 2017 Jun;43(6):774–784. doi: 10.1007/s00134-017-4699-y. doi: 10.1007/s00134-017-4699-y. Epub 2017 Feb 17. Erratum in: Intensive Care Med. 2017 Jun; 43(6): 956. [DOI] [PubMed] [Google Scholar]
- 65.Hinson JS, Ehmann MR, Fine DM, Fishman EK, Toerper MF, Rothman RE, Klein EY. Risk of Acute Kidney Injury After Intravenous Contrast Media Administration. Ann Emerg Med. 2017 May;69(5):577–586.e4. doi: 10.1016/j.annemergmed.2016.11.021. doi: 10.1016/j.annemergmed.2016.11.021. Epub 2017 Jan 25. [DOI] [PubMed] [Google Scholar]
- 66.Hassen GW, Hwang A, Liu LL, Mualim F, Sembo T, Tu TJ, Wei DH, Johnston P, Costea A, Meletiche C, Usmani S, Barber A, Jaiswal R, Kalantari H. Follow up for emergency department patients after intravenous contrast and risk of nephropathy. West J Emerg Med. 2014 May;15(3): 276–81. doi: 10.5811/westjem.2013.8.17915. doi: 10.5811/westjem.2013.8.17915. Epub 2014 Jan 7. PubMed PMID: 24868304; PubMed Central PMCID: PMC4025523. [DOI] [PMC free article] [PubMed] [Google Scholar]