Abstract
Background: Adherence to a healthy diet has been reported to be essential for the primary prevention of colorectal cancer, through a reduction of tissue inflammation, a low concentration of circulating lipoproteins and lower levels of serum cholesterol. Since an altered expression of the fatty acids pattern has been demonstrated to be a crucial event in colorectal carcinogenesis, lipidomic analysis is considered able to identify early diagnostic and prognostic biomarkers of complex diseases such as colorectal cancer. Methods: cell membrane fatty acid profile and serum lipoproteins pattern were evaluated by gas chromatography and electrophoresis method respectively. Results: There is a close association between diet and lipidomic profile in colorectal cancer, both in pre-clinical and clinical studies. A modified serum lipoproteins pattern has been demonstrated to be predominant in intestinal tumors. Conclusions: The study of fatty acids profile in cell membrane and the evaluation of serum lipoproteins subfractions could be useful to have an integrate vision on the interactions between lipids and the pathogenesis of colorectal cancer and to understand the mechanisms of action and the consequences of these interactions on human health status. (www.actabiomedica.it)
Keywords: nutrition, colorectal cancer, lipidomic analysis, fatty acids, lipoproteins
Introduction
Nutrition and cancer
Increasing evidence reports an important and significant association between nutrition and cancer, in particular as nutrition is a potentially modifiable risk factor for cancer. However, interest in the association of diet/ nutrition and cancer first appeared in the early 1800s and probably even before. Starting from this date, progress in understanding this association has been made over the past two centuries, even if often not leading to conclusive statements (1).
In this regard, several works reported that a healthy diet is associated with a lower incidence and prevalence of cancer, particularly when the gastrointestinal system is involved. For example, a higher adherence to a Mediterranean diet has been associated with a lower incidence of breast cancer (2), lung cancer (3), and prostate cancer (4).
This beneficial effect seems to be attributable to some important effects of the Mediterranean diet, which includes foods with anti-inflammatory properties (5) and anti-oxidant effects (6). Since inflammation and oxidative stress seem to be the basis of several cancers, the Mediterranean diet could potentially lower the incidence of cancer through these mechanisms.
Moreover, the Mediterranean diet seems to have important effects on the genetic patrimony. For example, older people having a higher adherence to the Mediterranean diet have significant higher telomere length than those having a lower adherence, therefore suggesting a potential role of this diet in maintaining a good genetic patrimony (7), protective for cancer.
Other studies have reported that also micronutrients may have a role in the prevention of cancer, both in terms of vitamins and minerals that have anti-oxidant and anti-inflammatory effects. For example, it has been reported that higher vitamin D levels are associated with a lower incidence of cancer and with better outcomes in people already affected by cancer (8), but the literature regarding this topic is still conflicting and not univocal. (9) Similarly, a higher magnesium intake seems to be associated with a lower incidence of pancreatic cancer in a large population followed-up for 7 years (10).
Altogether these findings suggest an important role of diet in cancer onset, that future research should better study.
Nutrition and colorectal cancer
Nutrition seems to play a pivotal role in the onset and in the progression of gastrointestinal cancers, particularly of colorectal cancer (CRC).
Regarding the Mediterranean diet, for example, it was reported in a large meta-analysis involving 11 cohort studies that a higher adherence to this dietary regimen significantly decreased the risk of CRC by 18%, independently from several potential confounders (11). In a recent umbrella review regarding Mediterranean diet and health outcomes, however, the strength of evidence was graded only as weak, mainly due to the presence of high heterogeneity of the studies available on the topic. (12) Therefore, other studies are needed in this direction in order to confirm these findings.
Among the food components present in the Mediterranean diet, some words should be spent on fibers and on meat.
It is widely known that a higher intake of dietary fibers is associated with a lower risk of CRC in several cohort longitudinal studies, and in people with pre-neoplastic lesion, such as adenomas (13, 14). In a recent umbrella review, a higher dietary fibers significantly lowered the risk of CRC of about 36%, but this evidence was, again, characterized by a high heterogeneity and therefore the strength of evidence was graded only a suggestive (15). In this regard, fibers seem to be able to decrease the risk of CRC by several mechanisms. Among them, the most important seems to be a cleaning effect on the colon, particularly of toxins (16). Another important effect seems to be associated with the viscosity of dietary fibers. Viscosity is a physicochemical property associated with dietary fibers, particularly soluble dietary fibers. Viscous dietary fibers thicken when mixed with fluids and include polysaccharides such as gums, pectins, psyllium, and β-glucans. Viscous fibers have been associated with beneficial physiological responses in human, animal, and animal-alternative in vitro models (17).
On the other hand, the introduction of meat seems to be deleterious for the onset of CRC. Red meat intake was identified as a probable risk factor for CRC, with research supporting that this may especially be true for tumors of the traditional adenoma-carcinoma pathway. Dietary heme intake shows a strong association with KRAS-mutated tumors, such as CRC (18). It has been reported that heme can enhance the endogenous formation of carcinogenic N-nitrose compounds (18). In this regard, the way of cooking meats seems to be relevant, since N-nitrose compounds are mainly produced by processed meats (19).
Altogether these findings support the idea that diet plays an important role in CRC and that the amounts of some foods (especially processed meats) should be strictly limited.
Nutrition, colorectal cancer and lipid profile
Different pre-clinical and clinical studies have confirmed the anti-cancer and cancer preventing action of diet (20-22). In particular, colorectal cancer (CRC) is considered a metabolic pathology where tumor growth and progression are affected by the complex interactions between cancer cells and the surrounding microenvironment (23). Environmental factors such as smoking, physical inactivity, overweight and obesity have been related to an increased risk of CRC (24). In fact, adherence to a healthy diet has been reported to be essential for the primary prevention of CRC. The link between adherence to the Mediterranean diet and a lower risk of cancer is mediated by several mechanisms, including reduction of tissue inflammation, low concentration of circulating lipoproteins and lower levels of serum cholesterol.
It is widely accepted that lipid and phospholipid metabolism plays a key role in cancer initiation, cellular invasion and tumor metastasis, and diet is considered as the major factor influencing fatty acid composition in tissue. The investigation of dietary intake can be useful to understand the relations between the patterns of fatty acid metabolism and specific diseases such as cancer (25,26).
Conflicting results have been reported on low-density (LDL-C) and high-density lipoprotein cholesterol (HDL-C) and serum triglyceride (TG) levels in different tumor types (27-29). Compared to subjects without cancer, in patients with malignancy LDL-C has been reported to be increased (27), normal (29) or decreased (30). The tumor types may explain the inconsistency in the results. Apparently, the effect on serum lipoprotein patterns differs among tumor types. However, the same type of tumor has been associated with different lipoprotein levels. The stage of disease is another possible contributory factor, although abnormal serum cholesterol levels have not consistently been linked to the progression of cancer (30,31). Studies of serum cholesterol levels in patients with gastrointestinal cancer have provided conflicting results, reporting either no (32), negative (33) or positive correlations (34). Recently, an association has been found between serum cholesterol and colorectal adenoma, a well-established precursor lesion of colorectal cancer (35). Moreover, experimental evidence of tumor cell accumulation in lesions caused by endothelial inflammation and injury has suggested an increased incidence of metastases in patients with increased LDL-C levels (36). In addition, recent studies showed that lovastatin, an 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitor, has anti-metastatic effects on human colon adenocarcinoma cell lines as well as on other cell lines (37).
Increased serum lipid levels are associated with the presence of metastases in patients with colorectal cancer (38). Although the exact reason is not clear, this is an interesting finding from an oncological point of view. In blood or interstitial fluids, most cholesterol or TG exists in a lipoprotein complex with apoproteins, and the major detrimental effect of serum cholesterol may be attributed to LDL, that can have various effects on tumor biology. There is much evidence to support the view that tumor cell growth is partially dependent on exogenous LDL-C, possibly through LDL receptors on tumor cells (39). However, experimental evidence shows that LDL receptors are frequently downregulated in malignant tumors (36, 40-42). In these cases, neoplastic proliferation may be stimulated by de novo synthesis of endogenous cholesterol through the HMG-CoA reductase pathway (36, 40,42). Indeed, pharmacological inhibition of HMG-CoA reductase has been shown to prevent the growth and invasion of some tumors (43). These findings suggest that increased LDL-C might be beneficial for the proliferation and invasion step of carcinoma cells.
The relationship between a metastatic phenotype and hyperlipidemia in colorectal cancer patients supports this hypothesis and is in line with other previous studies. It has already been demonstrated that 63.3% of colon cancer patients are LDL receptor negative, and the absence of the LDL receptor is associated with a poor prognosis and with enhanced HMG-CoA reductase activity (41,42). High serum TC and LDL-C levels in patients with metastases might be explained by an increased demand for cholesterol from neoplastic cells, resulting in an increased endogenous cholesterol synthesis. Moreover, recent data from literature have attributed a role to LDLC in cell growth and differentiation, with a possible involvement of hypercholesterolemia in cancer progression and metastatic spread (36). LDL-C, indeed, has been reported to affect host immune functions. LDL is required for the optimal expression of the Fc receptor or CD14, which mediates the phagocytosis of human monocytes, and a high-fat diet has been shown to decrease the antitumor activity of macrophages in mice (44-46). Moreover, high LDL levels have been reported to inhibit T-cell proliferation (47). These facts suggest that stimulation of cancer cells and suppression of the immune system by high LDLC levels might facilitate tumor invasion and survival of colorectal cancer cells in lymph nodes and distant metastases. In vivo studies suggest that there may be an association between cholesterol and metastatic cancer, i.e. colon cancer induced in rats by dimethylhydrazine was associated with a reduced incidence of metastases after deprivation of dietary cholesterol (48). Moreover, drugs that inhibit endogenous cholesterol synthesis, i.e. lovastatin or simvastatin, show anti-metastatic effects on colon, pancreatic and melanoma cancer cells in vitro and in vivo (37, 43, 49, 50). Moreover, in addition to its greater growth-inhibitory effect on metastatic cancer cells, lovastatin appears to universally reduce trans endothelial migration by acting on tumor cells, quiescent endothelial cells and LDL-stimulated cells (37). Based on these findings, repeated monitoring of serum lipid profiles in colorectal cancer patients may facilitate to predict tumor aggressiveness. The reduction in serum lipid levels might help to prevent metastases in certain cancers. Further studies including larger patient cohorts are warranted to evaluate the role of serum LDL-C as a predictive marker of recurrence of neoplasia in colorectal cancer patients.
A new approach to study the relationship between nutrition, colorectal cancer and lipid profile is the lipidomic approach. This new approach, emphasized in this review, is in agreement with recent studies considering lipidomic platforms able to provide an invaluable window to novel pathogenic mechanisms as well as helping to identify early diagnostic and prognostic biomarkers of complex diseases, such as CRC. The attractiveness of lipidomics is that they are strictly connected with nutritional elements and lipid supplementation. This offers an opportunity for prevention and treatment: in prevention, it is important to have nutritional directions in order to maintain the membrane lipid balance in the optimal values; in therapy, it is important to follow nutritional directions that keep membrane receptors and functions at their best, in order to improve the effects of the medical intervention.
Improvement of the comprehension at molecular level of factors derived from nutrition, metabolism and stress that influence the functioning of the membrane compartment is certainly useful to identify and validate membrane profiles to gain a global picture of human metabolic states.
Methods
Human blood samples
Human blood samples collected in tubes containing ethylenediamine-tetraacetic acid (K-EDTA) anticoagulant were layered on a Ficoll–Paque solution and centrifuged at 400x g for 40 min at room temperature. The lymphocytes and plasma were then removed and the erythrocytes were recovered from the bottom layer and washed with phosphate-buffered saline. Isolated red blood cells were stored at -80 °C until they were assayed.
Tissue samples
For the pre-clinical phase of the study, mice treated with specific enriched diets were killed by cervical dislocation. The entire intestinal tract was immediately removed, washed and fresh tissue samples of intestine were collected and stored at -80°C until assayed. All animal experiments were carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
For the clinical phase of the study, patients with histologically proven colorectal cancer were enrolled in the study. At surgery, colorectal mucosa and cancer tissue were obtained from each patient and the specimens were stored at -80°C until assayed. Informed consent was obtained from each patient and the study was approved by the Ethics Committee of IRCCS “S. de Bellis”.
To extract cell membrane fatty acids from tissue samples, about 20 mg of mice and human intestinal tissue were used and the method of Folch with slight modifications was performed for cell membrane fatty acids preparation (51,52).
Fatty Acids preparation and quantification
Samples of isolated red cells blood and intestinal tissue were treated with 0.9 mL of a salt solution acidified with sulfuric acid. All samples received 5.0 mL of chloroform:methanol (2:1, v/v) and the samples were centrifuged at 1000xg for 10 min. The lower layer, containing fatty acids, was removed with care, replaced in a new tube and dried by a centrifugal evaporator. The FAME were obtained by adding toluene and BF3 with MeOH 14% and incubating for 2 h at 80°C. After the addition of toluene and 5% aqueous sodium chloride solution, the samples were centrifuged and fatty acid methyl esters contained in the upper layer of the tubes were collected, transferred into a vial and analyzed by gas chromatography equipment with auto-sampler, a split/splitless injector, FID detector and a hydrogen gas. A BPX 70 capillary column SGE Analytical Science, P/N SGE054623, 60 m x 0.25 mm ID, BPX70 0.25UM was used and the amount injected was 1 uL in splitless mode (split flow 50 mL x min-1, splitlesstime 1 min). Quantification of fatty acid methyl esters was performed using a mixture of standards (Supelco 37-Component FAME Mix, Sigma-Aldrich, Milan, Italy).
Small dense LDL analysis
Blood samples were obtained by venous puncture, after 12 h fasting, and collected in tubes containing Ethylenediaminetetraacetic Acid (EDTA-K2) anticoagulant. The samples were then centrifuged at 2000x g for 10 min at 4 °C to obtain serum and stored at -80°C until use. Small dense Lipoproteins (sdLDL) were assayed using Lipoprint LDL System (Quantimetrix, USA). Each serum sample was applied on a high resolution polyacrylamide gel tube in order to separate LDL fractions and subfractions by electrophoresis. The resolved lipoproteins bands were scanned and analyzed.
Fatty acids profile and CRC
Lipidomic analysis aimed to identify and quantify cellular lipids and their interactions with other cellular components such as proteins and gene expression, as it is known to be a powerful tool to predict cancer progression and the development of metastases (53,54). Previous reports have shown that metabolic perturbation of phospholipids is associated with various cancer types (55-57), indicating that the composition of phospholipids may be critical for deciding the fate of tumor cells.
An altered expression of the fatty acids pattern has been demonstrated to be a crucial event in colorectal carcinogenesis (58-60). Fatty acid synthase (FAS) activity levels, the key enzyme in the fatty acids biosynthesis pathway, as well as its mRNA expression, are upregulated in colorectal cancer tissues (59). Recently, in Apc Min/+ mouse model, we showed that a possible molecular mechanism by which omega-3-polyunsaturated fatty acids (n-3-PUFAs) and olive oil in the diet were able to reduce intestinal cell proliferation was the reduction of lipogenic enzymes activity and gene expression, such as Fatty Acid Synthase (FAS) (61). Fatty acids and their polyunsaturated derivatives influence cell membranes fluidity and their physiological functions.
We demonstrated the presence of an altered fatty acid profile in patients with CRC compared to control subjects; a reduction of total n-3-PUFAs levels and consequently a higher n-6-PUFAs/n-3-PUFAs ratio was detected in cancer patients compared to control subjects (55). In addition, our recent study confirmed that not only several modifications in lipid metabolism occur in colorectal cancer, but that the presence of synchronous metastases was associated with a different tissue fatty acids profile, demonstrating the ability of tissue fatty acids analysis to identify lipid metabolism alterations associated with CRC and with synchronous metastasis (62).
Moreover, in an animal model of colon carcinogenesis, we demonstrated that cancer cell progression is affected by dietary natural compounds, which are able to control and to improve the environmental conditions in which tumors develop (63). The investigation of changes in the lipidomic profile of cell membrane is important to understand the complete scenario of the metabolic transformations which can occur in cancer. These changes may lead to alter the hydration levels and fluidity of cell membranes and to affect the proteins transduction involved in cell proliferation, apoptosis and differentiation (64).
In this context, we have demonstrated that olive oil and omega-3 PUFAs in the diet differently affect the fatty acid profile in intestinal tissue from ApcMin/+ mice, an animal model of CRC (65). Our data support evidences demonstrating the effects of dietary components, such as olive oil and omega-3 PUFAs, in counteracting intestinal carcinogenesis in vitro and in vivo. These protective effects seem to be due to the olive oil capacity to control the tissue inflammatory status, and to the omega-3 PUFAs ability to keep the cell membrane saturation index (known as stearic acid/oleic acid ratio) at high levels. Moreover, Eicosapentanoic acid (EPA), an omega-3-PUFA, has been demonstrated to affect cell proliferation through the regulation of lipogenic enzymes belonging to the cholesterol biosynthetic pathway (66,67). These enzymes interact with both intracellular signaling pathways and extracellular microenvironmental stresses. Hypoxia, low pH and nutrient starvation could activate several intracellular signaling pathways to promote lipogenic enzymes expression (68-70).
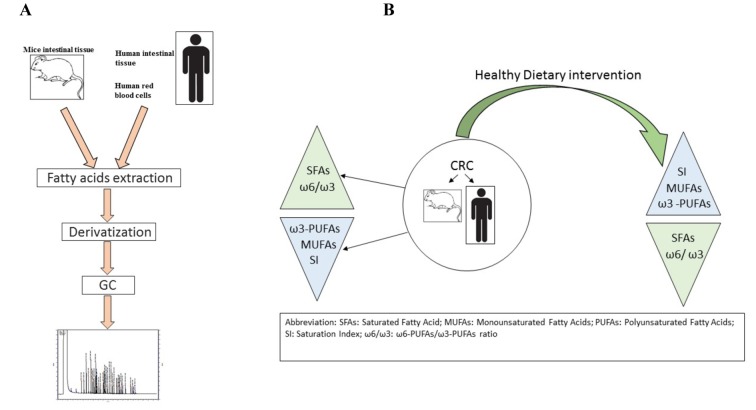
Figure 1 summarizes the main steps of the lipidomic analysis performed in animals and clinical studies, as well as the effects of dietary intervention on CRC.
Figure 1.
Panel A: Main steps of lipidomic analysis; Panel B: Dietary intervention effects
The anti-proliferative effects of omega-3 PUFAs seem to be also correlated with the over-expression of cell membrane receptors, which are considered negative modulators of cell proliferation, such as Low Density Lipoprotein (LDL) receptor and Cannabinoid type 1 (CB1) receptor (63,67,71). Different experimental evidences have reported that a CB1 receptor down-regulation is present in different types of cancer (72,73).
The expression of CB1-R seems to be modulated by bioactive natural components (74,75) such as flavonoids. The daily administration of quercetin in an animal model of induced colon cancer, exerted a protective effect against tumor formation by regulating the protein and gene expression level of CB1-R (76). The role of quercetin as an anti-cancer molecule has been confirmed in both in vitro and in vivo studies, demonstrating its ability to modulate p-STAT3 expression, a biomarker of cell proliferation and aggressiveness.
Low-density Lipoproteins and CRC
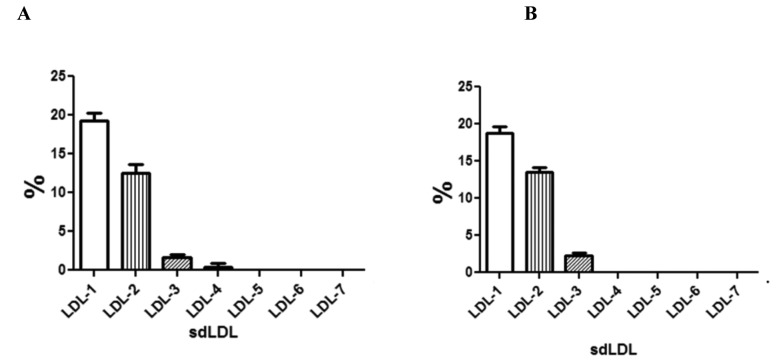
Low-density Lipoproteins are composed of sub-fractions that differ in particle size and density one from the other. Among these LDL subfractions, small dense LDL particles are more atherogenic than larger particles and are considered biochemical markers associated with metabolic syndrome (77). The increased prevalence of smaller LDL particles is certainly related to higher body mass indexes (BMIs), increased visceral adiposity and likely to an inflammatory status consistent with an altered metabolic profile. We have studied the small dense LDL levels in serum from subjects with CRC, demonstrating that a modified LDL pattern was associated with CRC and with the presence of metastasis. The smaller LDL subfractions (LDL-4) were observed in serum of CRC patients with synchronous metastasis whereas these particles were absent in CRC patients without metastasis (Figure 2).
Figure 2.
Serum levels of small dense LDL (sdLDL) of 51 cases of CRC patients with (panel A) and without (panel B) synchronous metastasis. All values are expressed as mean ± standard deviation
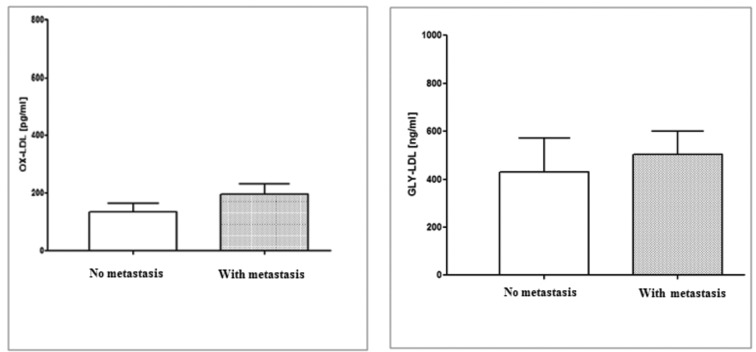
Literature data indicates that modified lipids, particularly oxidized and glycated low-density lipoprotein (ox-LDL and gly-LDL), are predominant in intestinal tumors, resulting in an increased pro-inflammatory cytokine expression (78-80). Inflammation and oxidative stress due to an increase in reactive oxygen species and a decrease of antioxidant defenses seem to be involved in the molecular mechanisms of colonic tissue carcinogenesis. Therefore, we investigated the levels of ox-LDL and gly-LDL in serum of CRC patients with and without synchronous metastasis. Figure 3 shows that higher levels of ox-LDL and gly-LDL were detected in CRC patients with metastasis in comparison with patients without metastasis, even if the difference was not statistically significant.
Figure 3.
Serum ox-LDL and gly-LDL levels in 51 cases of CRC patients with and without synchronous metastasis. All values are expressed as mean ± standard deviation
Conclusions
We confirm the tight link between diet and lipidomic profile in CRC, as well as the therapeutic role of dietary components on human health. In fact, the marked effect of diet interventions in absence of toxicity can make some of the Mediterranean Diet components excellent candidates for the prevention and treatment of subjects with a high risk for metabolic diseases and CRC. The lipidomic approach could be useful to evaluate the onset and progression of CRC and for the development of nutraceutical lines aimed at membrane balance restoring. In order to connect the lipidomic analysis with the patients clinical status and with canonical biochemical parameters an integrate vision is needed. This vision could better clarify the role of cell membranes fatty acids profiles in the pathogenesis and treatments of CRC.
References
- 1.Campbell TC. The Past, Present, and Future of Nutrition and Cancer: Part 1-Was A Nutritional Association Acknowledged a Century Ago? Nutr Cancer. 2017;69(5):811–7. doi: 10.1080/01635581.2017.1317823. [DOI] [PubMed] [Google Scholar]
- 2.Toledo E, Salas-Salvado J, Donat-Vargas C, et al. Mediterranean Diet and Invasive Breast Cancer Risk Among Women at High Cardiovascular Risk in the PREDIMED Trial: A Randomized Clinical Trial. JAMA Intern Med. 2015;175(11):1752–60. doi: 10.1001/jamainternmed.2015.4838. [DOI] [PubMed] [Google Scholar]
- 3.Anic GM, Park Y, Subar AF, Schap TE, Reedy J. Index-based dietary patterns and risk of lung cancer in the NIH-AARP diet and health study. Eur J Clin Nutr. 2016;70(1):123–9. doi: 10.1038/ejcn.2015.122. [DOI] [PubMed] [Google Scholar]
- 4.Lopez-Guarnido O, Alvarez-Cubero MJ, Saiz M, et al. Mediterranean diet adherence and prostate cancer risk. Nutr Hosp. 2014;31(3):1012–9. doi: 10.3305/nh.2015.31.3.8286. [DOI] [PubMed] [Google Scholar]
- 5.Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean diet attenuates inflammation and coagulation process in healthy adults: The ATTICA Study. Journal of the American College of Cardiology. 2004;44(1):152–8. doi: 10.1016/j.jacc.2004.03.039. [DOI] [PubMed] [Google Scholar]
- 6.Chatzianagnostou K, Del Turco S, Pingitore A, Sabatino L, Vassalle C. The Mediterranean Lifestyle as a Non-Pharmacological and Natural Antioxidant for Healthy Aging. Antioxidants (Basel, Switzerland) 2015;4(4):719–36. doi: 10.3390/antiox4040719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boccardi V, Esposito A, Rizzo MR, Marfella R, Barbieri M, Paolisso G. Mediterranean diet, telomere maintenance and health status among elderly. PLoS One. 2013;8(4):e62781. doi: 10.1371/journal.pone.0062781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaughan-Shaw PG, O’Sullivan F, Farrington SM, et al. The impact of vitamin D pathway genetic variation and circulating 25-hydroxyvitamin D on cancer outcome: systematic review and meta-analysis. Br J Cancer. 2017;116(8):1092–110. doi: 10.1038/bjc.2017.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis JPA. Vitamin D and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ : British Medical Journal. 2014:348. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dibaba D, Xun P, Yokota K, White E, He K. Magnesium intake and incidence of pancreatic cancer: the VITamins and Lifestyle study. Br J Cancer. 2015;113(11):1615–21. doi: 10.1038/bjc.2015.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwingshackl L, Schwedhelm C, Galbete C, Hoffmann G. Adherence to Mediterranean Diet and Risk of Cancer: An Updated Systematic Review and Meta-Analysis. Nutrients. 2017;9(10) doi: 10.3390/nu9101063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta-analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72(1):30–43. doi: 10.1038/ejcn.2017.58. [DOI] [PubMed] [Google Scholar]
- 13.Ben Q, Sun Y, Chai R, Qian A, Xu B, Yuan Y. Dietary fiber intake reduces risk for colorectal adenoma: a meta-analysis. Gastroenterology. 2014;146(3):689–99.e6.. doi: 10.1053/j.gastro.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Yao Y, Suo T, Andersson R, et al. Dietary fibre for the prevention of recurrent colorectal adenomas and carcinomas. Cochrane Database Syst Rev. 2017;1:CD003430. doi: 10.1002/14651858.CD003430.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veronese N, Solmi M, Caruso MG, et al. Dietary fiber and health outcomes: an umbrella review of systematic reviews and meta-analyses. The American Journal of Clinical Nutrition. 2018;107(3):436–44. doi: 10.1093/ajcn/nqx082. [DOI] [PubMed] [Google Scholar]
- 16.Jacobs LR. Relationship between dietary fiber and cancer: metabolic, physiologic, and cellular mechanisms. Proceedings of the Society for Experimental Biology and Medicine. 1986;183(3):299–310. doi: 10.3181/00379727-183-42423. [DOI] [PubMed] [Google Scholar]
- 17.Dikeman CL, Fahey GC., Jr Viscosity as related to dietary fiber: a review. Critical reviews in food science and nutrition. 2006;46(8):649–63. doi: 10.1080/10408390500511862. [DOI] [PubMed] [Google Scholar]
- 18.Gilsing AM, Fransen F, de Kok TM, et al. Dietary heme iron and the risk of colorectal cancer with specific mutations in KRAS and APC. Carcinogenesis. 2013;34(12):2757–66. doi: 10.1093/carcin/bgt290. [DOI] [PubMed] [Google Scholar]
- 19.Lijinsky W. N-Nitroso compounds in the diet. Mutat Res. 1999;443(1-2):129–38. doi: 10.1016/s1383-5742(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 20.Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: Meta-analysis of randomized controlled trials. Metab Syndr Relat Disorders. 2011;9:1–12. doi: 10.1089/met.2010.0031. [DOI] [PubMed] [Google Scholar]
- 21.Urpi-Sarda M, Casas R, Chiva-Blanch G, et al. Virgin oil and nuts as key foods of the Mediterranean diet effects on inflammatory biomarkers related to atherosclerosis. Pharmacol Res. 2012;65:577–583. doi: 10.1016/j.phrs.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 22.Marin C, Ramirez R, Delgado-Lista J, et al. Mediterranean diet reduces endothelial damage and improbe between adherence to the Mediterranean diet and oxidative stress. Am J Clin Nutr. 2011;88:1364–1370. [Google Scholar]
- 23.Zhang F, Du G. Dysregulated lipid metabolism in cancer. World J Biol Chem. 2012;3:167–174. doi: 10.4331/wjbc.v3.i8.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hernaez A, Castaner O, Elousa R, et al. Mediterranean diet improves High-Density Lipoprotein function in high-cardiovascular risk individuals. Circulation. 2017;135:633–643. doi: 10.1161/CIRCULATIONAHA.116.023712. [DOI] [PubMed] [Google Scholar]
- 25.Kuhaiada F.P. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 26.Van de Sande T, Roskams T, Lerut E, et al. High levels expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J Pathol. 2005;206:214–219. doi: 10.1002/path.1760. [DOI] [PubMed] [Google Scholar]
- 27.Alexopoulos CG, Blatsios B, Avgerinos A. Serum lipids and lipoprotein disorders in cancer patients. Cancer. 1987;60:3065–3070. doi: 10.1002/1097-0142(19871215)60:12<3065::aid-cncr2820601234>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Borrelli R, del Sordo G, De Filippo E, et al. High serum HDLcholesterol in pre- and post-menopausal women with breast cancer in southern Italy. Adv Exp Med Biol. 1993;348:149–153. doi: 10.1007/978-1-4615-2942-2_17. [DOI] [PubMed] [Google Scholar]
- 29.Dessì S, Batetta B, Pulisci D, et al. Cholesterol content in tumor tissues is inversely associated with high-density lipoprotein cholesterol in serum in patients with gastrointestinal cancer. Cancer. 1994;73:253–258. doi: 10.1002/1097-0142(19940115)73:2<253::aid-cncr2820730204>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 30.Knapp ML, Al-Sheibani S, Riches PG. Alterations of serum lipids in breast cancer: Effects of disease activity, treatment and hormonal factors. Clin Chem. 1991;37:2093–2101. [PubMed] [Google Scholar]
- 31.Henriksson P, Eriksson M, Ericsson S, et al. Hypocholesterolemia and increased elimination of low density lipoproteins in metastatic cancer of the prostate. Lancet. 1989;ii:1178–1180. doi: 10.1016/s0140-6736(89)91790-x. [DOI] [PubMed] [Google Scholar]
- 32.Neugut AI, Johnsen CM, Daniel JF. Serum cholesterol levels in adenomatous polyps and cancer of the colon. JAMA. 1986;255:365–367. [PubMed] [Google Scholar]
- 33.Forones NM, Falcao JB, Mattos D, Barone B. Cholesterolemia in colorectal cancer. Hepatogastroenterology. 1998;45:1531–1534. [PubMed] [Google Scholar]
- 34.Kitayama J, Hatano K, Kaisaki H, Suzuki H, Fujiii S, Nagawa H. Hyperlipidemia is positively correlated with lymph node metastasis in men with early gastric cancer. Br J Surg. 2001;91:191–198. doi: 10.1002/bjs.4391. [DOI] [PubMed] [Google Scholar]
- 35.Meance S, Boutron-Ruault MC, Myara A, et al. Fecal primary bile acids and serum cholesterol are associated with colorectal adenomas. Dig Dis Sci. 2003;48:1751–1757. doi: 10.1023/a:1025443012049. [DOI] [PubMed] [Google Scholar]
- 36.Mehta N, Hordines J, Volpe C, Doerr R, Cohen SA. Cellular effects of hypercholesterolemia in modulation of cancer growth and metastasis: A review of the evidence. Surg Oncol. 1997;6:179–185. doi: 10.1016/s0960-7404(97)00027-3. [DOI] [PubMed] [Google Scholar]
- 37.Mehta N, Hordines J, Donald S, Doerr R, Cohen SA. Low density lipoproteins and Lovastatin modulate the organ-specifi c transendothelial migration of primary and metastatic human colon adenocarcinoma cell lines in vitro. Clin Exp Metastasis. 1998;16:587–594. doi: 10.1023/a:1006548902592. [DOI] [PubMed] [Google Scholar]
- 38.Notarnicola M, Altomare DF, Correale M, et al. Serum Lipid Profile in Colorectal Cancer Patients with and without Synchronous Distant Metastases. Oncology. 2005;68:371–374. doi: 10.1159/000086977. [DOI] [PubMed] [Google Scholar]
- 39.Vitols S, Norgren S, Juliusson G, Tatidis L, Luthman H. Multilevel regulation of low density lipoprotein and 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in normal and leukemic cells. Blood. 1994;84:2689–2698. [PubMed] [Google Scholar]
- 40.Rao KN. The significance of the cholesterol biosynthetic pathway in cell growth and carcinogenesis. Anticancer Res. 1995;15:309–314. [PubMed] [Google Scholar]
- 41.Caruso MG, Osella AR, Notarnicola M, et al. Prognostic value of low density lipoprotein receptor expression in colorectal carcinoma. Oncol Rep. 1998;5:927–930. doi: 10.3892/or.5.4.927. [DOI] [PubMed] [Google Scholar]
- 42.Caruso MG, Notarnicola M, Santillo M, Cavallini A, Di Leo A. Enhanced 3-HMGCoA reductase activity in human colorectal cancer not expressing low density lipoprotein receptor. Anticancer Res. 1999;19:451–454. [PubMed] [Google Scholar]
- 43.Kusama T, Mukai M, Iwasaki T, et al. 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors reduce human pancreatic cancer cell invasion and metastases. Gastroenterology. 2002;122:308–317. doi: 10.1053/gast.2002.31093. [DOI] [PubMed] [Google Scholar]
- 44.Bigler RD, Khoo M, Lund-Katz S, Scerbo L, Esfahani M. Identification of low density lipoprotein as a regulator of Fc receptor-mediated phagocytosis. Proc Natl Acad Sci USA. 1990;87:4981–4985. doi: 10.1073/pnas.87.13.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Esfahani M, Bigler RD, Alfieri JL, Lund-Katz S, Baum JD, Scerbo L. Cholesterol regulates the cell surface expression of glycophospholipid anchored CD14 antigen on human monocytes. Biochem Biophys Acta. 1993;1149:217–223. doi: 10.1016/0005-2736(93)90204-d. [DOI] [PubMed] [Google Scholar]
- 46.Stewart-Phillips JL, Lough J, Phillips NC. The effect of a high-fat diet on murine macrophage activity. Int J Immunopharmacol. 1991;13:325–332. doi: 10.1016/0192-0561(91)90001-n. [DOI] [PubMed] [Google Scholar]
- 47.McCarthy BM, Okano Y, Nakayasu T, Macy M, Watson SR, Harmony JA. Plasma lipoprotein and transferrin regulate the proliferation of a continuous T lymphocyte cell line. J Lipid Res. 1987;28:1067–1077. [PubMed] [Google Scholar]
- 48.Cruse JP, Lewin MR, Clark CJ. Dietary cholesterol deprivation improves survival and reduces incidence of metastatic colon cancer in dimethylhydrazine-pretreated rats. Gut. 1982;23:594–599. doi: 10.1136/gut.23.7.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Notarnicola M, Messa C, Pricci M, et al. Up-regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity in left-sided human colon cancer. Anticancer Res. 2004;24:3837–3842. [PubMed] [Google Scholar]
- 50.Jani JP, Specht S, Sternmler N, et al. Metastasis of B16F10 mouse melanoma inhibited by lovastatin, an inhibitor of cholesterol biosynthesis. Invasion Metastasis. 1993;13:314–324. [PubMed] [Google Scholar]
- 51.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 52.Fisk HL, West AL, Childs CE, Burdge GC, Calder PC. The use of gas chromatography to analyze compositional changes of fatty acids in rat liver tissue during pregnancy. J Vis Exp. 2014;85:e51445. doi: 10.3791/51445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim HY, Lee KM, Kim SH, et al. Comparative metabolic and lipidomic profiling of human breast cancer cells with different metastatic potentials. Oncotarget. 2016;41:67111–67128. doi: 10.18632/oncotarget.11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baenke F, Peck B, Miess H, Schulze A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis Model Mech. 2013;6:1353–1363. doi: 10.1242/dmm.011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Coviello G, Tutino V, Notarnicola M, Caruso MG. Erythrocyte membrane fatty acids profile in colorectal cancer patients: a preliminary study. Anticancer Res. 2014;34:4775–4779. [PubMed] [Google Scholar]
- 56.Janovská A, Hatzinikolas G, Mano M, Wittert GA. The effect of dietary fat content on phospholipid fatty acid profile is muscle fiber type dependent. Am J Physiol Endocrinol Metab. 2010;298:E779–E786. doi: 10.1152/ajpendo.00356.2009. [DOI] [PubMed] [Google Scholar]
- 57.Andrade Fde O, de Assis S, Jin L, et al. Lipidomic fatty acid profile and global gene expression pattern mammary gland of rats that were exposed to lard-based high fat diet during fetal and lactation periods associated to breast cancer risk in adulthood. Chem Biol Interact. 2015;239:118–128. doi: 10.1016/j.cbi.2015.06.035. [DOI] [PubMed] [Google Scholar]
- 58.Bartsch H, Nair J, Owen RW. Dietary polyunsaturated fatty acids and cancers of the breast and colorectum: Emerging evidence for their role as risk modifiers. Carcinogenesis. 1999;20:2209–2218. doi: 10.1093/carcin/20.12.2209. [DOI] [PubMed] [Google Scholar]
- 59.Notarnicola M, Altomare DF, Calvani M, et al. Fatty Acid Synthase hyperactivation in human colorectal cancer: relationship with tumor side and sex. Oncology. 2006;71:327–332. doi: 10.1159/000107106. [DOI] [PubMed] [Google Scholar]
- 60.Azrad M, Turgeon C, Demark-Wahnefried W. Current evidence linking polyunsaturated fatty acids with cancer risk and progression. Front Oncol. 2013;3:224. doi: 10.3389/fonc.2013.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barone M, Notarnicola M, Caruso MG, et al. Olive oil and omega-3 polyunsaturated fatty acids suppress intestinal polyp growth by modulating the apoptotic process in ApcMin/+ mice. Carcinogenesis. 2014;35:1613–1619. doi: 10.1093/carcin/bgu068. [DOI] [PubMed] [Google Scholar]
- 62.Notarnicola M, Lorusso D, Tutino V, et al. Differential tissue fatty acids profiling between colorectal cancer patients with and without synchronous metastasis. Int J Molecular Sciences. 2018;19(4) doi: 10.3390/ijms19040962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Notarnicola M, Tutino V, De Nunzio V, Dituri F, Caruso MG, Giannelli G. Dietary omega-3 Polyunsaturated Fatty Acids Inhibit Tumor Growth in Transgenic ApcMin/+ Mice, Correlating with CB1 Receptor Up-Regulation. Int J Mo Sci. 2017;18(3) doi: 10.3390/ijms18030485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibarguren M, López DJ, Escribá PV. The effect of natural and synthetic fatty acids on membrane structure, microdomain organization, cellular functions and human health. Biochim Biophys Acta. 2014;1838:1518–1528. doi: 10.1016/j.bbamem.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 65.Tutino V, Caruso MG, De Leonardis G, De Nunzio V, Notarnicola M. Tissue Fatty Acid Profile Is Differently Modulated From Olive Oil and Omega-3 Polyunsaturated Fatty Acids in ApcMin/+ Mice. Endocr Metab Immune Disord Drug Targets. 2017;17(4):303–308. doi: 10.2174/1871530317666170911161623. [DOI] [PubMed] [Google Scholar]
- 66.Notarnicola M, Tutino V, Caruso MG, Francavilla A. n-3 polyunsaturated fatty acids reverse the development of polyps in ApcMin/+transgenic mice. Oncol. Rep. 2016;35:504–510. doi: 10.3892/or.2015.4359. [DOI] [PubMed] [Google Scholar]
- 67.Notarnicola M, Messa C, Refolo MG, Tutino V, Miccolis A, Caruso MG. Synergic effect of eicosapentaenoic acid and lovastatin on gene expression of HMGCoA reductase and LDL receptor in cultured HepG2 cells. Lipids Health Dis. 2010;9:135. doi: 10.1186/1476-511X-9-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mashima T, Seimiya H, Tsuro T. De novo fatty-acid synthesis and related pathways as molecular targets for cancer therapy. Br J Cancer. 2009;100:1369–1372. doi: 10.1038/sj.bjc.6605007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Furuta E, Pai SK, Zhan R, et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003–1011. doi: 10.1158/0008-5472.CAN-07-2489. [DOI] [PubMed] [Google Scholar]
- 70.Menendez JA, Decker JP, Lupu R. In support of fatty acid synthase (FAS) as metabolic oncogene: extracellular acidosis acts in an epigenetic fashion activating FAS gene expression in cancer cells. J Cell Biochem. 2005;94(1):1–4. doi: 10.1002/jcb.20310. [DOI] [PubMed] [Google Scholar]
- 71.Notarnicola M, Tutino V, Tafaro A, Amatulli F, Caruso MG. Antitumorigenic effect of dietary natural compounds via lipid metabolism modulation in ApcMin/+mice. Anticancer Res. 2013;33:3739–3744. [PubMed] [Google Scholar]
- 72.Ligresti A, Bisogno T, Matias I, et al. Possible endocannabinoid control of colorectal cancer growth. Gastroenterology. 2003;125:677–687. doi: 10.1016/s0016-5085(03)00881-3. [DOI] [PubMed] [Google Scholar]
- 73.Linsalata M, Notarnicola M, Tutino V, et al. Effects of Anandamide on Polyamine Levels and cell growth in human colon cancer cells. Anticancer Res. 2010;30:2583–2590. [PubMed] [Google Scholar]
- 74.Refolo MG, D’alessandro R, Malerba N, et al. Anti proliferative and pro apoptotic effects of flavonoid quercetin are mediated by CB1 receptor in human colon cancer cell lines. J Cell Physiol. 2015;230:2973–2980. doi: 10.1002/jcp.25026. [DOI] [PubMed] [Google Scholar]
- 75.Di Francesco A, Falconi A, Di Germanio C, et al. Extravirgin olive oil up-regulates CB1 tumor suppressor gene in human colon cancer cells and in rat colon via epigenetic mechanisms. J Nutr Biochem. 2015;26:250–258. doi: 10.1016/j.jnutbio.2014.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Tutino V, De Nunzio V, Tafaro A, et al. Cannabinoid receptor-1 up-regulation in the Azoxymethane (AOM) treated mice after dietary treatment with quercetin. Anticancer Res. 2018;38(8):4485–4491. doi: 10.21873/anticanres.12752. [DOI] [PubMed] [Google Scholar]
- 77.Gentile M, Iannuzzo G, Mattiello A, Rubba F, Panico S, Rubba P. Association between body shape index and small dense LDL particles in a cohort of Mediterranean women: findings from Profetto ATENA. J Clin Biochem Nutr. 2017;61:130–134. doi: 10.3164/jcbn.17-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Castro-Barquero S, Lamuela-Raventós RM, Doménech M, Estruch R. Relationship between Mediterranean Dietary Polyphenol Intake and Obesity. Nutrients. 2018;10:1523. doi: 10.3390/nu10101523. doi:10.3390/nu10101523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al Saudi RM, Kasabri V, Naffa R, Bulatova N, Bustanji Y. Glycated LDL-C and glycated HDL-C in association with adiposity, blood and atherogenicity indices in metabolic syndrome patients with and without prediabetes. Ther Adv Endocrinol Metab. 2018;14:9(10):311–323. doi: 10.1177/2042018818788198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Abidi M, Khan MS, Ahmad S, et al. Biophysical and biochemical studies on glycoxidatively modified human low density lipoprotein. Arch Biochem Biophys. 2018;1(645):87–99. doi: 10.1016/j.abb.2018.02.019. doi: 10.1016/j.abb.2018.02.019. [DOI] [PubMed] [Google Scholar]