Abstract
Introduction:
In China, rotavirus is the leading cause of diarrhea hospitalizations among children aged <5 years. A locally manufactured rotavirus vaccine is available for private market use, but little is known about its coverage. Given the impending availability of newer rotavirus vaccines, we evaluated intussusception rates among children aged <2 years to better understand intussusception epidemiology for future vaccine safety monitoring.
Methods:
We conducted a retrospective review at 4 hospitals in Chenzhou City of Hunan Province and Kaifeng City of Henan Province. We identified intussusception cases admitted during 2009–2013 by reviewing medical records with the ICD-10 discharge code for intussusception and extracting demographic and clinical information from the electronic clinical record systems.
Results:
During 2009–2013, 1715 intussusception hospitalizations among 1,487,215 children aged <2 years occurred in both cities. The average annual intussusception hospitalization incidence was 112.9 per 100,000 children aged <2 years (181.8 per 100,000 children <1 year; 56 per 100,000 children 1 to <2 years). Intussusception incidence was low among infants aged <3 months and peaked at age 6–8 months. No clear seasonality was observed. Ultrasound was used to diagnose 95.9% of cases. Enema reduction was performed in 80% cases; 25% of cases in Chenzhou and 16% in Kaifeng required surgical intervention. No deaths were reported. The median time between symptom onset and admission was 1 day.
Conclusions:
This study provides information on intussusception incidence and epidemiology in two cities of China during 2009–2013. Monitoring intussusception rates in this population will be important in the post-rotavirus vaccine era.
Keywords: intussusception, incidence, rotavirus vaccine safety, China
INTRODUCTION
Rotavirus is the most common cause of severe diarrhea worldwide among children under 5 years of age [1,2]. In 1999, the first licensed rotavirus vaccine, Rotashield, was withdrawn from the market less than 1 year after its introduction into the US childhood immunization program due to an association with intussusception, an intestinal condition that can cause bowel obstruction [3]. In 2006, two new rotavirus vaccines, RotaTeq (Merck and Co., Inc.) and Rotarix (GlaxoSmithKline), were licensed and subsequently introduced into the routine child immunization programs of many countries worldwide [4,5]. Although clinical trials of these two vaccines did not show an increased risk of intussusception [6,7], recent post-marketing data have shown a potential, small elevated risk with both vaccines, particularly after the first dose [8–12].
Intussusception is the most common cause of acute intestinal obstruction in infants [13–15], with peak occurrence typically between 4 and 10 months of age [16]. Most (60%) cases of intussusception occur in children <1 year of age, and a greater proportion (about two-thirds) of cases occurs in males [17]. Reported baseline incidence of intussusception varies between 9 and 328 per 100,000 children <1 year of age in different countries, with the highest rates reported in Asian countries including Korea (328 cases per 100,000) [17] and Vietnam (302 cases per 100,000) [18]. Reported intussusception incidence can also vary by ethnic group, geographical area and different study periods, even within the same country.
In China, rotavirus is the most common cause of acute diarrhea, causing an estimated 35–40% of acute diarrhea hospitalizations among children <5 years of age, or ~330,000 hospitalizations annually [19]. A locally manufactured rotavirus vaccine (Lanzhou Lamb Rotavirus vaccine [LLR], Lanzhou Institute of Biological Products) was licensed in 2000 and is available in the private market. Little is known about LLR vaccination coverage, however, >40 million doses have been distributed around the country to date. More recently, clinical trials for RotaTeq and Rotarix have been completed in China, and additional rotavirus vaccines are under development, one of which will likely be available on the market in the next 3 to 5 years.
Given the impending availability of many rotavirus vaccines in China, it is important to understand the baseline epidemiology and incidence of intussusception to help future vaccine safety monitoring. We conducted a retrospective study in two cities in China to determine the baseline incidence of intussusception by age and location and to examine the seasonal patterns, clinical characteristics, and treatment patterns of intussusception.
METHODS
Data sources
Hospitalization Data
We conducted a retrospective medical record review of intussusception hospitalizations among children <2 years of age during 2009–2013 at 3 hospitals in Chenzhou City, which is located in Hunan Province in Southern China and has an average annual population of ~150,000 children aged <2 years, and at 1 hospital in Kaifeng City, which is located in Henan Province in Northern China and has an average annual population of ~152,000 children aged <2 years (Figure 1). These 4 hospitals (The First Renmin Hospital of Chenzhou, The Third Renmin Hospital of Chenzhou, and Xiangnan Hospital in Chengzhou; Children’s Hospital of Kaifeng in Kaifeng) treat the majority of pediatric intussusception cases in the two cities [20]. At these hospitals, intussusception cases present to the Emergency Room or Outpatient Department and are subsequently admitted to the Inpatient Department.
Figure 1.
Map of hospitals included in the retrospective intussusception hospitalization review
We identified intussusception cases by discharge diagnosis using the International Classification of Disease, Tenth Revision, Clinical Modification (ICD-10-CM) code for intussusception (K56.1). We then reviewed the electronic medical records of these intussusception cases and used a standardized form to collect information on the child’s residence, date of birth, sex, date of admission, and clinical course, including date of symptom onset, signs and symptoms, diagnostic criteria met for case confirmation, treatment, complications, and outcome.
Population data
Population data from 2009 through 2013 for Chenzhou City and Kaifeng City were obtained from the National Statistics Bureau of China [21]. The populations of both cities remained relatively stable during the study period as immigrant populations are not common in these cities.
Statistical analysis
We calculated the annual incidence of intussusception hospitalizations per 100,000 children by age group, sex, and city using the number of intussusception cases as the numerator and the total number of children per corresponding age group as the denominator. Because the 4 hospitals included are the major centers with treatment capability for pediatric intussusception in these two cities, we assumed that all children from Chenzhou and Kaifeng with intussusception were treated at these hospitals and excluded patients who were not residents of the two cities from the numerator. We used Microsoft Excel (version 2010) and SPSS (version 17, SPSS Inc., Chicago, IL, USA) for all statistical analyses.
This retrospective review was determined to be public health non-research by the China CDC.
RESULTS
During 2009–2013, 1715 hospitalizations for intussusception among 1,487,215 children <2 years of age occurred in Chenzhou and Kaifeng cities (583 among 751,770 children in Chenzhou and 1132 among 735,445 children in Kaifeng). (Table 1). By age group, the average annual incidence rates of intussusception for both cities combined were 181.8 per 100,000 children <1 year of age, 56 per 100,000 children 1 to <2 years of age, and 112.9 per 100,000 all children <2 years of age. Comparing incidence rates for each city, intussusception hospitalization rates were higher for all age groups in Kaifeng versus Chenzhou.
Table 1.
Characteristics of the infants with intussusception, by city – 2009–2013
| Characteristic | City | Both cities (n=1715) | |
|---|---|---|---|
| Chenzhou (n=583) | Kaifeng (n=1132) | ||
| Age in months, median(range) | 8(0–23) | 8(0–23) | 8(0–23) |
| Average annual intussusception rate per 100 000, by age | |||
| < 1 year | 122.1 | 257.7 | 181.8 |
| 1–2 years | 35.3 | 75.3 | 56 |
| <2 years | 77.4 | 148.8 | 112.9 |
| Average annual intussusception rate per 100 000, by sex | |||
| Male | 102 | 189.2 | 144.7 |
| Female | 48.3 | 103.2 | 74.9 |
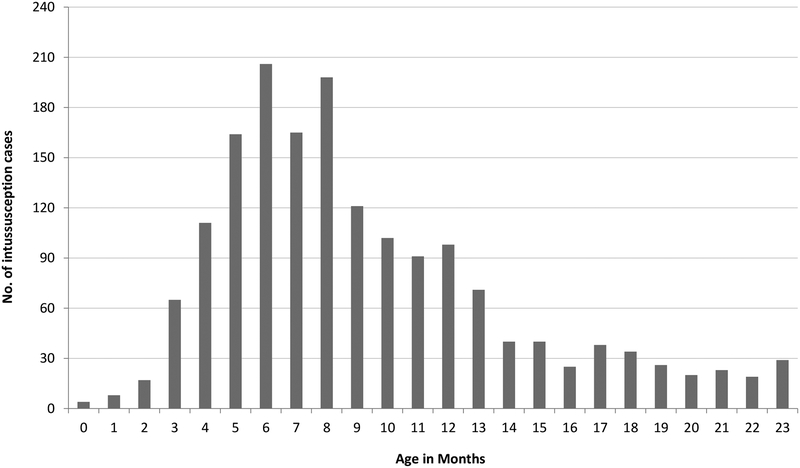
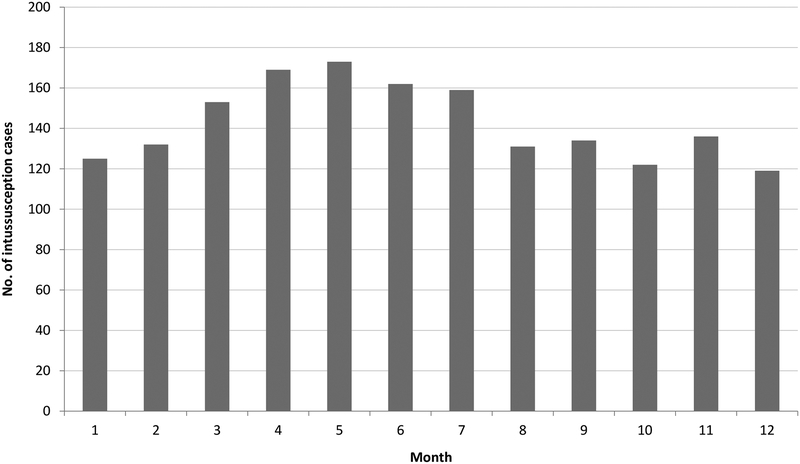
Of the 1715 hospitalized intussusception cases, nearly 70% were male. The median age of cases was 8 months (range: 0 to 23 months), with peak incidence occurring between 6 and 8 months (Figure 2). Although the monthly occurrence of intussusception hospitalizations varied during the study period, no clear seasonality was observed (Figure 3). In total, 78 (4.5%) children were hospitalized for multiple episodes of intussusceptions (Table 2); 64 (82.1%) of these 78 children had experienced 2 episodes of intussusception, and 14 (17.9%) had experienced 3 or more episodes of intussusception.
Figure 2.
Age distribution of intussusception hospitalizations among children <2 years of age in Chenzhou and Kaifeng, China, 2009–2013
Figure 3.
Monthly distribution of intussusception cases among children <2 years of age in Chenzhou and Kaifeng, China, 2009–2013
Table 2.
Clinical course for infants with intussusception, by city – 2009–2013
| Clinical course | City | Both cities (n=1715) | |
|---|---|---|---|
| Chenzhou (n=583) | Kaifeng (n=1132) | ||
| No. (%) of children with multiple episodes | |||
| 2 episodes | 22 (3.8) | 42 (3.7) | 64 (3.7) |
| >2 episodes | 5 (0.9) | 9 (0.8) | 14 (0.8) |
| Total | 27 (4.6) | 51 (4.5) | 78 (4.5) |
| Clinical symptoms, no. (%) | |||
| Diarrhea | 72 (13.3) | 35 (3.1) | 107(6.2) |
| Vomiting | 455 (78.0) | 891 (78.7) | 1346 (78.5) |
| Fever | 121 (20.8) | 122 (10.8) | 243 (14.2) |
| Bloody stool | 372 (63.8) | 683 (60.3) | 1055(61.5) |
| Abdominal distension | 185 (31.7) | 98 (8.7) | 283 (16.5) |
| Abdominal mass | 208 (35.7) | 962 (85.0) | 1170(68.2) |
| Diagnosis, no. (%) | |||
| Ultrasound | 155 (26.6) | 325 (28.7) | 480(28.0) |
| Radiography | 5 (0.9) | 22 (1.9) | 27 (1.6) |
| Contrast enema | 9 (1.5) | 1 (0.1) | 10 (0.6) |
| Ultrasound+radiography | 21 (3.6) | 782 (69.1) | 803(46.8) |
| Ultrasound+contrast enema | 324 (55.6) | 2 (0.2) | 326(19.0) |
| Radiography+contrast enema | 2 (0.3) | 0 (0) | 2(0.1) |
| Ultrasound+radiography+contrast enema | 59 (10.1) | 0 (0) | 59 (3.4) |
| Other* | 8 (1.4) | 0 (0) | 8 (0.5) |
| Treatment, no. (%) | |||
| Reduction by air enema | 315 (54.0) | 928 (82.0) | 1243 (72.5) |
| Reduction by liquid enema | 113 (19.4) | 21 (1.9) | 134 (7.8) |
| Surgery without resection | 127 (21.8) | 152(13.4) | 279(16.3) |
| Surgery with resection | 19 (3.3) | 25 (2.2) | 44 (2.6) |
| Outcome, no. (%) | |||
| Discharged | 574 (98.5) | 1132 (100) | 1706 (99.5) |
| Transferred | 5 (0.9) | 0 (0) | 5 (0.3) |
| Died | 0 (0) | 0 (0) | 0 (0) |
| Unknown | 4 (0.7) | 0 (0) | 4 (0.2) |
| Duration of hospitalization in days, median(range) | 4 (0–77) | 3 (0–24) | 4 (0–77) |
| Time between onset of symptoms and admission in days, median (range) | 1 (0–9) | 1 (0–13) | 1 (0–13) |
Intussusception cases diagnosis by ‘other’ methods included 4 cases identified by ultrasound+contrast enema+CT, 2 identified by ultrasound+CT, 1 identified by ultrasound+radiography+contrast enema+CT, 1 identified by contrast enema+CT
Signs and symptoms observed at the time of presentation included diarrhea, vomiting, fever, bloody stool, abdominal distension and abdominal mass (Table 2). Vomiting, abdominal mass, and bloody stool were the most frequently reported, observed in 78.5%, 68.2% and 61.5% of all cases, respectively. Frequencies of reported signs and symptoms were similar between the two cities, except that a greater proportion of children were noted to have abdominal masses in Kaifeng compared with Chenzhou (85% vs. 35.7%). Over 95% of intussusception cases were diagnosed by ultrasound alone or by ultrasound with a combination of other diagnostic methods, such as radiography, contrast enema, and/or computed tomography (CT); only 30% of cases were diagnosed by a single method (Table 2). The median time between the onset of symptoms and admission was 1 day (range: 0 to 13 days), and the median length of hospitalization was 4 days (range: 0 to 77 days). Of the 1715 cases, the majority (80.3%) was successfully treated by air or fluid enema; 279 (16.3%) required surgery without intestinal resection, and 44 (2.6%) required surgery with intestinal resection. Two cases in Kaifeng experienced complications of wound sepsis. No deaths occurred in either city (Table 2)
DISCUSSION
This study provides contemporary data on the baseline incidence and epidemiology of intussusception in two Chinese cities before the introduction of rotavirus vaccine into the Chinese routine immunization program. The baseline intussusception rate that we found in this review among children <1 year of age in the two cities (181.8 per 100,000 children) was higher than rates of intussusception reported in most other settings of the world (global median: 74 per 100,000 children) [22]. Compared with other low/very low childhood mortality locations in Asia, the intussusception rate for the two cities was higher than that reported for Taiwan (77 per 100,000) [23], Singapore (22.8–100.8 per 100,000) [24], Thailand (17.9–47.8 per 100,000) [25] and Malaysia, (17.8 per 100,000) [26], similar to that reported for Hong Kong (108 per 100,000) [27] and Japan [28] (185 per 100,000), and lower than that reported for South Korea (328 per 100,000) [17] and Vietnam (302 per 100,000) [18]. Furthermore, even in the two cities we examined, the intussusception rate in Kaifeng was almost 2-fold higher than that in Chenzhou. It has been suggested that genetic, dietary, and environmental factors may play an important role in the variation of incidence of intussusception worldwide [16,25,29–30]. However, differences in the study methodology and availability of diagnostic and treatment facilities for intussusception might also have contributed to the observed variation.
In both Chenzhou and Kaifeng, intussusception was most common in infants 4 to 9 months of age, peaking around 6 months of age. This age pattern is similar to that reported in studies from other parts of the world [29,31,32]. The very low incidence of intussusception in the first three months of life has been consistently described in many studies; these data support recommendations for timely administration of rotavirus vaccines at the recommended ages of 2 and 4 months (Rotarix) or 2, 4 and 6 months (RotaTeq). If rotavirus vaccine administration were delayed, first doses which have been associated with intussusception in other countries would be administered at an age with higher background rates of intussusception. Consequently, the number of natural intussusception cases that would be temporally related to vaccination by chance alone would be greater.
We observed no seasonal pattern in the distribution of intussusception cases in Chenzhou and Kaifeng. Although some settings have reported a peak season of intussusception during winter (Thailand, UK) [25, 33] or warmer months (Taiwan and Hong Kong) [34,35], most studies have not identified any seasonality of intussusception [36–39]. Previous surveillance indicates that the rotavirus season in these two cities occurs during the months of October through February, and the lack of any seasonal increase in intussusception during these months suggests that rotavirus is not a major cause of intussusception.
The most common clinical symptoms of intussusception reported in Chenzhou and Kaifeng were consistent with classic features of intussusception reported in other studies [18]. The abdominal mass was reported predominantly in Kaifeng but not the case in Chenzhou, which might be caused by the documentation. The vast majority (83.6%) of intussusception cases were successfully reduced by either air or liquid enema with only 18% requiring surgical intervention; no deaths were reported. The low rates of surgical intervention and fatality observed are likely explained by the timely presentation for intussusception treatment. Studies conducted in Thailand, Australia, Vietnam and Saudi Arabia have indicated that the proportion of intussusception cases requiring surgical intervention increased with the length of the time between the onset of intussusception and admission to the hospital [25, 40, 41]. In regions of the world with suboptimal access to health care and where children often present late for treatment of intussusception, such as in sub-Saharan Africa, nearly all patients require surgical intervention, and fatality rates of up to 25%−33% among hospitalized intussusception cases have been reported [42].
Some study limitations should be considered. First, it is possible that some children who live in Chenzhou or Kaifeng went to other hospitals to seek treatment for intussusception instead of the 4 hospitals in our study. This may have led us to underestimate intussusception incidence to some extent, although the hospitals we surveyed are the major centers in the area that would see most cases of intussusception. Second, since we conducted a retrospective study, our data were influenced by the quality of medical record keeping in the participating hospitals. Intussusception cases were only identified by ICD-10 code, and, aside from what was available in the medical record, no further effort could be made to validate the diagnosis. Third, because of resource constraints, we conducted our study in only two cities of China that are likely not representative of the entire country, but may be representative of other similar cities in China. Finally, although there is a locally licensed rotavirus vaccine (LLR) available in the private market in China, we could not determine the vaccination status of all intussusception cases due to insufficient record keeping of private market vaccine administration. Vaccination coverage is estimated to be approximately 20% in the larger cities, but data are lacking for other areas. Unpublished data from local CDCs [43] show that LLR coverage in Chenzhou in 2013 was about 20% and in Kaifeng from 2010–2013 was about 7%. Further investigation should be conducted to evaluate this issue.
This retrospective review provides important baseline information of current incidence and epidemiological characteristics of intussusception in two Chinese cities during 2009–2013 which will be important for evaluating rotavirus vaccine safety should it be introduced to the routine immunization program in China.
ACKNOWLEDGEMENTS
The authors would like to thank Zhiyi Xie, Weihao Luo in The First Renmin Hospital of Chenzhou, Dr. Li and Dr. Jin in Pediatric Hospital of Kaifeng, Xiaojing Shen in Henan CDC, Dr. Liu and Xi Wang in Kaifeng CDC for their assistance in conducting this study.
Footnotes
Conflicts of interest: The authors indicate that they have no conflicts of interest relevant to this article to disclose.
Publisher's Disclaimer: Disclaimer: The findings and conclusions of this report are those of the authors and do not necessarily represent the official positions of the Chinese Center for Disease Control and Prevention and US Centers for Disease Control and Prevention.
REFERENCES
- [1].Parashar UD, Hummelman EG, Bresee JS, et al. Global illness and deaths caused by rotavirus disease in children. Emerg Infect Dis. 2003;9:565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tate JE, Burton AH, Boschi-Pinto C, et al. WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. [DOI] [PubMed] [Google Scholar]
- [3].Murphy TV, Gargiullo PM, Massoudi MS, et al. Intussusception among infants given an oral rotavirus vaccine. N Engl J Med. 2001; 344:564–72. [DOI] [PubMed] [Google Scholar]
- [4].Parashar UD, Johnson H, Steele AD, Tate JE. Health Impact of Rotavirus Vaccination in Developing Countries: Progress and Way Forward. Clin Infect Dis. 2016;62 Suppl 2:S91–S95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Vesikari T, Van Damme P, Giaquinto C, et al. European Society for Paediatric Infectious Diseases consensus recommendations for rotavirus vaccination in Europe: update 2014. Pediatr Infect Dis J. 2015;34(6):635–643. [DOI] [PubMed] [Google Scholar]
- [6].Ruiz-Palacios GM, Perez-Schael I, Velazquez FR, et al. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. [DOI] [PubMed] [Google Scholar]
- [7].Vesikari T, Matson DO, Dennehy P, et al. Safety and efficacy of a pentavalent human-bovine (WC3) reassortantrotavirus vaccine. N Engl J Med. 2006;354:23–33. [DOI] [PubMed] [Google Scholar]
- [8].Haber P, Parashar UD, Haber M, DeStefano F. Intussusception after monovalent rotavirus vaccine-United States, Vaccine Adverse Event Reporting System (VAERS), 2008–2014. Vaccine. 2015;33(38):4873–4877. [DOI] [PubMed] [Google Scholar]
- [9].Leino T, Ollgren J, Strömberg N, Elonsalo U. Evaluation of the Intussusception Risk after Pentavalent Rotavirus Vaccination in Finnish Infants. PLoS One 2016;11(3):e0144812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yung CF, Chan SP, Soh S, Tan A, Thoon KC. Intussusception and Monovalent Rotavirus Vaccination in Singapore: Self-Controlled Case Series and Risk-Benefit Study. J Pediatr. 2015;167(1):163–168. [DOI] [PubMed] [Google Scholar]
- [11].Bauchau V, Van Holle L, Mahaux O, et al. Post-marketing monitoring of intussusception after rotavirus vaccination in Japan. Pharmacoepidemiol Drug Saf. 2015;24(7):765–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Escolano S, Hill C, Tubert-Bitter P. Intussusception risk after RotaTeq vaccination: evaluation from worldwide spontaneous reporting data using a self-controlled case series approach. Vaccine. 2015;33(8):1017–1020. [DOI] [PubMed] [Google Scholar]
- [13].Pepper VK, Stanfill AB, Pearl RH. Diagnosis and management of pediatric appendicitis, intussusception, and Meckel diverticulum. Surg Clin North Am 2012, 92:505–526. vii. [DOI] [PubMed] [Google Scholar]
- [14].Bines JE, Patel M, Parashar U. Assessment of postlicensure safety of rotavirus vaccines, with emphasis on intussusception. J Infect Dis. 2009, 200(Suppl 1):S282–S290. [DOI] [PubMed] [Google Scholar]
- [15].Huppertz HI, Soriano-Gabarro M, Grimprel E, et al. Intussusception among young children in Europe. Pediatr Infect Dis J. 2006, 25:S22–S29. [DOI] [PubMed] [Google Scholar]
- [16].World Health Organization. Acute intussusception in infants and children Incidence, clinical presentation and management: a global perspective. Geneva, Switzerland: World Health Organization; 2002. p. 1–98. [Google Scholar]
- [17].Jo DS, Nyambat B, Kim JS, et al. Population-based incidence and burden of childhood intussusceptions in Jeonbuk Province, South Korea. Int J Infect Dis. 2009;13: e383–8. [DOI] [PubMed] [Google Scholar]
- [18].Bines JE, Liem NT, Justice FA, et al. Intussusception Study Group. Risk factors for intussusception in infants in Vietnam and Australia: adenovirus implicated, but not rotavirus. J Pediatr. 2006;149:452–60. [DOI] [PubMed] [Google Scholar]
- [19].Liu N, Yen C, Fang ZY, et al. Projected health impact and cost-effectiveness of rotavirus vaccination among children <5 years of age in China. Vaccine. 2012;30(48):6940–6945. [DOI] [PubMed] [Google Scholar]
- [20].Personal communication, Liqing Li from Kaifeng Children’s Hospital, Zhiyi Xie from the First Renmin Hospital of Chenzhou.
- [21].National Statistics Bureau of China. Network reporting system of infectious diseases population database. 2014. Accessed on 2 March 2015.
- [22].Jiang J, Jiang B, Parashar U, et al. Childhood intussusception: a literature review. PLoS One. 2013;8(7):e68482. doi: 10.1371/journal.pone.0068482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen SC, Wang JD, Hsu HY, et al. Epidemiology of childhood intussusception and determinants of recurrence and operation: analysis of national health insurance data between 1998 and 2007 in Taiwan. Pediatr Neonatol. 2010;51: 285–291. [DOI] [PubMed] [Google Scholar]
- [24].Tan N, Teoh YL, Phua KB, et al. An update of paediatric intussusception incidence in Singapore: 1997–2007, 11 years of intussusception surveillance. Ann Acad Med Singapore. 2009;38: 690–692. [PubMed] [Google Scholar]
- [25].Khumjui C, Doung-ngern P, Sermgew T, et al. Incidence of intussusception among children 0–5 years of age in Thailand, 2001–2006. Vaccine. 2009;27 Suppl 5: F116–119. [DOI] [PubMed] [Google Scholar]
- [26].Giak CL, Singh HS, Nallusamy R, et al. Epidemiology of intussusception in Malaysia: a three-year review. Southeast Asian J Trop Med Public Health. 2008;39: 848–855. [PubMed] [Google Scholar]
- [27].Hong Kong Intussusception Study Group. Intussusception trends in Hong Kong children. Hong Kong Med J. 2007;13: 279–283. [PubMed] [Google Scholar]
- [28].Nakagomi T, Takahashi Y, Arisawa K, Nakagomi O. A high incidence of intussusception in Japan as studied in a sentinel hospital over a 25-year period (1978–2002). Epidemiol Infect. 2006;134: 57–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tate JE, Simonsen L, Viboud C, et al. Trends in intussusception hospitalizations among US infants, 1993–2004: implications for monitoring the safety of the new rotavirus vaccination program. Pediatrics. 2008; 121: e1125–e1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Justice F, Carlin J, Bines J. Changing epidemiology of intussusceptions in Australia. J Paediatr Child Health. 2005; 41: 475–478. [DOI] [PubMed] [Google Scholar]
- [31].Fischer TK, Bihrmann K, Perch M, et al. Intussusception in early childhood: a cohort study of 1.7 million children. Pediatrics. 2004;114(3):782–785. [DOI] [PubMed] [Google Scholar]
- [32].Parashar UD1, Holman RC, Cummings KC, et al. Trends in intussusception-associated hospitalizations and deaths among US infants. Pediatrics. 2000;106(6):1413–1421. [DOI] [PubMed] [Google Scholar]
- [33].Samad L, Cortina-Borja M, Bashir HE, et al. Intussusception incidence among infants in the UK and Republic of Ireland: a pre-rotavirus vaccine prospective surveillance study. Vaccine. 2013;31(38):4098–4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ho WL, Yang TW, Chi WC, et al. Intussusception in Taiwanese in children: analysis of incidence, length of hospitalization and hospital cost in different age groups. J Formos Med Assoc. 2005;104(6): 398–401. [PubMed] [Google Scholar]
- [35].Nelson EA, Tam JS, Glass RI, et al. Incidence of Rotavirus Diarrhea and Intussusception in Hong Kong Using Standardized Hospital Discharge Data. Pediatr Infect Dis J. 2002;21(7): 701–703. [DOI] [PubMed] [Google Scholar]
- [36].Buettcher M, Baer G, Bonhoeffer J, et al. Three-Year Surveillance of Intussusception in Children in Switzerland. Pediatrics. 2007;120(3): 473–480. [DOI] [PubMed] [Google Scholar]
- [37].Jenke AC, Klaassen-Mielke R, Zilbauer M, et al. Intussusception: incidence and treatment-insights from the nationwide German surveillance. J Pediatr Gastroenterol Nutr. 2011;52(4):446–51. [DOI] [PubMed] [Google Scholar]
- [38].Chen YE, Beasley S, Grimwood K; New Zealand Rotavirus Study Group. Intussusception and rotavirus associated hospitalisation in New Zealand. Arch Dis Child. 2005;90(10):1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Weiß S, Streng A, Kries Rv, Liese J, Wirth S, Jenke AC. Incidence of intussusception in early infancy: a capture-recapture estimate for Germany. Klin Padiatr. 2011;223(7):419–23. [DOI] [PubMed] [Google Scholar]
- [40].Blanch AJ, Perel SB, Acworth JP. Paediatric intussusception: epidemiology and outcome. Emerg Med Australas. 2007;19(1):45–50. [DOI] [PubMed] [Google Scholar]
- [41].Crankson SJ1, Al-Rabeeah AA, Fischer JD, et al. Idiopathic intussusception in infancy and childhood. Saudi Med J. 2003;24 Suppl:S18–20. [PubMed] [Google Scholar]
- [42].Mpabalwani EM, Chitambala P, Chibumbya JN, et al. Intussusception incidence rates in 9 Zambian hospitals, 2007–2011: prerotavirus vaccine introduction. Pediatr Infect Dis J. 2014;33 Suppl 1:S94–8. [DOI] [PubMed] [Google Scholar]
- [43].Chenzhou CDC and Kaifeng CDC, rotavirus vaccine usage data from 2009–2013.