Abstract
There is a paucity of primary literature directly comparing tongue-lip adhesion (TLA) versus mandibular distraction osteogenesis (MDO) in surgical treatment of patients with Pierre Robin Sequence (PRS). This study comprehensively reviews the literature to evaluate and compare the effectiveness of MDO and TLA in improving airway and feeding outcomes. A search was performed using the MEDLINE (PubMed interface) and Embase databases for publications between 1960 through June 2017. English-language, original studies involving MDO or TLA in treatment of PRS subjects were included. Extracted data included prevention of tracheostomy (primary airway outcome) and ability to feed exclusively by mouth (primary feeding outcome). Sixty-seven studies total were included in the review. Ninety-five percent (657/693) of subjects treated with MDO avoided tracheostomy. Eighty-nine percent (289/323) of subjects treated with TLA avoided tracheostomy. Eighty-seven percent (323/370) of subjects treated with MDO achieved full oral feeds at latest follow-up. Seventy percent (110/157) of subjects treated with TLA achieved full oral feeds at latest follow up. The rate of second intervention for recurrent obstruction ranged from 4 to 6% in MDO studies, compared to range of 22 to 45% in TLA studies. Variability of patient selection, surgical techniques, outcomes measurement methods, and follow up length across studies precluded meta-analysis of the data. Both MDO and TLA are effective alternatives to tracheostomy for patients who fail conservative management, improve feeding and promote weight gain. MDO may be superior to TLA in long-term resolution of airway obstruction and avoidance of gastrostomy, but is associated with notable complications.
Introduction
Pierre Robin sequence (PRS), first described in the French literature in 19341, refers to the triad of micrognathia, glossoptosis and airway obstruction (Figure 1). In 73–90% of cases2–5, infants also present with a U-shaped or V-shaped cleft of the secondary palate. The incidence reported in the literature ranges from 1/31206 live births to 1/8060 live births4. PRS can be an isolated finding, or can be associated with a genetic syndrome in approximately 50% of cases2,7–10.
Figure 1.
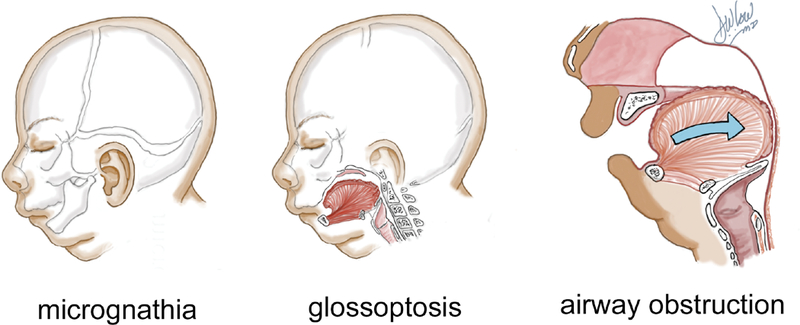
Pierre Robin Sequence triad: micrognathia, glossoptosis, upper airway obstruction
The two primary clinical sequalae of PRS are tongue-based airway obstruction and feeding difficulties. The small jaw contributes to the tendency for the tongue to fall back into the pharynx, occluding the upper airway11. The airway obstruction, as well as the associated cleft palate, contributes to poor weight gain and feeding difficulties. An estimated 40–70% of PRS cases need nasogastric tube feeding for up to several months, and may even require gastrostomy tube feeding12–14.
Treatment of airway obstruction begins with non-invasive techniques. Placing the infant in a prone position allows for the tongue to fall forward and reduce airway obstruction. In 40–70% of cases, positioning alone is successful in relieving obstruction12,15,16. If positioning fails to relieve airway obstruction, placement of a nasopharyngeal airway (NPA) can serve as a temporizing measure while awaiting mandibular growth17,18. Infants requiring additional respiratory support may require continuous positive airway pressure (CPAP)19.
Although conservative management is sufficient to relieve airway obstruction for many neonates with PRS20, those who fail will ultimately require surgical intervention. Historically, tracheostomy was the only option for long-term airway stabilization21 and the preferred intervention among otolaryngologists22. However, it is associated with significant morbidity and mortality23. Carr et al110 reported a 43% rate of serious complications and 0.7% mortality rate, mostly due to accidental decannulation or obstructions. Tracheostomy is burdensome for caretakers111, requiring regular maintenance and suctioning to prevent mucous obstruction112. 60% of parents of children with non-syndromic and 80% of parents of children with syndromic PRS surveyed described the overall experience with tracheostomy as difficult and cited prolonged tracheostomy as a major concern111. Furthermore, children with PRS who underwent tracheostomy were not decannulated until an average age of 3.1 years21. The prolonged dependence on tracheostomy may contribute to significant delays in speech production and language development113.
Previously, the only surgical alternative to tracheostomy was glossopexy or tongue-lip adhesion (TLA). Popularized by Douglas in 194624, with subsequent revisions in technique25–27, the procedure anchors the tongue to the lower lip and mandible, securing the tongue in an anterior lingual position, preventing occlusion of the upper airway (Figure 2). It is typically performed in the first months of life and reversed at around 12 months of age, often at the time of cleft palate repair.
Figure 2.
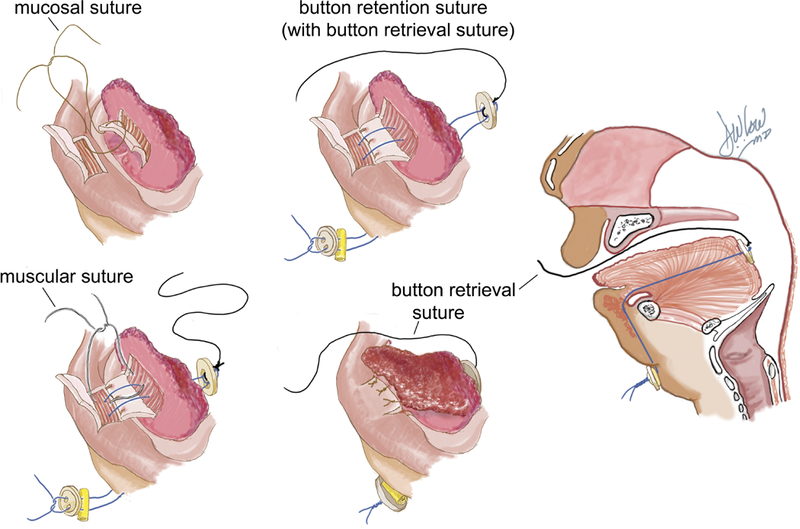
Tongue-lip adhesion technique showing mucosal, button retention, muscular and button retrieval sutures
More recently, mandibular distraction has gained popularity as an alternative method of treating this form of airway obstruction. Initially applied to patients who had already undergone tracheostomy and/or TLA28,29, over the past decade it has been adopted by many centers as the primary surgical intervention for tongue-based airway obstruction in PRS patients. Lengthening the mandible brings the tongue forward through its attachments to the lingual surface of the mandible, addressing both glossoptosis and micrognathia30, relieving occlusion of the airway and creating more space for the tongue (Figure 3) (See Video, Supplemental Digital Content 1, which demonstrates our technique for mandibular distraction osteogenesis for patients with PRS, available in the “Related Videos” section of the Full-Text article on PRSJournal.com or, for Ovid users, available at INSERT HYPER LINK) (Video Graphic 1).
Figure 3.
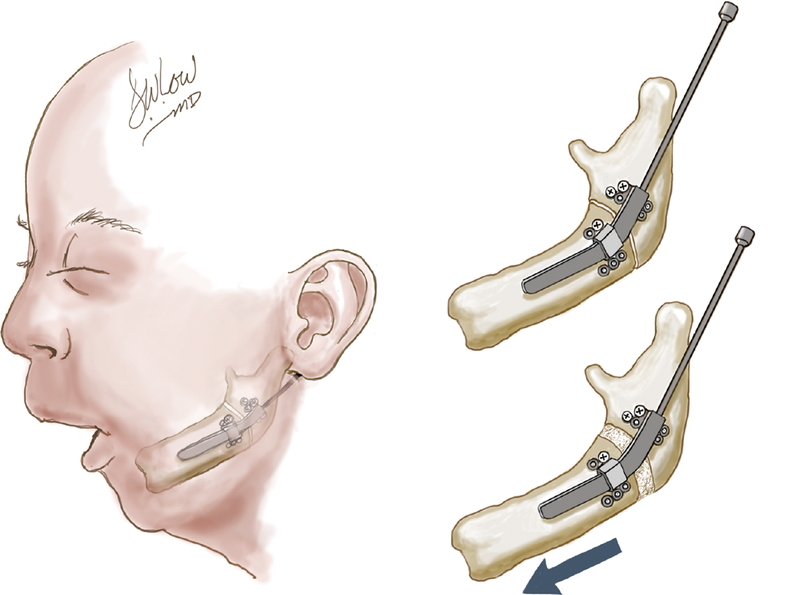
Mandibular distraction osteogenesis in the micrognathic patient.
In addition to avoiding the complications associated with tracheostomy, both MDO114–116 and TLA6 have a lower cost burden. However, it is important to emphasize that tracheostomy may be the optimal and life-saving intervention for patients with multiple sites of airway obstruction (i.e. subglottic stenosis, tracheomalacia), significant secondary respiratory abnormalities and central apnea85,109.
The decision to undergo either MDO or TLA is influenced greatly by the surgeon’s specialty and experience7,22,31–33. A number of studies have proposed algorithms and patient selection guidelines34–39, but these rely largely on expert opinion or results from small studies. Furthermore, there is a paucity of primary literature directly comparing outcomes of TLA versus MDO.
Given the limited number of original research studies, this review aims to comprehensively compile data on specific defined endpoints from the existing literature. Evaluation of the data on airway and feeding outcomes in MDO and TLA will assist surgeons caring for PRS patients with their choice of intervention and in counseling of families.
Methodology
A search was performed using the MEDLINE (PubMed interface) and Embase databases for publications between 1960 through June 2017. Selected articles were analyzed with regard to level of evidence40, patient demographics, length of follow up, diagnosis, distractor type, airway and feeding outcomes. With the exception of two studies41,42, all studies included a mixed cohort of syndromic and isolated PRS subjects. We present our search criteria in Table 1, and literature selection and outcomes measurement methodology in Figure 4.
TABLE 1.
Database and search terms used to identify relevant articles
| Database | Date Searched | Search Terms |
|---|---|---|
| Medline | 1960 to June 2017 | MeSH terms: “Pierre Robin syndrome”, “airway obstruction” combined with “retrognathia” or “micrognathia”, “osteogenesis, distraction” combined with “airway obstruction”; Free text keywords: “glossopexy”, “tongue lip adhesion” |
| Embase | 1960 to June 2017 | Disease search term: “pierre robin syndrome” queried with subheadings “surgery”, “disease management” and “therapy” |
Figure 4.
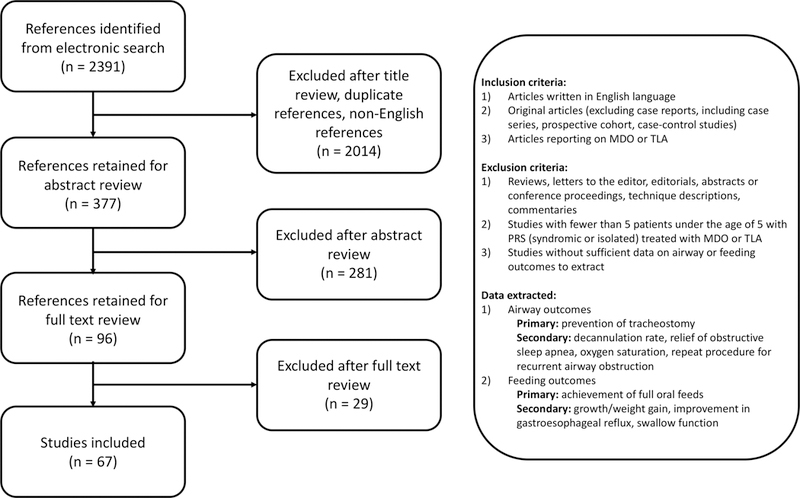
Flowchart of literature selection criteria and strategy
Results
Airway outcomes and feeding outcomes extracted from individual studies are presented in Table 2 and Table 3, respectively, along with pertinent patient demographics and reported complications. Table 4 summarizes the aggregated data for the primary outcomes, specifically avoidance of tracheostomy and achievement of full oral feeds at latest follow up. Given that most studies were single intervention case series that did not directly compare MDO and TLA, the four comparison studies, the highest level of evidence available in the literature, are presented in detail in Table 5.
Table 2.
Comparison of Airway Outcomes
| MDO article |
Type of Distractor |
Patient selection/surgical indication |
Tracheostomy prevented /Decannulation |
OSA | Perioperative Complications |
Follow up length |
Level of Evidence/ MINORS |
Notes |
|---|---|---|---|---|---|---|---|---|
| Al-Samkari HT et al.60 | external | 42% syndromic, failure of conservative management | Prevented: 91.67% (11/12) | N/A | Not reported | not reported, available in only 67% (8/12) patients | IV/10 | emergency tracheostomy 10 days after distractor removal in syndromic patient |
| Andrews BT et al.85 | internal nonresorbable | 29% syndromic, exclude central sleep apnea, severe GER, or concomitant airway anomalies | Prevented: 97.26% (71/73) | N/A | dental injuries (6%), neck scarring (8%), wound infection (5%), temporomandibular joint ankyloses (10%) | 64.8 months | IV/12 | 1 subject required tracheostomy for unrecognized central sleep apnea, 1 subject had emergent tracheostomy for loss of endotracheal tube on transport after surgery |
| Bangiyev JN et al.88 | external | Syndrome not specified, 52% laryngomalacia, 24% tracheomalacia, 8% bronchomalacia | N/A | 73.1% demonstrated severe pre-MDO sleep apnea (AHI>10); AHI: preoperative 30.3, postoperative 8.7 (p<0.001) | Not reported | at least 1 year | IV/12 | |
| Breugem C et al.89 | internal resorbable | 17% syndromic, failed conservative therapy, exclude other airway obstruction | Prevented: 100.00% (11/11) | N/A | 18% (1 cellulitis, 1 lost pin during consolidation) | not reported | IV/10 | |
| Burstein FD et al.80 | internal resorbable | 25% syndromic, require supplemental O2, CPAP, intubation | Prevented: 100.00% (14/14); Decannulated: 83.33% (5/6) | preoperative RDI 15.34; postoperative RDI 1.11 | 28.5% (4 pin site infections) | 24 months | IV/12 | |
| Cascone P et al.90 | external | 7% syndromic, respiratory crisis at birth and endotracheal intubation | N/A | AHI: preoperative 80.1, postoperative 2.1 | 14% (4 loss of external pin during consolidation) | 1 month | IV/12 | |
| Chigurupati R et al.91 | internal nonresorbable | 40% syndromic, fail conservative therapy | Prevented: 100.00% (2/2) Decannulated: 66.67% (2/3) | N/A | 25% (pin site infection requiring early removal) | not reported | IV/10 | |
| Ching JA et al.83 | external | 8% syndromic, no central apnea, laryngomalacia or tracheal stenosis | Prevented: 93.94% (31/33) | N/A | 30% (4 pin site infection, 6 operative replacement of pin or device) | range 6 months to 10 years, mean 77.4 months | IV/12 | 1 failed due to laryngomalacia diagnosed postoperatively, previously not visualized |
| Denny A, Amm C59 | external | 63% syndromic, no secondary sites of airway obstruction | Prevented: 100.00% (11/11) | obtained in 7 patients, all previously severely impaired, normal 1 week to 1 month after operation | None | 60 months | IV/12 | 1 patient previously failed TLA |
| Denny A, Kalantarian B58 | external | Syndromic status not specified, excluded patients with lower airway anomalies | Prevented: 100% (5/5) | all discharged home on apnea monitors, discontinued use after 90 days due to no apneic events | 25% (1 device failure needing replacement) | range 9–22 months | IV/12 | |
| Denny AD, Talisman R, Hanson PR et al.29 | external | 40% syndromic, fail conservative therapy | Decannulated: 66.67% (2/3) | 10 subjects with predistraction apnea, none had triggering of apnea monitor 3 months post distraction | 10% (1 pin displacement) | at least 6 months | IV/12 | 1 subject with Treacher Collins failed to distract because of premature consolidation and continued to require cannula |
| Flores RL, Greathouse ST, Costa M et al.79 | internal nonresorbable | 31% syndromic, include laryngomalacia, no central apnea, no other secondary airway anomaly | Prevented: 91.36% (74/81), 1 death due to apneic disease | 8.11% (6) had AHI >20 postoperatively | Not reported | at least 1 year | IV/12 | overlapping cohort with Tahiri et al, Murage et al., Tholpady et al., Flores RL, Tholpady SS, Sati S et al |
| Genecov DG et al.66 | external and internal | 4% syndromic, exclude laryngomalacia, tracheomalacia, subglottic/supraglottic stenosis, neurologic impairment, untreated GERD with vocal cord/epiglottic edema | Prevented: 96.15% (25/26) Decannulated: 92.00% (38/42) | preoperative RDI 35–50 to postoperative RDI 5–15 in 65 patients; in 2 patients RDI remained above 35 postoperatively | 13% (9 pin site infections) 13% (9 device failure), 9% (6 asymmetric movement of depressor anguli oris muscle) | not reported | IV/10 | 2 failed decannulations due to previously undiagnosed swallowing abnormalities and inability to handle secretions |
| Goldstein JA et al.77 | internal nonresorbable | 46% syndromic, fail conservative therapy | Prevented: 87.50% (21/24) Decannulated: 100.00% (2/2) | AHI: preoperative PSGs in 20 subjects, 39.3±22.0; postoperative PSGs in 14 subjects, 3.0±1.5 (p < 0.0001) | 4%(1 transient facial nerve paralysis), 8% (2 pin site infections), 4%(1 major submental abscess) | not reported | IV/10 | 1 patient underwent TLA after MDO |
| Gozu A et al.92 | external | 25% syndromic, fail conservative therapy | Prevented: 100% (4/4) | N/A | Not reported | 44.4 months | IV/12 | |
| Hammoudeh J et al.93 | internal nonresorbable | 25% syndromic, no lower airway anomaly | Prevented: 96.55% (28/29), 1 patient death | 8 subjects requiring preoperative intubation: AHI preoperative 39.7, postoperative 3.13; remaining 20 subjects: AHI preoperative 39.7, postoperative 5.8. | 14% (1 device failure, 1 transient facial nerve palsy, 1 device exposure, 1 pin site infection) | 18.7 months | IV/12 | 1 tracheostomy in patient with interstitial lung disease and central apnea, 1 patient expired soon after surgery, (had multiple comorbidities) |
| Hong P et al.65 | internal nonresorbable | 67% syndromic, all had GERD, no central apnea, no lower airway anomaly | Prevented: 100.00% (6/6) | N/A | 33% (2 local erythema) | 18 months | IV/12 | |
| Izadi K et al.94 | internal nonresorbable | 47% syndromic, central apnea, severe reflux, other airway lesions | Prevented: 93.33% (14/15) | N/A | None reported | 14 months | IV/12 | |
| Konas E et al.95 | internal nonresorbable | 8% syndromic, fail conservative therapy | Prevented: 100.00% (13/13) | N/A | 30% (1 facial nerve palsy, 1 reintubation, 1extension rod fracture, 2 pin site infections) | at least 1 year | IV/12 | |
| Lam DJ et al.39 | primarily external, few had internal | 56% syndromic, fail conservative therapy | Prevented: 83.61% (51/61) Decannulation: 67.74% (42/62) | N/A | 27% (14 premature consolidation, 9 open bite deformity, 5 TMJ ankylosis) | 30 months | IV/12 | poorer odds of success associated with craniofacial microsomia or Goldenhar syndrome (OR, 0.07 [95% CI, 0.009–0.52]) |
| Lin SY et al.96 | internal nonresorbable | None syndromic, all have OSA | Prevented: 100.00% (4/4) Decannulated: 100.00% (1/1) | 3/5 had resolution of airway obstruction documented by PSG, AHI<1.5 and no snoring, 4th child had primary snoring without apnea, 5th child severe OSA with AHI 20.2 | Not reported | median 47.5 months | IV/12 | |
| Mahrous MA et al.47 | internal nonresorbable | None syndromic, all had severe OSA | Decannulated: 100.00% (4/4) | preoperative RDI 14 to postoperative RDI 1 | 36% (incomplete osteotomy on one side, facial nerve palsy, pin site infection) | range 12–24 months | IV/12 | |
| Mandell DL et al.44 | external and internal | 50% syndromic, exclude secondary airway anomaly, central sleep apnea, severe GERD | Prevented: 100.00% (8/8) Decannulated: 16.67% (2/12) | 85% (5) had improvement of OSA, 3/6 had postoperative sleep studies showing resolution | 23% (3 premature consolidation, 2 cheek abscess, 1 lip erosion, 1 cellulitis, 1 facial nerve palsy, 2 TMJ ankylosis) | 13 months | IV/12 | |
| Meyer AC et al.97 | external | 33% syndromic, fail conservative therapy | Prevented: 100.00% (18/18) | N/A | Not reported | 79 months | IV/12 | overlapping cohort with Tibesar et al. and Scott AR et al. |
| Mingo KM et al.41 | external | only isolated PRS or Stickler’s, compared patients with or without preoperative CT | Prevented: 98.04% (50/51) Decannulated: 100.00% (1/1) | N/A | 12% (2 fracture between 2 posterior pins 1 bilateral hardware loosening, 1 temporary reintubation, 2 pin site infections, 1 unilateral marginal mandibular nerve weakness) | range 1–8 years | III/12 | |
| Mitsukawa N et al.98 | internal nonresorbable | 30% syndromic, fail conservative therapy, have OSA | Prevented: 100.00% (8/8) Decannulated: 100.00% (2/2) | AHI: preoperative range 9.6 to 18.8, postoperative range 0.6 to 3.6 | 10% (device dislodged and reoperation) | range 6 months to 4 years | IV/12 | |
| Monasterio FO, Drucker M, Molina F et al.28 | external | Syndrome not specified, fail conservative therapy | Decannulated: 100.00% (3/3) | preoperative apnea index 20.5, hypopnea index 7.4; all resolved postoperatively | 13% (1 device dislodged, 1 abscess) | 3 months | IV/10 | |
| Monasterio FO, Molina F, Berlanga F et al.45 | external | 6% syndromic, fail conservative therapy | N/A | Preoperative apnea index 18.3; preoperative hypopnea index 8.5; both disappeared postoperatively | 33% (5 pin site infection, 1 incomplete corticotomy requiring reoperation) | not reported | IV/8 | |
| Morovic CG, Monasterio L99 | internal nonresorbable | 29% syndromic, exclude central apnea and other airway anomalies | Prevented: 100% (5/5) Decannulated: 100.00% (2/2) | postoperative apnea index range 8 to 18 | 29% (2 pin extrusions) | range 1 to 45 months | IV/14 | Prospective study |
| Mudd PA et al.53 | internal nonresorbable | 29% syndromic, fail conservative therapy | Prevented: 100.00% (24/24) | N/A | 42% (1 facial paralysis due to compression of posteriorly displaced distraction arm, 2 facial nerve injury, 1 marginal mandibular weakness, 6 cellulitis) | 28.8 months | IV/12 | |
| Murage KP et al.55 | internal nonresorbable | 22% syndromic, fail conservative therapy | Prevented: 92.00% (46/50) | AHI: preoperative 37.8±25.6, postoperative 6.5±8.03 (p<0.05) | Device failure (2%), surgical site infection (22%), transient facial nerve palsy (2%). | 37 months | IV/12 | 3 required repeat MDO |
| Rachmiel A, Emodi O, Rachmiel D et al.100 | internal nonresorbable | Syndrome not specified, exclude tracheomalacia | Prevented: 100.00% (11/11) Decannulated: 100.00% (7/7) | Apnea index: preoperative >20, postoperative <2 | 5% pin site infection, 11% damage to mandibular branch of facial nerve | 12 months | IV/12 | |
| Rachmiel A, Srouji S, Emodi O et al.46 | external | 72% syndromic, severe OSA and tracheostomy | Decannulated: 100.00% (11/11) | Preoperative RDI>25, postoperative <2 | Not reported | not reported | IV/8 | |
| Scott AR et al.101 | external | 26% syndromic, fail conservative therapy | Prevented: 100.00% (17/17) Decannulated: 50.00% (1/2) | N/A | 5% (1 anterior open-bite deformity), 21% (4 long-term tooth loss/malformation), 16% (injury to marginal mandibular branch of facial nerve), 16% (3 hypertrophic scars), 5% (1 repeat MDO) | 67.2 months | IV/12 | overlapping cohort with Tibesar et al. and Meyer et al. |
| Senders CW et al.102 | external | 23% syndromic, fail conservative therapy | Prevented: 90.91% (10/11) Decannulated: 100.00% (2/2) | N/A | 15% (2 pin site infection) 8% (1 greenstick fracture), 8% (1 loose pins), 8% (1 mandibular branch of facial nerve palsy) | 32.7 months | IV/12 | subject requiring tracheostomy later diagnosed with central hypoventilatory syndrome |
| Shen W et al.103 | internal nonresorbable | Syndrome not specified, fail conservative therapy | Prevented: 100.00% (6/6) | N/A | None reported | 6 months | IV/10 | |
| Schoemann MB et al.104 | internal resorbable | 18% syndromic, fail conservative therapy | Prevented: 100.00% (18/18) | N/A | 5% (1 pin site infection) | not reported | IV/8 | immediate 5mm at time of distractor placement |
| Sidman Jd et al.105 | external | Isolated PRS only, fail conservative therapy | Prevented: 100.00% (2/2) | N/A | N/A | more than 3 years | IV/13 | |
| Tahiri Y et al.106 | internal nonresorbable | 34% syndromic, exclude central apnea, include laryngomalacia | Prevented: 93.82% (76/81) Decannulated: 63.64% (7/11) | AHI: preoperative, 41.5; 1 month postoperative, 12.1; 1 year postoperative, 5.8 | 9% surgical site infection, 7.5% ventilator-associated pneumonia, 1.2% hematoma, 1.2% facial nerve neurapraxia | 21.6 months | III/12 | all infants <4kg, overlapping cohort with Flores RL, Greathouse ST, Costa M et al, Tholpady et al, and Murage et al. |
| Tholpady SS et al.86 | internal nonresorbable | 18% syndromic, all patients had laryngomalacia | Prevented: 100% (10/10) Decannulated: 100.00% (1/1) | AHI: preoperative 46.1±31.8, postoperative 4.1±3.0 (p = 0.002) | 36% surgical site infection | 28 months | IV/12 | overlapping cohort with Flores RL, Greathouse ST, Costa M et al, Murage et al, and Flores RL, Tholapdy SS, Sati et al. |
| Tibesar RJ et al.54 | external | Syndrome percent not specified, fail conservative therapy | Prevented: 100.00% (32/32) | N/A | 28% (9 open bite deformity), 16% (5 tooth malformation/loss), 9% (3 facial nerve injury) | 91.2 months | IV/12 | overlapping cohort with Meyer AC et al. and Scott AR et al. |
| Wittenborn W et al.81 | external and internal | 18% syndromic, exclude other airway anomalies | Prevented: 82.35% (14/17) | 10 patients with both preoperative and postoperative PSG had 55% improvement in episodes per hour | None reported | 16.5 months | IV/12 | 1 internal MDO required tracheostomy 4 months after surgery. 2 external MDO failed extubations, both had previously undiagnosed tracheal stenosis and required tracheostomy. 1 underwent correction and subsequent internal MDO and successful decannulation. |
| Zellner EG et al.84 | internal nonresorbable | Syndrome not specified, fail conservative therapy | Prevented: 95.00% (19/20) | AHI: preoperative 51.3, postoperative 5.5 (p<0.1) | 10% (2 device infection), 10% (2 facial nerve injuries) | not reported | III/12 | multicenter study, 1 patient needed tracheostomy for continued sleep apnea |
| TLA studies | Distractor Type | Patient selection/surgical indication | Tracheostomy prevented | OSA | Complications | Follow up length | Level of Evidence/ MINORS | Notes |
| Abramowicz S et al.78 | N/A | 15% syndromic, fail conservative therapy | 90.00% (18/20) | N/A | Not reported | not reported | IV/11 | Odds of requiring tracheostomy 5 times greater if GILLS score >3; both subjects requiring tracheostomy were syndromic |
| Bijnen CL et al.63 | N/A | 41% syndromic, fail conservative therapy | 90.00% (18/20) | 5/8 patients showed improvement in PSG | 23% (5 partial dehiscence), 30% (6 small abscesses) | 12 months (range 1 to 9 years) | IV/12 | |
| Bull MJ et al.82 | N/A | 43% syndromic, fail conservative therapy | 85.71% (6/7) | N/A | Not reported | range 8 to 30 months | IV/12 | subject had multiple congenital anomalies with tracheobronchomalacia (found subsequently), required tracheostomy and CPAP for prolonged ventilatory management |
| Caouette-Laberge L et al.2 | N/A | 46% syndromic, fail conservative therapy | 81.82% (9/11) | N/A | 4 subjects deceased | not reported | IV/10 | |
| Evans AK et al.7 | N/A | 46% syndromic, fail conservative therapy | 83.87% (26/31) | N/A | 6% (2 wound dehiscence) | not reported | IV/12 | overlapping cohort with Rogers et al., 3 subjects requiring tracheostomy due to GER |
| Fujii M et al.107 | N/A | 18% syndromic, fail conservative therapy | 89.47% (17/19) | N/A | Not reported | at least 1 year | IV/12 | 2 subjects (1 with Treacher Collins) required tracheostomy at age 2 months; 1 for prolonged swelling of tongue on POD20; 1 required tracheal intubation soon after TLA and tracheostomy performed on POD24 |
| Hoffman W48 | N/A | 70% syndromic, fail conservative therapy | 91.30% (21/23) | N/A | 26% (6 wound dehiscence) | 39.6 months | IV/12 | both patients requiring tracheostomy were syndromic, 1 subject did not have preoperative bronchoscopy, requiring tracheostomy for laryngomalacia; 1 subject required tracheostomy after recurrent respiratory infections and pneumonia, decannulated 7.5 years after tracheostomy |
| Huang F et al.52 | N/A | Syndrome status not specified, fail conservative therapy | Prevented: 91.67% (11/12) Decannulated: 50.00% (1/2) | N/A | 29% (4 wound dehiscence) | not reported | IV/8 | subject requiring tracheostomy had multiple brain infarctions |
| Kirschner RE et al.34 | N/A | 50% syndromic, fail conservative therapy | 79.31% (23/29) | no obstructive apnea seen after takedown | 41.6% dehiscence rate if mucosal adhesion alone, none if muscular sutures | 29 months | IV/12 | 5/6 subjects requiring tracheostomy were syndromic; due to wound dehiscence (2), persistent glossoptosis, laryngomalacia, laryngeal granulation tissue, failure of later elective intubation |
| Li HY et al.87 | N/A | Syndrome not specified, fail conservative therapy, require endotracheal intubation | 42.86% (3/7) | N/A | 4 failures of TLA due to wound dehiscence | at least 1 year | IV/12 | |
| Mann RJ et al.108 | N/A | All isolated PRS, fail conservative therapy, exclude tracheomalacia | 95.45% (21/22) | N/A | 13.6% (3 stitch abscesses), 4.5% (1 tongue mucocele) | 96 months | IV/12 | subject requiring tracheostomy had wound dehiscence secondary to traumatic postoperative extubation, eventually requiring tracheostomy for subglottic stenosis |
| Resnick CM et al.49 | N/A | 72% syndromic, fail conservative therapy, rule out other airway anomalies | 100.00% (18/18) | AHI: preoperative 15.1±4.3, postoperative 9.9±4.1 (p=0.307) | Not reported | range 1 to 31 months | IV/11 | |
| Rogers GF et al.36 | N/A | 45% syndromic, exclude other airway anomalies | 88.68% (47/53) | N/A | Not reported | not reported | IV/11 | overlapping cohort with Evans AK et al.; all subjects requiring tracheostomy were syndromic, 2 subjects due to early partial dehiscence and failed extubation, 1 due to laryngeal web and failed subsequent MDO, 2 due to repeated aspiration, 1 due to GER and failure to wean off vent |
| Schaefer RB et al.35 | N/A | All isolated PRS, rule out other airway anomalies | 88.89% (8/9) | N/A | Not reported | median 33 months | IV/12 | isolated PRS subject requiring tracheostomy due to previously unrecognized tracheomalacia |
| Sedaghat AR et al.50 | N/A | Syndrome not specified, fail conservative therapy | 87.50% (7/8) | AHI: preoperative AHI 52.6, postoperative 34.5; 1 subject had increase in AHI after TLA | Not reported | range 5 to 32 days | IV/11 | subject requiring tracheostomy also failed MDO, had persistent respiratory issues and hypotonia |
| Comparison studies | Type of Distractor | Patient selection/surgical indication | Tracheostomy prevented | OSA | Perioperative Complications | Follow up length | Level of Evidence/ MINORS | Notes |
| Flores RL, Tholpady SS, Sati S et al.42 | internal nonresorbable | Nonsyndromic only, sleep study results indicating an AHI >20, significant CO2 retention, absence of other significant airway anomalies, absence of TMJ abnormality |
MDO: 100.00% (24/24); TLA: 73.33% (11/15), no p value provided | Preoperative AHI: MDO 47 vs TLA 37.6 (p < 0.05). Postoperative AHI: at 1 month, MDO 10.9 vs TLA 21.6 (p < 0.05), at 1 year, MDO 2.5 vs TLA 22. (p < 0.05) | MDO: 16.67% (1 equipment failure, 3 infections); TLA: 40% (3 wound dehiscence, 3 scar contractures); no p-value | at least 1 year | III/18 | Retrospective, historical comparison, MDO (2004 to 2009) TLA (1994 to 2004) |
| Khansa I et al.43 | internal nonresorbable | 32% syndromic, unsustainable weight gain without tube feeds, unstable airway with positioning alone, no lower airway anomalies, no central sleep apnea, surgeon and family preference for MDO vs TLA | MDO: 100.00% (10/10); TLA: 100.00% (8/8) | Preoperative AHI: MDO 27.7, TLA 15.2 (p=0.01); Postoperative AHI: MDO 1.5, TLA 2.8 (p=0.8); mean decrease MDO 94.6 vs TLA 81.6% (p=0.01); Residual moderate OSA (AHI>5) in 0% MDO, 12.5% TLA (p=0.4) | MDO: 40% (4 cellulitis); TLA: 25% (2 temporary reintubations); p=0.6 | 1 year | II/19 | prospective comparison, MDO subjects had highest baseline AHI (27.7), TLA subjects had intermediate baseline AHI (15.2) |
| Papoff P et al.56 | external | 56% MDO patients syndromic, 0% TLA patients syndromic, fail positioning and non-invasive ventilation, physical signs of respiratory distress associated with obstructive apneas, prolonged feeding difficulties, no distal airway anomalies |
MDO: 88.89% (8/9); TLA 100% (9/9), no p value provided | surgery completely resolved airway obstruction in 3/9 TLA subjects, 8/9 MDO subjects (p=0.050); residual respiratory distress (opisthotonus, pectus excavatum, desaturations, need for prone positioning), diagnosed more commonly after TLA than after MDO (6/9 vs 1/9, P=0.050). | MDO: 44% (4 lost pin in consolidation requiring repositioning), normal dental development; TLA: 22% (2 wound adhesions requiring repeat TLA), no p value | until age of palatoplasty (22.7±4.1 months in TLA group, 9.2±1.3 months in MDO group) | III/16 | Retrospective; historical comparison, MDO (2006–2014), TLA (before 2002–2005) |
| Susarla SM et al.57 | internal nonresorbable | 29.5% syndromic, continued feeding abnormalities (aspiration, penetration, or inability to control secretions) excluded patients needing pretreatment gastrostomy (global hypotonia, severe developmental delay) and multilevel airway lesions needing tracheostomy | MDO: 100.00% (30/30); TLA: 96.77% (30/31), no p value provided | N/A | MDO: 17% (4 surgical-site infections, 1 temporary marginal mandibular nerve palsy); TLA: 10% (3 wound dehiscence); p nonsignificant | 3–4 years | III/18 | 2-institution retrospective; historical comparison, MDO (2010–2013), TLA (before 2007–2009) |
MDO, mandibular distraction osteogenesis; TLA, tongue-lip adhesion.; N/A, not applicable; OR, odds ratio; CI, confidence interval; OSA, obstructive sleep apnea; RDI, respiratory distress index; AHI, apnea-hypoapnea index; PSG, polysomnogram; CPAP, continuous positive airway pressure; GER, gastroesophageal reflux
Table 3.
Comparison of Feeding Outcomes
| Article | Type of Distractor | Patient selection/surgical indication | Full oral feeds | Growth / Weight | Complications | Follow up length | Level of Evidence/ MINORS | Notes |
|---|---|---|---|---|---|---|---|---|
| MDO studies | ||||||||
| Al-Samkari HT et al.60 | external | 42% syndromic, failure of conservative management | 66.67% (8/12) | average daily weight gain 16.5 g | Not reported | not reported, available in only 67% (8/12) patients | III/10 | 60% of syndromic PRS needed gastrostomy compared to 0% of isolated PRS |
| Breugem C et al.89 | internal resorbable | 17% syndromic, failed conservative therapy, exclude other airway obstruction | 90.91% (10/11) | N/A | 18% (1 cellulitis, 1 lost pin during consolidation) | not reported | IV/8 | 6 subjects achieved full oral feeds at discharge, 4 more subjects within 4 weeks of discharge |
| Denny A, Amm C59 | external | 63% syndromic, no secondary sites of airway obstruction | 100.00% (11/11) | growth above 50th percentile in all patients, trend of average or above-average weight gain continues in 4 patients with longest 3–5 year follow up | None | 60 months | IV/12 | 1 patient previously failed TLA; 54.5% full oral feeds at 1 month, 100% at 1 year |
| Denny A, Kalantarian B58 | external | Syndromic status not specified, excluded patients with lower airway anomalies | N/A | all subjects met or exceeded average 500g per month weight gain | 25% (1 device failure needing replacement) | range 9–22 months | IV/12 | |
| Genecov DG et al.66 | external and internal | 4% syndromic, exclude laryngomalacia, tracheomalacia, subglottic/supraglottic stenosis, neurologic impairment, untreated GERD with vocal cord/epiglottic edema | 91.04% (61/67) | N/A | 13% (9 pin site infections) 13% (9 device failure), 9% (6 asymmetric movement of depressor anguli oris muscle) | not reported | IV/10 | |
| Goldstein JA et al.77 | internal nonresorbable | 46% syndromic, fail conservative therapy | 83.33% (20/24) | N/A | 4%(1 transient facial nerve paralysis), 8% (2 pin site infections), 4%(1 major submental abscess) | not reported | IV/10 | 1 patient underwent TLA after MDO |
| Izadi K et al.94 | internal nonresorbable | 47% syndromic, central apnea, severe reflux, other airway lesions | 91.67% (22/24) | N/A | None reported | 14 months | IV/12 | |
| Hong P et al.65 | internal nonresorbable | 67% syndromic, all had GERD, no central apnea, no lower airway anomaly | 100.00% (6/6) | N/A | 33% (2 local erythema) | 18 months | IV/12 | |
| Konas E et al.95 | internal nonresorbable | 8% syndromic, fail conservative therapy | 100.00% (13/13) | N/A | 30% (1 facial nerve palsy, 1 reintubation, 1extension rod fracture, 2 pin site infections) | at least 1 year | IV/12 | |
| Mandell DL et al.44 | external and internal | 50% syndromic, exclude secondary airway anomaly, central sleep apnea, severe GERD | 84.50% (7/8) | N/A | 23% (3 premature consolidation, 2 cheek abscess, 1 lip erosion, 1 cellulitis, 1 facial nerve palsy, 2 TMJ ankylosis) | 13 months | IV/12 | 1 patient required gastrostomy tube |
| Mingo KM et al.41 | external | isolated PRS or Stickler’s syndrome, compared patients with or without preoperative CT | 86.54% (25/30) | N/A | 12% (2 fracture between 2 posterior pins 1 bilateral hardware loosening, 1 temporary reintubation, 2 pin site infections, 1 unilateral marginal mandibular nerve weakness) | range 1–8 years | IV/12 | |
| Mudd PA et al.53 | internal nonresorbable | 29% syndromic, fail conservative therapy | 91.67% (22/24) | weight percentile: 25th after birth, significantly lower than norm during 3 month postoperative period, then steady growth curve through 1 year of age, most remain below 50th | 42% (1 facial paralysis due to compression of posteriorly displaced distraction arm, 2 facial nerve injury, 1 marginal mandibular weakness, 6 cellulitis) | 28.8 months | IV/12 | 50% discharged full oral feeds, 4 pts required gastrostomy tube, 3 were placed prior to MDO, 1 within 1 month of MDO for failure to thrive, at latest follow up 2 subjects continue to require supplemental feeding by gastrostomy tube (have associated neurologic sequelae of associated syndrome) |
| Scott AR et al.101 | external | 26% syndromic, fail conservative therapy | 84.21% (16/19) | N/A | 5% (1 anterior open-bite deformity), 21% (4 long-term tooth loss/malformation), 16% (injury to marginal mandibular branch of facial nerve), 16% (3 hypertrophic scars), 5% (1 repeat MDO) | 67.2 months | IV/12 | overlapping cohort with Tibesar et al. and Meyer et al. |
| Spring MA, Mount DL61 | internal nonresorbable | 90% syndromic, fail conservative therapy | 70.00% (7/10) | 7/10 subjects showed early decline in growth rate following distraction, persistent to 12 months; other 3 subjects had steady growth rate percentile | 20% (2 mandibular nerve weakness) 30% (3 early limited mandibular range of motion), 10% (incisional scar hypertrophy) | range 12–28 months | IV/12 | 2 subjects required gastric tube, 1 gastric gavage; statistically significant relationship between younger age of subject and growth percentile rank decline |
| Tibesar RJ et al.54 | external | Syndrome percent not specified, fail conservative therapy | 78.13% (25/32) | N/A | 28% (9 open bite deformity), 16% (5 tooth malformation/loss), 9% (3 facial nerve injury) | 91.2 months | IV/12 | overlapping cohort with Meyer AC et al. and Scott AR et al. |
| TLA studies | Distractor Type | Patient selection/surgical indication | Full oral feeds | Growth / Weight | Complications | Follow up length | Level of Evidence | Notes |
| Bijnen CL et al.63 | N/A | 41% syndromic, fail conservative therapy | N/A | Catch up growth in 10 subjects, other 11 subjects remained on same growth percentile | 23% (5 partial dehiscence), 30% (6 small abscesses) | 12 months (range 1 to 9 years) | IV/12 | |
| Caouette-Laberge L et al.2 | N/A | 46% syndromic, fail conservative therapy | 100.00% (11/11) | N/A | 4 subjects deceased | not reported | IV/10 | |
| TLA Article | Type of Distractor | Patient selection/surgical indication | Full oral feeds | Growth / Weight | Complications | Follow up length | Level of Evidence | Notes |
| Cozzi F et al.62 | N/A | Proportion syndromic not specified, fail conservative therapy | N/A | Body weight percentile: preoperative 9.7±2.6, postoperative 17.5± 4.6 (p>0.05); Weight velocity: preoperative 19.1±4.9, postoperative 74.2± 4.7 (p< 0.001) | 19% (9 adhesion dehiscence) | 68.4 months | IV/12 | |
| Cruz MJ et al.109 | N/A | 17% syndromic, fail conservative therapy | 83.33% (10/12) | N/A | 17% (2 postoperative desaturations and bradycardia), 8% (1 chin abscess), 50% (6 dehiscences) | minimum 6 months | IV/12 | |
| Denny AD, Amm CA, Schaefer RB51 | N/A | 64% syndromic, fail conservative therapy | 45.45% (5/11) | N/A | 27% (3 wound dehiscence, 2 of which require repeat TLA) | 94.8 months | IV/12 | subjects gastrostomy dependent for more than a year |
| Hoffman W.48 | N/A | 70% syndromic, fail conservative therapy | 65.22% (15/23) | N/A | 26% (6 wound dehiscence) | 39.6 months | IV/8 | 10 subjects weaned to oral feeds by day 21, 4 subjects discharged with gastrostomy tube, 9 with nasogastric tubes (5 converted to PO, 1 lost to follow up, and 3 required gastrostomy due to myopathy, aspiration on swallow study and severe oral aversion) |
| Huang F et al.52 | N/A | Syndrome status not specified, fail conservative therapy | 78.57% (11/14) | N/A | 29% (4 wound dehiscence) | not reported | IV/10 | |
| Kirschner RE et al.34 | N/A | 50% syndromic, fail conservative therapy | 62.07% (18/29) | N/A | 41.6% dehiscence rate if mucosal adhesion alone, none if muscular sutures | 29 months | IV/12 | |
| Schaefer RB et al.35 | N/A | All isolated PRS, rule out other airway anomalies | 100.00% (9/9) | N/A | Not reported | median 33 months | IV/12 | those requiring nasogastric feeding in infancy discontinued by age 8 weeks |
| Comparison studies | Type of Distractor | Patient selection/surgical indication | Full oral feeds | Growth / Weight | Complications | Follow up length | Level of Evidence | Notes |
| Khansa I et al.43 | internal nonresorbable | 32% syndromic,unsustainable weight gain without tube feeds, unstable airway with positioning alone, no lower airway anomalies, no central sleep apnea, surgeon and family preference for MDO vs TLA | MDO: 90.00% (9/10), TLA: 87.50% (7/8), p=0.6 | Similar baseline weight percentiles; change at 2–3 months: MDO −3.8% vs TLA −10.5%; at 10–12 month: MDO 19.5% vs TLA 19.4%; at 16–20 months: MDO 25.3% vs TLA 17.9%; failure to thrive duration: MDO 150 vs 162 days; all p > 0.5 | MDO: 40% (4 cellulitis); TLA: 25% (2 temporary reintubations); p=0.6 | 1 year | II/19 | prospective comparison, more MDO subjects required non-oral feeds at baseline (70 vs 37.5, p = 0.3); both patients requiring gastrostomy were syndromic |
| Papoff P et al.56 | external | 56% MDO patients syndromic, 0% TLA patients syndromic,fail positioning and non-invasive ventilation, physical signs of respiratory distress associated with obstructive apneas, prolonged feeding difficulties, no distal airway anomalies | MDO: 100.00% (9/9), TLA: 100.00% (9/9) | N/A | MDO: 44% (4 lost pin in consolidation requiring repositioning), normal dental development; TLA: 22% (2 wound adhesions requiring repeat TLA), no p value | until age of palatoplasty (22.7±4.1 months in TLA group, 9.2±1.3 months in MDO group) | III/16 | retrospective comparison; 1 patient with velocardiofacial syndrome and neurologic impairment (hypotonia) underwent TLA after MDO failed and received trach; Infants resumed oral feeding sooner after MDO than after TLA (mean days after surgery to full oral feeds 44±24 vs 217±134, (p<0.003). |
| Susarla SM et al.57 | internal nonresorbable | 29.5% syndromic, continued feeding abnormalities (aspiration, penetration, or inability to control secretions) excluded patients needing pretreatment gastrostomy (global hypotonia, severe developmental delay) and multilevel airway lesions needing tracheostomy | MDO: 83.33% (25/30), TLA: 15/31 (48.39%), p=0.009 | N/A | MDO: 17% (4 surgical-site infections, 1 temporary marginal mandibular nerve palsy); TLA: 10% (3 wound dehiscence); p nonsignificant | 3–4 years | III/18 | 2-institution retrospective; historical comparison, MDO (2010–2013), TLA (before 2007–2009) |
Table 4.
Summary of TLA and MDO Outcomes
| Intervention | TLA | MDO |
|---|---|---|
| Avoidance of tracheostomy | 89.47% (289/323)* | 95.00% (657/693)* |
| Decannulation | N/A | 80.00% (165/206) |
| Full Oral Feeds at latest follow up | 70.06% (110/157) | 87.30% (323/370) |
| Reported rates of reoperation | 45% (5/11)51, 29% (4/14)52, 27% (3/11)2, 22% (2/9)56 | 6% (3/50)55, 5% (1/19)54, 4% (1/23)53 |
Five MDO studies, one TLA study and the MDO cohort of a comparison study were excluded from aggregation of results due to overlapping cohort of patients
MDO, mandibular distraction osteogenesis; TLA, tongue-lip adhesion.; N/A, not applicable.
Table 5.
Summary of Comparison Studies
| Study | Design | Indications | Type of Distractor | Tracheostomy Prevented | Full oral feeds | Complications | Follow up length | Level of Evidence/MINORS |
|---|---|---|---|---|---|---|---|---|
| Flores RL, Tholpady SS, Sati S et al.42 | Retrospective, historical comparison, MDO (2004 to 2009) TLA (1994 to 2004), nonsyndromic only, age<6 months, | sleep study results indicating an AHI >20, significant CO2 retention, absence of other significant airway anomalies, absence of TMJ abnormality | internal nonresorbable | MDO: 100.00% (24/24); TLA: 73.33% (11/15), no p value provided | N/A | MDO: 16.67% (1 equipment failure, 3 infections); TLA: 40% (3 wound dehiscence, 3 scar contractures); no p-value | at least 1 year | III/18 |
| Khansa et al.43 | Prospective, 32% syndromic, nonrandomized, MDO subjects had higher baseline AHI (27.7) vs TLA (15.2) | unsustainable weight gain without tube feeds, unstable airway with positioning alone, no lower airway anomalies, no central sleep apnea, surgeon and family preference for MDO vs TLA | internal nonresorbable | MDO: 100.00% (10/10); TLA: 100.00% (8/8) | MDO: 90.00% (9/10), TLA: 87.50% (7/8), p=0.6 | MDO: 40% (4 cellulitis); TLA: 25% (2 temporary reintubations); p=0.6 | 1 year | II/19 |
| Papoff et al.56 | Retrospective; historical comparison, MDO (2006–2014), TLA (before 2002–2005), 56% MDO patients and 0% TLA patients syndromic, no distal airway anomalies | fail positioning and non-invasive ventilation, physical signs of respiratory distress associated with obstructive apneas, prolonged feeding difficulties |
external | MDO: 88.89% (8/9), patient with veocardiofacial syndrome and neurologic impairment (hypotonia) underwent TLA after MDO failed then received tracheostomy; TLA 100% (9/9), no p value, 1 patient died (after TLA lysis, did not respond to resuscitation); residual respiratory distress diagnosed more commonly after TLA than after MDO (6/9 vs 1/9, P=0.050). | MDO: 100.00% (9/9), TLA: 100.00% (9/9), infants resumed oral feeding sooner after MDO than after TLA (44±24 vs 217±134 days, P<0.003). | MDO: 44% (4 lost pin in consolidation requiring repositioning), normal dental development; TLA: 22% (2 wound adhesions requiring repeat TLA), no p value | until age of palatoplasty (22.7±4.1 months in TLA group, 9.2±1.3 months in MDO group) | III/16 |
| Susarla et al.57 | 2-institution retrospective; historical comparison, MDO (2010–2013), TLA (before 2007–2009), groups statistically comparable in demographic and clinical factors, 29.5% syndromic, excluded patients needing pretreatment gastrostomy (global hypotonia, severe developmental delay) and multilevel airway lesions needing tracheostomy | fail conservative management, continued feeding abnormalities (aspiration, penetration, or inability to control secretions) | internal nonresorbable | MDO: 100.00% (30/30); TLA: 96.77% (30/31), no p value, 1 death in TLA cohort | MDO: 83.33% (25/30), TLA: 15/31 (48.39%), p=0.009 | MDO: 17% (4 surgical-site infections, 1 temporary marginal mandibular nerve palsy); TLA: 10% (3 wound dehiscence); p nonsignificant | 3–4 years | III/18 |
Airway Outcomes
Avoidance of tracheostomy:
Ninety-five percent (657/693) of subjects treated with MDO avoided tracheostomy. Eighty-nine percent (289/323) of subjects treated with TLA avoided tracheostomy. In a large MDO cohort study (34% syndromic), tracheostomy was avoided in 93% (76/81) of subjects after 22 months of follow up106. Tracheostomy was avoided in 89% of a large TLA cohort study (45% syndromic)36. One hundred percent of those treated with MDO, versus 73% of those treated with TLA, avoided tracheostomy in a retrospective historical comparison study of an isolated PRS cohort with at least 1 year of follow up (no p value provided)42. All subjects in both the MDO and TLA cohorts avoided tracheostomy in a prospective, nonrandomized study with at least one year of follow up43.
Decannulation:
Eighty percent (165/206) of subjects achieved decannulation following MDO. Notably, decannulation was achieved in only 17% (2/12) of a cohort where all subjects had complex congenital syndromic comorbidities44. However, decannulation was achieved in 100% of a cohort (11/11) that was 72% syndromic and had severe OSA46.
Relief of OSA:
Both a prospective nonrandomized study43 and a retrospective historical comparison study42 found that MDO subjects had significantly lower postoperative apnea-hypoapnea index (AHI) compared to TLA subjects, despite having significantly higher AHI preoperatively.
Improvement in oxygen saturation:
After MDO, oxygen saturation levels improved from a preoperative range of 72–76% to a postoperative range of 91–95%28,45–47. After TLA, oxygen saturation levels increased from a preoperative range of 63–73% to a postoperative range of 81–88%48–50. A retrospective historical comparison study found that patients undergoing MDO had significantly higher oxygen saturation levels than TLA patients both at 1 month (98.3 percent versus 87.5 percent; p < 0.05) and 1 year postoperatively (98.5 percent versus 89.2 percent; p < 0.05) despite having higher preoperative AHI42.
Repeat procedure:
Denny et al reported that 45% of patients (5/11) treated with TLA eventually required a secondary surgery for recurrent airway obstruction (four distractions and one repeat TLA)51. Three other studies reported 22% (2/9)56, 27% (3/11)2, and 29% (4/14)52 of subjects required repeat procedure due to early disruption from wound breakdown. In contrast, the rates of repeat distraction were 4% (1/23)53, 5% (1/19)54, and 6% (3/50)55.
Feeding Outcomes
Achievement of full oral feeds:
Eighty-seven percent (323/370) of subjects treated with MDO achieved full oral feeds at latest follow up. Eleven percent (42/370) still required gastrostomy for supplemental feeding at latest follow up. By comparison, 70% (110/157) of subjects treated with TLA achieved full oral feeds at latest follow up, with 20% (32/157) requiring gastrostomy for supplemental feeding. A retrospective historical comparison found that subjects undergoing TLA were 6.5 times more likely to require a gastrostomy tube for nutritional support, compared to subjects undergoing MDO57.
Of note, in a single-center retrospective study where all infants achieved full oral feeds, infants resumed oral feeding sooner after MDO than after TLA, despite the MDO cohort containing significantly more syndromic patients56. However, no significant difference in time needed to discontinue tube feeds was found in a prospective nonrandomized study43.
Growth/weight gain:
While most MDO studies53,58–60 reported improved weight gain following distraction, one study61 found an early decline in growth rate in 7 out of 10 subjects that persisted for 12 months. Cozzi et al found that infants had an increase in both body weight percentile and weight velocity percentile following TLA62. In contrast, Bijnen et al reported that 11 of the 21 subjects remained within the same growth percentile63. No significant difference in growth between MDO and TLA patients was found both in a prospective, nonrandomized study43.
Improvement in gastroesophageal reflux (GER):
No studies reviewed specifically reported on GER before and after TLA. Hong et al reported that all 6 subjects who underwent MDO had resolution of GER that was seen on preoperative upper GI series, and were able to discontinue anti-reflux medical therapy65. Similarly, Genecov et al found that only 3% of patients had GER postoperatively, a decrease from 67% with GER preoperatively66.
Swallow function:
Two MDO studies45,65 specifically evaluated swallowing function following surgical intervention. Both studies reported resolution of laryngeal penetration (passage of materials into the larynx) and aspiration (passage of materials through the vocal folds) following distraction.
Complications
While not the main outcome of comparison in this review, the relative risks associated with either intervention should be noted. Perioperative complications reported in individual studies are presented in Tables 2 and 3.
Complications of MDO include external scarring85, hypertrophic scarring101, infection45,47,65,80,89,, hardware exposure93, device dislodgment or pin loss28,90,98,99,102, facial nerve problems101,47,66 and tooth-bud damage101. Tibesar et al retrospectively reviewed and reported open bite deformity (28%), dental complications (16%) and facial nerve injuries (9%) as the leading complications in the long-term period54. While TLA causes minimal external scarring compared to MDO, its complications include wound dehiscence34,48,51,52, abscesses and mucoceles63,108, and injuries to Wharton’s ducts51,63.
Discussion
TLA and MDO, both adopted as alternatives to tracheostomy, have distinct inherent advantages and disadvantages. These features are presented in Table 6, along with the most commonly cited complications. TLA is a relatively simple procedure and provides immediate relief of obstruction by moving the tongue. However, the procedure is only a temporizing measure that relies on “catch-up growth” of the mandible to ultimately relieve airway obstruction. While some have reported subsequent normal growth of the mandible35,67, one study found various structures did not reach normal values compared to controls68. Certain cases of syndromic PRS, including Treacher Collins and hemifacial microsomia, have persistent micro/retrognathia compared to isolated PRS, Stickler or velocardiofacial syndrome69.
Table 6.
Comparison of TLA and MDO advantages and disadvantages
| Intervention | Advantages | Disadvantages | Commonly cited complications |
|---|---|---|---|
| TLA | immediate relief of airway obstruction, short operating time, shorter length of stay and overall cost6, minimal external scarring | reliance of “catch-up growth” of the mandible, dysphagia and early feeding difficulties51, interference with development of pre-speech skills and sound production110 | wound dehiscence, early release of retention sutures, tongue lacerations, injuries to Wharton’s ducts |
| MDO | lengthens mandible and increases mandibular volume, lower cost compared to tracheostomy111 | requires specialized training and equipment, parental compliance in turning device, second operation required for removal, gradual improvement of airway obstruction | hypertrophic scarring, device malfunction or infection, open bite deformity, dental damage, facial nerve injury |
MDO corrects micrognathia, in contrast to TLA, by lengthening the mandible and increasing mandibular volume29,70. But the procedure is technically more difficult and requires surgeons with specialized training and equipment, necessitates a secondary operation for removal of hardware, and is associated with complications such as hardware infection, dental damage and nerve injury. Furthermore, improvement of the airway obstruction is gradual, as the mandible is slowly lengthened through distraction. Thus, the airway may require monitoring in an intensive care setting during the distraction period.
Prior reviews
Several reviews have been published on the management of PRS, MDO and TLA. A systematic review by Viezel-Mathieu et al71 found TLA was successful in relieving airway obstruction in 81.3% patients. Our review of the literature yielded a greater success rate of 89% in TLA subjects. However, we specifically defined the primary endpoint as avoidance of tracheostomy. A recent review by Tahiri et al.72 found that MDO successfully treated airway obstruction in 89.3% of cases. Ow and Cheung conducted a meta-analysis of MDO studies published between 1966–200673. Their analysis yielded a 91% rate of prevention of tracheostomy and a 78% rate of decannulation. Our findings (95% prevention of tracheostomy and 90% decannulation) may differ due to inclusion of more recently published studies, and inclusion of only subjects with diagnosis of PRS. Finally, Breik et al.74 found that MDO led to 82% of children feeding exclusively orally after surgery, and resolution of GERD in 66/70 patients. Our review found that 87% of subjects reported in the literature achieved exclusive oral feeding. We included subjects who initially required supplemental feeding but later discontinued at latest follow up, which may contribute to our higher rate.
Few systematic reviews have included both TLA and MDO interventions. Bookman et al. 75 conducted a systematic review on neonates with tongue-based airway obstruction, and found a lack of consistency in diagnosis, treatment protocols and reporting of outcomes. The review did not include more recent prospective studies or studies comparing both MDO and TLA patients. Almajed et al76 systematically compared reported PSG outcomes following MDO and TLA. MDO was associated with the lowest percentage of significant airway obstruction postoperatively (3.6%) compared to 50% for infants who underwent TLA. No prior reviews have directly compared feeding outcomes following TLA versus following MDO.
Airway Outcomes
TLA is effective for relief of severe upper airway obstruction, with 89% of subjects reported in the literature being able to avoid a tracheostomy. Similarly, tracheostomy was prevented in 95% of MDO subjects. Furthermore, MDO can improve airway obstruction even in patients who previously required tracheostomy, with successful decannulation in 80% of subjects. Aggregate data from the literature reviewed and comparison studies suggest that MDO promotes greater resolution of OSA and higher oxygen saturation, compared to TLA. MDO may provide more stable and long-term relief, as a higher percentage of infants treated with TLA required a repeat procedure for recurrent airway obstruction.
Feeding Outcomes
Both TLA and MDO can relieve feeding difficulties, with 70% and 88% of subjects, respectively, resuming full oral feeds. Improved feeding function promotes continued weight gain and growth following MDO and TLA, although infants may continue to be at lower weight percentiles. No significant difference in long-term weight gain or growth was found between MDO and TLA64. However, the higher percentage of MDO subjects achieving full oral feeds seen in the aggregate data, as well as findings from two retrospective comparison studies56,57, suggest that MDO has an advantage over TLA with earlier return to oral feeding and discontinuation of supplemental feeding, and lower rates of gastrostomy placement. The avoidance of an additional surgical intervention and decreasing time needed to care for a feeding tube are important potential benefits for patient quality of life and caretaker burden. Moreover, although limited studies are available, MDO may also promote normal feeding by relieving GER and improving swallowing function.
Recommendations for clinical practice
Regardless of the intervention, careful patient selection is necessary to anticipate potential complications and achieve optimal outcomes. In both MDO53,60,61,77 and TLA36,48,78 syndromic patients tended to have poorer airway and feeding outcomes. Subjects who still required gastrostomy after either MDO or TLA often had an associated syndrome 43,53,56,60. Poorer odds of success of tracheostomy avoidance or decannulation with MDO were associated with having a diagnosis of craniofacial microsomia or Goldenhar syndrome (OR, 0.07 [95% CI, 0.009–0.52])39. Syndromic diagnosis is similarly associated with an increased risk of failure of TLA, in addition to gastroesophageal reflux disease, preoperative intubation, late surgical intervention, and low birth weight (GILLS acronym)36. Gastroesophageal reflux and age >30 days were also associated with failure of MDO to avoid tracheostomy79. Definitive conclusions regarding the choice of intervention for PRS with associated syndromes or comorbidities cannot be drawn from this review.
In several instances, patients who failed initial intervention, ultimately requiring tracheostomy, were found to have concomitant airway anomalies, such as laryngomalacia, tracheal webs and vascular rings36,48,52,79–83. Patients with central apnea or neurologic disabilities also were likely to fail either MDO or TLA36,56,66,79,84,85. Most studies excluded patients with these comorbidities from their analysis, although one study on subjects with layrngomalacia treated with MDO reported 100% success in avoidance of tracheostomy or decannulation86. Thorough preoperative airway evaluation (i.e. nasopharyngoscopy or bronchoscopy to identify other causes of obstruction other than glossoptosis42,43) and polysomnogram62, as well as assessment of other comorbidities suggesting a syndrome, allows for the most appropriate selection of intervention. The results of this review suggest that patients with laryngomalacia may still be candidates for MDO, but TLA should be avoided86,87. Furthermore, patients with significant lower airway anomalies, who are likely to fail either MDO or TLA, may preferably undergo tracheostomy primarily34,85.
Future Directions
Despite the possible advantages MDO may have over TLA in long-term relief of airway obstruction and feeding difficulties, it has the notable disadvantage of requiring a second operation for removal and complications such as nerve and tooth bud damage. Additional studies are required to identify risk factors for MDO perioperative complications. Of particular interest would be whether syndromic PRS subjects are more prone to perioperative complications, given that several studies have shown they have poorer feeding and airway outcomes39,53,60,61,77. Expanded knowledge of risk factors and continued advances in operative technique can help to reduce complications, increasing the benefit to risk ratio of MDO over TLA.
The comprehensive literature search performed for this review yielded primarily retrospective, lower-level evidence studies (case series and cohort studies). Only five studies compared outcomes of MDO and TLA subjects, of which one study was prospective43. Parents were offered both interventions, but the authors suggest that surgeons may have unintentionally counseled parents of neonates with more severe phenotypes towards MDO, given that the MDO cohort had more severe baseline characteristics than the TLA cohort. In all four retrospective comparisons42,56,57, all TLA subjects were treated prior to the adoption of MDO at the institution. The results may be confounded by other factors that may have changed between the beginning and end of the study period.
Before reaching a consensus on optimal treatment modality for PRS patients, more carefully designed prospective studies comparing the two interventions need to be conducted. These studies should institute a standardized protocol for defining and diagnosing PRS, preoperative evaluation to assess for comorbidities, and objective measurements of short and long-term airway function. Subjects should be carefully followed for nutritional requirements and growth.
Limitations
This review assesses the best evidence available at the time of publication to compare airway and feeding outcomes of MDO and TLA subjects. However, the ability to rigorously compare outcomes was limited by variations in the selection of cohorts, and the severity of phenotype. Most studies included mix of syndromic and nonsyndromic isolated PRS. The majority excluded patients with central apnea, neurological disabilities and/or concomitant airway anomalies, but a few included these potentially higher risk patients. Surgical techniques also varied between institutions, which could affect rate of surgical complications and success in improving outcomes. Studies varied in length of follow up. We are not able to account for the possibility of higher success rates simply because some cohorts were followed for shorter periods of time, before patients could develop recurrence in airway obstruction. Finally, the lack of standardization in reporting outcomes after surgical intervention creates difficulties in direct comparisons of MDO and TLA outcomes. The strength of this review is that it included only studies where defined endpoints could be extracted. Furthermore, we have highlighted variations in patient selection, technique and methods of reporting outcomes, as well as level of evidence grading, in summary tables of individual studies.
Conclusion
A comprehensive review of the literature regarding surgical management of PRS and airway and feeding outcomes has led to several determinations: 1) Both MDO and TLA are effective alternatives to tracheostomy for patients who fail conservative management 2) Both interventions improve feeding and promote weight gain 3) MDO may be superior to TLA in long-term resolution of airway obstruction and avoidance of gastrostomy. 4) MDO has a higher rate of reported long-term complications, including scarring, dental damage and facial nerve injuries. 5) Definitive conclusions on which intervention yields better outcomes cannot be made due to variability of patient selection, surgical techniques, and follow up length.
Supplementary Material
Video Graphic 1, supplemental digital content 1, - See Video, which demonstrates our technique for mandibular distraction osteogenesis for patients with Pierre Robin Sequence, available in the "Related Videos" section of the Full-Text article on PRSJournal.com or, for Ovid users, available at INSERT HYPER LINK.
Footnotes
Disclosure: None of the authors listed have any conflicts of interest to report.
Conflicts of Interest:
The authors report no relevant financial disclosures related to this current work.
IRB:
This study does not contain primary data involving human subjects at the Children’s Hospital of Philadelphia and so did not undergo Institutional Review Board review.
References
- 1.Robin P. Glossoptosis due to atresia and hypotrophy of the mandible. Am J Dis Child 1934;48(3):541–547. [Google Scholar]
- 2.Caouette-Laberge L, Bayet B, Larocque Y. The Pierre Robin Sequence: Review of 125 cases and evolution of treatment modalities. Plast Reconstr Surg 1994;93(5):934–942. [PubMed] [Google Scholar]
- 3.Bütow K-W, Hoogendijk C, Zwahlen RA. Pierre Robin sequence: Appearances and 25 years of experience with an innovative treatment protocol. J Pediatr Surg 2009;44(11):2112–2118. [DOI] [PubMed] [Google Scholar]
- 4.Vatlach S, Maas C, Poets CF. Birth prevalence and initial treatment of Robin sequence in Germany: a prospective epidemiologic study. Orphanet J Rare Dis 2014;9(1):1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa MA, Tu MM, Murage KP, Tholpady SS, Engle WA, Flores RL. Robin Sequence: Mortality, causes of death, and clinical outcomes. Plast Reconstr Surg 2014;134(4):738–745. [DOI] [PubMed] [Google Scholar]
- 6.Scott AR, Mader NS. Regional variations in the presentation and surgical management of Pierre Robin sequence. Laryngoscope 2014;124(12):2818–2825. [DOI] [PubMed] [Google Scholar]
- 7.Evans A, Rahbar R, Rogers GF, Mulliken JB, Volk MS. Robin sequence: A retrospective review of 115 patients. Int J Pediatr Otorhi 2006;70(6):973–980. [DOI] [PubMed] [Google Scholar]
- 8.Marques I, de Sousa T, Carneiro A, Barbieri M, Bettiol H, Gutierrez M. Clinical experience with infants with Robin Sequence: A prospective study. Cleft Palate-craniofacial J 2001;38(2):171–178. [DOI] [PubMed] [Google Scholar]
- 9.Van den Elzen APM, Semmekrot BA, Bongers EMHF, Huygen PLM, Marres HAM. Diagnosis and treatment of the Pierre Robin sequence: results of a retrospective clinical study and review of the literature. Eur J Pediatr 2001;160(1):47–53. [DOI] [PubMed] [Google Scholar]
- 10.Tan T, Kilpatrick N, Farlie PG. Developmental and genetic perspectives on Pierre Robin sequence. Am J Med Genet Part C Semin Med Genet 2013;163(4):295–305. [DOI] [PubMed] [Google Scholar]
- 11.Evans KN, Sie KC, Hopper RA, Glass RP, Hing AV, Cunningham ML. Robin sequence: from diagnosis to development of an effective management plan. Pediatrics 2011;127(5):936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith MC, Senders CW. Prognosis of airway obstruction and feeding difficulty in the Robin sequence. Int J Pediatr Otorhi 2005;70(2):319–324. [DOI] [PubMed] [Google Scholar]
- 13.Glynn F, Fitzgerald D, Earley MJ, Rowley H. Pierre Robin sequence: an institutional experience in the multidisciplinary management of airway, feeding and serous otitis media challenges. Int J Pediatr Otorhi 2011;75(9):1152–1155. [DOI] [PubMed] [Google Scholar]
- 14.Lidsky ME, Lander TA, Sidman JD. Resolving feeding difficulties with early airway intervention in Pierre Robin Sequence. Laryngoscope 2008;118(1):120–123. [DOI] [PubMed] [Google Scholar]
- 15.Anderson ICW, Sedaghat AR, BM M. Prevalence and severity of obstructive sleep apnea and snoring in infants with Pierre Robin sequence. Cleft Palate-craniofacial J 2011; 48(5):614–618. [DOI] [PubMed] [Google Scholar]
- 16.Schaefer RB, Gosain AK. Airway management in patients with isolated Pierre Robin sequence during the first year of life. J Craniofac Surg 2003;14(4):462–467. [DOI] [PubMed] [Google Scholar]
- 17.Abel F, Bajaj Y, Wyatt M, Wallis C. The successful use of the nasopharyngeal airway in Pierre Robin sequence: an 11-year experience. Arch Dis in Child 2012; 97(4):331–334. [DOI] [PubMed] [Google Scholar]
- 18.Wagener S, Rayatt SS, Tatman AJ. Management of infants with Pierre Robin sequence. Cleft Palate-craniofacial J 2003;40(2):180–185. [DOI] [PubMed] [Google Scholar]
- 19.Daniel M, Bailey S, Walker K, Hensley R. Airway, feeding and growth in infants with Robin sequence and sleep apnoea. Int J Pediatr Otorhi 2013;77(4):499–503. [DOI] [PubMed] [Google Scholar]
- 20.Côté A, Fanous A, Almajed A, Lacroix Y. Pierre Robin sequence: Review of diagnostic and treatment challenges. Int J Pediatr Otorhi 2015;79(4):451–464. [DOI] [PubMed] [Google Scholar]
- 21.Tomaski SM, Zalzal GH, Saal HM. Airway obstruction in the Pierre Robin sequence. Laryngoscope 1995;105(2):111–114. [DOI] [PubMed] [Google Scholar]
- 22.Myer CM, Reed JM, Cotton RT, Willging JP, Shott SR. Airway management in Pierre Robin sequence. Otolaryngol Head Neck Surg 1998;118(5):630–635. [DOI] [PubMed] [Google Scholar]
- 23.Gianoli GJ, Miller RH, Guarisco JL. Tracheotomy in the first year of life. Ann Otol Rhinol Laryngol 1990;99(11):896–901. [DOI] [PubMed] [Google Scholar]
- 24.Douglas B. The treatment of micrognathia associated with obstruction by a plastic procedure. Plast Reconstr Surg 1946;1(3):300–308. [DOI] [PubMed] [Google Scholar]
- 25.Routledge RT. The Pierre-Robin syndrome: a surgical emergency in the neonatal period. Br J Plast Surg 1960;13:204–218. [DOI] [PubMed] [Google Scholar]
- 26.Smith JD. Treatment of airway obstruction in Pierre Robin syndrome. A modified lip-tongue adhesion. Arch Otolaryngol 1981;107(7):419–421. [DOI] [PubMed] [Google Scholar]
- 27.Argamaso RV. Glossopexy for Upper Airway Obstruction in Robin Sequence. Cleft Palate-craniofacial J 1992;29(3):232–238. [DOI] [PubMed] [Google Scholar]
- 28.Monasterio FO, Drucker M, Molina F, Ysunza A. Distraction osteogenesis in Pierre Robin sequence and related respiratory problems in children. J Craniofac Surg 2002;13(1):79–83. [DOI] [PubMed] [Google Scholar]
- 29.Denny AD, Talisman R, Hanson PR, Recinos RF. Mandibular distraction osteogenesis in very young patients to correct airway obstruction. Plast Reconstr Surg 2001;108(2):302–311. [DOI] [PubMed] [Google Scholar]
- 30.Rachmiel A, Aizenbud D, Pillar G, Srouji S. Bilateral mandibular distraction for patients with compromised airway analyzed by three-dimensional CT. Int J Pediatr Otorhi 2005;34(1),9–18. [DOI] [PubMed] [Google Scholar]
- 31.Basart H, Kruisinga FH, Breugem CC, Griot PJW, Hennekam RC, der Horst C. Will the right Robin patient rise, please? Definitions and criteria during management of Robin sequence patients in the Netherlands and Belgium. J Cranio Maxill Surg 2015;43(1):92–96. [DOI] [PubMed] [Google Scholar]
- 32.van Lieshout M, Joosten K, Mathijssen I, et al. Robin sequence: A European survey on current practice patterns. J Cranio Maxill Surg 2015;43(8):1626–1631. [DOI] [PubMed] [Google Scholar]
- 33.Collins B, Powitzky R, Robledo C, Rose C, Glade R. Airway Management in Pierre Robin Sequence: Patterns of Practice. Cleft Palate-craniofacial J 2014;51(3):283–289. [DOI] [PubMed] [Google Scholar]
- 34.Kirschner RE, Low DW, Randall P, et al. Surgical airway management in Pierre Robin sequence: is there a role for tongue-lip adhesion? Cleft Palate Craniofac J 2003;40(1):13–18. [DOI] [PubMed] [Google Scholar]
- 35.Schaefer RB, Stadler JA, Gosain AK. To distract or not to distract: an algorithm for airway management in isolated Pierre Robin sequence. Plast Reconstr Surg 2004;113(4):1113–1125. [DOI] [PubMed] [Google Scholar]
- 36.Rogers GF, Murthy AS, A LR, Mulliken JB. The GILLS Score: Part I. Patient selection for tongue-lip adhesion in Robin Sequence. Plast Reconstr Surg 2011;128(1):243–251. [DOI] [PubMed] [Google Scholar]
- 37.Denny AD. Distraction osteogenesis in Pierre Robin neonates with airway obstruction. Clin in Plast Surg 2004;31(2):221–229. [DOI] [PubMed] [Google Scholar]
- 38.Paes EC, van Nunen DPF, Speleman L, et al. A pragmatic approach to infants with Robin sequence: a retrospective cohort study and presence of a treatment algorithm. Clin Oral Invest 2015;19(8):2101–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lam D, Tabangin M, Shikary T, et al. Outcomes of mandibular distraction osteogenesis in the treatment of severe micrognathia. Jama Otolaryngol Head Neck Surg 2014;140(4):338–345. [DOI] [PubMed] [Google Scholar]
- 40.Sullivan D, Chung K, Eaves F, Rohrich R. The level of evidence pyramid: Indicating levels of evidence in Plastic and Reconstructive Surgery articles. Plast Reconstr Surg 2011;128(1):311–314. [DOI] [PubMed] [Google Scholar]
- 41.Mingo K, Sidman J, Sampson D, Lander T, Tibesar R, Scott A. Use of external distractors and the role of imaging prior to mandibular distraction in infants with isolated Pierre Robin Sequence and Stickler Syndrome. Jama Facial Plast Surg 2015;18(2):95–100. [DOI] [PubMed] [Google Scholar]
- 42.Flores RL, Tholpady SS, Sati S, et al. The surgical correction of Pierre Robin Sequence: Mandibular distraction osteogenesis versus tongue-lip adhesion. Plast Reconstr Surg 2014;133(6):1433–1439. [DOI] [PubMed] [Google Scholar]
- 43.Khansa I, Hall C, Madhoun LL, et al. Airway and feeding outcomes of mandibular distraction, tongue-lip adhesion, and conservative management in Pierre Robin Sequence: A prospective study. Plast Reconstr Surg 2017;139(4), 975e–983e. [DOI] [PubMed] [Google Scholar]
- 44.Mandell DL, Yellon RF, Bradley JP, Izadi K, Gordon CB. Mandibular distraction for micrognathia and severe upper airway obstruction. Arch Otolaryngol Head Neck Surg 2004;130(3):344–348. [DOI] [PubMed] [Google Scholar]
- 45.Monasterio FO, Molina F, Berlanga F, et al. Swallowing disorders in Pierre Robin sequence: its correction by distraction. J Craniofac Surg 2004;15(6):934–941. [DOI] [PubMed] [Google Scholar]
- 46.Rachmiel A, Srouji S, Emodi O, Aizenbud D. Distraction Osteogenesis for Tracheostomy Dependent Children With Severe Micrognathia. J Craniofac Surg 2012;23(2):459–463. [DOI] [PubMed] [Google Scholar]
- 47.Mahrous Mohamed A, Al Bishri A, Haroun Mohamed A. Distraction osteogenesis as followed by CTf scan in Pierre Robin sequence. J Craniomaxillofac Surg 2011;39(6):412–419. [DOI] [PubMed] [Google Scholar]
- 48.Hoffman W. Outcome of tongue-lip plication in patients with severe Pierre Robin sequence. J Craniofac Surg 2003;14(5):602–608. [DOI] [PubMed] [Google Scholar]
- 49.Resnick CM, Dentino K, Katz E, Mulliken JB, Padwa BL. Effectiveness of tongue-lip adhesion for obstructive sleep apnea in infants with Robin Sequence measured by polysomnography. Cleft Palate-craniofacial J 2015;53(5):584–588. [DOI] [PubMed] [Google Scholar]
- 50.Sedaghat AR, Anderson IC, McGinley MB, Rossberg MI, Redett RJ, Ishman SL. Characterization of obstructive sleep apnea before and after tongue-lip adhesion in children with micrognathia. Cleft Palate Craniofac J 2012;49(1):21–26. [DOI] [PubMed] [Google Scholar]
- 51.Denny AD, Amm CA, Schaefer RB. Outcomes of tongue-lip adhesion for neonatal respiratory distress caused by Pierre Robin sequence. J Craniofac Surg 2004; 15(5):819–823. [DOI] [PubMed] [Google Scholar]
- 52.Huang F, Lo L-J, Chen Y-R, Yang JC, Niu C-K, Chung M-Y. Tongue-lip adhesion in the management of Pierre Robin sequence with airway obstruction: technique and outcome. Chang Gung Med J 2005;28(2):90–96. [PubMed] [Google Scholar]
- 53.Mudd PA, Perkins JN, Harwood JEF, Valdez S, Allen GC. Early Intervention. Otolaryngol Head Neck Surg 2011;146(3):467–472. [DOI] [PubMed] [Google Scholar]
- 54.Tibesar RJ, Scott AR, C M. Distraction osteogenesis of the mandible for airway obstruction in children: long-term results. Otolaryngol Head Neck Surg 2010;143(1):90–96. [DOI] [PubMed] [Google Scholar]
- 55.Murage KP, Costa MA, Friel MT, Havlik RJ, Tholpady SS, Flores RL. Complications associated with neonatal mandibular distraction osteogenesis in the treatment of Robin Sequence. J Craniofac Surg 2014;25(2):383–387. [DOI] [PubMed] [Google Scholar]
- 56.Papoff P, Guelfi G, Cicchetti R, et al. Outcomes after tongue–lip adhesion or mandibular distraction osteogenesis in infants with Pierre Robin sequence and severe airway obstruction. Int J Oral Max Surg 2013;42(11):1418–1423. [DOI] [PubMed] [Google Scholar]
- 57.Susarla SM, Mundinger GS, Chang CC, et al. Gastrostomy placement rates in infants with Pierre Robin Sequence: A comparison of tongue-lip adhesion and mandibular distraction osteogenesis. Plast Reconstr Surg 2017:149–154. [DOI] [PubMed]
- 58.Denny A, Kalantarian B. Mandibular distraction in neonates: a strategy to avoid tracheostomy. Plast Reconstr Surg 2002;109(3):896–904. [DOI] [PubMed] [Google Scholar]
- 59.Denny A, Amm C. New technique for airway correction in neonates with severe Pierre Robin sequence. J Pediatr 2005;147(1):97–101. [DOI] [PubMed] [Google Scholar]
- 60.Al-Samkari HT, Kane AA, Molter DW, Vachharajani A. Neonatal outcomes of Pierre Robin sequence: an institutional experience. Clin Ped 2010;49(12):1117–1122. [DOI] [PubMed] [Google Scholar]
- 61.Spring M, Mount D. Pediatric feeding disorder and growth decline following mandibular distraction osteogenesis. Plast Reconstr Surg 2006;118(2):476. [DOI] [PubMed] [Google Scholar]
- 62.Cozzi F, Totonelli G, Frediani S, Zani A, Spagnol L, Cozzi DA. The effect of glossopexy on weight velocity in infants with Pierre Robin syndrome. J Pediatr Surg 2008;43(2):296–298. [DOI] [PubMed] [Google Scholar]
- 63.Bijnen CL, Don Griot PJ, Mulder WJ, Haumann TJ, Van Hagen AJ. Tongue-lip adhesion in the treatment of Pierre Robin sequence. J Craniofac Surg 2009;20(2):315–320. [DOI] [PubMed] [Google Scholar]
- 64.Paes EC, de Vries I, Penris WM, et al. Growth and prevalence of feeding difficulties in children with Robin sequence: a retrospective cohort study. Clin Oral Invest 2017;21(6):2063–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hong P, Brake MK, Cavanagh JP, Bezuhly M, Magit AE. Feeding and mandibular distraction osteogenesis in children with Pierre Robin sequence: a case series of functional outcomes. Int J Pediatr Otorhinolaryngol 2012;76(3):414–418. [DOI] [PubMed] [Google Scholar]
- 66.Genecov DG, Barceló CR, Steinberg D, Trone T, Sperry E. Clinical experience with the application of distraction osteogenesis for airway obstruction. J Craniofac Surg 2009;20 Suppl 2(8):1817–1821. [DOI] [PubMed] [Google Scholar]
- 67.Pasyayan HM, Lewis MB. Clinical experience with the Robin sequence. Cleft Palate J 1984;21(4):270–6. [PubMed] [Google Scholar]
- 68.Figueroa AA, Glupker TJ, Fitz MG, EA B. Mandible, tongue, and airway in Pierre Robin sequence: a longitudinal cephalometric study. Cleft Palate Craniofac J 1991;28(4):425–434. [DOI] [PubMed] [Google Scholar]
- 69.Rogers GF, Rogers G, Lim AA, Mulliken JB, Padwa BL. Effect of a syndromic diagnosis on mandibular size and sagittal position in Robin sequence. J Oral Maxillofac Surg 2009;67(11):2323–2331. [DOI] [PubMed] [Google Scholar]
- 70. Pfaff MJ, Metzler P, Kim Y, Steinbacher DM. Mandibular volumetric increase following distraction osteogenesis. J Plast Reconstr Aesthetic Surg 2014;67(9):1209–1214. [DOI] [PubMed] [Google Scholar]
- 71.Viezel-Mathieu A, Safran T, Gilardino MS. A Systematic Review of the Effectiveness of Tongue Lip Adhesion in Improving Airway Obstruction in Children With Pierre Robin Sequence. J Craniofac Surg 2016;27(6):1453. [DOI] [PubMed] [Google Scholar]
- 72.Tahiri Y, Viezel-Mathieu A, Aldekhayel S, Lee J, Gilardino M. The effectiveness of mandibular distraction in improving airway obstruction in the pediatric population. Plast Reconstr Surg 2014;133(3):352e–359e. [DOI] [PubMed] [Google Scholar]
- 73.Ow AT, Cheung LK. Meta-analysis of mandibular distraction osteogenesis: clinical applications and functional outcomes. Plast Reconstr Surg 2008;121(3):54e–69e. [DOI] [PubMed] [Google Scholar]
- 74.Breik O, Umapathysivam K, Tivey D, Anderson P. Feeding and reflux in children after mandibular distraction osteogenesis for micrognathia: A systematic review. Int J Pediatr Otorhi 2016;85:128–135. [DOI] [PubMed] [Google Scholar]
- 75.Bookman LB, Melton KR, Pan BS, et al. Neonates with tongue-based airway obstruction: a systematic review. Otolaryngol Head Neck Surg 2012;146(1):8–18. [DOI] [PubMed] [Google Scholar]
- 76.Almajed A, Viezel-Mathieu A, Gilardino MS, Flores RL, Tholpady SS, Côté A. Outcome following surgical interventions for micrognathia in infants With Pierre Robin Sequence: A systematic review of the literature. Cleft Palate Craniofac J 2017;54(1):32–42. [DOI] [PubMed] [Google Scholar]
- 77.Goldstein JA, Chung C, Paliga TJ, et al. Mandibular distraction osteogenesis for the treatment of neonatal tongue-based airway obstruction. J Craniofac Surg 2015;26(3):634–641. [DOI] [PubMed] [Google Scholar]
- 78.Abramowicz S, Bacic JD, Mulliken JB, Rogers GF. Validation of the GILLS score for tongue-lip adhesion in Robin sequence patients. J Craniofac Surg 2012;23(2):382–386. [DOI] [PubMed] [Google Scholar]
- 79.Flores RL, Greathouse TS, Costa M, Tahiri Y, Soleimani T, Tholpady SS. Defining failure and its predictors in mandibular distraction for Robin sequence. J Cranio Maxill Surg 2015;43(8):1614–1619. [DOI] [PubMed] [Google Scholar]
- 80.Burstein FD, Williams JK. Mandibular distraction osteogenesis in Pierre Robin sequence: application of a new internal single-stage resorbable device. Plast Reconstr Surg 2005;115(1):61–69. [PubMed] [Google Scholar]
- 81.Wittenborn W, Panchal J, Marsh JL, Sekar KC, Gurley J. Neonatal distraction surgery for micrognathia reduces obstructive apnea and the need for tracheotomy. J Craniofac Surg 2004;15(4):623–630. [DOI] [PubMed] [Google Scholar]
- 82.Bull MJ, Givan DC, Sadove AM, Bixler D, Hearn D. Improved outcome in Pierre Robin sequence: effect of multidisciplinary evaluation and management. Pediatrics 1990;86(2):294–301. [PubMed] [Google Scholar]
- 83.Ching JA, Daggett JD, Alvarez SA, Conley CL, Ruas EJ. A Simple Mandibular Distraction Protocol to Avoid Tracheostomy in Patients With Pierre Robin Sequence. Cleft Palate-craniofacial J 2015;54(2):210–215. [DOI] [PubMed] [Google Scholar]
- 84.Zellner E, Mhlaba J, Reid R, et al. Does Mandibular Distraction Vector Influence Airway Volumes and Outcome? J Oral Maxillofac Surg 2017;75(1):167–177. [DOI] [PubMed] [Google Scholar]
- 85.Andrews BT, Fan KL, Roostaeian J, Federico C, Bradley JP. Incidence of concomitant airway anomalies when using the university of California, Los Angeles, protocol for neonatal mandibular distraction. Plast Reconstr Surg 2013;131(5):1116–1123. [DOI] [PubMed] [Google Scholar]
- 86.Tholpady SS, Costa M, Hadad I, et al. Mandibular distraction for Robin Sequence associated with laryngomalacia. J Craniofac Surg 2015;26(3):826–830. [DOI] [PubMed] [Google Scholar]
- 87.Li H-Y, Lo L-J, Chen K-S, Wong K-S, Chang K-P. Robin sequence: review of treatment modalities for airway obstruction in 110 cases. Int J Pediatr Otorhi 2002;65(1):45–51. [DOI] [PubMed] [Google Scholar]
- 88.Bangiyev JN, Traboulsi H, Abdulhamid I, et al. Sleep architecture in Pierre-Robin sequence: The effect of mandibular distraction osteogenesis. Int J Pediatr Otorhi 2016;89:72–75. [DOI] [PubMed] [Google Scholar]
- 89.Breugem C, Paes E, Kon M. Bioresorbable distraction device for the treatment of airway problems for infants with Robin sequence. Clin oral invest 2012;16(4):1325–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cascone P, Papoff P, Arangio P, Vellone V, Calafati V, Silvestri A. Fast and early mandibular osteodistraction (FEMOD) in severe Pierre Robin Sequence. J Cranio Maxill Surg 2014;42(7):1364–1370. [DOI] [PubMed] [Google Scholar]
- 91.Chigurupati R, Massie J, Dargaville P, Heggie A. Internal mandibular distraction to relieve airway obstruction in infants and young children with micrognathia. Pediatr Pulm 2004;37(3):230–235. [DOI] [PubMed] [Google Scholar]
- 92.Gözü A, Genç B, Palabiyik M, et al. Airway management in neonates with Pierre Robin sequence. Turk J Pediatr 2010;52(2):167–172. [PubMed] [Google Scholar]
- 93.Hammoudeh J, Bindingnavele VK, Davis B, et al. Neonatal and infant mandibular distraction as an alternative to tracheostomy in severe obstructive sleep apnea. Cleft Palate Craniofac J 2012;49(1):32–38. [DOI] [PubMed] [Google Scholar]
- 94.Izadi K, Yellon R, Mandell DL, et al. Correction of upper airway obstruction in the newborn with internal mandibular distraction osteogenesis. J Craniofac Surg 2003;14(4):493–499. [DOI] [PubMed] [Google Scholar]
- 95.Konaş E, Çalış M, Bitik O, et al. Functional outcomes of mandibular distraction for the relief of severe airway obstruction and feeding difficulties in neonates with Pierre Robin sequence. Turk J Pediatr 2016;58(2):159–167. [DOI] [PubMed] [Google Scholar]
- 96.Lin SY, Halbower AC, Tunkel DE, Vanderkolk C. Relief of upper airway obstruction with mandibular distraction surgery: Long-term quantitative results in young children. Arch Otolaryngol Head Neck Surg 2006;132(4):437–441. [DOI] [PubMed] [Google Scholar]
- 97.Meyer AC, Lidsky ME, Sampson DE, Lander TA, Liu M, Sidman JD. Airway interventions in children with Pierre Robin Sequence. Otolaryngol - Head Neck Surg 2008;138(6):782–787. [DOI] [PubMed] [Google Scholar]
- 98.Mitsukawa N, Satoh K, Suse T, Hosaka Y. Clinical success of mandibular distraction for obstructive sleep apnea resulting from micrognathia in 10 consecutive Japanese young children. J Craniofac Surg 2007;18(4):948–953. [DOI] [PubMed] [Google Scholar]
- 99.Morovic C, Monasterio L. Distraction osteogenesis for obstructive apneas in patients with congenital craniofacial malformations. Plast Reconstr Surg 2000;105(7):2324–2330. [DOI] [PubMed] [Google Scholar]
- 100.Rachmiel A, Emodi O, Rachmiel D, Aizenbud D. Internal mandibular distraction to relieve airway obstruction in children with severe micrognathia. Int J Oral Max Surg 2014;43(10):1176–1181. [DOI] [PubMed] [Google Scholar]
- 101.Scott AR, Tibesar RJ, Lander TA, Sampson DE, Sidman JD. Mandibular distraction osteogenesis in infants younger than 3 months. Arch Facial Plast Surg 2011;13(3):173–179. [DOI] [PubMed] [Google Scholar]
- 102.Senders CW, Kolstad CK, Tollefson TT, Sykes JM. Mandibular distraction osteogenesis used to treat upper airway obstruction. Arch Facial Plast Surg 2010;12(1):11–15. [DOI] [PubMed] [Google Scholar]
- 103.Shen W, Jie C, Chen J, Zou J, Ji Y. Mandibular distraction osteogenesis to relieve Pierre Robin severe airway obstruction in neonates: Indication and operation. J Craniofac Surg 2009;20(8):1812–1816. [DOI] [PubMed] [Google Scholar]
- 104.Schoemann M, Burstein F, Bakthavachalam S, Williams J. Immediate mandibular distraction in mandibular hypoplasia and upper airway obstruction. J Craniofac Surg 2012;23(7):1981–1984. [DOI] [PubMed] [Google Scholar]
- 105.Sidman JD, Sampson D, Templeton B. Distraction Osteogenesis of the Mandible for Airway Obstruction in Children. Laryngoscope 2001;111(7):1137–1146. [DOI] [PubMed] [Google Scholar]
- 106.Tahiri Y, Greathouse TS, Tholpady SS, Havlik R, Sood R, Flores RL. Mandibular distraction osteogenesis in low-weight neonates with Robin Sequence: Is it safe? Plast Reconstr Surg 2015;136(5):1037–1044. [DOI] [PubMed] [Google Scholar]
- 107.Fujii M, Tachibana K, Takeuchi M, Nishio J, Kinouchi K. Perioperative management of 19 infants undergoing glossopexy (tongue‐lip adhesion) procedure: a retrospective study. Pediatr Anesth 2015;25(8):829–833. [DOI] [PubMed] [Google Scholar]
- 108.Mann RJ, Neaman KC, Hill B, Bajnrauh R, Martin MD. A novel technique for performing a tongue-lip adhesion—the tongue suspension technique. Cleft Palate Craniofac J 2012;49(1):27–31. [DOI] [PubMed] [Google Scholar]
- 109.Cruz MJ, Kerschner JE, Beste DJ, Conley SF. Pierre Robin Sequence: Secondary respiratory difficulties and intrinsic feeding abnormalities. Laryngoscope 1999;109(10):1632–1636. [DOI] [PubMed] [Google Scholar]
- 110.Carr MM, Poje CP, Kingston L, Kielma D. Complications in pediatric tracheostomies. Laryngoscope 2001; 111(11):1925–1928. [DOI] [PubMed] [Google Scholar]
- 111.Demke J, Bassim M, Patel MR, Dean S. Parental perceptions and morbidity: tracheostomy and Pierre Robin sequence. Int J Pediatr Otorhi 2008;72(10),1509–1516. [DOI] [PubMed] [Google Scholar]
- 112.Kremer B, AI B-K, Eckel HE. Indications, complications, and surgical techniques for pediatric tracheostomies—an update. J Pediatr Surg 2002;37(11),1556–1562. [DOI] [PubMed] [Google Scholar]
- 113.Kaslon K, Stein R. Chronic pediatric tracheotomy: assessment and implications for habilitation of voice, speech and language in young children. Int J Pediatr Otorhi 1985;9(2):165–171. [DOI] [PubMed] [Google Scholar]
- 114.Runyan CM, Armando U-R, Karlea A, et al. Cost Analysis of Mandibular Distraction versus Tracheostomy in Neonates with Pierre Robin Sequence. Otolaryngol -- Head Neck Surg 2014;151(5):811–818. [DOI] [PubMed] [Google Scholar]
- 115.Paes EC, Fouché JJ, Muradin M, Speleman L, Kon M, Breugem CC. Tracheostomy versus mandibular distraction osteogenesis in infants with Robin sequence: a comparative cost analysis. Br J Oral Maxillofac Surg 2014;52(3):223–229. [DOI] [PubMed] [Google Scholar]
- 116.Hong P, Bezuhly M, Taylor MS, Hart RD, Kearns DB, Corsten G. Tracheostomy versus mandibular distraction osteogenesis in Canadian children with Pierre Robin sequence: a comparative cost analysis. J Otolaryngol - Head Neck Surg 2012;41(3):207–214. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Graphic 1, supplemental digital content 1, - See Video, which demonstrates our technique for mandibular distraction osteogenesis for patients with Pierre Robin Sequence, available in the "Related Videos" section of the Full-Text article on PRSJournal.com or, for Ovid users, available at INSERT HYPER LINK.


