Abstract
Background:
The diagnostic and prognostic value of appropriate use criteria (AUC) for coronary artery disease (CAD) is well established. Whether the diagnostic yield of AUC for predicting CAD is preserved among the elderly is not known.
Methods:
We analyzed a multi-site prospective cohort of 1511 consecutive patients (mean age 59 ±13 years, 57% males) who underwent outpatient, community-based SPECT myocardial perfusion imaging (MPI). Appropriateness of the studies was determined based on the 2013 multimodality AUC for detection and risk assessment of stable ischemic heart disease. An abnormal SPECT MPI was defined by either a summed stress score ≥ 4 or a summed difference score ≥ 2.
Results:
Abnormal SPECT MPI was present in 190 patients (12.5%), while ischemia on MPI alone was present in 122 (8%). In multivariate logistic regression analysis, age ≥ 60 years, male gender, hypertension, diabetes mellitus, and known CAD were independent predictors of an abnormal SPET MPI, while appropriate indication of testing was not. Age ≥ 60 years was also an independent predictor of inducible myocardial ischemia, while appropriate indication for testing was not. Among elderly (≥ 60 year), regardless of appropriateness of testing, there was no difference in the prevalence of an abnormal SPECT (19% vs. 14%, p=0.14) or prevalence of SPECT ischemia (11% vs. 11%, p=1.00). Among younger patients however, appropriate testing predicted a greater prevalence of an abnormal SPECT (12% vs. 7%, p=0.013).
Conclusion:
In this multi-site cohort, testing based on AUC did not discriminate the risk of an abnormal SPECT MPI among the elderly. Caution is advised when relying on AUC for referring elderly patients for SPECT MPI.
Keywords: appropriate use criteria, myocardial perfusion imaging, age, coronary artery disease, SPECT
INTRODUCTION
Age is a strong determinant of coronary artery disease (CAD) risk. According to the Diamond and Forrester classification, the pretest likelihood of CAD is significantly greater among elderly patients, regardless of typicality of symptoms1. While CAD risk factors, especially diabetes mellitus2, are strong determinants of CAD risk as well, the value of the burden of CAD risk factors in predicting myocardial ischemia seems limited among the elderly3. The appropriate use criteria (AUC) guide physicians to refer patients for single photon emission computed tomography (SPECT) myocardial perfusion imaging (MPI) and are based on the likelihood of the test to provide meaningful diagnostic information4. We have previously reported the diagnostic and prognostic value of an appropriate SPECT MPI from a community-based cohort of patients5. We previously reported that there is no demonstrable gender-based differential impact of AUC on the diagnostic or prognostic utility of SPECT MPI6. However, the impact AUC on the diagnostic yield of MPI among elderly patients, who have a high likelihood of CAD, has not been studied. In this study, we sought to investigate the impact of AUC on the diagnostic yield of SPECT MPI among the elderly.
METHODS
This report is a sub-analysis of a previously published multi-site prospective cohort study of 1,511 consecutive patient, all of whom were referred for a clinically indicated SPECT MPI in the outpatient setting5. The detailed methodology of the study, including study population and MPI testing have been previously published5. Briefly, 1707 consecutive patients who underwent SPECT-MPI between August 15, 2007 and May 15, 2011, were identified. These patients were referred from the offices of 22 physicians (20 primary care and 2 cardiologists) in 11 private practices. Among these patients, 182 met one or more exclusion criteria and 14 (0.9%) subjects were lost to follow-up. The remaining 1,511 (99.1%) eligible subjects were prospectively followed for 27 ± 10 months. The study was approved by the Institutional Review Board of Rush University Medical Center (Chicago, IL).
Clinical Data and Appropriate Use Criteria Determination
Patient demographics, referral diagnosis, risk factors and cardiovascular history were recorded prior to stress MPI. Known CAD was defined as a history of myocardial infarction (MI), prior percutaneous or surgical revascularization or anatomical stenosis ≥ 50% on a coronary angiography. Patient medical record, including the referring physician clinical evaluation, preceding MPI were reviewed to determine patient symptoms, indication, and rationale for testing, including type of surgery if MPI was performed for preoperative risk assessment. Among patients with ischemic equivalent symptoms but no prior CAD, pretest likelihood of CAD was determined using the Diamond and Forrester criteria1. Since Diamond and Forrester tables do not provided estimates for CAD prevalence beyond age 69 years, the pre-test likelihood of CAD among symptomatic patients ≥70 years of age was determined using highest age category in (60 – 69 years) in these tables1. The 10-year risk of ischemic heart disease among asymptomatic patients was determined using the Framingham Risk Score7. In the original report, the appropriateness of SPECT-MPI was determined according to the 2009 AUC for cardiac radionuclide imaging.8 For the purpose of this report, the appropriateness of MPI use was reclassified according to the 2013 multimodality AUC for the detection and risk assessment of stable ischemic heart disease using initially collected clinical data elements4. Each MPI study was categorized as appropriate, maybe appropriate or rarely appropriate. To study the impact of rarely appropriate testing on the diagnostic yield of stress MPI, studies with appropriate and maybe appropriate indications were grouped together as being “appropriate”. The study cohort was further divided into two subgroups based on age: < 60 and ≥ 60 years of age. Age 60 was selected to classify age subgroup since it defines the highest age category in the Diamond and Forrester tables1.
Stress Myocardial Perfusion Imaging
SPECT MPI was performed using a one-day, rest/stress technetium-99m sestamibi protocol9. Stress modality (exercise vs. adenosine vs. adenosine with low-level exercise) was selected as clinically appropriate10,11. All MPI studies were acquired using a dual-head, cardiac SPECT camera (MAIcam 180® - Mid-Atlantic Imaging, Inc. – Columbia, MD) without attenuation correction.12,13 SPECT MPI was interpreted using QPS/QGS software (Cedars-Sinai Cardiac Suite, Los Angeles, CA), by an expert nuclear cardiologist who was blinded to clinical data. Segmental radiotracer activity was semiquantitatively scored on a 17-segment model of the left ventricle (LV), using a five-point scoring system (0= normal to 4= absent)14. The segmental scores within the stress and rest scans were summed to generate summed stress scores (SSS) and summed rest scores, respectively. The summed difference score (SDS) was calculated from the sum of the segmental difference scores between stress and rest scans. An abnormal MPI was defined as a SSS ≥ 4 or SDS ≥ 2.
Statistical Analysis
Statistical analyses were conducted using STATA version 12 (College Station, TX). Student’s t-test was used to analyze differences in continuous data. The chi-square test was used to compare categorical data. Univariate and multivariate logistic regression analyses were performed to determine the predictors of an abnormal SPECT MPI. Multivariate analysis was adjusted for clinical covariates of age (by cut-off of 60 years), gender, CAD risk factors (diabetes mellitus, hypertension, dyslipidemia, tobacco use, and family history), known CAD status, and test appropriateness. The multivariable regression model was tested for collinearity between the variables, and none was observed. A p-value of < 0.05 was considered to represent statistical significance.
RESULTS
The mean age of the study population (n=1,511) was 59 ± 13 years, with 57% being males. A total of 664 patients (44%) were older than 60 years of age. SPECT MPI was abnormal in 190 (12.5%) patients, and 271 patients (18%) had a history of known CAD. Ischemia on SPECT MPI was noted in 122 patients (8%). Six-hundred and ninety-nine studies (54%) were performed for indications that were determined to be appropriate (or may be appropriate). Demographic and clinical data of the study population, stratified by an abnormal SPECT MPI, is provided in table 1. Patients with an abnormal MPI were older and more often males. They also had a greater prevalence of known CAD, hypertension, diabetes, and were more often smokers. Indication for stress testing was more often appropriate or maybe appropriate among patients who had an abnormal MPI. On univariate logistic regression analysis, all clinical and demographic variables were significantly associated with an abnormal MPI, except history of smoking and family history of CAD (Table 2). Appropriate indication for testing was also associated with an abnormal MPI on univariate analysis. However, on multivariate regression analysis, age ≥ 60 years, male gender, hypertension, diabetes and known CAD were the only independent predictors of an abnormal MPI. Appropriateness of testing was not an independent predictor of an abnormal MPI. When analyzing age as a continuous variable, there was a significant increased risk of an abnormal MPI with every 10-year increment in age (odds ratio [OR], 1.52; 95% confidence interval, 1.24 – 1.85; p-value: <0.0001), after adjusting for all variables (Table 2), including appropriateness criteria. Similar results were noted for ischemia on SPECT MPI, which was independently predicted by age ≥ 60, hypertension and known CAD (Table 3). Again, among patients ≥ 60 years of age, appropriateness of testing did not predict ischemia on SPECT MPI.
Table 1:
Distribution of Demographic and Clinical Variables of the Study Population Stratified by Abnormal SPECT Myocardial Perfusion Imaging (n=1511).
| Variable | Abnormal MPI (n=190) |
Normal MPI (n=1321) |
p-value |
|---|---|---|---|
| Age (years) | 65 ± 13 | 58 ±13 | <0.0001 |
| Male gender (%) | 76% | 54% | <0.0001 |
| Hypertension (%) | 71% | 53% | <0.0001 |
| Diabetes (%) | 35% | 20% | <0.0001 |
| Hyperlipidemia (%) | 59% | 44% | <0.0001 |
| Smoking (%) | 15% | 12% | 0.14 |
| Family history of CAD (%) | 35% | 36% | 0.82 |
| Known CAD (%) | 52% | 13% | <0.0001 |
| Appropriate or Maybe appropriate testing (%) | 68% | 52% | <0.0001 |
Table 2:
Predictors of an Abnormal Stress Myocardial Perfusion SPECT.
| Variable | Univariate Predictors | Multivariate Predictors | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age ≥ 60 years | 2.06 | 1.49 – 2.86 | <0.0001 | 1.67 | 1.06 – 2.64 | 0.03 |
| Male gender | 2.69 | 1.86 – 3.91 | <0.0001 | 2.17 | 1.47 – 3.19 | <0.0001 |
| Hypertension | 1.83 | 1.30 – 2.57 | 0.001 | 1.55 | 1.07 – 2.24 | 0.02 |
| Diabetes | 2.26 | 1.61 – 3.19 | <0.0001 | 1.58 | 1.10 – 2.28 | 0.01 |
| Hyperlipidemia | 1.73 | 1.24 – 2.39 | 0.001 | 1.11 | 0.79 – 1.58 | 0.53 |
| Smoking | 1.49 | 0.96 – 2.32 | 0.08 | 1.38 | 0.85 – 2.21 | 0.19 |
| Family history of CAD | 1.03 | 0.73 – 1.43 | 0.89 | 1.05 | 0.74 – 1.49 | 0.78 |
| Known CAD | 7.51 | 5.33 – 10.6 | <0.0001 | 5.17 | 3.65 – 7.31 | <0.0001 |
| Appropriate/May be Appropriate indication | 2.04 | 1.45 – 2.86 | <0.0001 | 1.10 | 0.66 – 1.87 | 0.70 |
Table 3:
Predictors of Ischemia on Stress Myocardial Perfusion SPECT.
| Variable | Univariate Predictors | Multivariate Predictors | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Age ≥ 60 years | 2.01 | 1.38 – 2.93 | <0.0001 | 1.58 | 1.03 – 2.42 | 0.04 |
| Male gender | 1.63 | 1.11 – 2.42 | 0.014 | 1.46 | 0.96 – 2.24 | 0.08 |
| Hypertension | 2.09 | 1.39 – 3.14 | <0.0001 | 1.64 | 1.06 – 2.52 | 0.03 |
| Diabetes | 1.75 | 1.17 – 2.61 | 0.006 | 1.37 | 0.90 – 2.01 | 0.15 |
| Hyperlipidemia | 1.65 | 1.13 – 2.39 | 0.009 | 1.19 | 0.80 – 1.77 | 0.39 |
| Smoking | 1.49 | 0.96 – 2.32 | 0.08 | 1.35 | 0.78 – 2.33 | 0.28 |
| Family history of CAD | 1.04 | 0.71 – 1.52 | 0.83 | 1.09 | 0.74 – 1.64 | 0.64 |
| Known CAD | 3.13 | 2.11 – 4.63 | <0.0001 | 2.31 | 1.52 – 3.53 | <0.0001 |
| Appropriate/May be Appropriate indication | 1.51 | 1.04 – 2.28 | 0.03 | 1.13 | 0.73 – 1.75 | 0.57 |
When stratifying the prevalence of an abnormal MPI by appropriateness of indication and by age, patients older than 60 years of age had a greater prevalence of an abnormal MPI regardless of the appropriateness of indication (Figure 1). Among patients tested for a rarely appropriate indication, patients older than 60 years of age had twice the prevalence of an abnormal SPECT MPI, when compared to patients younger than 60 years of age (14% vs. 7%, p=0.006). The prevalence of an abnormal MPI among elderly patients tested for rarely appropriate indications was similar to that among elderly tested for appropriate indications (14% vs. 19%, respectively, p=0.14). A similar trend was also noted for ischemia on SPECT MPI (Figure 2). Stress induced myocardial ischemia was more prevalent among the elderly, with no difference in the prevalence of ischemia among the elderly (11%) regardless of appropriateness criteria (p=1.00).
Figure 1:
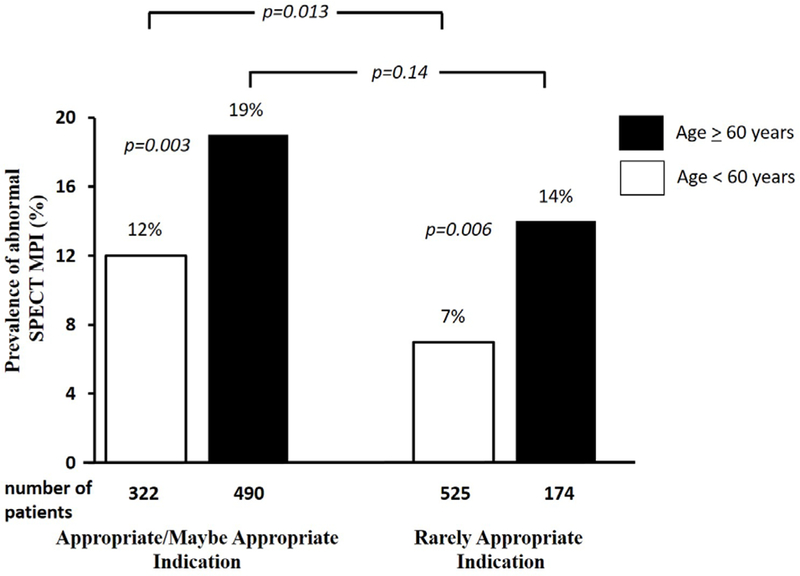
Prevalence of an Abnormal Stress Myocardial Perfusion SPECT Stratified by Age and Appropriateness of Indication.
Figure 2:
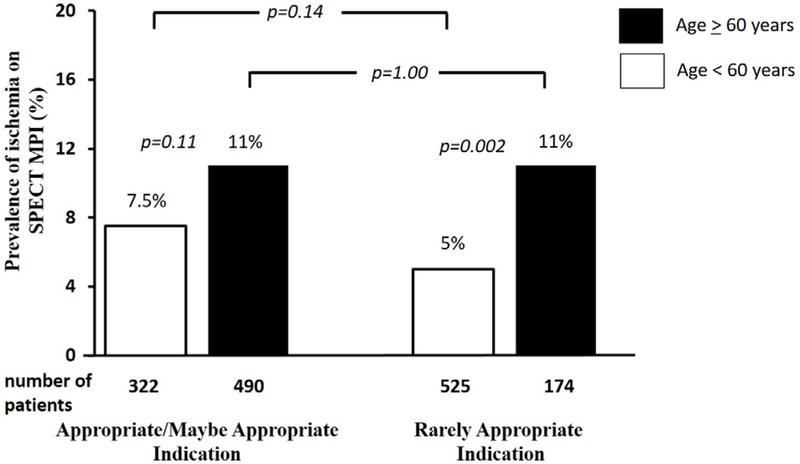
Prevalence of an Abnormal Stress Myocardial Perfusion SPECT Stratified by Age and Appropriateness of Indication.
DISCUSSION
In this study, we report the impact of AUC on the relationship between age and abnormal SPECT MPI. In this broad-based, multi-site cohort, application of AUC did not effectively stratify the risk of an abnormal MPI or ischemia on MPI among the elderly. We identified a similarly high proportions of an abnormal SPECT MPI and ischemia on SPECT MPI among elderly patients whether testing was performed for an appropriate or a rarely appropriate indication. On the contrary, AUC did stratify the risk of an abnormal SPECT MPI in patients younger than 60 years of age, as appropriate testing was associated with a greater prevalence of an abnormal MPI (12%) when compared to rarely appropriate testing (7%, p=0.013). However, such a relationship only attained borderline statistical significance for the prevalence of ischemia on SPECT MPI among patients younger than 60 years of age (7.5 vs. 5%, p=0.14).
AUC are intended to aid in effective application of MPI and improve the value of testing. Yield of testing is an important determinant of value in decision making. AUC suggest the appropriateness of a cardiovascular test for diagnosis of suspected CAD, but do not preferentially recommend one appropriate imaging test over the other. Additionally, AUC does not classify the utility of a test by age, which is a strong predictor of CAD. In a study of asymptomatic patients (who would otherwise be rarely appropriate for SPECT MPI) referred for stress testing, increasing age was an independent predictor of ischemia on SPECT MPI3. Age >74 years was the strongest independent predictor of ischemia (OR and p-value: 3.65 and 0.03), in comparison to other independent predictors – smoking (OR and p-value: 1.72 and 0.01) and diabetes (OR and p-value: 1.41 and 0.001). Though an increasing number of CAD risk factors predicted an increasing prevalence of ischemia, this effect was lost among those >74 years of age, suggesting a significantly greater impact of increasing age on myocardial ischemia when compared to other risk factors. Similarly, in a separate study of symptomatic patients undergoing exercise stress MPI, increasing age was an independent predictor of ischemic ECG response, while other CAD risk factors were not predictive15.
Though AUC do not classify the appropriateness level of an indication for MPI by age, AUC are likely heavily weighted by age, especially among those with no known CAD. Diamond and Forrester criteria is often used as a guide for determining the pretest probability of CAD. Based on this criteria, the prevalence of CAD increases with age, for both men and women, across typicality of symtoms1. Based on Diamond and Forrester criteria, the pretest probability of CAD is for men and women ≥ 60 years of age with typical angina is greater than 90%. A similar trend of increasing anatomical CAD on coronary computed tomography angiography (CCTA) with increasing age has been reported from the analysis of data from the COroNary CT Angiography Evaluation For Clinical Outcomes: An InteRnational Multicenter (CONFIRM) registry16. A recent pooled analysis of the data from Rule Out Myocardial Infarction/Ischemia Using Computer Assisted Tomography-II (ROMICAT-II) and American College of Radiology Imaging Network-Pennsylvania (ACRIN-PA) study revealed a significantly greater prevalence of anatomical stenosis among those ≥ 60 years of age17. These studies further underscore the fact that the elderly represent a high-risk population. Though AUC generally advise against routine testing for rarely appropriate indications, this may not hold true across all age categories, as a substantial proportion of elderly tested for rarely appropriate indications in our cohort had an abnormal SPECT MPI. Our results do not discount the value of AUC, and in fact, among patients younger than 60 years of age, application of AUC identifies a significantly higher proportion of abnormal MPI among those tested for appropriate indication.
LIMITATOINS
Our study population represents a relatively lower-risk community-based population, and thus is not representative of higher-risk tertiary care center populations. Additionally, the lack of outcomes data limits our ability to determine if high prevalence of abnormal SPECT MPI among elderly tested for rarely appropriate indications translates into a high rate of cardiovascular outcomes. The prevalence of testing for inappropriate indications is high in our study cohort and is likely not reflective of current practice. However, the study was conducted to investigate the impact of AUC on results of SPECT MPI and not to report adherence rates with AUC.
CONCLUSIONS
Age is a strong independent predictor of an abnormal SPECT MPI and inducible myocardial ischemia. The value of age in predicting an abnormal SPECT MPI is maintained across MPI test appropriateness categories. Appropriate testing identifies a greater prevalence of abnormal MPI in patients younger than 60 years of age, though it does not provide such a diagnostic discrimination among elderly. Cautious reliance on AUC is advised when considering elderly patients for MPI.
Acknowledgments
FUNDING: The original study leading to this cohort was funded by Astellas Pharma Global Development (Northbrook, IL). The present analysis and report were internally funded.
Footnotes
DECLARATIONS OF INTEREST: Rami Doukky receives research funding grants from Astellas Pharma (Northbrook, IL). Saurabh Malhotra has nothing to disclose.
REFERENCES
- 1.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300(24):1350–1358. [DOI] [PubMed] [Google Scholar]
- 2.Rivera JJ, Nasir K, Choi EK, et al. Detection of occult coronary artery disease in asymptomatic individuals with diabetes mellitus using non-invasive cardiac angiography. Atherosclerosis. 2008. [DOI] [PubMed] [Google Scholar]
- 3.Malhotra S, Sharma R, Kliner DE, Follansbee WP, Soman P. Relationship between silent myocardial ischemia and coronary artery disease risk factors. J Nucl Cardiol. 2013;20(5):731–738. [DOI] [PubMed] [Google Scholar]
- 4.Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2014;63(4):380–406. [DOI] [PubMed] [Google Scholar]
- 5.Doukky R, Hayes K, Frogge N, et al. Impact of appropriate use on the prognostic value of single-photon emission computed tomography myocardial perfusion imaging. Circulation. 2013;128(15):1634–1643. [DOI] [PubMed] [Google Scholar]
- 6.Doukky R, Hayes K, Frogge N. Appropriate use criteria for SPECT myocardial perfusion imaging: Are they appropriate for women? J Nucl Cardiol. 2016;23(4):695–705. [DOI] [PubMed] [Google Scholar]
- 7.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. [DOI] [PubMed] [Google Scholar]
- 8.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Endorsed by the American College of Emergency Physicians. J Am Coll Cardiol. 2009;53(23):2201–2229. [DOI] [PubMed] [Google Scholar]
- 9.Henzlova MJ, Duvall WL, Einstein AJ, Travin MI, Verberne HJ. ASNC imaging guidelines for SPECT nuclear cardiology procedures: Stress, protocols, and tracers. J Nucl Cardiol. 2016;23(3):606–639. [DOI] [PubMed] [Google Scholar]
- 10.Doukky R Pharmacologic Stress testing in myocardial perfusion imaging: Technical applications In: Mann A, Heller GV, Hendel RC, eds. Nuclear Cardiology: Technical Applications. New York: McGraw-Hill; 2007:107–124. [Google Scholar]
- 11.Poulin MF, Alexander S, Doukky R. Prognostic implications of stress modality on mortality risk and cause of death in patients undergoing office-based SPECT myocardial perfusion imaging. J Nucl Cardiol. 2016;23(2):202–211. [DOI] [PubMed] [Google Scholar]
- 12.Chawla D, Rahaby M, Amin AP, et al. Soft tissue attenuation patterns in stress myocardial perfusion SPECT images: a comparison between supine and upright acquisition systems. J Nucl Cardiol. 2011;18(2):281–290. [DOI] [PubMed] [Google Scholar]
- 13.Ballany W, Mansour K, Morales Demori R, Al-Amoodi M, Doukky R. The impact of regimented aminophylline use on extracardiac radioisotope activity in patients undergoing regadenoson stress SPECT myocardial perfusion imaging: a substudy of the ASSUAGE trial. J Nucl Cardiol. 2014;21(3):496–502. [DOI] [PubMed] [Google Scholar]
- 14.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. [DOI] [PubMed] [Google Scholar]
- 15.Malhotra S, Follansbee WP, Soman P. Predictors of an ischemic electrocardiographic response in patients with exercise-induced myocardial ischemia. J Nucl Cardiol.2011;18(4):678–684. [DOI] [PubMed] [Google Scholar]
- 16.Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM).Circulation.2011;124(22):2423–2432, 2421–2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bamberg F, Mayrhofer T, Ferencik M,et al. Age- and sex-based resource utilisation and costs in patients with acute chest pain undergoing cardiac CT angiography: pooled evidence from ROMICAT II and ACRIN-PA trials.Eur Radiol.2018;28(2):851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]


