Abstract
An undergraduate organic chemistry laboratory experiment involving the breakage of amide C—N bonds is reported. Whereas amides are typically considered stable species due to well-established resonance effects, this experiment allows students to cleave the amide C—N bond in a nickel-catalyzed esterification process. Moreover, students perform the experiment on the benchtop using a commercially available paraffin wax capsule containing the necessary nickel precatalyst and N-heterocyclic carbene ligand. The laboratory procedure introduces students to several modern topics in organic chemistry that are not otherwise well-represented in typical undergraduate organic chemistry curricula, such as amide bond cleavage, transition metal-catalyzed cross-coupling reactions, and nonprecious-metal catalysis.
Keywords: Second-Year Undergraduate, Upper-Division Undergraduate, Organic Chemistry, Laboratory Instruction, Hands-On Learning/Manipulatives, Organometallics, Synthesis, NMR Spectroscopy, Chromatography, Amides
Graphical Abstract
INTRODUCTION
Transition metal-catalyzed cross-coupling reactions have become an indispensible tool in academic and industrial settings.1 Although the field of cross-couplings has traditionally been dominated by aromatic electrophiles, the use of acyl electrophiles has gained traction in recent years. One particular area of excitement is the activation of amide C—N bonds2 using transition metal catalysis.3 Interest in this field has been driven not only by the largely untapped synthetic potential of amides, but also by the inherent challenge and intellectual curiosity associated with cleaving amide C—N bonds. Thanks to the efforts of several research groups, a host of catalytic amide cross-coupling reactions have been developed since 2015 using either palladium or nickel.3 The latter has been especially attractive, given that nickel is an inexpensive, nonprecious metal. Nickel-catalyzed esterifications,4 transamidations,5 Suzuki—Miyaura couplings,6 Negishi couplings,7 and Heck reactions8 of amide derivatives have now been developed. Typically, an electron-withdrawing group, such as a phenyl ring or a carbamate, is placed on the amide nitrogen, presumably weakening the amide C—N bond.
Although the Ni-catalyzed methodologies noted above are typically performed under inert, glovebox conditions due to catalyst and ligand sensitivities, the advent of new technologies has allowed for these reactions to be performed entirely on the benchtop. Specifically, the necessary Ni(cod)2 precatalyst and the key N-heterocyclic carbene (NHC) ligand, SIPr, can be encapsulated in paraffin wax, analogous to Buchwald’s encapsulation of palladium catalysts,9 to enable benchtop delivery.10 Prompted by the commercialization of these user-friendly capsules,11 an undergraduate laboratory experiment that would introduce students to modern catalytic amide cross-coupling reactions was pursued. Several metal-catalyzed cross-coupling experiments for undergraduate organic chemistry laboratories have been developed,12 though no pedagogical experiments regarding amide cross-coupling with C—N bond cleavage have been reported.
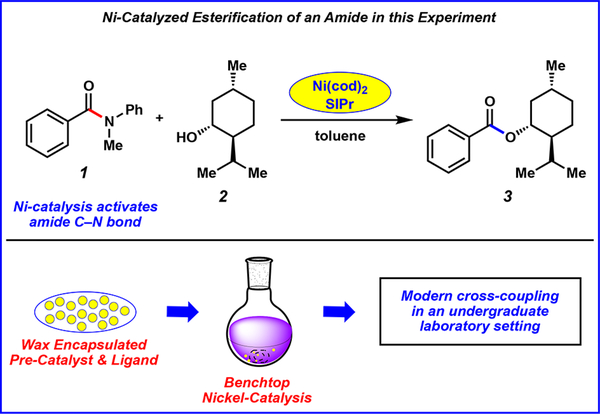
An advanced undergraduate organic chemistry laboratory experiment that allows students to perform the nickel-catalyzed coupling of an amide with an alcohol to generate an ester product has been developed (Figure 1). The procedure is performed on the benchtop by employing the aforementioned commercially available paraffin capsules containing Ni(cod)2 and SIPr.11 In addition to familiarizing students with the history and importance of cross-couplings, the lab writeup also introduces new frontiers in the field, including the growing use of non-precious-metal catalysis and unconventional electrophiles (i.e., amides), as well as the development of user-friendly synthetic technologies (i.e., paraffin encapsulation of catalysts). Additionally, the experimental protocol provides an opportunity for students to hone laboratory skills, such as air-free reaction setup, advanced purification strategies, and spectroscopic analysis.
Figure 1.
Esterification of amides being performed in this undergraduate laboratory experiment.
PEDAGOGICAL GOALS
Student excitement in the undergraduate organic chemistry laboratory can be driven by exposure to modern organic chemistry research.13 This laboratory experiment leverages recent advances in reagent delivery methods and new frontiers in cross-coupling research to spur student engagement and meet the following learning goals:
To introduce students to transition metal-catalyzed cross-coupling reactions, an important class of transformations used routinely by industrial and academic chemists.
To capture student attention by teaching them several aspects of contemporary cross-coupling research, including nonprecious-metal catalysis, amide C—N bond activation, and technologies that allow for air-sensitive catalysts and ligands to be handled on the benchtop.
To help students hone laboratory techniques such as reaction setup, reaction monitoring, purification, and spectroscopic characterization.
OVERVIEW OF LABORATORY EXPERIMENT
This laboratory experiment is designed for a second-year or upper-division undergraduate laboratory course. The procedure requires 4–5 h to complete; thus, it fits well in either one long laboratory period or two shorter laboratory periods. Before entering the laboratory, each student completes a prelaboratory worksheet (Supporting Information, p S17) that ensures their comprehension of relevant concepts and safety considerations. After completing the experiment, each student completes a postlaboratory worksheet (p S20). This provides a forum for students to analyze their data and conceptualize their findings within the field of organic chemistry.
Experiment
Students complete a prelaboratory worksheet prior to performing the experiment individually. Following an instructor-led overview of the experimental procedure, students charge a 1-dram vial with N-methyl-N-phenylbenzamide (1, prepared by the laboratory support personnel prior to the laboratory period), the alcohol nucleophile (i.e., menthol (2)), the commercially available paraffin—Ni(cod)2/SIPr capsule, and toluene. The reaction vessel is then flushed with N2 for several minutes before being swiftly capped with a Teflon-lined, plastic cap and sealed with Teflon tape. Once sealed, the reaction is heated to 110 °C for 30 min using a heated aluminum block. After the reaction mixture is cooled to ambient temperature, thin-layer chromatography (TLC) analysis is performed, and the crude reaction mixture is dry-loaded onto Celite using slow rotary evaporation. The dry-loaded material is stored at room temperature if the experiment is completed on a second day. Otherwise, the dry-loaded crude material is purified using silica gel flash chromatography. A yield is obtained for the isolated product, and 1H NMR spectroscopy is used to characterize menthyl benzoate (3) and assess its purity. Data interpretation and analysis is performed in a postlaboratory worksheet. A detailed description of the experiment is provided in the Supporting Information.
HAZARDS
Closed-toed shoes, long pants covering the ankles, safety glasses, gloves, and flame-resistant laboratory coats should be worn at all times. All hazardous materials should be handled and disposed of in accordance with the recommendation of the materials’ safety data sheet and environmental health and safety (EH&S). The amide substrate, menthol, and ester products are irritants. The wax capsule containing Ni(cod)2 and SIPr is an irritant, is a flammable solid, and may cause cancer. Toluene is flammable and is an irritant and a reproductive toxin. N-Methylaniline is flammable and is an irritant and an acute toxin. Dichloromethane is an acute toxin, an irritant, and a regulated carcinogen. Ethyl acetate and hexanes are flammable and volatile organic solvents. The n-hexane in hexanes is a neurotoxin. Deuterated chloroform is a cancer suspect agent and mutagen. Stirring-hot plates should be used inside the fume hood and kept away from flammable solvents, especially given the high temperature utilized in this laboratory experiment. The size of the reaction vessel, the volume of reaction media, and temperature of the heating block should be strictly adhered to in order to avoid pressure buildup in the reaction vessel.
RESULTS AND DISCUSSION
The use of paraffin-encapsulated Ni(cod)2 and SIPr to enable amide cross-coupling reactions, including the esterification of amides, was recently disclosed.10 In this communication, esterification could be achieved utilizing a variety of alcohol coupling partners. For this laboratory experiment, we selected menthol as a nucleophile as it is highly crystalline and available in high purity. Several adaptations were made to the experimental protocol before its implementation in undergraduate instructional laboratories. These are described in the Instructor’s Notes in the Supporting Information (p S24), but can be summarized as follows: (A) The original reaction was performed at 80 °C for 16 h.4a Upon optimization, it was found that the reaction could be performed much more quickly (i.e., 30 min) if heated to 110 °C. (B) Reactions are typically performed under rigorously dry and deoxygenated conditions. However, the esterification proceeded smoothly without flame-drying the reaction vessel, deoxygenating the solvent by sparging, or recrystallizing the menthol nucleophile. (C) It was also necessary to optimize how the crude product would be stored between lab periods in a manner that facilitated purification. It was found that the crude ester could be dry-loaded onto Celite, stored at room temperature, and then purified using flash column chromatography on silica gel.
With a suitable procedure in hand, the experimental protocol was implemented in eight sections of an undergraduate organic chemistry laboratory course across two academic terms consisting primarily of chemistry and biochemistry majors. The course instructor and teaching assistants introduced students to the many topics at hand, such as cross-coupling reactions, nonprecious-metal catalysis, the importance and stability of amides, the recent discovery of amides as cross-coupling partners, and glovebox-free catalysis enabled by wax-encapsulation. This knowledge was furthered by students reading the laboratory handout, which also provided the students with an understanding of the experimental details. Students were required to complete a prelaboratory worksheet before performing the experiment. This ensured that students understood the chemistry, its context in the broader field of transition metal catalysis, and the experimental protocol, including safety considerations. Satisfactory completion of the prelaboratory worksheet was required for students to perform the experiment, as judged by the instructor or a graduate student teaching assistant.
Students then performed the experiment over two laboratory periods. The first period was devoted to reaction setup, analysis by TLC, and dry-loading of the crude product onto Celite. During the second period, students performed a purification, followed by analysis.
Overall, of the 65 students who carried out the experiment in the eight sections across two quarters of enrollment, 37 were able to isolate desired ester product 3 as a white solid. Isolated yields ranged from 17% to over 100% after flash column chromatography. Low yields or failure to obtain product were attributed to experimental error that may have occurred during N2 purging or capping, leading to catalyst deactivation. Thus, it is critical that instructors stress to students that oxygen must not be allowed to enter the reaction vial to ensure experimental success. Some students reported obtaining mixed fractions (likely due to coelution with ligand byproducts) during chromatography, which also contributed to some of the lower yields. Yields in excess of 100% were attributed to impurities or residual solvent present in the isolated material following purification. Common impurities observed were N-methylaniline and unreacted amide substrate, N-methyl-N-phenylbenzamide (1). In addition, students assigned peaks in their 1H NMR spectra, identified impurities, and assessed the purity of their purified samples qualitatively on the basis of their 1H NMR spectra (student spectra are given in the Supporting Information, p S48). The students identified diagnostic product signals in the 1H NMR spectra, including those of the aryl protons (8.04 (2H), 7.55 (1H), and 7.44 (2H) ppm) as well as the proton α to the oxygen of the ester (4.94 ppm). With regard to common impurities, the protons of the N-methyl group in the amide substrate (3.51 ppm) and the N-methylaniline byproduct (2.79 ppm) were easily identifiable and diagnostic in student 1H NMR spectra. In addition, students discussed any unexpected peaks in the 1H NMR spectrum to assess the purity of their product. In cases where students did not obtain product, they were given access to another student’s spectrum so that they could practice their interpretation of 1H NMR spectra and complete the postlaboratory handout.
Students completed a postlaboratory worksheet to facilitate data analysis and further expand on key concepts. There were 61 students who correctly calculated their percent yield of the desired product 3 and identified reasonable causes for deviations from the theoretical maximum. In addition, the students were asked to assign peaks in their 1H NMR spectra, identify impurities, and assess the purity of their desired product. All students who submitted a postlaboratory worksheet correctly addressed these prompts and received full credit for this question.
Students were also asked several questions to gauge pedagogical outcomes of this laboratory experiment. Out of 65 students, 60 convincingly communicated their understanding of why amide C—N bond activation using catalysis represents an important scientific advance. Of 65 students, 63 successfully used online resources to find another cross-coupling of amides other than esterification. Additionally, 61 of 65 were able to explain the importance of having one or more electron-withdrawing N-substituents on the amide in order to facilitate transition metal-catalyzed amide C—N bond activation. Generally, student performance during the experiment and on the postlaboratory worksheet demonstrated significant student learning.
Students were given the opportunity to leave comments. These were largely positive, emphasizing the excitement of performing cutting-edge research in an undergraduate laboratory course, the desire for greater exposure to modern research in general, and the enjoyment of learning different laboratory techniques. One student simply noted: “it was very cool to experience working with this new capsule technique.” Additional student comments about this experiment are available in the Supporting Information (p S28).
Several modifications to this experiment can be envisioned. These are described in the Supporting Information (p S24) and include the following: the use of alternate amide or alcohol coupling partners, the use of heptane in place of hexanes for chromatography, and suggestions for making the experiment more inquiry-focused.
Discussion Topics
This laboratory experiment provided a platform for the discussion of many topics in the realm of modern organic chemistry. Discussion topics included the stability of amides, the use of amides in chemical synthesis, transition-metal-catalyzed cross-couplings, non-precious-metal catalysis, the growing field of amide C—N bond activation, and how practical limitations (i.e., air-sensitivity) can be overcome to enable the widespread use of new methodologies. More broadly speaking, the experiment exposed students to new frontiers in research, green chemistry, the relevance of synthetic methodology to pharmaceutical chemistry, and Nobel Prize-winning reactions. Finally, the laboratory experiment provided a forum to discuss many experimental techniques, including reaction setup, the handling of air-sensitive catalysts, column chromatography (with dry-loading), thin-layer chromatography, and spectroscopic analysis.
CONCLUSIONS
A modern undergraduate organic chemistry laboratory experiment was developed involving the breakage of amide C—N bonds. The experimental procedure, which involves the use of a wax-encapsulated nickel precatalyst and NHC ligand, enabled students to perform an unconventional esterification of an amide substrate on the benchtop. The laboratory experiment provided a forum for students to hone their experimental techniques, while also introducing them to several modern topics in organic chemistry that are not typically emphasized in undergraduate organic chemistry coursework, such as transition-metal-catalyzed couplings using nonprecious metals, amide C—N bond activation, and new technologies for the handling of air-sensitive catalysts.
Supplementary Material
ACKNOWLEDGMENTS
We thank the students and teaching assistants (Alex Bagdasarian, Sydnee Green, Zach Hern, Vincent Hipwell, Wendell Scott, Brian Shao, Luke Sisto, Stasik Popov, and Yiyi Yao) of the Chem 30CL organic chemistry laboratory course for their feedback on this experiment. The authors also thank TCI Chemicals Inc. for helpful discussions and for providing paraffin wax capsules containing Ni(cod)2 and SIPr. The authors are grateful to the NIH-NIGMS (R01 GM117016 for N.K.G and F31-GM121016 for L.A.M), the NSF (DGE-1144087 for J.E.D.), the Foote family (L.A.M. and J.E.D.), and the University of California, Los Angeles, for financial support. M.M.N. acknowledges the UCLA Department of Chemistry and Biochemistry for an undergraduate research fellowship. These studies were supported by shared instrumentation grants from the NSF (CHE-1048804) and the National Center for Research Resources (S10RR025631).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available on the ACS Publications website at DOI: 10.1021/acs.jchemed.8b00489.
Detailed student handout, a prelaboratory worksheet, a postlaboratory worksheet, notes for instructors, and spectra of products, including spectra from students (PDF, DOCX)
REFERENCES
- (1).(a) Metal-Catalyzed Cross-Coupling Reactions; Diederich F, de Meijere A, Eds.; Wiley-VCH: Weinheim, 2004. [Google Scholar]; (b) Hassan J; Sévignon M; Gozzi C; Schulz E; Lemaire M Aryl—Aryl Bond Formation One Century after the Discovery of the Ulmann Reaction. Chem. Rev 2002, 102 (5), 1359–1469. [DOI] [PubMed] [Google Scholar]; (c) Topics in Current Chemistry; Miyaura N, Ed.; Springer-Verlag: New York, 2002; Vol. 219. [Google Scholar]; (d) Corbet J-P; Mignani G Selected Patented Cross-Coupling Reaction Technologies. Chem. Rev 2006, 106 (7), 2651–2710. [DOI] [PubMed] [Google Scholar]; (e) Negishi E Transition Metal-Catalyzed Organometallic Reactions that Have Revolutionized Organic Synthesis. Bull. Chem. Soc. Jpn 2007, 80 (2), 233–257. [Google Scholar]; (f) Application of Transition Metal Catalysis in Drug Discovery and Development: An Industrial Perspective; Shen HC, Crawley ML, Trost BM, Eds.; John Wiley & Sons, Inc.: Hoboken, 2012. [Google Scholar]; (g) Johansson Seechurn C; Etching MO; Colacot TJ; Snieckus V Palladium-Catalyzed Cross-Coupling: A Historical Contextual Perspective to the 2010 Nobel Prize. Angew. Chem., Int. Ed 2012, 51 (21), 5062–5085. [DOI] [PubMed] [Google Scholar]
- (2).(a) The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science; Greenberg A, Breneman CM, Liebman JF, Eds.; John Wiley & Sons, Inc: Hoboken, NJ, 2003. [Google Scholar]; (b) Pauling L; Corey RB; Branson HR The Structure of Proteins: Two Hydrogen-Bonded Helical Configurations of the Polypeptide Chain. Proc. Natl. Acad. Sci. U. S. A 1951, 37 (4), 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).For recent reviews on cross-couplings of amides, see:Dander JE; Garg NK Breaking Amides Using Nickel Catalysis. ACS Catal. 2017, 7 (2), 1413–1423.Meng G; Shi S; Szostak M Cross-Coupling of Amides by N—C Bond Activation. Synlett 2016, 27 (18), 2530–2540.Liu C; Szostak M Twisted Amides: From Obscurity to Broadly Useful Transition Metal-Catalyzed Reactions by N—C Amide Bond Activation. Chem. - Eur. J 2017, 23 (30), 7157–7173.Takise R; Muto K; Yamaguchi J Cross-Coupling of Aromatic Esters and Amides. Chem. Soc. Rev 2017, 46 (19), 5864–5888.
- (4).(a) Hie L; Fine Nathel NF; Shah TK; Baker EL; Hong X; Yang Y-F; Liu P; Houk KN; Garg NK Conversion of Amides to Esters by the Nickel-Catalyzed Activation of Amide C—N Bonds. Nature 2015, 524, 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Hie L; Baker EL; Anthony SM; Desrosiers J-N; Senanayake C; Garg NK Nickel-Catalyzed Esterification of Aliphatic Amides. Angew. Chem. Int. Ed 2016, 55 (48), 15129–15132. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Weires NA; Caspi DD; Garg NK Kinetic Modeling of the Nickel-Catalyzed Esterification of Amides. ACS Catal 2017, 7 (7), 4381–4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).(a) Baker EL; Yamano MM; Zhou Y; Anthony SM; Garg NK A Two-Step Approach to Achieve Secondary Amide Transamidation Enabled by Nickel Catalysis. Nat. Commun 2016, 7, 11554. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dander JE; Baker EL; Garg NK Nickel-Catalyzed Transamidation of Aliphatic Amide Derivatives. Chem. Sci 2017, 8 (9), 6433–6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).(a) Weires NA; Baker EL; Garg NK Nickel-Catalysed Suzuki-Miyaura Coupling of Amides. Nat. Chem 2016, 8, 75–79. [DOI] [PubMed] [Google Scholar]; (b) Boit TB; Weires NA; Kim J; Garg NK Nickel-Catalyzed Suzuki—Miyaura Coupling of Aliphatic Amides. ACS Catal. 2018, 8 (2), 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shi S; Meng G; Szostak M Synthesis of Biaryls via Nickel Catalyzed Suzuki—Miyaura Coupling of Amides by Carbon—Nitrogen Cleavage. Angew. Chem. Int. Ed 2016, 55 (24), 6959–6963. [DOI] [PubMed] [Google Scholar]
- (7).(a) Simmons BJ; Weires NA; Dander JE; Garg NK Nickel-Catalyzed Alkylation of Amide Derivatives. ACS Catal. 2016, 6 (5), 3176–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shi S; Szostak M Nickel-Catalyzed Negishi Cross-Coupling of N-Acylsuccinimides: Stable, Amide-Based, Twist-Controlled Acyl-Transfer Reagents via N—C Activation. Synthesis 2017, 49 (16), 3602–3608. [Google Scholar]; (c) Shi S; Szostak M Nickel-Catalyzed Diaryl Ketone Synthesis by N—C Cleavage: Direct Negishi Cross-Coupling of Primary Amides by Site-Selective N,N-Di-Boc Activation. Org. Lett 2016, 18 (22), 5872–5875. [DOI] [PubMed] [Google Scholar]; (d) Shi S; Szostak M Efficient Synthesis of Diaryl Ketones by Nickel-Catalyzed Negishi Cross-Coupling of Amides via Carbon-Nitrogen Bond Cleavage at Room Temperature Accelerated by a Solvent Effect. Chem. - Eur. J 2016, 22 (30), 10420–10424. [DOI] [PubMed] [Google Scholar]
- (8).(a) Medina JM; Moreno J; Racine S; Du S; Garg NK Mizoroki—Heck Cyclizations of Amide Derivatives for the Introduction of Quaternary Centers. Angew. Chem. Int. Ed 2017, 56 (23), 6567–6571. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Walker JA; Vickerman KL; Humke JN; Stanley LM Ni-Catalyzed Alkene Carboacylation via Amide C—N Bond Activation. J. Am. Chem. Soc 2017, 139 (30), 10228–10231. [DOI] [PubMed] [Google Scholar]
- (9).Sather AC; Lee HG; Colombe JR; Zhang A; Buchwald SL Dosage Delivery of Sensitive Reagents Enables Glove Box-Free Synthesis. Nature 2015, 524, 208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Dander JE; Weires NA; Garg NK Benchtop Delivery of Ni(cod)2 using Paraffin Capsules. Org. Lett 2016, 18 (15), 3934–3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).The necessary paraffin-wax capsules containing Ni(cod)2 and SIPr are commercially available from TCI Chemicals Inc. (product number B5418) or can be readily prepared (see ref 10). Amide substrate 1 will soon be commercially available from TCI Chemicals Inc.
- (12).For recent examples of undergraduate laboratory experiments incorporating transition-metal-catalyzed cross-coupling reactions, see:Costa NE; Pelotte AL; Simard JM; Syvinski CA; Deveau AM Discovering Green, Aqueous Suzuki Coupling Reactions: Synthesis of Ethyl (4-Phenylphenyl)acetate, a Biaryl with Anti-Arthritic Potential. J. Chem. Educ 2012, 89 (8), 1064–1067.Aktoudianakis E; Chan E; Edward AR; Jarosz I; Lee V; Mui L; Thatipamala SS; Dicks AP Greening Up” the Suzuki Reaction. J. Chem. Educ 2008, 85 (4), 555–557.Hamilton AE; Buxton AM; Peeples CJ; Chalker JM An Operationally Simple Aqueous Suzuki-Miyaura Cross-Coupling Reaction for an Undergraduate Organic Chemistry Laboratory. J. Chem. Educ 2013, 90 (11), 1509–1513.Callam CS; Lowary TL Suzuki Cross-Coupling Reactions: Synthesis of Unsymmetrical Biaryls in the Organic Laboratory. J. Chem. Educ 2001, 78 (7), 947–948.Hoogenboom R; Meier MAR; Schubert US The Introduction of High-Throughput Experimentation Methods for Suzuki-Miyaura Coupling Reactions in University Education. J. Chem. Educ 2005, 82 (11), 1693–1696.Herrmann WA; Bohm VPW; Reisinger C-P Introduction to Homogenous Catalysis: Carbon—Carbon Bond Formation Catalyzed by a Defined Palladium Complex. J. Chem. Educ 2000, 77 (1), 92–95.Hie L; Chang JJ; Garg NK Nickel-Catalyzed Suzuki—Miyaura Cross-Coupling in a Green Alcohol Solvent for an Undergraduate Organic Chemistry Laboratory. J. Chem. Educ 2015, 92 (3), 571–574.Thananatthanachon T; Lecklider MR Synthesis of Dichlorophosphinenickel-(II) Compounds and Their Catalytic Activity in Suzuki Cross-Coupling Reactions: A Simple Air-Free Experiment for Inorganic Chemistry Laboratory. J. Chem. Educ 2017, 94 (6), 786–789.Ong J-Y; Chan S-C; Hoang T-G Empowering Students To Design and Evaluate Synthesis Procedures: A Sonogashira Coupling Project for Advanced Teaching Lab. J. Chem. Educ 2018, 95 (6), 1078–1081.
- (13).Clark IE; Romero-Calderón R; Olson JM; Jaworski L; Lopatto D; Banerjee U “Deconstructing” Scientific Research: A Practical and Scalable Pedagogical Tool to Provide Evidence-Based Science Instruction. PLoS Biol 2009, 7 (12), e1000264. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.