Abstract
Background/Objectives
Although total body skin examination (TBSE) is the primary screening mechanism for melanoma, there is no consensus on which anatomic sites a screening TBSE should include. We sought to establish which anatomic sites are examined during routine (>90%) TBSEs of patients at high risk for skin cancer.
Methods
A Google survey was emailed to 173 international dermatologist skin cancer specialists.
Results
More than 75% of participants reported routinely examining the scalp, ears, face and neck, trunk, breasts, inframammary areas, axillae, extremities, palms and soles, nails, interdigital spaces, and buttocks. The least frequently inspected anatomic sites included genitalia, with male genitalia more frequently examined than female (penis n = 39; 52%; labia majora n = 21; 28%; P = 0.003), the perianal region (n = 26; 34.7%), and the ocular conjunctiva and oral mucosa (n = 35; 46.7%). Participants cited not screening these areas because of perceived patient discomfort, low prevalence of malignancy, and the expectation that other specialists examine the area.
Conclusions
The role of routine surveillance of neglected anatomic sites is unclear and warrants further discussion weighing potential mortality benefit against the incidence of melanoma in obscure sites, morbidity of intervention in sensitive sites, cost-effectiveness, and potential for patient discomfort.
Keywords: total body skin examination, skin cancer screening, melanoma, nonmelanoma skin cancer, cutaneous oncology
Introduction
Skin cancer is the most common human malignancy, with more than 5 million cases diagnosed in the United States annually [1]. The visible nature of cutaneous malignancies presents a theoretical opportunity for secondary prevention of disease not possible for many other malignancies. Studies have shown that receiving a screening total body skin examination (TBSE) independently increases the likelihood of identifying melanoma and reduces the incidence of thick melanomas. Furthermore, detection of early melanoma results in more treatable disease and better prognosis, with 5-year survival of thin melanoma nearing 99% [2,3]. However, skin cancer screening, even in the highest risk populations, is controversial, as no prospective randomized controlled trial has been conducted that shows demonstrable mortality benefit. Though controversial, screening TBSEs by dermatologists present the critical potential for dermatologists to detect early disease.
Although most dermatologists recognize the important role for TBSEs and its teaching is incorporated as a fundamental in postgraduate dermatology education, there has been little published with regard to what anatomic sites should reproducibly constitute a TBSE. While textbooks suggest in-depth, thorough screening of the total body including obscure anatomic sites, there is no accepted standard within the dermatologic community of which sites are routinely examined in practice, and moreover no consensus of which sites should be routinely examined. The authors’ anecdotal evidence suggests that inspection of certain obscure anatomic sites including genitalia, intergluteal clefts, perianal areas, and oral and ocular mucosa is not always performed on routine TBSEs because of perceived patient embarrassment, low prevalence of malignancy, and the perception that particular anatomic sites are likely examined by other specialists. The rationale for our study was therefore to begin to better understand baseline TBSE practices, by first studying the practices of dermatologist skin cancer specialists. The primary objective of our study was to identify which anatomic sites are routinely examined during TBSEs (>90% of the time) in patients at high risk for skin cancer. The secondary objective was to identify the rationale for not examining particular anatomic sites. We hypothesized that obscure anatomic sites are likely infrequently examined during TBSEs even amongst dermatologist skin cancer specialists.
Methods
Participants
The contact information for 173 international dermatologists deemed skin cancer specialists was compiled from (1) board members and executive board members of the International Dermoscopy Society (IDS), (2) the United States Pigmented Lesion Clinic Group, and (3) the Skin Cancer Foundation. Email addresses were collected using the IDS website in conjunction with the American Academy of Dermatology directory.
Survey Administration
The study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board without the requirement for written informed consent in accordance with the Helsinki Declaration. Participation in the survey study was voluntary; therefore, completion of the survey was considered to be tacit consent. No identifying information on participants was collected (eg, name, email). The survey was hosted on Google survey, and a link to the survey was emailed to all participants. Participants were contacted 3 times: an initial email in September 2016 and 2 follow-up emails, the first in October 2016 and the second in December 2016. The survey was closed to participants at the end of January 2017.
The survey was designed to capture only the responses of dermatologists who participated in the care of high-risk skin cancer patients. If a participant answered that their practice did not meet this criterion, the survey automatically ended and their responses were not included. Furthermore, all questions were worded specifically to target the dermatologist’s clinic dedicated to the care of high-risk patients. The survey instrument can be found in the online supplementary material.
Statistical Analysis
Descriptive and relative frequencies along with cross-classifications were used to describe the survey responses. To reduce the potential for type 1 errors based on multiple comparisons, we used the Bonferroni correction and divided the study α-level by the number of comparisons made α/20 ~= 0.002. Proportions were considered statistically different at an α-level of <0.002.
Results
Seventy-seven of the 173 (44.5%) participants in the target sample completed the survey. Analysis was subsequently performed on 75 of the original 77 participants, as 1 respondent stated their clinic did not have a portion of care dedicated to the care of patients at high risk for skin cancer, and 1 respondent was excluded, as he/she was a nurse practitioner and not a practicing dermatologist. Table 1 displays demographics and baseline practices of survey respondents. Of the 75 participants, 43 (57.3%) were male, 35 (46.7%) were from the USA, and 36 (48%) recorded practicing in countries in Europe, South America, Asia, and Australia. A majority of participants (n = 47; 62.7%) had >40% of their clinic devoted to the care of high-risk skin cancer patients.
Table 1.
Demographics and Baseline Practices of Survey Respondents (N = 75)
| Sex | |
| Female | 31 (41.3%) |
| Country of practice | |
| Asia | 4/73 (5.5%) |
| Australia | 2/73 (2.7%) |
| Europe | 26/73 (35.6%) |
| North America | 37/73 (50.7%) |
| South America | 4/73 (5.5%) |
| Median years practicing post-residency | 20 yrs |
| Percentage of clinic dedicated to care of high-risk patients | |
| 1%–20% | 11/75 (14.7%) |
| 21%–40% | 17/75 (22.7%) |
| 41%–60% | 16/75 (21.3%) |
| 61%–80% | 10/75 (13.3%) |
| 81%–100% | 21/75 (28%) |
| Time to perform TBSE | |
| 0–10 minutes | 51/75 (68%) |
| 10+ minutes | 24/75 (32%) |
| Time allocated for new visit | |
| 0–20 minutes | 32/74 (43.2%) |
| 21–30 minutes | 27/74 (36.5%) |
| 30+ minutes | 15/74 (20.3%) |
| Time allocated for follow-up visit | |
| 0–20 minutes | 49/73 (67.1%) |
| 21–30 minutes | 17/73 (23.3%) |
| 30+ minutes | 7/73 (9.6%) |
| Do you/office staff ask patients to disrobe including undergarments (ie, underpants and/or bra)? | |
| Always | 44/75 (58.7%) |
| Sometimes | 24/75 (32%) |
| Never | 7/75 (9.3%) |
| For those who ask their patients, how often do patients comply? | |
| 0%–40% | 6/68 (8.8%) |
| 41%–80% | 26/68 (38.2%) |
| 81%–100% | 36/68 (52.9%) |
| Equipment in room | |
| Good overhead lighting | 69/75 (92%) |
| Auxiliary lighting | 49/75 (65.3%) |
| Monitors for viewing photos/digital dermoscopy | 60/75 (80%) |
| Stirrups | 16/75 (21.3%) |
| Vaginal specula | 5/75 (6.7%) |
| Hair dryer | 1/75 (1.3%) |
| Positions in which patients are examined | |
| Sitting | 2/75 (2.7%) |
| Standing | 9/75 (12%) |
| Lying | 22/75 (29.3%) |
| Two of the above positions | 13/75 (17.3%) |
| All 3 (sitting, standing, and lying) | 26/75 (34.7%) |
A majority of survey respondents reported always using dermoscopy for TBSEs (n = 64; 85.3%). Three respondents reported never using dermoscopy for TBSEs. While 20 participants (26.7%) reported using total body photography for comparison during TBSEs in >40% of their high-risk patients, 60 (80%) reported that their examination rooms were equipped with monitors for viewing baseline photos and/or digital dermoscopy. Nearly all participants (n = 69; 92%) stated their examination rooms were equipped with good overhead lighting, while a minority reported their examination rooms were equipped with stirrups, vaginal specula, and hair dryers for the inspection of scalp lesions (n = 16, 21.3%; n = 5, 6.7%; n = 1, 1.3%, respectively).
In preparation for the TBSE, the majority of dermatologists surveyed (n = 44; 58.7%) stated patents are always told to remove all their clothing, including undergarments, and the majority of high-risk patients comply >60% of the time (n = 51; 68%).
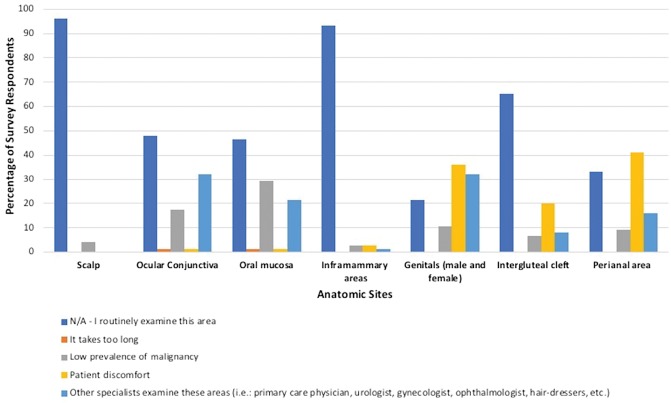
Table 2 presents the frequency by which survey respondents reported routinely examining each anatomic site. Greater than 75% of participants reported routinely examining the following areas during TBSEs: scalp, ears, face and neck, trunk, breasts, inframammary areas, axillae, extremities, palms and soles, finger and toenails, interdigital spaces, and buttocks. The scalp was reported to be routinely examined by 84% of participants (n = 63). Of those who do not examine the scalp, the primary reason cited was low prevalence of malignancy. The ocular conjunctiva and oral mucosa were routinely examined by fewer than half of participants (n = 35; 46.7%). The main reasons for not examining these sites included the expectation that other specialists examine these areas and low prevalence of malignancy, which is depicted in graphical form in Figure 1.
Table 2.
Frequency of Survey Respondents Examining Each Anatomic Location
| Anatomic Location | % of Survey Respondents Who Reported Routinely Examining Site, % (n) | P Valuea,b |
|---|---|---|
| Scalp* | 84% (63/75) | 0.001 |
| Ears | 94.7% (71/75) | 0.17 |
| Face and neck | 98.7% (74/75) | 1 |
| Ocular conjunctiva* | 46.7% (35/75) | <0.00001 |
| Oral mucosa* | 46.7% (35/75) | <0.00001 |
| Trunk (abdomen, chest, and back) | 98.7% (74/75) | – |
| Breast | 93.3% (70/75) | 0.09 |
| Inframammary areas | 96% (72/75) | 0.31 |
| Axillae | 93.3% (70/75) | 0.09 |
| Extremities | 98.7% (74/75) | 1 |
| Palms and soles | 97.3% (73/75) | 0.56 |
| Interdigital spaces | 85.3% (64/75) | 0.002 |
| Fingernails | 96% (72/75) | 0.31 |
| Toenails | 96% (72/75) | 0.31 |
| Scrotum* | 50.7% (38/75) | <0.00001 |
| Penis* | 52% (39/75) | <0.00001 |
| Labia majora* | 28% (21/75) | <0.00001 |
| Labia minora* | 10.7% (8/75) | <0.00001 |
| Vagina* | 2.7% (2/75) | <0.00001 |
| Buttocks | 93.3% (70/75) | 0.09 |
| Intergluteal cleft* | 70.7% (53/75) | <0.00001 |
| Perianal region* | 34.7% (26/75) | <0.00001 |
P value comparing likelihood of examining site vs not examining site using trunk (abdomen/chest/back) as reference; calculated using chi-square test.
To account for multiple comparisons, a P value of <0.002 was considered significantly different.
Statistically significant result.
Figure 1.
Rationale for not examining particular anatomic sites. [Copyright: ©2019 Bajaj et al.]
The perianal region (n = 26; 34.7%), labia majora (n = 21; 28%), and labia minora (n = 8; n = 10.7%) were infrequently examined. Survey respondents reported routinely screening the scrotum (n = 38; 50.7%) and penis (n = 39; 52%) more often than the female genitalia. The vagina was examined least frequently (n = 2; 2.7%). The primary reason cited for not examining the male/female genitalia included patient discomfort (n = 27/59; 45.7%), with a similar number of participants citing that other specialists examine this area (n = 24/59; 40.7%) and a few also citing low prevalence of malignancy (n = 8/59; 13.6%).
Discussion
To our knowledge, our study is the first to delve deeply into the practices of dermatologists when performing TBSEs, including the content, technique, and rationale. In reviewing the literature pertaining to screening TBSEs, only a few studies explicitly state in their methodology which anatomic sites were screened during TBSEs, and when detailed, anatomic sites examined were not standardized among different studies [3–5]. Our international survey study showed that both in the United States and abroad, the majority of dermatologists screening patients at high risk for skin cancer reported not routinely examining the genitalia, perianal area, oral mucosa, and ocular conjunctiva. We hope that our findings stimulate thought-provoking debate within the dermatology community about whether these sites should be routinely examined during screening TBSEs.
Within the medical community there is growing recognition of the harms posed by broad-stroked, intuition-based cancer screening where there has been no demonstrable effect on mortality [6]. For melanoma, there is no doubt that finding early-stage localized thin disease reduces mortality [7]. The 5-year melanoma-specific survival for mucosal melanoma is in staunch contrast to that of cutaneous melanoma, 61% vs 91%, respectively. This raises the inevitable question of whether increased screening of such neglected sites would improve prognosis by identifying early-stage disease. However, before recommending nuanced screening for specific target populations, such as for acral lentiginous melanoma in dark-skinned populations, or in this case for increased screening of neglected anatomic sites, we caution readers to consider the potential harms of increased surveillance [8,9].
To date there is no evidence that surveillance of obscure anatomic sites would result in decreased mortality from melanoma. Mucosal melanoma, as compared to cutaneous melanoma, has a unique mutation signature, distinct pathogenesis, and may be an independently more aggressive disease process. Furthermore, increased surveillance would likely result in an increased number of surgical procedures and increased morbidity. When considering the atypical clinical, dermoscopic, and histopathological spectrum surrounding “nevi of special sites,” the expected morbidity and rate of excess surgical intervention would be exacerbated in the genital and perianal sites in specific [10]. As such, clinicians examining these areas must be aware of the degree of clinical atypia present in even benign melanosis [11]. With sensitive anatomic sites, we could expect both functional and cosmetic impairment from repetitive intervention. Such interventions are equally fraught with patient anxiety.
It is equally our burden to weigh the surmounting health care costs associated with increased procedures against the exceedingly low incidence of melanoma in the anatomic sites that our study suggests are infrequently being examined, even in patients at the highest risk for skin cancer. In 2010, statistics from the Surveillance, Epidemiology, End Results Program database showed the incidence for mucosal melanoma (including the nasal cavity, accessory sinuses, oral cavity, anorectal area, genital tract) was approximately 2 per 1 million [12]. This low incidence is disproportionate when compared to our tendency to over-biopsy; as one study found, more than 1,000 benign nevi were biopsied between 2009 and 2013 in order to detect 1 melanoma in patients under 19 years of age [13]. The low incidence of melanoma in these sites, when contextualized with both the expected morbidity from increased screening and with the already high biopsy rate, raises important questions regarding surveillance recommendations.
In addition to citing low prevalence of malignancy as rationale for not examining oral mucosa, ocular mucosa, and genitalia, many dermatologists in our study also reported that they avoided examination of the genitalia because of patient discomfort. Perceived patient discomfort with examination of sensitive areas may be exacerbated when the gender of the physician differs from that of the patient as studies have corroborated for pelvic examinations, colonoscopies, and even most recently for TBSEs that female patients have a preference toward female physicians [14–16]. Significantly, studies have shown that patients who were educated on the importance of TBSEs were found to have decreased discomfort when receiving a genital examination [17].
Perhaps a more effective method for surveillance of such anatomic sites lies in empowering patients to be vigilant in their own skin self-examination. In one study of patients with genital melanoma, more than 65% of patients had a 6-month delay before consulting a medical professional, citing embarrassment as the primary reason for this delay [18]. Focusing increased attention toward educating patients on alarm signs, and mitigating patient embarrassment toward alerting a medical professional when there is a suspicious lesion in a sensitive anatomic area, warrants further study and may prove to be a higher yield intervention than recommending broad-based increased screening of these anatomic sites [18].
In addition, our survey confirmed that clinicians rationalize the decision not to examine specific anatomic sites based on the expectation of examination by other specialists, notably the oral and ocular mucosa and genitalia. This ambiguity of who, if any, clinician should be screening particular anatomic sites was highlighted by Krathen et al, who found that while 83% of dermatologists, compared with 55% of gynecologists, reported being good or very good at evaluating pigmented lesions of the vulva, only 66% of dermatologists felt diagnosing a vulvar melanoma was their role, compared with 81% of gynecologists who agreed or strongly agreed it was their role [19]. Should these sites be deemed important for surveillance, it is equally important to bridge the gap with other specialties, as well as to educate patients to seek outside examination, to ensure adequate and complete screening.
Our preliminary survey study is notably limited by a small sample of dermatologists who dedicate a portion of their care to high-risk skin cancer screening and is not a random sample of the dermatology community at large. These findings may in fact be accentuated in a general dermatology clinic. We recognize that with a response rate of 77 (44.5%), our findings are exposed to nonresponse bias, meaning that nonrespondents could be categorically less or more likely to examine obscure sites. Furthermore, given the nature of a retrospective survey study, our results could be exposed to potential response bias. To this point, however, one would assume that in a survey study, providers would be more likely to overestimate, rather than underestimate, the frequency by which they routinely examine obscure areas in their practice. In the future, it may be helpful to retest a subgroup of respondents to gauge reproducibility of findings.
Our study attempts to reflect the anatomic sites routinely inspected by dermatologists specializing in skin cancer and evaluating patients at increased risk. It highlights the ambiguities that exist regarding what sites should be examined during a routine surveillance examination, and also the variation in how different practitioners conduct TBSEs, and what equipment they have available to aid their examination. We hope our findings stimulate further investigation on which anatomic sites should be routinely evaluated to best reduce melanoma mortality while minimizing morbidity from unnecessary procedures.
Conclusions
Our study sheds insight into the screening habits of skin cancer specialists when performing routine TBSEs on patients at high risk for skin cancer. Overall, we found that the genitalia, perianal regions, ocular conjunctiva, and oral mucosa are not routinely examined with the rationale that these anatomic sites are associated with a low prevalence of malignancy and that other experts examine these sites. Further studies that investigate the morbidity, cost-effectiveness, patient preference, and potential mortality benefit of examining particular anatomic sites will help to establish what a routine TBSE should include.
Footnotes
Funding: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748. The study was approved by the Memorial Sloan Kettering Cancer Center Institutional Review Board.
Competing interests: The authors have no conflicts of interest to disclose.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151(10):1081–1086. doi: 10.1001/jamadermatol.2015.1187. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg MS, Doucette JT, Lim HW, Spencer J, Carucci JA, Rigel DS. Risk factors for presumptive melanoma in skin cancer screening: American Academy of Dermatology National Melanoma/Skin Cancer Screening Program experience 2001–2005. J Am Acad Dermatol. 2007;57(1):60–66. doi: 10.1016/j.jaad.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Aitken JF, Janda M, Elwood M, Youl PH, Ring IT, Lowe JB. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54(1):105–114. doi: 10.1016/j.jaad.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 4.Lookingbill DP. Yield from a complete skin examination: findings in 1157 new dermatology patients. J Am Acad Dermatol. 1988;18(1 Pt 1):31–37. doi: 10.1016/s0190-9622(88)70004-3. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock MA, Martin RA, Risica PM, et al. Thorough skin examination for the early detection of melanoma. Am J Prev Med. 1999;17(3):169–175. doi: 10.1016/s0749-3797(99)00077-x. [DOI] [PubMed] [Google Scholar]
- 6.Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122(20):3152–3156. doi: 10.1002/cncr.30177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542(7639):115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marchetti MA, Chung E, Halpern AC. Screening for acral lentiginous melanoma in dark-skinned individuals. JAMA Dermatol. 2015;151(10):1055–1056. doi: 10.1001/jamadermatol.2015.1347. [DOI] [PubMed] [Google Scholar]
- 9.Tsai MS, Chiu MW. Patient-reported frequency of acral surface inspection during skin examination in white and ethnic minority patients. J Am Acad Dermatol. 2014;71(2):249–255. doi: 10.1016/j.jaad.2014.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mason AR, Mohr MR, Koch LH, Hood AF. Nevi of special sites. Clin Lab Med. 2011;31(2):229–242. doi: 10.1016/j.cll.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Haugh AM, Merkel EA, Zhang B, et al. A clinical, histologic, and follow-up study of genital melanosis in men and women. J Am Acad Dermatol. 2017;76(5):836–840. doi: 10.1016/j.jaad.2016.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Bishop KD, Olszewski AJ. Epidemiology and survival outcomes of ocular and mucosal melanomas: a population-based analysis. Int J Cancer. 2014;134(12):2961–2971. doi: 10.1002/ijc.28625. [DOI] [PubMed] [Google Scholar]
- 13.Oliveria SA, Selvam N, Mehregan D, et al. Biopsies of nevi in children and adolescents in the United States, 2009 through 2013. JAMA Dermatol. 2015;151(4):447–448. doi: 10.1001/jamadermatol.2014.4576. [DOI] [PubMed] [Google Scholar]
- 14.Rifkin JI, Shapiro H, Regensteiner JG, Stotler JK, Schmidt B. Why do some women refuse to allow male residents to perform pelvic exams? Acad Med. 2002;77(10):1034–1038. doi: 10.1097/00001888-200210000-00020. [DOI] [PubMed] [Google Scholar]
- 15.Menees SB, Inadomi JM, Korsnes S, Elta GH. Women patients’ preference for women physicians is a barrier to colon cancer screening. Gastrointest Endosc. 2005;62(2):219–223. doi: 10.1016/s0016-5107(05)00540-7. [DOI] [PubMed] [Google Scholar]
- 16.Houston NA, Secrest AM, Harris RJ, et al. Patient preferences during skin cancer screening examination. JAMA Dermatol. 2016;152(9):1052–1054. doi: 10.1001/jamadermatol.2016.1005. [DOI] [PubMed] [Google Scholar]
- 17.Leffell DJ, Berwick M, Bolognia J. The effect of pre-education on patient compliance with full-body examination in a public skin cancer screening. J Dermatol Surg Oncol. 1993;19(7):660–663. doi: 10.1111/j.1524-4725.1993.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 19.Krathen MS, Liu CL, Loo DS. Vulvar melanoma: a missed opportunity for early intervention? J Am Acad Dermatol. 2012;66(4):697–698. doi: 10.1016/j.jaad.2011.10.006. [DOI] [PubMed] [Google Scholar]