Abstract
Background
Mucous membrane pemphigoid (MMP) is a heterogeneous group of blistering disorders affecting the mucosae with or without skin involvement, characterized by the presence of autoantibodies to components of the basement membrane zone, including the bullous pemphigoid antigen BP180 and β4 integrin. Current literature has shown that a minority of patients present circulating antibodies to laminin-332 and this population seems to be associated with a relatively high risk of malignancy.
Objective
To present our personal case series of patients with MMP-associated malignancy from a dermatology university hospital.
Methods
Twenty-two patients affected by MMP were seen in the period between 2001 and 2016; in 4 patients (18%) an associated cancer was detected.
Results
These patients were 2 men and 2 women, with a mean age of 69.7 years (range, 48–83). The associated malignancies included a breast cancer, a pancreatic adenocarcinoma, a metastatic laryngeal carcinoma, and a hepatic carcinoma. All patients had negative results for both BP180 and laminin-332 autoantibodies.
Conclusion
We confirm that MMP patients have a relatively high possibility of developing a solid cancer, but the autoantibody detection is not mandatory and is probably correlated with the severity of the disease.
Keywords: mucous membrane pemphigoid, malignancy, autoimmune bullous disorders, paraneoplastic pemphigus, laminin-332, blistering diseases, laminin-5, anti-epiligrin, cicatricial pemphigoid, adenocarcinoma
Introduction
Mucous membrane pemphigoid (MMP), formerly cicatricial pemphigoid, is a rare heterogeneous group of autoimmune subepithelial blistering disorders involving the mucous membranes and occasionally the skin with a chronic course and tendency toward scarring [1]. Previously, other names have been used to describe these conditions, including benign MMP, anti-laminin-5 cicatricial pemphigoid, oral pemphigoid, and ocular pemphigoid. Because it is difficult to clinically distinguish the various MMP subgroups, the collective term MMP is now accepted [2].
MMP commonly affects the oral mucosa, but ocular and nasal epithelia, the first aerodigestive tract and the genitals may be involved. Cutaneous involvement may be absent or limited. MMP usually affects patients aged between 60 and 80 years, with a female prevalence [1–3]. The disease is mainly controlled with corticosteroids and other immunosuppressive drugs, including cyclosporine, mycophenolate, cyclophosphamide, and azathioprine [3].
Most of the MMP autoantibody profile shares the same target antigens of other autoimmune blistering diseases from the pemphigoid group and paraneoplastic pemphigus, namely circulating IgG and/or IgA autoantibodies targeting the basement membrane zone (BMZ) components, including the bullous pemphigoid antigens BP180 and BP230, and the β4 integrin [1–6]. Current evidence demonstrates that a minority of MMP patients with autoantibodies to IgG anti-laminin-332 (formerly anti-laminin-5 or anti-epiligrin) have an increased relative risk of cancer [1,7]. Previously, this subgroup was named anti-epiligrin cicatricial pemphigoid (AECP), to differentiate it from the majority of cicatricial pemphigoid patients without these specific autoantibodies. Finally, there is no report of MMP-associated malignancies with autoantibodies to BP180 [1,2,8–14].
We present a case series of MMP patients with associated malignancies and a brief overview of the current literature.
Case Series
During the period between 2001 and 2016, at the Department of Dermatology at the University of Bologna, we diagnosed and followed up 22 patients affected by MMP. From this group, we selected 4 individuals (18%) who developed a solid tumor (a breast cancer, a pancreatic adenocarcinoma, a metastatic laryngeal carcinoma, and a hepatocarcinoma) before the MMP diagnosis or during the MMP follow-up. They were 2 men and 2 women, with a mean age of 69.7 years (range, 48–83). In general, our patients had mainly oral and/or genital mucous involvement. Only patient 2 had concomitant skin lesions, characterized by blisters and erosions on the trunk and limbs, and patient 4 had exclusive conjunctival involvement, undergoing a complete loss of eyelashes due to synechiae. Cancer detection was preceding in case 1 (diagnosed 1 year before MMP) and metachronous in the remaining cases: 1 year after MMP in cases 2 and 4, and 3 years after in case 3. All patients’ data, treatment, and follow-up are summarized in Table 1. Another patient, a 73-year-old woman, received the diagnosis of breast cancer 6 years preceding MMP occurrence. She is still under follow-up in our clinic, but was excluded from the study because of the long interval between cancer occurrence and the MMP diagnosis.
Table 1.
Clinical and Serological Data From MMP Patients With Associated Malignancies at the University of Bologna
| No. | Sex/Age at Diagnosis of MMP (yr) | Sites Involved | Interval Between MMP Onset and Tumor Diagnosis | Associated Malignancy | BP Antigens BP180 and BP230 | Anti-laminin-332 | Treatment and Follow-up |
|---|---|---|---|---|---|---|---|
| 1 | F/66 (2014) | Oral/genital, ocular | 1 yr before MMP onset | Breast cancer | Negative | Negative | MMP developed after 1 yr of surgery for breast cancer. Follow-up treatment with dapsone 200 mg/d and prednisone 7.5 mg/d without recurrence. |
| 2 | M/82 (2014) | Skin/oral/ocular | 1 yr after MMP onset | Metastatic laryngeal SCC | Negative | Negative | During methylprednisolone treatment, the patient developed laryngeal SCC that became metastatic after 1 yr. The steroid was discontinued and treatment with dapsone 200 mg/d in addition to radiotherapy and chemotherapy was started. He died after 6 mos. |
| 3 | M/48 (2013) | Oral | 3 yrs after MMP onset | Prostatic AC | Negative | ND | Oral MMP, involving exclusively the hard palate and the gums. Systemic steroids and dapsone with favorable results until complete remission. Prostatic AC after 3 yrs. Follow-up without MMP relapses. |
| 4 | F/83 (2016) | Ocular | 1 yr after MMP onset | Hepatic cancer | Negative | Negative | Breast cancer at age 72, mastectomy, and liver cirrhosis for chronic hepatitis, with eye MMP, under treatment with methylprednisolone. Hepatocarcinoma developed recently during follow-up. |
AC = adenocarcinoma; BP = bullous pemphigoid; ND = not done; SCC = squamous cell carcinoma.
An MMP diagnosis was made by combining the clinical and histopathological findings. In particular, clinical findings included active mucous membrane involvement characterized by blisters and/or erosions. In addition, direct and indirect immunofluorescence from perilesional mucosa revealed a linear deposition of IgG and/or C3 at the BMZ and circulating IgG anti-BMZ autoantibodies, respectively (Figure 1). Immunoblot analysis was negative for anti-BP180 and anti-BP230 autoantibodies, as well as anti-purified human laminin-332.
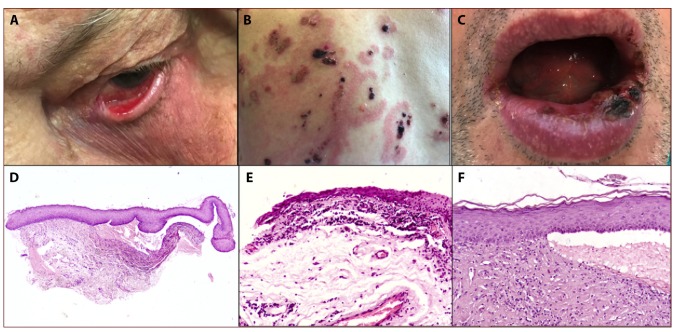
Figure 1.
(A) Ocular MMP with conjunctival involvement, with symblepharon formation. (B) Polymorphic manifestation of MMP with cutaneous involvement, characterized by tense, serous, or hemorrhagic bullae. (C) Oral MMP with hemorrhagic crusting and lip erosions. (D) Low magnification of subepidermal blister with inflammatory cell infiltrate (hematoxylin and eosin [H&E], ×4). (E,F) MMP inflammatory infiltrate of the upper dermis, with numerous eosinophils (H&E, ×10; H&E, ×20). [Copyright: ©2019 La Placa et al.]
The investigation described was carried out on residual biopsy sections following diagnostic analysis during the course of institutional diagnostic services, and the study was exempted from institutional review board review.
Discussion
MMP is an autoimmune bullous disorder with predominant or exclusive mucosal involvement. As reported for paraneoplastic pemphigus and bullous pemphigoid, MMP is a pathogenetic example of cell-mediated immunity involvement, characterized by the presence of different autoantibody responses to basement membrane antigens, including the BP180 and BP230, or β4 integrin [3]. Moreover, whereas most of MMP patients share BP180 autoantigen and oral or cutaneous involvement [12], a minority of individuals express laminin-332 antigen, which is a glycoprotein that interacts with other BMZ molecules to stabilize the extracellular matrix [1,2,6–9]. These individuals cannot be distinguished clinically from those with other variants of MMP [10]. Because it has been reported that these patients with anti-laminin-332 have an increased relative risk of developing cancer, in particular adenocarcinoma, prompt diagnosis and treatments are crucial [8–12]. Various pathogenetic hypotheses have been put forward, including the theory that tumor cells secrete laminin-332 with the consequent loss of keratinocyte adhesion and blister formation [10].
A literature search revealed several case reports and retrospective cohort studies regarding MMP and malignancy [8,12–36]. In particular, Egan et al described a cohort of 35 patients affected by AECP followed up in a period of 12 years; 10 patients (28.6%) developed a solitary cancer [8]. Eight of these patients had cancer after the onset of AECP, most within 12 to 14 months, and all deaths were cancer-related. The associated malignancies included 3 lung, 3 stomach, 2 colon, and 2 endometrial cancers. They estimated a relative risk of 6.8% for solid cancer, but 15.4% if diagnosed within the first year of blister formation. They also documented the short interval occurring between the onset of AECP and cancer (average 14 months). This was the first cohort study regarding MMP and associated malignancies. Later, Letko et al have published a retrospective study of 79 patients affected by MMP [11]. Only in 3 patients was a cancer associated with MMP; a breast ductal carcinoma in situ, a lung squamous cell carcinoma, and a colon adenocarcinoma. Three other oncology patients had been excluded from the study because the cancer diagnosis preceded the MMP (13, 5, and 4 years, respectively). In another report, Sadler et al described a patient with mycosis fungoides treated for 12 years with topical clobetasol ointment who developed oral and nasal erosions; histopathology led to the diagnosis of AECP [9]. This is the first association between MMP and lymphoma. Moreover, the authors provided a summary overview of all MMP (or AECP) cancer-associated cases published until 2007, including a letter from Shannon and colleagues [14], reporting a case of cicatricial pemphigoid and non-Hodgkin lymphoma. More recently, Bernard and colleagues analyzed a large cohort of 154 MMP patients, and an associated neoplasia was found in 18 (11.7%) of them [13]. In this French study, the prevalence and significance of anti-laminin-332 was analyzed, showing that the presence of these antibodies is directly correlated with the severity of the disease, but not exclusively with the presence of a neoplasm. In fact, of these 18 patients, they showed that only 2 (6.4%) were laminin-332 positive, while 16 (13%) were laminin-332 negative, concluding that this frequency does not differ from that of the general population in the same age range. It is noteworthy, finally, that in most of these cases the interval between cancer detection and MMP occurrence was very long (more than 8 years), and the cancer diagnosis frequently preceded the MMP occurrence.
On the other hand, case reports published to date include 23 MMP or AECP patients with 25 solid tumors, in particular lung, gastric, ovarian, cervical, and pancreatic adenocarcinomas; and renal, prostatic, thyroid, hepatocellular, and esophageal carcinomas [8,14–36]. In addition, a case of large B-cell non-Hodgkin lymphoma and a case of mycosis fungoides are cited above [8,14]. Although it is sometimes impossible to clarify the time of cancer detection, in 16 cases the diagnosis of MMP preceded the tumor diagnosis or was synchronous (11 and 5 cases, respectively), whereas in 9 patients the bullous disorder occurred after the cancer detection (metachronous) (Table 2).
Table 2.
Case Reports of MMP-Associated Malignancies From the Literature
| Study | Study Design | MMP Antibodies | Diagnosis of Malignancy | Time of Malignancy Diagnosis | Treatment and Follow-up |
|---|---|---|---|---|---|
| Taniuchi et al, 1999 [15] | AECP case report | Laminin-5 | Gastric AC | S | Minocycline 200 mg/d and nicotinamide 1,500 mg/d without improvement. Bullous lesions disappeared after gastrectomy, but metastases occurred after 8 mos. |
| Chamberlain et al, 2004 [16] | Case report (2 cases) | Laminin-5 by indirect immunofluorescence | Uterine CA and ovarian CA (both metastatic) | M (both cases) | In both patients, remission of bullous disease was obtained after hysterectomy. |
| Yamada et al, 2012 [17] | Case report | Laminin-332 | Thyroid CA, kidney CA and retroperitoneal sarcoma | S | Skin and mucosal lesions healed after surgery and treatment with pulse steroid therapy and plasmapheresis. |
| Fukushima et al, 2008 [18] | AECP case report | NA | Prostatic CA | M | Skin lesions were resistant to therapy (oral prednisolone 30 mg, minocycline hydrochloride 100 mg, and nicotinamide 1,500 mg), but improved after administration of dapsone 100 mg/d. |
| Matsushima et al, 2004 [19] | AECP case report | Laminin-5 by immunoblot | Lung carcinoma | M | MMP improved after prednisone and minocycline, but the patient died after tracheostomy. |
| Setterfield et al, 1999 [20] | Case report | Laminin-5 | Non-small-cell lung CA | P | Prednisolone 40 mg/d and azathioprine 50 mg/d and thereafter dapsone 100 mg/d and cyclosporine 3 mg/kg/d did not improve until the time of death. |
| Shibuya et al, 2012 [21] | Case report | Laminin-332 γ2 subunit by immunoblot | Ovarian CA | S | Prednisolone 30 mg/d was partially effective. |
| Mitsuya et al, 2008 [22] | Case report | Laminin-5 γ2 subunit | Metastatic ovarian CA | M | Prednisolone 40 mg/d and minocycline 200 mg/d, then intravenous immunoglobulins without improvement. |
| Saravanan et al, 2006 [23] | Case report | NA | Renal CA | P | Treatment with dapsone, prednisolone, and azathioprine, but disease progressed. |
| Young et al, 2011 [24] | Case report | Laminin-332 | Metastatic prostate CA | M | Orchiectomy and hormone deprivation therapy. Mycophenolate mofetil 1,500 mg and prednisone cured the blisters. |
| Ostlere et al, 1992 [25] | Case report | ND | Metastatic pancreas CA | P | No response with prednisolone and dapsone, and then prednisolone and cyclophosphamide. |
| Uchiyama et al, 2000 [26] | AECP case report | Laminin-5 serum immunoprecipitation | Gastric CA | P | MMP resolved after gastrectomy. |
| Dainichi et al, 2011 [27] | Case report | Laminin-332 | Hepatocellular CA in cirrhosis | M | MMP not responsive to minocycline 200 mg/d, dapsone 75 mg/d, and oral prednisolone 30 mg/d. Patient died. |
| Polliack, 1968 [28] | Case report | ND | Thyroid AC | P | MMP not responsive to prednisolone and sulfapyridine until fatal outcome. |
| Hope-Ross et al, 1990 [29] | Case report | ND | Esophageal CA | P | Exclusive ocular involvement treated with topical dexamethasone, acetyl cysteine, and cyclosporine with good clinical response. |
| Greer et al, 1980 [30] | Case report | ND | Metastatic lung CA | P | After radiotherapy, MMP was well controlled with prednisone. |
| Kilby, 1965 [31] | Case report | ND | Metastatic pancreas CA | P | Unsuccessful treatment with 20 mg/d of prednisolone. |
| Gibson et al, 1997 [32] | AECP case report | Laminin-5 by immunoprecipitation | Lung CA | P | Good response of MMP with multiple courses of prednisone, cyclophosphamide, and dapsone. |
| Fujimoto et al, 1998 [33] | AECP case report | ND | Gastric CA | S | After gastrectomy, splenectomy, and cholecystectomy, MMP healed and corticosteroids were discontinued. Few weeks after betamethasone was stopped, blisters relapsed and the patient died of metastases. |
| Ding et al, 2014 [34] | AECP case report | Negative | Cervical AC | P | MMP healed after hysteroannessiectomy and lymphadenectomy. |
| Fukuchi et al, 2013 [35] | Case report | Laminin-332 and BP230 positive | Unknown origin AC | P | MMP remission with prednisone 60 mg/d, but the patient died and diagnosis of low-grade AC of unknown primary site was made after autopsy. |
| Demitsu et al, 2009 [36] | Case report | Laminin-332 and BP180 | Pancreas CA | S | Betamethasone 4 mg and plasma exchange gave benefit to MMP resolution, but patient died of intrahepatic cholangitis followed by sepsis. |
| Shannon et al, 2003 [14] | Case report | Laminin-5 by immunoblot | Large B-cell non-Hodgkin lymphoma | M | MMP developed after 2 cycles of chemotherapy with remission of lymphoma. High-dose systemic steroids and azathioprine controlled the pemphigoid up to resolution. |
| Sadler et al, 2007 [9] | AECP case report and reported cases review | Laminin-5 by immunoblot | Mycosis fungoides | M | Cutaneous lymphoma controlled with topical clobetasol for 12 years before occurrence of oral and nasal MMP. |
AC = adenocarcinoma; BP = bullous pemphigoid; CA = carcinoma; M = metachronous; NA = not available; ND = not detected; P = preceding the cancer diagnosis; S = synchronous.
Determining a true paraneoplastic syndrome is often impossible. In fact, most neoplasms are initially occult and asymptomatic. In particular, a true paraneoplastic syndrome comprises the contemporaneous presence (or detection) of both dermatosis and cancer, with resolution of symptoms after cancer healing, and possible recurrence after cancer relapse. Therefore, the term “paraneoplastic” is used only for a subtype of pemphigus, while the term “associated malignancies” must be preferred in the other autoimmune blistering disorders, including MMP [37].
In our case series, 3 patients developed the malignancy 1 to 3 years after the MMP diagnosis. A woman had breast cancer 1 year before MMP occurrence. Although 1 of our patients died from metastases 1.5 year after MMP diagnosis (case 2), the others cleared their bullous disease after cancer removal and are still under follow-up (range, 2–5 years) in our clinic without recurrences.
Conclusions
The clinical importance of determining specific antibodies in autoimmune blistering disorders is well known. For example, the presence of BP180 and/or BP230 in bullous pemphigoid may represent an active disease also in the absence of clinical symptoms [38]. In this regard, various studies demonstrate the correlation between laminin-332 MMP and cancer, underscoring the importance of immunological diagnosis of these autoantibodies [7,10].
On the other hand, and in accordance with the recent study by Bernard and coworkers [13], our data demonstrate the relatively frequent association of MMP with a solid cancer, but no significant correlation with autoantibody detection. However, it is possible that laminin-332 reactivity is correlated with the severity of the disease, but needs to be determined by further studies.
Footnotes
Funding: None.
Competing interests: The authors have no conflicts of interest to disclose.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Kartan S, Shi VY, Clark AK, Chan LS. Paraneoplastic pemphigus and autoimmune blistering diseases associated with neoplasm: characteristics, diagnosis, associated neoplasms, proposed pathogenesis, treatment. Am J Clin Dermatol. 2017;18(1):105–126. doi: 10.1007/s40257-016-0235-z. [DOI] [PubMed] [Google Scholar]
- 2.Chan LS, Ahmed AR, Anhalt GJ, et al. The first international consensus on mucous membrane pemphigoid: definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol. 2002;138(3):370–379. doi: 10.1001/archderm.138.3.370. [DOI] [PubMed] [Google Scholar]
- 3.Broussard KC, Leung TG, Moradi A, Thorne JE, Fine JD. Autoimmune bullous diseases with skin and eye involvement: cicatricial pemphigoid, pemphigus vulgaris, and pemphigus paraneoplastica. Clin Dermatol. 2016;34(2):205–213. doi: 10.1016/j.clindermatol.2015.11.006. [DOI] [PubMed] [Google Scholar]
- 4.Anhalt GJ, Kim SC, Stanley JR, et al. Paraneoplastic pemphigus: an autoimmune mucocutaneous disease associated with neoplasia. N Engl J Med. 1990;323(25):1729–1735. doi: 10.1056/NEJM199012203232503. [DOI] [PubMed] [Google Scholar]
- 5.Mutasim DF, Pelc NJ, Anhalt GJ. Paraneoplastic pemphigus, pemphigus vulgaris, and pemphigus foliaceous. Clin Dermatol. 1993;11(3):173–181. doi: 10.1016/0738-081x(93)90106-m. [DOI] [PubMed] [Google Scholar]
- 6.Bhol KC, Colon JE, Ahmed AR. Autoantibody in mucous membrane pemphigoid binds to an intracellular epitope on human beta4 integrin and causes basement membrane zone separation in oral mucosa in an organ culture model. J Invest Dermatol. 2003;120(4):701–702. doi: 10.1046/j.1523-1747.2003.12081.x. [DOI] [PubMed] [Google Scholar]
- 7.Lazarova Z, Salato VK, Lanschuetzer CM, Janson M, Fairley JA, Yancey KB. IgG anti-laminin-332 autoantibodies are present in a subset of patients with mucous membrane, but not bullous, pemphigoid. J Am Acad Dermatol. 2008;58(6):951–958. doi: 10.1016/j.jaad.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Egan CA, Lazarova Z, Darling TN, Yee C, Coté T, Yancey KB. Anti-epiligrin cicatricial pemphigoid and relative risk of cancer. Lancet. 2001;357(9271):1850–1851. doi: 10.1016/S0140-6736(00)04971-0. [DOI] [PubMed] [Google Scholar]
- 9.Sadler E, Lazarova Z, Sarasombath P, Yancey KB. A widening perspective regarding the relationship between anti-epiligrin cicatricial pemphigoid and cancer. J Dermatol Sci. 2007;47(1):1–7. doi: 10.1016/j.jdermsci.2007.02.012. [DOI] [PubMed] [Google Scholar]
- 10.Egan CA, Lazarova Z, Darling TN, Yee C, Yancey KB. Anti-epiligrin cicatricial pemphigoid: clinical findings, immunopathogenesis, and significant associations. Medicine (Baltimore) 2003;82(3):177–186. doi: 10.1097/01.md.0000076003.64510.00. [DOI] [PubMed] [Google Scholar]
- 11.Letko E, Gürcan HM, Papaliodis GN, Christen W, Foster CS, Ahmed AR. Relative risk for cancer in mucous membrane pemphigoid associated with antibodies to the beta4 integrin subunit. Clin Exp Dermatol. 2007;32(6):637–641. doi: 10.1111/j.1365-2230.2007.02463.x. [DOI] [PubMed] [Google Scholar]
- 12.Cozzani E, Di Zenzo G, Calabresi V, et al. Autoantibody profile of a cohort of 78 Italian patients with mucous membrane pemphigoid: correlation between reactivity profile and clinical involvement. Acta Derm Venereol. 2016;96(6):768–773. doi: 10.2340/00015555-2311. [DOI] [PubMed] [Google Scholar]
- 13.Bernard P, Antonicelli F, Bedane C, et al. Prevalence and clinical significance of anti-laminin 332 autoantibodies detected by a novel enzyme-linked immunosorbent assay in mucous membrane pemphigoid. JAMA Dermatol. 2013;149(5):533–540. doi: 10.1001/jamadermatol.2013.1434. [DOI] [PubMed] [Google Scholar]
- 14.Shannon JF, Mackenzie-Wood A, Wood G, Goldstein D. Cicatricial pemphigoid in non-Hodgkin’s lymphoma. Intern Med J. 2003;33(8):396–397. doi: 10.1046/j.1445-5994.2003.t01-1-00430.x. [DOI] [PubMed] [Google Scholar]
- 15.Taniuchi K, Takata M, Matsui C, et al. Antiepiligrin (laminin 5) cicatricial pemphigoid associated with an underlying gastric carcinoma producing laminin 5. Br J Dermatol. 1999;140(4):696–700. doi: 10.1046/j.1365-2133.1999.02773.x. [DOI] [PubMed] [Google Scholar]
- 16.Chamberlain AJ, Cooper SM, Allen J, et al. Paraneoplastic immunobullous disease with an epidermolysis bullosa acquisita phenotype: two cases demonstrating remission with treatment of gynaecological malignancy. Australas J Dermatol. 2004;45(2):136–139. doi: 10.1111/j.1440-0960.2004.00068.x. [DOI] [PubMed] [Google Scholar]
- 17.Yamada H, Nobeyama Y, Matsuo K, et al. A case of paraneoplastic pemphigus associated with triple malignancies in combination with antilaminin-332 mucous membrane pemphigoid. Br J Dermatol. 2012;166(1):230–231. doi: 10.1111/j.1365-2133.2011.10520.x. [DOI] [PubMed] [Google Scholar]
- 18.Fukushima S, Egawa K, Nishi H, et al. Two cases of anti-epiligrin cicatricial pemphigoid with and without associated malignancy. Acta Derm Venereol. 2008;88(5):484–487. doi: 10.2340/00015555-0506. [DOI] [PubMed] [Google Scholar]
- 19.Matsushima S, Horiguchi Y, Honda T, et al. A case of anti-epiligrin cicatricial pemphigoid associated with lung carcinoma and severe laryngeal stenosis: review of Japanese cases and evaluation of risk for internal malignancy. J Dermatol. 2004;31(1):10–15. doi: 10.1111/j.1346-8138.2004.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 20.Setterfield J, Shirlaw PJ, Lazarova Z, et al. Paraneoplastic cicatricial pemphigoid. Br J Dermatol. 1999;141(1):127–131. doi: 10.1046/j.1365-2133.1999.02933.x. [DOI] [PubMed] [Google Scholar]
- 21.Shibuya T, Komatsu S, Takahashi I, et al. Mucous membrane pemphigoid accompanied by ovarian cancer: a case with autoantibodies solely against γ(2)-subunit of laminin-332. J Dermatol. 2012;39(10):882–884. doi: 10.1111/j.1346-8138.2011.01482.x. [DOI] [PubMed] [Google Scholar]
- 22.Mitsuya J, Hara H, Ito K, Ishii N, Hashimoto T, Terui T. Metastatic ovarian carcinoma-associated subepidermal blistering disease with autoantibodies to both the p200 dermal antigen and the gamma 2 subunit of laminin 5 showing unusual clinical features. Br J Dermatol. 2008;158(6):1354–1357. doi: 10.1111/j.1365-2133.2008.08483.x. [DOI] [PubMed] [Google Scholar]
- 23.Saravanan K, Baer ST, Meredith A, Dyson A, von der Werth J. Benign mucous membrane pemphigoid of the upper aero-digestive tract: rare paraneoplastic syndrome presentation in renal cell carcinoma. J Laryngol Otol. 2006;120(3):237–239. doi: 10.1017/S0022215106000193. [DOI] [PubMed] [Google Scholar]
- 24.Young AL, Bailey EE, Colaço SM, Engler DE, Grossman ME. Anti-laminin-332 mucous membrane pemphigoid associated with recurrent metastatic prostate carcinoma: hypothesis for a paraneoplastic phenomenon. Eur J Dermatol. 2011;21(3):401–404. doi: 10.1684/ejd.2011.1360. [DOI] [PubMed] [Google Scholar]
- 25.Ostlere LS, Branfoot AC, Staughton RC. Cicatricial pemphigoid and carcinoma of the pancreas. Clin Exp Dermatol. 1992;17(1):67–68. doi: 10.1111/j.1365-2230.1992.tb02541.x. [DOI] [PubMed] [Google Scholar]
- 26.Uchiyama K, Yamamoto Y, Taniuchi K, Matsui C, Fushida Y, Shirao Y. Remission of antiepiligrin (laminin-5) cicatricial pemphigoid after excision of gastric carcinoma. Cornea. 2000;19(4):564–566. doi: 10.1097/00003226-200007000-00033. [DOI] [PubMed] [Google Scholar]
- 27.Dainichi T, Hirakawa Y, Ishii N, et al. Mucous membrane pemphigoid with autoantibodies to all the laminin 332 subunits and fatal outcome resulting from liver cirrhosis and hepatocellular carcinoma. J Am Acad Dermatol. 2011;64(6):1199–1200. doi: 10.1016/j.jaad.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 28.Polliack A. Benign mucous membrane pemphigoid with laryngeal stenosis in a patient with thyroid carcinoma. Arch Pathol. 1968;86(1):48–51. [PubMed] [Google Scholar]
- 29.Hope-Ross M, Benedict-Smith A, Hillery M, Mullaney P, Condon P, Collum LM. Ocular cicatricial pemphigoid and oesophageal carcinoma. Acta Ophthalmol (Copenh) 1990;68(3):361–363. doi: 10.1111/j.1755-3768.1990.tb01941.x. [DOI] [PubMed] [Google Scholar]
- 30.Greer KE, Beacham BE, Askew FC., Jr Benign mucous membrane pemphigoid in association with internal malignancy. Cutis. 1980;25(2):183–185. [PubMed] [Google Scholar]
- 31.Kilby PE. Carcinoma of the pancreas presenting with “benign mucous membrane pemphigoid.”. Cancer. 1965;18:847–850. doi: 10.1002/1097-0142(196507)18:7<847::aid-cncr2820180711>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Gibson GE, Daoud MS, Pittelkow MR. Anti-epiligrin (laminin 5) cicatricial pemphigoid and lung carcinoma: coincidence or association? Br J Dermatol. 1997;137(5):780–782. [PubMed] [Google Scholar]
- 33.Fujimoto W, Ishida-Yamamoto A, Hsu R, et al. Anti-epiligrin cicatricial pemphigoid: a case associated with gastric carcinoma and features resembling epidermolysis bullosa acquisita. Br J Dermatol. 1998;139(4):682–687. [PubMed] [Google Scholar]
- 34.Ding DC, Chu TY, Hsu YH. Remission of anti-epiligrin cicatricial pemphigoid after excision of cervical adenocarcinoma. J Cutan Pathol. 2014;41(8):692–693. doi: 10.1111/cup.12348. [DOI] [PubMed] [Google Scholar]
- 35.Fukuchi O, Suko A, Matsuzaki H, et al. Anti-laminin-332 mucous membrane pemphigoid with autoantibodies to α3, β3 and γ2 subunits of laminin-332 as well as to BP230 and periplakin associated with adenocarcinoma from an unknown primary site. J Dermatol. 2013;40(1):61–62. doi: 10.1111/j.1346-8138.2012.01645.x. [DOI] [PubMed] [Google Scholar]
- 36.Demitsu T, Yoneda K, Iida E, et al. A case of mucous membrane pemphigoid with IgG antibodies against all the α3, β3 and γ2 subunits of laminin-332 and BP180 C-terminal domain, associated with pancreatic cancer. Clin Exp Dermatol. 2009;34(8):e992–e994. doi: 10.1111/j.1365-2230.2009.03646.x. [DOI] [PubMed] [Google Scholar]
- 37.Balestri R, Magnano M, La Placa M, et al. Malignancies in bullous pemphigoid: a controversial association. J Dermatol. 2016;43(2):125–133. doi: 10.1111/1346-8138.13079. [DOI] [PubMed] [Google Scholar]
- 38.Schmidt E, Obe K, Bröcker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol. 2000;136(2):174–178. doi: 10.1001/archderm.136.2.174. [DOI] [PubMed] [Google Scholar]