Abstract
Objective
Anemia of chronic disease (ACD) refers to hypoproliferative anemia in the context of acute or chronic activation of the immune system. There is a paucity of prospective data addressing the risk factors for ACD development. An association between common chronic diseases and ACD was examined cross-sectionally and longitudinally.
Method
A cohort of 265,459 healthy participants without ACD at baseline were prospectively followed annually or biennially.
Results
During average follow-up period of 62 months, 4,906 participants developed ACD (incidence rate 3.58 per 1000 person-years). Multivariable-adjusted hazard ratio (HR) [95% confidence interval (CI)] for incident ACD comparing estimated glomerular filtration rate 30–60 and < 30 vs. ≥ 60 ml/min/1.73 m2 were 3.93 [3.18–4.85] and 39.11 [18.50–82.69]; HRs [95% CI] for ACD comparing prediabetes and diabetes vs. normal were 1.19 [1.12–1.27] and 2.46 [2.14–2.84], respectively. HRs [95% CI] for incident ACD comparing body-mass-index (BMI) of < 18.5, 23–24.9 and ≥ 25 vs. 18.5–22.9 kg/m2 were 0.89 [0.78–1.00], 0.89 [0.80–0.99] and 0.78 [0.66–0.91], respectively. HRs [95% CI] for incident ACD comparing prehypertension and hypertension vs. normal were 0.79 [0.73–0.86] and 1.10 [0.99–1.23], respectively. Metabolic syndrome, hypertension, chronic liver disease, and chronic obstructive pulmonary disease were not associated with incident ACD.
Conclusions
The severity of chronic kidney disease and diabetic status were independently associated with an increased incidence of ACD, whereas prehypertension and an increasing BMI were significantly associated with decreased risk of ACD.
Introduction
Anemia of chronic disease (ACD) refers to normochromic, normocytic, hypoproliferative anemia in the context of acute or chronic inflammatory states, including infections, cancers, and autoimmune conditions.[1, 2] Some epidemiological studies have reported that ACD also occurs in clinical conditions accompanied by mild but persistent inflammation including chronic kidney disease (CKD), diabetes mellitus, and aging.[3–5] The prevalence of anemia from most causes has decreased globally between 1990 and 2010, but ACD is expected to increase as population ages.[6–8]
Although the underlying pathophysiology of ACD is multifactorial, hepcidin may play a central role in ACD.[9] Chronic inflammation elevates pro-inflammatory cytokines, including interleukin-6, which centrally mediates hepcidin synthesis. Hepcidin inhibits iron absorption in the intestine and release of recycled iron from macrophages, resulting in reduced efficiency of iron recycling from red blood cells. This functional iron deficiency leads to impaired proliferation of erythroid progenitor cells in the marrow, resulting in iron-restrictive anemia.[3]
ACD is common but often overlooked in actual clinical practice and the risk factors of ACD is not fully understood. CKD leads to dysfunction of renal erythropoietin-producing cells resulting in normocytic normochromic anemia, which was present in nearly half of patients with CKD.[10, 11] Type 2 diabetes increases the risk for anemia by two or three times, which affects 10–15% of patients with type 2 diabetes.[12–14] In these studies, anemia in diabetic patients can be considered as ACD, including the exclusion of iron deficiency anemia and other causes of secondary influences on hemoglobin levels.[14] ACD is also frequently diagnosed in the elderly (>65 years); a few population-based studies have shown that 17% of the elderly are anemic,[15] and 70% of hospitalized elderly patients with anemia were found to have ACD.[5] However, most studies focused on specific single disease or elderly population and were cross-sectional studies limited by the temporal ambiguity between risk factors and anemia. Until now, there is a paucity of prospective cohort study to demonstrate the risk factors for the development of ACD in general population. We examined a prospective relationship of common chronic diseases and their severity with the development of ACD in a large cohort of young and middle-aged Korean adults who underwent a regular health screening examination.
Patients and methods
Study population
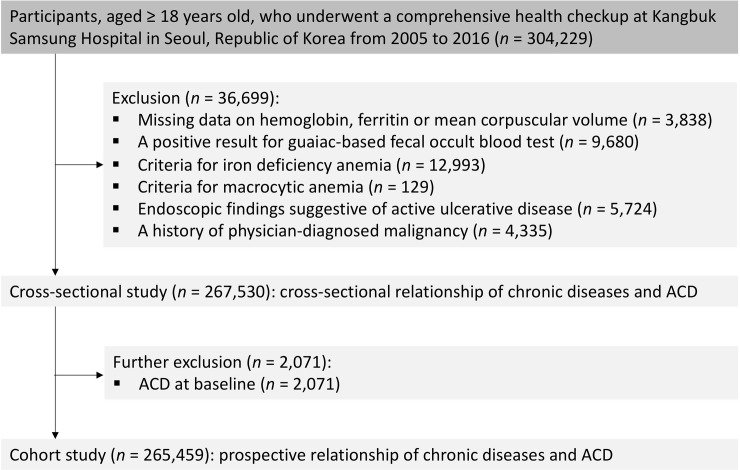
The Kangbuk Samsung Health Study (KSHS) is a cohort study of Korean men and women men and women ≥ 18 years of age who underwent a comprehensive regular (annual or biennial) health examination at Kangbuk Samsung Hospital Total Healthcare Centers in Republic of Korea.[16] The current analyses included all study participants with at least one follow-up visit who underwent a comprehensive health examination between 2005 and 2015 and were followed annually or biennially until December 2016 (n = 304,229). ACD was defined as having anemia without evidence of nutritional anemia or gastrointestinal blood loss.[9] The selection process of study participants is shown in Fig 1. We excluded subjects with missing data on hemoglobin, ferritin, or mean corpuscular volume (MCV) at baseline (n = 3,838), subjects with positive for fecal occult blood tests (n = 9,680), subjects with iron deficiency anemia (n = 12,993) or macrocytic anemia (n = 129), subjects with endoscopic findings including gastric ulcers, duodenal ulcer, inflammatory bowel disease, angiodysplasia, or other gastrointestinal malignancies (esophageal cancer, gastric cancer, or colorectal cancer) (n = 5,724), or subjects with a history of physician-diagnosed malignancy (n = 4,335). After excluding 36,699 subjects, 267,530 participants were included in the baseline cross-sectional study. The cohort participants were not registered at once, but were made up of KSHS cohort in the form of new subjects being added each year. (S1 Table) For cohort study, we further excluded an additional 2,071 subjects with ACD at baseline and finally analyzed 265,459 subjects.
Fig 1. Flow chart of study population.
Measurements
Anthropometric measurements and procedures for obtaining blood samples were described previously.[16, 17] In accordance with WHO criteria, anemia was defined as a hemoglobin level < 13.0 g/dL in men and < 12.0 g/dL in women.[18, 19] Iron deficiency was defined as anemia with a transferrin saturation rate < 16%, or a serum ferritin concentration < 30 ng/mL. Transferrin saturation was calculated by dividing serum iron by total iron-binding capacity. Macrocytic anemia was defined as anemia with MCV > 100 fL.[20] CKD was defined as estimated glomerular filtration rate (eGFR) was <60 ml/min/1.73 m2. Body mass index (BMI) was categorized according to Asian-specific criteria;[21] underweight, BMI < 18.5 kg/m2; normal weight, BMI of 18.5–22.9 kg/m2; overweight, BMI of 23–24.9 kg/m2; and obese, BMI ≥ 25 kg/m2. Metabolic syndrome (MetS) was defined using harmonized criteria.[22, 23] Blood pressure (BP) was categorized into normal BP (BP of <120/<80mmHg), prehypertension (systolic BP of 120–139 mmHg or diastolic BP of 80–89 mmHg) and hypertension (systolic BP of ≥140mmHg or diastolic BP of ≥90 mmHg). Diabetes mellitus was defined as either fasting serum glucose ≥126 mg/dL, glycated hemoglobin (HbA1c) ≥ 6.5%, or the use of blood glucose-lowering drugs, and prediabetes was defined as either fasting serum glucose 100–125 mg/dL or HbA1c 5.7–6.4%. Subjects were considered to have chronic liver disease if either serum hepatitis B surface antigen or anti-hepatitis C antibody was positive, or they had an ultrasonographic diagnosis of fatty liver, or liver cirrhosis. Chronic obstructive pulmonary disease (COPD) was defined as forced expiratory volume in 1 sec/forced vital capacity ratio <0.7.
Statistical analyses
We first evaluated the cross-sectional relationship between comorbidities and ACD at baseline, and then we analyzed the prospective relationship of comorbidities and incident ACD in a cohort study. To compare the characteristics of the study participants between the groups, we used an independent t-tests for continuous variables or χ2 tests for categorical variables. To determine the cross-sectional relationship between comorbidities and ACD, we used a logistic regression model to estimate odds ratios (ORs) with 95% confidence interval (CI). Then, the hazard ratio (HR) and 95% CI were calculated for incident ACD according to comorbidities. Each participant was followed from baseline exam until either development of ACD or the last health exam conducted prior to December 31, 2016, whichever came first. The incidence rate was calculated as number of incident cases divided by number of person-years of follow-up. Since ACD was known to have developed between the two visits, but the precise time at which it developed was unknown, a parametric proportional hazard model was used to account for this type of interval censoring (stpm command in Stata). To determine linear trends of incidence, the number of categories was used as a continuous variable and tested on each model.
The models were initially adjusted for age and sex, and then were adjusted for smoking, alcohol intake, physical activity, education level, center, and year of screening examination. All analyses were performed using STATA version 15.0 (StataCorp, College Station, Texas, USA).
Ethics
This study was approved by the Institutional Review Board of Kangbuk Samsung Hospital (KBSMC 2015-07-019). The acquisition of informed consent was waived, as we retrospectively accessed only data that were de-identified. All data were fully anonymized before our analyses.
Results
Baseline cross-sectional study
A total of 267,530 participants were included in analyses for evaluation of cross-sectional relationship between chronic diseases and prevalent ACD. The participants who made up of study cohort are presented in S1 Table by the year of registration. Of these, 2,071 (0.77%) had ACD at baseline. The baseline characteristics of the study participants by prevalence of prevalent ACD are presented in S2 Table. Age, HDL-C, and high sensitivity C-reactive protein were positively associated with the prevalent ACD, whereas BMI, uric acid, eGFR, fasting glucose, total cholesterol, LDL-C, triglycerides, alanine aminotransferase, aspartate aminotransferase, gamma glutamyl transaminase, and HOMA-IR were negatively associated with prevalent ACD. The proportions of male, current smoking, alcohol intake, vigorous exercise, higher education level, MeS, hypertension, chronic liver disease, and obesity were also negatively associated with prevalent ACD. The proportion of patients with CKD was positively associated with prevalent ACD.
S3 Table shows the baseline cross-sectional relationships between chronic diseases and prevalent ACD. The baseline severity of eGFR and diabetic status were significantly associated with an increased prevalence of ACD (P-trend < 0.001). In contrast, the baseline number of MetS traits, BP category, and low BMI category were significantly associated with a decreased prevalence of ACD (P-trend < 0.001). The chronic liver disease and COPD were not significantly associated with the prevalence of ACD.
In a multivariate model adjusted for age, sex, smoking, alcohol intake, physical activity, education level, center, and year of screening examination, decreased baseline eGFR and severe diabetic status were positively associated with an increased prevalence of ACD; number of MetS traits and higher levels of BP and BMI were inversely associated with prevalent ACD.
Prospective cohort study
After excluding 2,071 subjects with baseline ACD, 265,459 subjects were included in cohort study to investigate the risk factors for incident ACD. The baseline characteristics of the cohort study participants by incident ACD are presented in Table 1. Table 2 shows the prospective associations between the chronic diseases and their severity with ACD among subjects without ACD at baseline. During 1,368,691.2 person-years of follow-up, 4,906 participants developed ACD (incidence rate 3.58 per 1,000 person-years). The average follow-up period for subjects who did not develop ACD was 5.2 years.
Table 1. Baseline characteristics of the study participants by incidence of anemia of chronic disease (ACD).
| Characteristics | Overall | No incident ACD | Incident ACD | P value |
|---|---|---|---|---|
| Number | 265,459 | 260,553 | 4,906 | <0.001 |
| Age (years)a | 37.7 (8.0) | 37.7 (8.0) | 38.5 (9.0) | <0.001 |
| Male (%) | 58.3 | 59.0 | 20.1 | <0.001 |
| Current smoker (%) | 24.7 | 25.0 | 8.8 | <0.001 |
| Alcohol intake (%)c | 17.4 | 17.6 | 8.0 | <0.001 |
| Vigorous exercise (%)d | 14.7 | 14.7 | 13.7 | 0.046 |
| High education level (%) | 82.5 | 82.6 | 76.7 | <0.001 |
| Chronic kidney disease (%) | 0.5 | 0.5 | 2.1 | <0.001 |
| Diabetes (%) | 3.1 | 3.0 | 4.8 | <0.001 |
| Metabolic syndrome (%) | 13.7 | 13.8 | 8.6 | <0.001 |
| Hypertension (%) | 11.5 | 11.5 | 9.3 | <0.001 |
| Chronic liver disease | 30.4 | 30.7 | 15.6 | <0.001 |
| COPD (%) | 1.7 | 1.7 | 1.2 | 0.009 |
| Obesity (%) | 28.3 | 28.6 | 14.1 | <0.001 |
| BMI (kg/m2) | 23.3 (3.2) | 23.3 (3.2) | 22.0 (2.9) | <0.001 |
| Systolic BP (mmHg)a | 111.4 (13.5) | 111.5 (13.5) | 106.6 (14.4) | <0.001 |
| Diastolic BP (mmHg)a | 71.9 (9.9) | 72.0 (9.8) | 68.1 (10.1) | <0.001 |
| Uric acid (mg/dl)a | 5.4 (1.4) | 5.4 (1.4) | 4.6 (1.3) | <0.001 |
| eGFR | 93.8 (15.4) | 93.8 (15.4) | 94.9 (17.6) | <0.001 |
| Hb (mg/dl)a | 14.7 (1.4) | 14.7 (1.4) | 13.0 (0.9) | <0.001 |
| Glucose (mg/dl)a | 94.3 (14.1) | 94.3 (14.0) | 94.0 (20.6) | 0.164 |
| Total cholesterol (mg/dl)a | 191.7 (33.4) | 191.8 (33.4) | 185.6 (33.5) | <0.001 |
| LDL-C (mg/dl)a | 113.5 (30.2) | 113.6 (30.2) | 104.8 (29.0) | <0.001 |
| HDL-C (mg/dl)a | 56.3 (13.8) | 56.2 (13.8) | 60.3 (14.1) | <0.001 |
| Triglycerides (mg/dl)b | 95 (67–142) | 96 (67–142) | 76 (57–109) | <0.001 |
| Ferritin (mg/dl)b | 89.0 (40.7–165.7) | 90.2 (41.1–167.2) | 49.9 (29.8–84.4) | <0.001 |
| ALT (U/l)b | 20 (14–29) | 20 (14–29) | 15 (12–21) | <0.001 |
| AST (U/l)b | 21 (18–26) | 21 (18–26) | 20 (16–24) | <0.001 |
| GGT (U/l)b | 20 (13–35) | 20 (13–35) | 13 (10–21) | <0.001 |
| HOMA-IRb | 1.59 (1.05–2.22) | 1.59 (1.05–2.22) | 1.51 (0.94–2.06) | <0.001 |
| hsCRP (mg/l)b | 0.4 (0.2–0.9) | 0.4 (0.2–0.9) | 0.3 (0.2–0.8) | <0.001 |
Data are
ameans (standard deviation)
bmedians (interquartile range), or percentages.
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; BP, blood pressure; HDL-C, high-density lipoprotein-cholesterol; hsCRP, high sensitivity C-reactive protein; HOMA-IR, homeostasis model assessment of insulin resistance.
c ≥ 20 g of ethanol per day
d ≥ 3 time per week
Table 2. Development of anemia of chronic disease (ACD) based on chronic diseases in the prospective cohort study.
| Person-years | Incident ACD | Incidence Density (per 1000 person-years) | Age and sex-adjusted HR (95% CI) |
Multivariate adjusted HR a (95% CI) |
|
|---|---|---|---|---|---|
| eGFR | |||||
| ≥60 | 1,362,098.0 | 4,801 | 3.5 | 1.00 (reference) | 1.00 (reference) |
| 30~60 | 6,501.0 | 98 | 15.1 | 3.24 (2.63–4.00) | 3.93 (3.18–4.85) |
| <30 | 72.8 | 7 | 96.2 | 30.85 (14.66–64.93) | 39.11 (18.50–82.69) |
| P for trend | <0.001 | <0.001 | |||
| Diabetic status | |||||
| Normal | 908,913.4 | 3,102 | 3.4 | 1.00 (reference) | 1.00 (reference) |
| Prediabetes | 422,096.3 | 1,569 | 3.7 | 1.19 (1.12–1.26) | 1.19 (1.12–1.27) |
| Diabetes | 37,638.8 | 235 | 6.2 | 2.24 (1.95–2.57) | 2.46 (2.14–2.84) |
| P for trend | <0.001 | <0.001 | |||
| Number of Metabolic syndrome trait | |||||
| 0 | 577,599.1 | 2,846 | 4.9 | 1.00 (reference) | 1.00 (reference) |
| 1 | 367,303.6 | 1,155 | 3.1 | 0.82 (0.76–0.88) | 0.93 (0.87–1.00) |
| 2 | 231,672.2 | 479 | 2.1 | 0.67 (0.60–0.74) | 0.83 (0.74–0.92) |
| ≥3 | 190,890.7 | 420 | 2.2 | 0.78 (0.70–0.87) | 1.06 (0.93–1.20) |
| P for trend | <0.001 | 0.261 | |||
| BP category | |||||
| Normal | 814,636.7 | 3,648 | 4.5 | 1.00 (reference) | 1.00 (reference) |
| Prehypertension | 398,740.4 | 796 | 2.0 | 0.70 (0.65–0.76) | 0.79 (0.73–0.86) |
| Hypertension | 154,289.7 | 458 | 3.0 | 0.96 (0.86–1.07) | 1.10 (0.99–0.23) |
| P for trend | <0.001 | 0.223 | |||
| Chronic liver disease | |||||
| No | 1,314,000.0 | 4,748 | 3.6 | 1.00 (reference) | 1.00 (reference) |
| Yes | 54,691.2 | 158 | 2.9 | 0.87 (0.74–1.02) | 0.90 (0.77–1.05) |
| COPD | |||||
| No | 1,335,104.6 | 4,776 | 3.6 | 1.00 (reference) | 1.00 (reference) |
| Yes | 21,248.0 | 58 | 2.7 | 0.84 (0.65–1.09) | 0.87 (0.67–1.12) |
| BMI category | |||||
| <18.5 | 66,554.5 | 418 | 6.3 | 1.02 (0.92–1.13) | 0.89 (0.78–1.00) |
| 18.5~22.9 | 587,934.8 | 2,952 | 5.0 | 1.00 (reference) | 1.00 (reference) |
| 22.9~24.9 | 322,610.4 | 844 | 2.6 | 0.80 (0.74–0.86) | 0.89 (0.80–0.99) |
| ≥25 | 391,407.6 | 691 | 1.8 | 0.64 (0.59–0.70) | 0.78 (0.66–0.91) |
| P for trend | <0.001 | 0.044 |
a Estimated from the logistic regression model.
Multivariate model 1 was adjusted for age, sex, center, year of screening exam, smoking status, alcohol intake, physical activity and education level: Model 2: model 1 plus adjustment for obesity, chronic kidney disease, diabetes, hypertension, COPD, metabolic syndrome, and chronic liver disease Abbreviations: BMI, body mass index; CI, confidence intervals; OR, odd ratios.
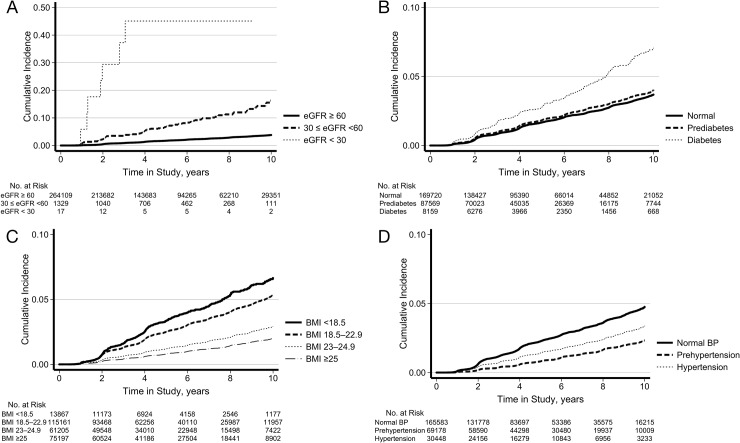
Baseline severity of eGFR and diabetic status were associated with an increased risk of incident ACD in a graded and dose-response manner (P for trend < 0.001). Multivariable-adjusted HR (95% CI) for ACD comparing eGFR 30–60 and < 30 vs. ≥ 60 ml/min/1.73 m2 were 3.93 (3.18–4.85) and 39.11 (18.50–82.69), respectively (P for trend < 0.001). And the multivariable adjusted HR (95% CI) for ACD comparing prediabetes and diabetes vs. normal were 1.19 (1.12–1.27) and 2.46 (2.14–2.84), respectively (P for trend < 0.001). Increasing BMI was inversely associated with incident ACD. HR (95% CI) for ACD comparing BMIs of <18.5, 23–24.9, and >25 vs. 18.5–22.9 kg/m2 were 0.89 (0.78–1.00), 0.89 (0.80–0.99) and 0.78 (0.66–0.91), respectively. Prehypertension was associated with a decreased risk of ACD with corresponding HR (95% CI) of 0.79 (0.73–0.86). The cumulative incidence of ACD was displayed in Fig 2 by CKD groups (Fig 2A), diabetes categories (Fig 2B), BMI groups (Fig 2C) and BP categories (Fig 2D). Metabolic syndrome, hypertension, chronic liver disease, and COPD were not associated with the incidence of ACD in multivariate analyses.
Fig 2.
Cumulative incidence of anemia of chronic disease according to chronic kidney disease groups (A), diabetes categories (B), BMI groups (C) and BP categories (D).
Discussion
In this large cohort study of young and middle-aged Korean men and women, both the cross-sectional and cohort analyses demonstrated that decreased eGFR and severe diabetic status were associated with increased risk of ACD. Conversely, higher BMI categories were associated with a decreased risk of developing ACD in dose-response manner. These associations persisted even after adjusting for possible confounders. Our results provide information about the relative risk for developing ACD among patients with various common chronic diseases.
In our study of 267,530 participants, the proportion of those with ACD was 0.77% in overall population, 0.9% of participants with diabetes and 6.7% of participants with eGFR < 60 ml/min/1.73 m2. However, previous studies of patients with diabetes or CKD reported a higher prevalence of anemia of 10–20%.[10–13] Our lower prevalence of ACD is partly explained by the following reasons: previous studies included nutritional anemia in the anemia outcome and reported a higher prevalence, and chronic diseases in hospital populations from other studies were more severe than in the general population of our study.
Previous studies have shown an association between eGFR, diabetes status and ACD.[10–13] Diabetes and CKD are considered chronic inflammatory states characterized by an increased level of pro-inflammatory cytokines involved in erythroid progenitor cells.[24, 25] In our study, subjects with eGFR < 30 ml/min/1.73 m2 had almost 40 fold increased risk of developing ACD compared with those with a eGFR ≥ 60 ml/min/1.73 m2. The risk of ACD in prediabetic and diabetic participants increased by 1.19 and 2.46 times compared to euglycemic participants, respectively. In CKD or diabetes, incident ACD increased as severity of these diseases increased. Contrary to our expectation, our study found that obese subjects and/or those with prehypertension had a lower risk of ACD. Given that obesity is characterized by chronic low-grade inflammation,[26, 27] a previous study also hypothesized that hemoglobin concentration might be lower in individuals with overweight or obesity.[28] However, this cross-sectional study using data from the third National Health and Nutrition Examination Survey (NHANES III) in a US population reported that overweight (BMI 25–29.9 kg/m2) and obese (BMI > 30 kg/m2) subjects were not associated with anemia compared with normal-weight subjects, while increasing BMI was associated with reduced transferrin saturation and higher serum ferritin, suggesting mechanisms of obesity-related inflammation. A recent systematic review summarized that obese subjects tend to have higher hemoglobin levels than non-obese subjects,[29] which is consistent with our findings.
The mechanisms underlying the inverse association between obesity and the risk of developing ACD are unknown. Therefore, we hypothesized the following reasons. First, subjects who might have developed obesity-induced iron deficiency anemia (IDA) were not included in our study population. Because obesity-associated inflammation affects iron homeostasis and results in an iron deficiency[30] and our study population excluded subjects with IDA. In turn, obese subjects were less likely to experience anemic outcomes. Second, obese subjects have more comorbidities, which may increase hemoglobin. Obese subjects are more likely to have obstructive sleep apnea and other obesity-related respiratory disorders, which result in chronic tissue hypoxia and lead to polycythemia.[31, 32] Third, obese subjects are less likely to be malnourished, because excessive caloric intake can develop into obesity.[26] Therefore, adequate or overnutrition in obese subjects might be associated with a reduced risk of anemia.
Several limitations of this study should be discussed. First, although we identified ACD cases after excluding nutritional anemia or possible blood loss, we may have included unexplained anemia without a proven etiology or clonal anemia.[33] However, given the younger age of our study population, the proportion of unexplained or clonal anemia cases would be minimal. Second, we have no data regarding hepcidin, a key ACD regulatory hormone and proinflammatory factors. Further mechanistic studies are required to elucidate the association between chronic diseases and ACD. Third, our findings are limited by selection bias of case definition. For example, the definition of chronic liver disease could be incomplete without consideration of laboratory findings (liver enzymes, albumin, prothrombin time). The etiology of macrocytic anemia includes chronic illness other than nutritional anemia. [20] However, the number of macrocytic anemia was very low (0.04%) and the impact of selection bias would be minimal. Finally, our study data included young and middle-aged Korean subjects who regularly attended health screening examinations, which could limit the generalizability of our results to other populations with different characteristics of age and race/ethnicity. However, our study provides reliable estimates of ACD risk because of the large sample size, the use of well-defined measurements, and the prospective nature of the cohort study.
This is the one of the largest cohort studies demonstrating a prospective association between chronic diseases and the incidence of ACD. Our cross-sectional and cohort studies identified that prehypertension and increasing BMI are independently associated with a decreased risk of ACD. Although the relationship between CKD or diabetes and anemia is well known, the negative relationships of obesity and prehypertension for incident anemia are novel and interesting findings. Anemia is a non-negligible, but unrecognizable risk. These data will allow clinicians to identify at-risk subjects for intervention. Further studies are warranted to confirm our results.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Cullis JO. Diagnosis and management of anaemia of chronic disease: current status. Br J Haematol. 2011;154(3):289–300. 10.1111/j.1365-2141.2011.08741.x . [DOI] [PubMed] [Google Scholar]
- 2.Gangat N, Wolanskyj AP. Anemia of chronic disease. Semin Hematol. 2013;50(3):232–8. 10.1053/j.seminhematol.2013.06.006 . [DOI] [PubMed] [Google Scholar]
- 3.Roy CN. Anemia of inflammation. Hematology Am Soc Hematol Educ Program. 2010;2010:276–80. Epub 2011/01/18. 10.1182/asheducation-2010.1.276 . [DOI] [PubMed] [Google Scholar]
- 4.Anty R, Dahman M, Iannelli A, Gual P, Staccini-Myx A, Amor IB, et al. Bariatric surgery can correct iron depletion in morbidly obese women: a link with chronic inflammation. Obes Surg. 2008;18(6):709–14. Epub 2008/03/12. 10.1007/s11695-007-9276-y . [DOI] [PubMed] [Google Scholar]
- 5.Joosten E, Lioen P. Iron deficiency anemia and anemia of chronic disease in geriatric hospitalized patients: How frequent are comorbidities as an additional explanation for the anemia? Geriatr Gerontol Int. 2015;15(8):931–5. Epub 2014/09/27. 10.1111/ggi.12371 . [DOI] [PubMed] [Google Scholar]
- 6.Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, et al. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123(5):615–24. 10.1182/blood-2013-06-508325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Culleton BF, Manns BJ, Zhang J, Tonelli M, Klarenbach S, Hemmelgarn BR. Impact of anemia on hospitalization and mortality in older adults. Blood. 2006;107(10):3841–6. Epub 2006/01/13. 10.1182/blood-2005-10-4308 . [DOI] [PubMed] [Google Scholar]
- 8.Bach V, Schruckmayer G, Sam I, Kemmler G, Stauder R. Prevalence and possible causes of anemia in the elderly: a cross-sectional analysis of a large European university hospital cohort. Clin Interv Aging. 2014;9:1187–96. Epub 2014/08/06. 10.2147/CIA.S61125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352(10):1011–23. Epub 2005/03/11. 10.1056/NEJMra041809 . [DOI] [PubMed] [Google Scholar]
- 10.McClellan W, Aronoff SL, Bolton WK, Hood S, Lorber DL, Tang KL, et al. The prevalence of anemia in patients with chronic kidney disease. Curr Med Res Opin. 2004;20(9):1501–10. Epub 2004/09/24. 10.1185/030079904X2763 . [DOI] [PubMed] [Google Scholar]
- 11.Ryu SR, Park SK, Jung JY, Kim YH, Oh YK, Yoo TH, et al. The Prevalence and Management of Anemia in Chronic Kidney Disease Patients: Result from the KoreaN Cohort Study for Outcomes in Patients With Chronic Kidney Disease (KNOW-CKD). J Korean Med Sci. 2017;32(2):249–56. Epub 2017/01/04. 10.3346/jkms.2017.32.2.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kengne AP, Czernichow S, Hamer M, Batty GD, Stamatakis E. Anaemia, haemoglobin level and cause-specific mortality in people with and without diabetes. PLoS One. 2012;7(8):e41875 Epub 2012/08/10. 10.1371/journal.pone.0041875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gauci R, Hunter M, Bruce DG, Davis WA, Davis TME. Anemia complicating type 2 diabetes: Prevalence, risk factors and prognosis. J Diabetes Complications. 2017;31(7):1169–74. Epub 2017/04/24. 10.1016/j.jdiacomp.2017.04.002 . [DOI] [PubMed] [Google Scholar]
- 14.Thomas MC, Tsalamandris C, MacIsaac RJ, Jerums G. The epidemiology of hemoglobin levels in patients with type 2 diabetes. Am J Kidney Dis. 2006;48(4):537–45. Epub 2006/09/26. 10.1053/j.ajkd.2006.06.011 . [DOI] [PubMed] [Google Scholar]
- 15.Stauder R, Thein SL. Anemia in the elderly: clinical implications and new therapeutic concepts. Haematologica. 2014;99(7):1127–30. Epub 2014/07/06. 10.3324/haematol.2014.109967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chang Y, Ryu S, Choi Y, Zhang Y, Cho J, Kwon MJ, et al. Metabolically Healthy Obesity and Development of Chronic Kidney Disease: A Cohort Study. Ann Intern Med. 2016;164(5):305–12. Epub 2016/02/10. 10.7326/M15-1323 . [DOI] [PubMed] [Google Scholar]
- 17.Ryu S, Chang Y, Kim SG, Cho J, Guallar E. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men. Metabolism. 2011;60(6):860–6. Epub 2010/09/25. 10.1016/j.metabol.2010.08.005 . [DOI] [PubMed] [Google Scholar]
- 18.Organization WH. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. 2011. [Google Scholar]
- 19.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107(5):1747–50. Epub 2005/09/29. 10.1182/blood-2005-07-3046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aslinia F, Mazza JJ, Yale SH. Megaloblastic anemia and other causes of macrocytosis. Clin Med Res. 2006;4(3):236–41. Epub 2006/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Organization WH. The Asia-Pacific perspective: redefining obesity and its treatment. Sydney: Health Communications Australia; 2000. [Google Scholar]
- 22.Lorenzo C, Williams K, Hunt KJ, Haffner SM. The National Cholesterol Education Program—Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care. 2007;30(1):8–13. Epub 2006/12/29. 10.2337/dc06-1414 . [DOI] [PubMed] [Google Scholar]
- 23.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. Epub 2009/10/07. 10.1161/CIRCULATIONAHA.109.192644 . [DOI] [PubMed] [Google Scholar]
- 24.Means RT Jr., Krantz SB. Progress in understanding the pathogenesis of the anemia of chronic disease. Blood. 1992;80(7):1639–47. Epub 1992/10/11. . [PubMed] [Google Scholar]
- 25.Dai CH, Price JO, Brunner T, Krantz SB. Fas ligand is present in human erythroid colony-forming cells and interacts with Fas induced by interferon gamma to produce erythroid cell apoptosis. Blood. 1998;91(4):1235–42. Epub 1998/03/07. . [PubMed] [Google Scholar]
- 26.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111(11):1448–54. Epub 2005/03/23. 10.1161/01.CIR.0000158483.13093.9D . [DOI] [PubMed] [Google Scholar]
- 27.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010 Epub 2010/08/14. 10.1155/2010/289645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ausk KJ, Ioannou GN. Is obesity associated with anemia of chronic disease? A population-based study. Obesity (Silver Spring). 2008;16(10):2356–61. Epub 2008/08/23. 10.1038/oby.2008.353 . [DOI] [PubMed] [Google Scholar]
- 29.Cheng HL, Bryant C, Cook R, O'Connor H, Rooney K, Steinbeck K. The relationship between obesity and hypoferraemia in adults: a systematic review. Obes Rev. 2012;13(2):150–61. Epub 2011/10/11. 10.1111/j.1467-789X.2011.00938.x . [DOI] [PubMed] [Google Scholar]
- 30.Aigner E, Feldman A, Datz C. Obesity as an emerging risk factor for iron deficiency. Nutrients. 2014;6(9):3587–600. Epub 2014/09/13. 10.3390/nu6093587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz AR, Patil SP, Laffan AM, Polotsky V, Schneider H, Smith PL. Obesity and obstructive sleep apnea: pathogenic mechanisms and therapeutic approaches. Proc Am Thorac Soc. 2008;5(2):185–92. Epub 2008/02/06. 10.1513/pats.200708-137MG [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heinemann F, Budweiser S, Dobroschke J, Pfeifer M. Non-invasive positive pressure ventilation improves lung volumes in the obesity hypoventilation syndrome. Respir Med. 2007;101(6):1229–35. Epub 2006/12/15. 10.1016/j.rmed.2006.10.027 . [DOI] [PubMed] [Google Scholar]
- 33.Stauder R, Valent P, Theurl I. Anemia at older age: etiologies, clinical implications and management. Blood. 2017. Epub 2017/11/17. 10.1182/blood-2017-07-746446 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.