Abstract
Background
In patients with sepsis, an inflammatory response can lead to destruction of the glycocalyx. These alterations cause the progression of organ dysfunction. Destruction of the glycocalyx can also occur in chronic hyperglycemia. Glycated hemoglobin (HbA1c) is a reliable marker of premorbid hyperglycemia. We investigated the association between HbA1c level at admission and the degree of organ dysfunction progression 72 hours after admission and ICU mortality.
Methods and findings
This study was a retrospective observational study. Logistic regression and correlation analyses were performed to evaluate the association between the HbA1c level and the degree of organ dysfunction progression 72 hours after ICU admission. We applied survival analysis to examine the association between HbA1c level and ICU mortality. A total of 90 patients were included in this study. The association between HbA1c level and degree of organ dysfunction progression was significant (r = 0.320; P = 0.002). Multivariable logistic regression analysis showed that high HbA1c level (≥6.5%) (OR, 2.98; 95% CI, 1.033–8.567; P = 0.043) were significant, independent predictors of severe organ dysfunction progression. Patients with an HbA1c level ≥6.5% exhibited significantly greater liver and kidney dysfunction progression 72 hours after ICU admission compared with those with an HbA1c level <6.5%. Kaplan-Meier analysis showed that the survival period was significantly shorter in patients with an HbA1c level ≥6.5% than in those with an HbA1c level <6.5% (P < 0.001). Multivariable Cox proportional hazard analysis showed that HbA1c level ≥6.5% (HR, 3.49; 95% CI, 1.802–6.760; P <0.001) were significant, independent predictors of ICU mortality.
Conclusions
In patients with sepsis, the HbA1c level at ICU admission is associated with progression of organ dysfunction 72 hours later and with ICU mortality. It may be important to assess HbA1c level at ICU admission because it may be a predictor of ICU outcome. For patients with a high HbA1c level (≥6.5%), greater attention should be paid to the possibility of organ dysfunction progression.
Introduction
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1, 2]. Most patients with sepsis experience progressive organ dysfunction, and death may result from multi-organ failure. Therefore, predicting and preventing organ dysfunction are key to decreasing the risk of in-hospital mortality from sepsis.
International guidelines recommend that, within the first 3 hours after hospital admission, elevated lactic acid concentration should be decreased to a normal level and mean arterial blood pressure should be maintained at 65 mmHg or higher using fluid resuscitation and vasopressors, if needed [3]. These recommendations are designed to prevent progression of organ dysfunction by correcting an abnormal macrocirculation. However, according to recent studies, organ dysfunction progression may not be prevented completely by correcting an abnormal macrocirculation because organ dysfunction progression caused by sepsis is closely related to the microcirculation [4–10].
The microcirculation plays a major role in tissue oxygenation, exchange of nutrients and waste products, modulation of inflammation, and control of coagulation. Because the microcirculation is controlled by autoregulation, microcirculatory deterioration may persist even after correcting an abnormal macrocirculation [4–10]. In patients with sepsis, increased levels of reactive oxygen species and other inflammatory mediators can lead to destruction of the glycocalyx, an important component of the microcirculation. Degradation of the glycocalyx can lead to damage of the microcirculation and contribute to or cause progression of organ dysfunction [4–14]. Therefore, predicting the extent of endothelial glycocalyx damage is important for accurately predicting the patient’s progression of organ dysfunction.
Glycated hemoglobin (HbA1c) is an indicator of the mean plasma glucose level over the past 3 months (e.g., 6% equates to 126 mg/dL; 7% to 154 mg/dL; and 8% to 183 mg/dL) [15–17]. In addition, because the HbA1c level is unaffected by the onset of critical illness, it is a reliable marker of premorbid hyperglycemia [18]. A state of continuous hyperglycemia induces endothelial glycocalyx damage, as shown in patients with sepsis [19–23]. Therefore, increased HbA1c level, which indicates chronic hyperglycemia, is related to the magnitude of baseline glycocalyx destruction, which may then facilitate the progression of organ dysfunction in patients with sepsis.
We hypothesized that HbA1c level would be associated with organ dysfunction progression and intensive care unit (ICU) mortality in patients with sepsis. We investigated the association between HbA1c level at admission and the degree of organ dysfunction progression 72 hours after admission and ICU mortality.
Materials and methods
Study overview
This study was a retrospective observational study performed in a cohort of patients whose HbA1c level was measured at admission to the ICU between March 2008 and August 2017 at a South Korean academic tertiary care hospital with about 1,000 beds. Among patients whose HbA1c level was measured at admission to the ICU, those who met the inclusion criteria were enrolled. We analyzed the association between the HbA1c level at admission to the ICU and the degree of organ dysfunction progression 72 hours after admission. We applied survival analysis to examine the association between HbA1c level and ICU mortality. All patients in this study received standard treatments according to the guidelines of the Surviving Sepsis Campaign [3]. All data, including clinical and laboratory data, were acquired by review of the medical records.
Ethics statement
This study was performed in strict accordance with the principles expressed in the Declaration of Helsinki. The study was approved by the Institutional Review Board of the Korea Medical Center (IRB Number: 2018GR0266). We ensured protection of patient privacy and anonymity. The need for informed consent was waived because of the retrospective nature of the study.
Patients
The inclusion criteria were age ≥20 years, infected patients with Sequential Organ Failure Assessment (SOFA) score ≥2 points, HbA1c level measurement at ICU admission, and direct admission to the ICU. The exclusion criteria were any condition affecting the HbA1c level (e.g., chronic kidney disease, hemolysis, other anemias, recent blood transfusion, erythropoiesis-stimulating drugs, pregnancy) [15, 24]; having signed a do-not-resuscitate order (which could bias the mortality findings); receiving cardiopulmonary resuscitation at admission; or having a severe neurodegenerative disorder that could contribute to organ dysfunction (e.g., amyotrophic lateral sclerosis, Parkinson’s disease).
Definitions
Age and body mass index (calculated by weight divided by height squared) were included as continuous variables. Prior diagnosis of diabetes mellitus (DM) was defined as having been previously diagnosed with DM and taking noninsulin glucose-lowering agents to control DM. Causes of sepsis were based on the final laboratory, imaging, and clinical results. The laboratory findings at admission were collected. Use of vasopressors, steroids, or a ventilator, and continuous renal-replacement therapy (CRRT) were recorded only for the period of admission. ICU mortality was defined as a death during the interval from ICU admission to ICU discharge.
Sepsis-induced organ dysfunction progression was evaluated 72 hours after ICU admission for five systems (lung, cardiac, hepatic, coagulation, and kidney) using the following definitions. Lung dysfunction progression was defined as decreased partial pressure of arterial oxygen/fraction of inspired oxygen ratio as included in the SOFA score. Cardiac dysfunction progression was defined as sepsis-induced myocardial dysfunction diagnosed using echocardiography or an elevated cardiac muscle-specific creatine kinase level to more than five times the normal upper limit. Sepsis-induced myocardial dysfunction was defined as an ejection fraction (EF) <50% or a ≥10% decrease in baseline EF and return of EF to the baseline within 2 weeks [25–27]. Hepatic dysfunction progression was defined as an increased bilirubin level as included in the SOFA score. Coagulation dysfunction progression was defined as a decrease in platelet count as included in the SOFA score. Kidney dysfunction progression was defined as the need for CRRT because of acute kidney injury or an increased creatinine level as indicated in the SOFA score [28, 29].
The degree of organ dysfunction progression was calculated using a sum of dysfunction progression which was estimated at 1 score for each organ dysfunction progression. We classified the degree of organ dysfunction progression into two subsets: mild progression (score of 0–2 for organ dysfunction progression) and severe progression (score of 3–5 for organ dysfunction progression) according to the impact of the ICU mortality in this study.
Statistical analysis
Fisher’s exact tests were used to analyze categorical data, and Mann–Whitney U tests were used to analyze continuous data. Correlational analysis using Spearman’s rank correlation coefficients was performed to evaluate the association between the HbA1c level at ICU admission and the degree of organ dysfunction progression at 72 hours. HbA1c levels were analyzed using receiver-operating characteristic curves to identify the cutoff levels for the optimal predictive accuracy for severe organ dysfunction progression. Survival was evaluated using a Kaplan–Meier approach and log-rank tests. Logistic regression analysis and Cox proportional hazard analysis were used to identify independent factors for severe organ dysfunction progression at 72 hours and mortality. Variables without very high intercorrelations or associations between the independent variables and having a P-value <0.1 in the univariate analysis were included in the multivariable analyses. The data are presented as the adjusted odds ratios (ORs) or hazard ratios (HRs) and 95% confidence intervals (CIs). We considered P values <0.05 in two-sided tests to indicate significance. All statistical analyses were performed using SPSS software (ver. 20.0; IBM Corp., Armonk, NY, USA).
Results
Clinical characteristics of patients in this study
The clinical characteristics of patients in this study are shown in Table 1. The median age of these patients was 77 years, and 49 (54.4%) were men. Their median Acute Physiology and Chronic Health Evaluation II (APACHE II) and SOFA scores at ICU admission were 24 and 9, respectively. Fifty-two (57.8%) of these patients had been previously diagnosed with DM and were taking noninsulin glucose-lowering agents to control DM. During ICU admission, no patients transferred to other hospitals.
Table 1. Clinical characteristics of patients in this study.
| Variables | Total (N = 90) |
Mild (N = 57) |
Severe (N = 33) |
P value |
|---|---|---|---|---|
| Age (years)* | 77 (70–82) | 77 (72–83) | 77 (61–81) | 0.135 |
| Male gender | 49 (54.4) | 32 (56.1) | 17 (51.5) | 0.826 |
| Body Mass Index (Kg/m2)* | 20 (18–23) | 20 (18–22) | 21 (18–23) | 0.143 |
| APACHE II score at admission* | 24 (20–28) | 23 (20–28) | 25 (20–31) | 0.472 |
| SOFA score at admission* | 9 (7–11) | 8 (7–11) | 9 (7–12) | 0.303 |
| Charlson Comorbidity Index* Prior diagnosis of DM |
6 (5–8) 52 (57.8) |
7 (6–8) 29 (50.9) |
6 (5–7) 23 (69.7) |
0.164 0.121 |
| Diagnosis | ||||
| Pneumonia sepsis | 50 (55.6) | 38 (66.7) | 12 (36.4) | 0.008 |
| Biliary sepsis | 10 (11.1) | 1 (1.8) | 9 (27.3) | <0.001 |
| UTI sepsis | 23 (25.6) | 13 (22.8) | 10 (30.3) | 0.460 |
| Other | 7 (7.7) | 5 (8.7) | 2 (6.1) | 1.000 |
| Classification of cultured specimen | ||||
| Blood culture | 35 (38.9) | 17 (29.8) | 18 (54.5) | 0.026 |
| Sputum culture | 59 (65.6) | 45 (78.9) | 14 (42.4) | 0.001 |
| Urine culture | 29 (32.2) | 15 (26.3) | 14 (42.4) | 0.160 |
| Other | 3 (3.3) | 0 (0) | 3 (9.1) | 0.046 |
| Laboratory findings* | ||||
| C-reactive protein (mg/L) | 174 (93–252) | 207(104–249) | 157 (76–262) | 0.277 |
| Procalcitonin (ng/mL) | 7 (2–26) | 4 (1–7) | 11 (3–63) | 0.007 |
| Aspartate transaminase (IU/L) | 44 (28–115) | 39(24–64) | 74 (35–360) | 0.021 |
| Alanine transaminase (IU/L) | 24 (15–67) | 22 (13–35) | 34 (18–101) | 0.036 |
| Glucose (mg/dL) | 169 (120–250) | 161 (122–209) | 209 (118–295) | 0.148 |
| Lactic acid (mmol/L) | 4.5 (2.3–7.1) | 3.7 (2.0–6.1) | 5.3 (3.4–9.9) | 0.006 |
| Hemoglobin (g/dL) | 11 (9–13) | 11 (9–13) | 11 (10–13) | 0.925 |
| Platelet (x109/L) | 182 (112–264) | 199 (131–288) | 151 (91–240) | 0.038 |
| Vasopressor use | ||||
| Norepinephrine | 82 (91.1) | 49 (86) | 33 (100) | 0.025 |
| Vasopressin | 31 (34.4) | 10 (17.5) | 21 (63.6) | <0.001 |
| Dobutamine | 30 (33.3) | 19 (33.3) | 11 (33.3) | 1.000 |
| Dopamine | 27 (30) | 13 (22.8) | 14 (42.4) | 0.060 |
| Epinephrine | 9 (10) | 1 (1.8) | 8 (24.2) | 0.001 |
| Steroid use | 35 (38.9) | 18 (31.6) | 17 (51.5) | 0.075 |
| Ventilator use | 76 (84.4) | 48 (84.2) | 28 (84.8) | 1.000 |
| CRRT use | 23 (25.6) | 7 (12.3) | 16 (48.5) | <0.001 |
| Ventilator days* | 8 (2–14) | 9 (4–18) | 5 (1–11) | 0.020 |
| ICU mortality | 43 (47.8) | 18 (31.6) | 25 (75.8) | <0.001 |
| Glycated hemoglobin (%)* | 5.8 (5.3–7.2) | 5.7 (5.3–6.7) | 6.6 (5.6–8.7) | 0.014 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; DM, diabetes mellitus; UTI, urinary tract infection; CRRT, continuous renal replacement therapy; ICU, intensive care unit.
* Data are presented as median (25th percentile-75th percentile). Other variables are presented as number (percent).
At 72 hours after ICU admission, 33 of the 90 patients (36.7%) exhibited severe organ dysfunction progression. Procalcitonin, liver enzyme, and lactic acid levels were higher in patients with severe organ dysfunction progression than in those with mild organ dysfunction progression. About one-third of the patients with severe organ dysfunction progression exhibited sepsis of biliary tract origin. Patients with severe organ dysfunction progression had more severe sepsis than those with mild organ dysfunction progression because of higher percentage of those required vasopressor or steroid use. Twenty-five of the 33 patients with severe organ dysfunction progression (75.8%) died during ICU admission. HbA1c levels were significantly higher in patients with severe organ dysfunction progression than in those who had mild organ dysfunction progression (P = 0.014).
Association between HbA1c level at admission and organ dysfunction progression at 72 hours after ICU admission
We used Spearman’s correlational analysis to analyze the association between HbA1c level at admission and the degree of organ dysfunction progression at 72 hours. The association between HbA1c level and degree of organ dysfunction progression was significant (r = 0.320; P = 0.002).
Factors affecting the severity of organ dysfunction progression 72 hours after ICU admission
Thirty-three patients exhibited severe organ dysfunction progression during the 72 hours after ICU admission. The optimal cutoff HbA1c level to distinguish the severity of organ dysfunction progression was 6.5% (sensitivity: 54.5%, specificity: 73.7%). We used logistic regression analysis to identify the factors that predicted severe organ dysfunction progression at 72 hours after ICU admission. The univariate analysis showed that age, platelet count, levels of procalcitonin, lactic acid, glucose, and high glycated hemoglobin level (≥6.5%) were significantly associated with severe organ dysfunction progression. Multivariable logistic regression using backward elimination showed that high procalcitonin level (OR, 1.03; 95% CI, 1.005–1.047; P = 0.015), high lactic acid level (OR, 1.14; 95% CI, 1.007–1.284; P = 0.038), and high HbA1c level (≥6.5%) (OR, 2.98; 95% CI, 1.033–8.567; P = 0.043) were significant, independent predictors of severe organ dysfunction progression (Table 2).
Table 2. Factors affecting the severity of organ dysfunction progression at 72 hours after intensive care unit admission.
| Variables | Odds ratio | 95% confidence interval | P value |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 0.96 | 0.920–0.996 | 0.031 |
| Male gender | 0.83 | 0.351–1.962 | 0.671 |
| Body Mass Index (Kg/m2) | 1.10 | 0.973–1.237 | 0.129 |
| APACHE II score at admission | 1.02 | 0.960–1.090 | 0.482 |
| SOFA score at admission | 1.10 | 0.951–1.269 | 0.202 |
| Charlson Comorbidity Index | 0.84 | 0.656–1.070 | 0.156 |
| C-reactive protein (mg/L) | 1.00 | 0.994–1.002 | 0.350 |
| Procalcitonin (ng/mL) | 1.03 | 1.008–1.045 | 0.005 |
| Aspartate transaminase (IU/L) | 1.00 | 1.000–1.001 | 0.315 |
| Alanine transaminase (IU/L) | 1.00 | 0.999–1.001 | 0.868 |
| Glucose (mg/dL) | 1.00 | 1.000–1.006 | 0.096 |
| Lactic acid (mmol/L) | 1.17 | 1.039–1.322 | 0.010 |
| Hemoglobin (g/dL) | 0.98 | 0.802–1.197 | 0.843 |
| Platelet (x109/L) | 0.99 | 0.991–1.000 | 0.048 |
| Glycated hemoglobin ≥ 6.5% | 3.36 | 1.361–8.297 | 0.009 |
| Multivariable analysis | |||
| Procalcitonin (ng/mL) | 1.03 | 1.005–1.047 | 0.015 |
| Lactic acid (mmol/L) | 1.14 | 1.007–1.284 | 0.038 |
| Glycated hemoglobin ≥ 6.5% | 2.98 | 1.033–8.567 | 0.043 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment.
Multivariable logistic regression analysis using backward elimination was performed to investigate factors affecting the severe of organ dysfunction progression 72 hours post-ICU admission, after adjusting for 6 variables (age, procalcitonin, lactic acid, platelet, glucose and high glycated hemoglobin (≥6.5)) that were statistically significant in the univariate analysis.
Associations between HbA1c level ≥6.5% and individual organ dysfunction progression 72 hours after ICU admission
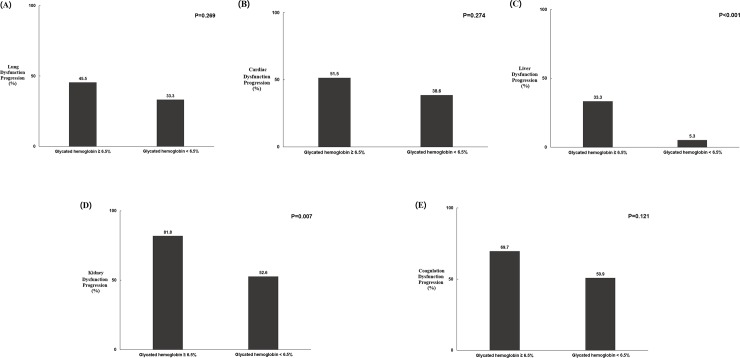
Patients with an HbA1c level ≥6.5% exhibited significantly greater liver and kidney dysfunction progression 72 hours after ICU admission compared with those with an HbA1c level <6.5%. In addition, there was a trend for those with HbA1c level ≥6.5% to exhibit greater progression of lung, cardiac, and coagulation dysfunction compared with those with an HbA1c level <6.5% (Fig 1).
Fig 1. Associations between HbA1c level and individual organ dysfunction progression 72 hours after intensive care unit admission.
(A) Lung dysfunction progression. (B) Cardiac dysfunction progression. (C) Liver dysfunction progression. (D) Kidney dysfunction progression. (E) Coagulation dysfunction progression.
Predictors of ICU mortality in patients with sepsis
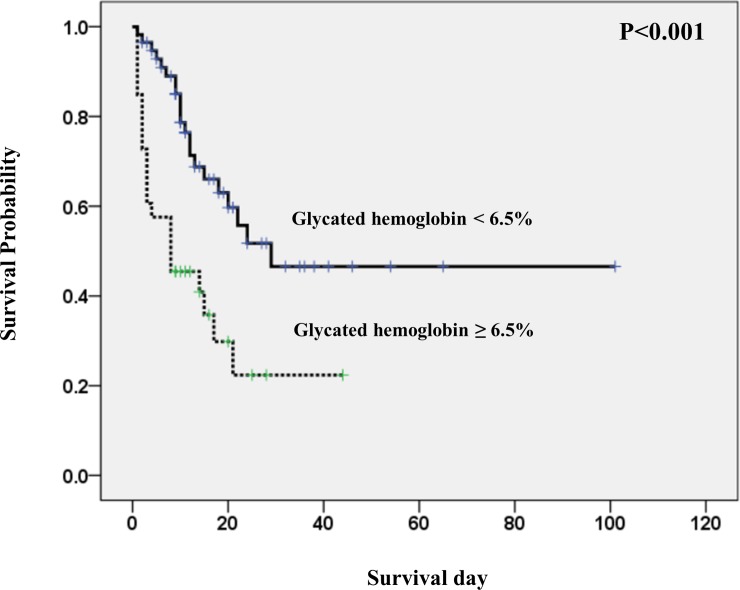
Forty-three of the 90 patients (47.8%) died during ICU admission. Their main cause of death was multi-organ failure caused by sepsis progression. Most deaths (34 patients, 79.1%) occurred during the 2 weeks after ICU admission. We used Kaplan–Meier analysis to estimate the probability of survival during ICU admission according to the HbA1c level. Kaplan–Meier analysis along with the log-rank test showed that the survival period was significantly shorter in patients with an HbA1c level ≥6.5% than in those with an HbA1c level <6.5% (P < 0.001; Fig 2).
Fig 2. The survival curve using the Kaplan Meier method, according to high glycated hemoglobin (≥6.5%) and low glycated hemoglobin (<6.5%) (Log rank test, p<0.001).
We then used Cox proportional hazard analysis to identify the mortality risk factors. The univariate analysis showed that an APACHE II score, SOFA score, procalcitonin level, aspartate transaminase level, alanine transaminase level, glucose level, platelet level, lactic acid level and HbA1c level ≥6.5% were all significantly associated with ICU mortality. Multivariable Cox proportional hazard analysis with backward elimination indicated that high aspartate transaminase level (HR, 1.00; 95% CI, 1.000–1.001; P = 0.007), high lactic acid level (HR, 1.07; 95% CI, 1.013–1.137; P = 0.017) and HbA1c level ≥6.5% (HR, 3.49; 95% CI, 1.802–6.760; P <0.001) were significant, independent predictors of ICU mortality (Table 3).
Table 3. Predictors of intensive care unit mortality in patients with sepsis.
| Risk factors | Hazard ratio | 95% CI | P value |
|---|---|---|---|
| Univariate analysis | |||
| Age (years) | 0.99 | 0.971–1.022 | 0.783 |
| Male sex | 1.12 | 0.611–2.055 | 0.714 |
| Body mass index (Kg/m2) | 0.97 | 0.889–1.062 | 0.522 |
| APACHE II score at admission | 1.06 | 1.011–1.108 | 0.016 |
| SOFA score at admission | 1.17 | 1.059–1.295 | 0.002 |
| Charlson Comorbidity Index | 0.95 | 0.804–1.112 | 0.499 |
| C-reactive protein (mg/L) | 0.99 | 0.996–1.001 | 0.272 |
| Procalcitonin (ng/mL) | 1.01 | 0.999–1.017 | 0.083 |
| Aspartate transaminase (IU/L) | 1.00 | 1.000–1.001 | 0.001 |
| Alanine transaminase (IU/L) | 1.00 | 1.000–1.001 | 0.005 |
| Glucose (mg/dL) | 1.00 | 1.000–1.004 | 0.022 |
| Lactic acid (mmol/L) | 1.08 | 1.022–1.134 | 0.006 |
| Hemoglobin (g/dL) | 1.02 | 0.893–1.175 | 0.734 |
| Platelet (x109/L) | 0.99 | 0.994–1.000 | 0.054 |
| Glycated hemoglobin ≥ 6.5% | 2.79 | 1.524–5.120 | 0.001 |
| Multivariable analysis | |||
| Aspartate transaminase (IU/L) | 1.00 | 1.000–1.001 | 0.007 |
| Lactic acid (mmol/L) | 1.07 | 1.013–1.137 | 0.017 |
| Glycated hemoglobin ≥ 6.5% | 3.49 | 1.802–6.760 | <0.001 |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment.
Multivariable Cox proportional hazard analysis using backward elimination was performed to evaluate risk factors of intensive care unit mortality, after adjusting for 9 variables (APACHE II score, SOFA score, procalcitonin, aspartate transaminase, alanine transaminase, glucose, platelet, lactic acid and high glycated hemoglobin (≥6.5%)) that were statistically significant in the univariate analysis.
Discussion
These findings support the idea that HbA1c level may be useful for predicting the degree of organ dysfunction progression 72 hours after ICU admission and ICU mortality in patients with sepsis. According to practice guidelines, patients with an HbA1c level ≥6.5% can be diagnosed with DM, and the HbA1c goal for many nonpregnant adults is <7% [15, 24]. However, our results suggest that an HbA1c level ≥6.5% increases the risk of progression of organ dysfunction and ICU mortality. Therefore, measuring the HbA1c level at ICU admission may be helpful for predicting the progression of organ dysfunction and mortality in patients with diagnosed DM and for diagnosing DM in patients with undiagnosed DM. However, it is not standard practice to measure the HbA1c level in patients with sepsis who were not previously diagnosed with DM. Our findings suggest that, when a patient with sepsis is admitted to the ICU, clinicians should consider measuring HbA1c level to help them predict the risk of organ dysfunction progression and mortality. Specifically, regardless of whether the patient has or has not been diagnosed with DM before admission, greater attention should be given to patients with a high HbA1c level (≥6.5%) because of the risk of organ dysfunction progression.
These results make several important contributions. To our knowledge, this is the first study to assess the association between HbA1c level and organ dysfunction in patients with sepsis. Although an association between the acute glycemic state and ICU outcome has been shown previously, the association between premorbid glycemic state and ICU outcome is unclear. In addition, only a few studies have shown an association between HbA1c level at admission and ICU mortality [30–33]. Our results suggest that a premorbid glycemic state predicts the degree of organ dysfunction progression 72 hours after ICU admission in patients with sepsis. For example, patients with a high HbA1c level had more severe organ dysfunction progression compared with those with a low HbA1c level.
Although the acute glycemic state may affect organ dysfunction progression during ICU admission, we believe that, for a short period of time (e.g. 72 hours after ICU admission), organ dysfunction progression is related to the premorbid glycemic state rather than to an acute glycemic state. For this reason, our study is relevant to clinical practice. However, a single parameter cannot be used to predict organ dysfunction progression in sepsis patients accurately. In this study, in addition to HbA1c level, procalcitonin and lactic acid levels were also associated with severe organ dysfunction progression after 72 hours. Therefore, several factors in addition to HbA1c level should be considered for predicting organ dysfunction progression accurately. Our findings provide support for a link between premorbid chronic hyperglycemia and progression of organ dysfunction, and suggest that HbA1c level has value as a predictor of organ dysfunction progression.
One possible explanation for these findings is that the chronic hyperglycemic state may damage the endothelial glycocalyx; this is considered to be the primary mechanism responsible for vascular complications in patients with DM [19–23]. Given that the HbA1c level reflects a patient’s premorbid glycemic state during the preceding 3 months, a high HbA1c level indicates a continuous hyperglycemic state [15]. The baseline glycocalyx damage may be more severe in patients with a high HbA1c level than in those with a low HbA1c level. The thickness of the glycocalyx in DM patients has been reported to be half of that in healthy controls [34, 35].
During sepsis, increased production of reactive oxygen species and other inflammatory mediators can also lead to glycocalyx damage [4–14]. This means that patients with a high HbA1c level who develop sepsis may experience more severe glycocalyx destruction compared with those with a low HbA1c level because of the effects of chronic hyperglycemia on the glycocalyx. From this perspective, HbA1c may not be a pathognomonic molecule in itself; rather, it reflects a chronic hyperglycemic state that may lead to baseline glycocalyx destruction.
HbA1c is a reliable marker of premorbid hyperglycemia because its level is unaffected by the onset of critical illness [18]. Degradation of the glycocalyx alters endothelial barrier permeability and may thus cause damage to the microcirculation, which contributes to organ dysfunction. Possible strategies for improving the microcirculation include performing appropriate fluid resuscitation, correcting electrolyte imbalance, maintaining glycemic control and effective insulin therapy, and considering administration of corticosteroid, albumin, and heparin or antithrombin [35]. However, further investigation of these strategies is needed to confirm whether they can improve the microcirculation and prevent or lessen the risk of organ dysfunction in patients with sepsis.
This study also revealed the most vulnerable organs in patients with sepsis and high HbA1c level. Progression of liver and kidney dysfunction was significantly higher in patients with an HbA1c level ≥6.5% at 72 hours after ICU admission (Fig 2). A previous study showed that patients with high HbA1c level are more likely to require CRRT than are those with low HbA1c level [32]. The difference in endothelial cell lining morphology and function between organs may explain the heterogeneity of organ damage seen in sepsis [4, 36–38]. The glomerular and peritubular beds in the kidney, hepatic sinusoids in the liver, and alveolar capillary barrier in the lungs are fragile under septic conditions [4, 36–38]. Our data provide evidence that the kidney and liver may be more easily injured by sepsis. However, patients with an HbA1c level ≥6.5% were more likely to exhibit progressive lung, cardiac, and coagulation dysfunction compared with those with an HbA1c level <6.5%. This issue requires further evaluation with a larger sample.
Finally, this study revealed a relationship between high HbA1c level and ICU mortality in patients with sepsis. Kaplan–Meier analysis showed that the survival period among patients was significantly shorter for patients with an HbA1c level ≥6.5% compared with those with an HbA1c level <6.5% (Fig 2). In addition, the lactic acid level and an HbA1c level ≥6.5% were significant independent predictors of ICU mortality (Table 3). We conclude that an HbA1c level ≥6.5% is associated with ICU mortality in patients with sepsis.
The present study has some limitations. First, it used a retrospective design. However, conducting a prospective study to explore this issue would be difficult because of the time and cost requirements. This study included patients with severe sepsis so that we could evaluate the relationship between HbA1c level and organ dysfunction. In total, 82 patients (91.1%) were initially injected with vasopressors because of shock, which had not been corrected by fluid resuscitation. For this reason, we were able to clarify the association between HbA1c level and organ dysfunction. Second, the total number of patients in this study (n = 90) was smaller than that in previous studies. However, measurement of HbA1c level is not routine in patients with sepsis, and we generally do not measure the HbA1c level in patients unless they have been previously diagnosed with DM. However, the total number of patients was sufficiently large to reveal an association between HbA1c level and organ dysfunction. Third, the study was not supported by experimental analysis of patients’ blood, but many previous studies nevertheless support our conclusions.
Despite some limitations, this study is the first to assess the association between HbA1c level and organ dysfunction progression in patients with sepsis. These results support the idea that HbA1c level along with other factors may be useful in clinical practice for predicting organ dysfunction progression in patients with sepsis.
Conclusions
In patients with sepsis, the HbA1c level at ICU admission is associated with progression of organ dysfunction 72 hours later and with ICU mortality. It may be important to assess HbA1c level at ICU admission because it may be a predictor of ICU outcome. For patients with a high HbA1c level (≥6.5%), greater attention should be paid to the possibility of organ dysfunction progression. Additional prospective, multicenter studies are needed to confirm these findings.
Supporting information
(DOCX)
(DOCX)
(TIF)
Abbreviations
- HbA1c
Glycated hemoglobin
- ICU
Intensive care unit
- SOFA
Sequential Organ Failure Assessment
- CPR
Cardiopulmonary resuscitation
- DNR
Do not resuscitate
- DM
Diabetes mellitus
- CRRT
Continuous renal replacement therapy
- EF
Ejection fraction
- APACHE II
Acute Physiology And Chronic Health Evaluation II
- OR
Odds ratio
- HR
Hazard ratio
- CI
Confidence interval
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Prescott HC, Angus DC. Enhancing Recovery From Sepsis: A Review. JAMA. 2018;319(1):62–75. Epub 2018/01/04. 10.1001/jama.2017.17687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801–10. Epub 2016/02/24. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017;43(3):304–77. Epub 2017/01/20. 10.1007/s00134-017-4683-6 . [DOI] [PubMed] [Google Scholar]
- 4.Ince C, Mayeux PR, Nguyen T, Gomez H, Kellum JA, Ospina-Tascon GA, et al. THE ENDOTHELIUM IN SEPSIS. Shock (Augusta, Ga). 2016;45(3):259–70. Epub 2016/02/13. 10.1097/shk.0000000000000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lipinska-Gediga M. Sepsis and septic shock-is a microcirculation a main player? Anaesthesiol Intensive Ther. 2016;48(4):261–5. Epub 2016/11/01. 10.5603/AIT.a2016.0037 . [DOI] [PubMed] [Google Scholar]
- 6.Kanoore Edul VS, Ince C, Dubin A. What is microcirculatory shock? Curr Opin Crit Care. 2015;21(3):245–52. Epub 2015/04/02. 10.1097/MCC.0000000000000196 . [DOI] [PubMed] [Google Scholar]
- 7.Ait-Oufella H, Maury E, Lehoux S, Guidet B, Offenstadt G. The endothelium: physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 2010;36(8):1286–98. Epub 2010/05/06. 10.1007/s00134-010-1893-6 . [DOI] [PubMed] [Google Scholar]
- 8.Ait-Oufella H, Bourcier S, Lehoux S, Guidet B. Microcirculatory disorders during septic shock. Curr Opin Crit Care. 2015;21(4):271–5. Epub 2015/06/24. 10.1097/MCC.0000000000000217 . [DOI] [PubMed] [Google Scholar]
- 9.Kara A, Akin S, Ince C. Monitoring microcirculation in critical illness. Curr Opin Crit Care. 2016;22(5):444–52. Epub 2016/09/02. 10.1097/MCC.0000000000000335 . [DOI] [PubMed] [Google Scholar]
- 10.Colbert JF, Schmidt EP. Endothelial and Microcirculatory Function and Dysfunction in Sepsis. Clin Chest Med. 2016;37(2):263–75. Epub 2016/05/28. 10.1016/j.ccm.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alphonsus CS, Rodseth RN. The endothelial glycocalyx: a review of the vascular barrier. Anaesthesia. 2014;69(7):777–84. Epub 2014/04/30. 10.1111/anae.12661 . [DOI] [PubMed] [Google Scholar]
- 12.Martin L, Koczera P, Zechendorf E, Schuerholz T. The Endothelial Glycocalyx: New Diagnostic and Therapeutic Approaches in Sepsis. Biomed Res Int. 2016;2016:3758278 Epub 2016/10/05. 10.1155/2016/3758278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerny V, Astapenko D, Brettner F, Benes J, Hyspler R, Lehmann C, et al. Targeting the endothelial glycocalyx in acute critical illness as a challenge for clinical and laboratory medicine. Crit Rev Clin Lab Sci. 2017;54(5):343–57. Epub 2017/09/30. 10.1080/10408363.2017.1379943 . [DOI] [PubMed] [Google Scholar]
- 14.Kottke MA, Walters TJ. Where's the Leak in Vascular Barriers? A Review. Shock (Augusta, Ga). 2016;46(3 Suppl 1):20–36. Epub 2016/07/13. 10.1097/shk.0000000000000666 . [DOI] [PubMed] [Google Scholar]
- 15.6. Glycemic Targets: Standards of Medical Care in Diabetes-2018. Diabetes care. 2018;41(Suppl 1):S55–s64. Epub 2017/12/10. 10.2337/dc18-S006 . [DOI] [PubMed] [Google Scholar]
- 16.Dorcely B, Katz K, Jagannathan R, Chiang SS, Oluwadare B, Goldberg IJ, et al. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab Syndr Obes. 2017;10:345–61. Epub 2017/09/02. 10.2147/DMSO.S100074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lyons TJ, Basu A. Biomarkers in diabetes: hemoglobin A1c, vascular and tissue markers. Transl Res. 2012;159(4):303–12. Epub 2012/03/20. 10.1016/j.trsl.2012.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luethi N, Cioccari L, Tanaka A, Kar P, Giersch E, Deane AM, et al. Glycated Hemoglobin A1c Levels Are Not Affected by Critical Illness. Crit Care Med. 2016;44(9):1692–4. Epub 2016/03/16. 10.1097/CCM.0000000000001656 . [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Friden V, Dasgupta I, Foster RR, Welsh GI, Tooke JE, et al. High glucose causes dysfunction of the human glomerular endothelial glycocalyx. Am J Physiol Renal Physiol. 2011;300(1):F40–8. Epub 2010/10/29. 10.1152/ajprenal.00103.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goligorsky MS. Vascular endothelium in diabetes. Am J Physiol Renal Physiol. 2017;312(2):F266–f75. Epub 2016/11/18. 10.1152/ajprenal.00473.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrin RM, Harper SJ, Bates DO. A role for the endothelial glycocalyx in regulating microvascular permeability in diabetes mellitus. Cell Biochem Biophys. 2007;49(2):65–72. Epub 2007/10/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salmon AH, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol. 2012;226(4):562–74. Epub 2011/11/22. 10.1002/path.3964 . [DOI] [PubMed] [Google Scholar]
- 23.10. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2018. Diabetes care. 2018;41(Suppl 1):S105–s18. Epub 2017/12/10. 10.2337/dc18-S010 . [DOI] [PubMed] [Google Scholar]
- 24.2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2018. Diabetes care. 2018;41(Suppl 1):S13–s27. Epub 2017/12/10. 10.2337/dc18-S002 . [DOI] [PubMed] [Google Scholar]
- 25.Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Curr Opin Crit Care. 2009;15(5):392–7. Epub 2009/07/28. 10.1097/MCC.0b013e3283307a4e . [DOI] [PubMed] [Google Scholar]
- 26.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Crit Care Med. 2007;35(6):1599–608. Epub 2007/04/25. 10.1097/01.CCM.0000266683.64081.02 . [DOI] [PubMed] [Google Scholar]
- 27.Jeong HS, Lee TH, Bang CH, Kim JH, Hong SJ. Risk factors and outcomes of sepsis-induced myocardial dysfunction and stress-induced cardiomyopathy in sepsis or septic shock: A comparative retrospective study. Medicine. 2018;97(13):e0263 Epub 2018/03/30. 10.1097/MD.0000000000010263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno R, Vincent JL, Matos R, Mendonca A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working Group on Sepsis related Problems of the ESICM. Intensive Care Med. 1999;25(7):686–96. Epub 1999/09/02. . [DOI] [PubMed] [Google Scholar]
- 29.Vincent JL, de Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: results of a multicenter, prospective study. Working group on "sepsis-related problems" of the European Society of Intensive Care Medicine. Crit Care Med. 1998;26(11):1793–800. Epub 1998/11/21. . [DOI] [PubMed] [Google Scholar]
- 30.Egi M, Bellomo R, Stachowski E, French CJ, Hart GK, Taori G, et al. The interaction of chronic and acute glycemia with mortality in critically ill patients with diabetes. Crit Care Med. 2011;39(1):105–11. Epub 2010/10/27. 10.1097/CCM.0b013e3181feb5ea . [DOI] [PubMed] [Google Scholar]
- 31.Gornik I, Gornik O, Gasparovic V. HbA1c is outcome predictor in diabetic patients with sepsis. Diabetes Res Clin Pract. 2007;77(1):120–5. Epub 2006/12/05. 10.1016/j.diabres.2006.10.017 . [DOI] [PubMed] [Google Scholar]
- 32.Kompoti M, Michalia M, Salma V, Diogou E, Lakoumenta A, Clouva-Molyvdas PM. Glycated hemoglobin at admission in the intensive care unit: clinical implications and prognostic relevance. J Crit Care. 2015;30(1):150–5. Epub 2014/09/23. 10.1016/j.jcrc.2014.08.014 . [DOI] [PubMed] [Google Scholar]
- 33.Mahmoodpoor A, Hamishehkar H, Shadvar K, Beigmohammadi M, Iranpour A, Sanaie S. Relationship between glycated hemoglobin, Intensive Care Unit admission blood sugar and glucose control with ICU mortality in critically ill patients. Indian J Crit Care Med. 2016;20(2):67–71. Epub 2016/04/15. 10.4103/0972-5229.175938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nieuwdorp M, Mooij HL, Kroon J, Atasever B, Spaan JA, Ince C, et al. Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes. 2006;55(4):1127–32. Epub 2006/03/29. . [DOI] [PubMed] [Google Scholar]
- 35.Iba T, Levy JH. Derangement of the endothelial glycocalyx in sepsis. J Thromb Haemost. 2018. Epub 2018/12/26. 10.1111/jth.14371 . [DOI] [PubMed] [Google Scholar]
- 36.Ma S, Evans RG, Iguchi N, Tare M, Parkington HC, Bellomo R, et al. Sepsis-induced acute kidney injury: A disease of the microcirculation. Microcirculation. 2018:e12483 Epub 2018/06/17. 10.1111/micc.12483 . [DOI] [PubMed] [Google Scholar]
- 37.Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14(7):417–27. Epub 2018/04/25. 10.1038/s41581-018-0005-7 . [DOI] [PubMed] [Google Scholar]
- 38.Pool R, Gomez H, Kellum JA. Mechanisms of Organ Dysfunction in Sepsis. Crit Care Clin. 2018;34(1):63–80. Epub 2017/11/19. 10.1016/j.ccc.2017.08.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.