Abstract
Pathogens are one of the factors driving pollinator declines. Diet can play an important role in mediating pollinator health and resistance to pathogens. Sunflower pollen (Helianthus annuus) dramatically reduced a gut pathogen (Crithidia bombi) of Bombus impatiens previously, but the breadth of this effect was unknown. We tested whether pollen from nine H. annuus cultivars, four wild H. annuus populations, H. petiolarus, H. argophyllus and two Solidago spp., reduced Crithidia in B. impatiens compared to mixed wildflower pollen and buckwheat pollen (Fagopyrum esculentum) as controls. We also compared hand- and honeybee-collected pollen (which contains nectar) to assess whether diet effects on pathogens were due to pollen or nectar. All Helianthus and Solidago pollen reduced Crithidia by 20–40-fold compared to buckwheat pollen, and all but three taxa reduced Crithidia compared to wildflower pollen. We found no consistent differences between hand- and bee-collected pollen, suggesting that pollen alone can reduce Crithidia infection. Our results indicate an important role of pollen diet for bee health and potentially broad options within the Asteraceae for pollinator plantings to manage bee disease.
Keywords: bumblebees, Bombus impatiens, Crithidia bombi, goldenrod, pollinator decline, sunflower
1. Background
Pollination services are critical in ecological and agricultural systems. In the United States, up to 90 crops are pollinated by bees [1] and worldwide, pollinators pollinate about one-third of food crops [2]. Pollinators also fill important ecological niches by aiding wild plant reproduction, contributing to the maintenance of a diverse landscape [3,4]. Since the turn of the twenty-first century, several pollinator taxa have declined, including some bee species [5–7]. With mounting concerns about these declines [8,9], research on pollinator diseases and their potential mitigation has become a pressing need [10,11].
Most bees rely solely on nectar and pollen as food sources, obtaining lipids and proteins from pollen and sugars from nectar [12]. Wildflower gardens and pollinator strips along agricultural lands are receiving increased attention as mechanisms to provide foraging habitat and nesting sites for pollinators [13]. Flowers can provide not only nutritional benefits but also play a role in mediating bee disease dynamics. Some floral rewards have properties that can reduce parasites [14], which we refer to hereafter as a ‘medicinal’ trait. If floral rewards of certain plant species are medicinal, this suggests potential benefits if these species are planted in wildflower gardens or pollinator strips. Thus, identifying plants with floral rewards that suppress pathogens could provide non-chemical options to improve pollinator health by incorporating target plant species into agroecosystems and natural habitats.
Studies of sunflower floral rewards (Helianthus annuus L.; Asteraceae) indicate that they may play a significant role in pathogen suppression. When compared to other monofloral pollen diets and a wildflower pollen mix, two cultivars of sunflower pollen (Helianthus annuus L.; Asteraceae) dramatically suppressed the trypanasomatid intestinal pathogen Crithidia bombi in the common eastern bumblebee Bombus impatiens and had less dramatic but still significant effects reducing the microsporidian pathogen Nosema ceranae in honeybees, Apis mellifera [15]. This discovery is consistent with two other studies suggesting that floral rewards from sunflower and related taxa have medicinal properties for bees. For example, ingestion of sunflower honey, which is made of primarily nectar with some pollen, reduced N. ceranae and increased survival in honeybees [16]. Additionally, some solitary bees are specialists on Asteraceae pollen [17] and it has been suggested that in Osmia, this may be due to pollen reducing brood parasitism [18], although other explanations may also explain these patterns [19]. These discoveries suggest that sunflower and possibly broader Asteraceae pollen have medicinal effects that could help bees resist pathogens or parasites, but the extent of this effect across plant taxa is unknown.
The goal of our study was to assess whether pollen from multiple cultivars and wild populations of H. annuus, its congeners and Asteraceae relatives significantly reduced C. bombi in B. impatiens. Additionally, we compared the effects of hand-collected (i.e. granular) and honeybee-collected pollen because honeybee-collected pollen contains nectar [20,21] and salivary enzymes [22,23] while hand-collected pollen does not. Thus, comparing hand- and honeybee-collected pollen allowed us to ascertain whether medicinal properties are due to pollen, nectar or both. Investigating medicinal effects from a wide range of plant species may provide options for pollinator diets.
2. Material and methods
2.1. Study system
The common eastern bumblebee, Bombus impatiens (Cresson), is a eusocial generalist pollinator with an annual colony life cycle [24], with colonies producing up to 400 workers [25]. Bombus impatiens is commonly found in eastern North America, from Maine to Ontario to the eastern Rocky Mountains and south through Florida [26]. Colonies of B. impatiens are commercially available.
The intestinal parasite Crithidia bombi (Kinetoplastea, Trypanosomatida) is found in wild and commercial B. impatiens populations and in other Bombus species worldwide [27,28]. Crithidia bombi can have varying ranges of parasitism [29], with 49% of bumblebee workers infected in wild colonies in the UK (Goulson et al. [30]) and up to 80% in western Massachusetts, USA populations [31]. Crithidia bombi is transmitted horizontally during floral visitation [32], and in the hive from one generation of workers to the next via contact with infected faeces [33]. Crithidia bombi can reduce Bombus terrestris early colony growth rate and successful emergence of hibernating queens [10,11], reduce the production of new daughter queens [30] and interact with starvation to increase mortality by 50% [34]. Furthermore, C. bombi reduced Bombus impatiens' motor learning rates of flower handling, and foraging rates [35], potentially reducing pollination and foraging efficiency.
Sunflower (Helianthus annuus) is a common early successional, self-compatible annual forb native to central North America [36] that is grown commercially for its oilseed and as a cover crop [37], with approximately 22 million ha of H. annuus grown for cultivation globally [38]. Moreover, sunflower is also planted on smaller farms in eastern North America for cut flowers and as a novelty or cover crop. Sunflower has relatively low protein compared to other pollen species [39,40] but is actively foraged on by a wide range of bee species [41] including B. impatiens [15].
2.2. Plant sources and cultivation
We used pollen from nine H. annuus cultivars, four populations of wild H. annuus, two Helianthus congeners, two Solidago species and two controls (buckwheat, Fagopyrum esculentum and a honeybee-collected wildflower mixed pollen). Hereafter, all 19 pollen treatments are referred to as ‘taxa’ for simplicity. Pollen from most taxa was collected from plants grown from seed obtained from the USDA Agricultural Research Service through the North Central Regional Plant Introduction Station, which is part of the US National Plant Germplasm System programme. The seeds were sown at the College of Natural Science's greenhouses at the University of Massachusetts-Amherst (electronic supplementary material, table S1) and were grown at the Crop and Animal Research and Education Center in South Deerfield, MA (42°28′45.53″ N 72°34′46.06″ W). We also collected pollen from three taxa outside our field site: H. annuus ‘Cobalt II’ cultivar and H. annuus ‘Black Oil Seed’ cultivar from farms in MA and wild-growing Solidago spp. from one population in MA (electronic supplementary material, table S1). For the taxa we did not grow, we used DNA barcoding following established protocols [42] to confirm species identity. Both yellow and orange-coloured Solidago pollen had 96% and 97% matches with Solidago rugosa and Solidago canadensis. Because of the close matches of both colours with both species, we will refer to these taxa as ‘Solidago yellow’ and ‘Solidago orange’ and both taxa will be considered as potentially both species. Helianthus annuus ‘Cobalt II’ and H. annuus ‘Black Oil Seed’ both produced yellow and orange pollen that we tested separately and were all 96–100% matches with H. annuus. We refer to them by their cultivar name and pollen colour. Buckwheat and one source of sunflower pollen used in our original research [15] were obtained from Changge Hauding Wax Industry, China, and the wildflower mix pollen was obtained from Koppert Biological Systems (Linden Apiaries, Howell, MN, USA). We used buckwheat as our single-species comparison to sunflower taxa because buckwheat has a similar protein content as sunflower pollen [39] but results in much higher C. bombi infection [15].
2.3. Pollen preparation
Pollen collection took place by hand only for five taxa, by honeybees only for eight taxa, and by both methods for three taxa (electronic supplementary material, table S1 and figure S1). Before starting the diet trials, hand- and honeybee-collected pollen was mixed with a 30% 1 : 1 glucose : fructose sugar solution, reflecting the concentration and sugar ratios in H. annuus nectar [43,44]. The ratio of sugar solution to pollen was different between hand- and honeybee-collected pollen to create a dough-like consistency similar across all taxa because hand-collected pollen contained no nectar and thus needed more liquid to reach the same consistency. For hand-collected pollen, we added 43–47% sugar solution by weight, compared to 7–24% sugar solution added to the honeybee-collected pollen. Honeybee-collected pollen can contain up to 40% more sugars by weight than hand-collected pollen [20,45], which roughly corresponds to the 20–40% more sugar solution added to hand-collected compared to honeybee-collected pollen in our experiment.
2.4. Inoculum preparation
Crithidia bombi were maintained in commercial B. impatiens ‘source’ colonies (Biobest Canada, Leamington, Ontario, Canada) infected with C. bombi from wild B. impatiens workers collected at Stone Soup Farm in Hadley, MA (42°21′51.93″ N, 72°33′55.88″ W). Every day that we inoculated bees, we prepared fresh C. bombi inoculum from 5 to 10 source colony workers using an established protocol [46]. Briefly, the inoculum was prepared by grinding mid- and hindguts in 1.5 ml Eppendorf tubes with 300 µl of one-fourth strength Ringer's solution (Fluka 96724, Sigma-Aldrich, St Louis, MO, USA). The solution was vortexed for 5 s and allowed to settle for 4–5 h at room temperature. After the solution settled, 10 µl samples of the supernatant were placed on a haemocytometer to count Crithidia cells. We then used 150 µl samples from 1 to 3 bees to make a mixture diluted with Ringer's solution to achieve 1200 C. bombi cells µl−1. This solution was mixed with an equal amount of 50% sucrose solution to prepare an inoculum with 600 C. bombi cells µl−1 in 25% sucrose, which falls within the natural C. bombi concentration range in infected faeces [47].
2.5. Laboratory trials
During the spring and summer of 2017, workers were isolated from commercially reared laboratory colonies that were confirmed to be free of C. bombi via biweekly subsamples of five bees. In total, 17 colonies were used, and each pollen taxon was assessed using at least three colonies. Before inoculation, worker bees were isolated in small vials and starved for 2–3 h. Bees were inoculated individually with 10 µl of fresh C. bombi inoculum. Bees were randomly assigned to one of the 19 different pollen treatments and housed individually in plastic 500 ml deli cups with approximately 50 mg (range 40–70 mg) of their treatment pollen and 10 ml of 30% sugar solution, made available by a cotton wick through a hole cut into the top of a 95 mm Petri dish (electronic supplementary material, figure S1C,D). Experimental bees were stored in the dark at 27°C in an incubator. Pollen and sugar solutions were replaced every other day. Crithidia bombi reaches a representative level 7 days post-inoculation [47]. Thus, after 7 days, bees were dissected and C. bombi was counted as described in ‘Inoculum preparation’ above. Radial cell length from the right forewing was measured as a proxy for bee size [48] because previous work suggests bee size affects C. bombi cell counts [49]. We ultimately included a total of 650 worker bees (253 bees died, 37 escaped and 13 had damaged wings; see electronic supplementary material, table S1, for treatment sample sizes).
2.6. Statistical analysis
All statistical analyses and graphing were conducted using R version 3.3.1 [50]. To examine the effects of pollen treatment on C. bombi raw cell counts (cells per 0.02 µl), we used generalized mixed linear models. Owing to the nature of our zero bounded data, we first tested the residuals with a Poisson distribution and checked for over-dispersion. Finding that the data were over-dispersed, we analysed data with a negative binomial error distribution with a log link function using the package lme4 [51], and calculated least-squares means and standard errors with the package lsmeans [52]. We included pollen treatment as a fixed effect, bee size (estimated by radial cell length) as a covariate and date of inoculation and colony of origin as random effects. Upon finding a significant overall effect of pollen treatment, we compared differences among pollen treatments using a Tukey's HSD post hoc test. In a separate analysis, we asked whether pollen species differed in their ability to reduce C. bombi by pooling pollen treatments into their respective species, or genera in the case of Solidago (H. annuus, H. petiolarus, H. argophyllus, Solidago spp.). Pollen species instead of taxon was used as a fixed predictor, bee size as a covariate and inoculation date and colony of origin as random effects. We also asked whether hand-collected versus honeybee-collected pollen differed in the ability to reduce C. bombi cell counts using a similar analysis, but with collection method as the predictor instead of species, including all taxa in one analysis and a separate analysis only for the three taxa which were collected using both methods. Finally, we used a survival analysis with the package survminer [53] to examine whether pollen treatment affected B. impatiens mortality rates by comparing our model with and without pollen treatment as the predictor; the model also included bee size, date of inoculation and colony of origin. We removed 50 bees that escaped or had wing damage from our survival analysis. Figures were made with ggplot2 [54] and cowplot [55].
3. Results
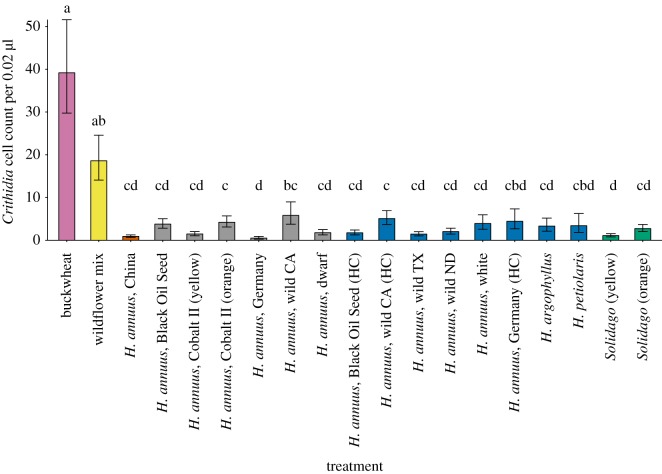
Crithidia bombi cell counts in B. impatiens were at least 90% lower in all Helianthus and Solidago pollen treatments compared to buckwheat pollen (figure 1). All but three taxa (H. annuus ‘Germany’ hand-collected, H. annuus, ‘wild California’ honeybee-collected and H. petiolaris) had at least 80% lower C. bombi cell counts than the wildflower pollen mix; these differences were significant. Some Asteraceae taxa, such as Solidago (yellow) and H. annuus ‘Germany’ (honeybee-collected) had significantly lower cell counts than others, such as H. annuus ‘wild California’ hand-collected and H. annuus ‘Cobalt II’ orange (figure 1). There was a negative relationship between bee size and C. bombi counts (, p < 0.001), such that larger bees had lower counts across all pollen taxa. In the survival analysis, neither pollen taxon (, p = 0.997) nor bee size (, p = 0.44) affected survival.
Figure 1.
Mean raw Crithidia count per 0.02 µl (±s.e.) for the 19 pollen taxa. Pollen treatments are: buckwheat (pink), wildflower mix (yellow), our positive control of H. annuus ‘China’ (orange), honeybee-collected taxa (grey), hand-collected taxa (blue) and Solidago spp. (green), which were honeybee-collected. Different letters associated with bars indicate statistically significant differences between pollen treatments after a post hoc Tukey's test. Full explanations for all taxa names are provided in electronic supplementary material, table S1; ‘HC’ refers to hand-collected for the three taxa where we had both honeybee and hand collection. Standard errors were calculated by back-transforming least-square means plus or minus least-square mean standard errors.
When we pooled taxa by species (H. annuus, H. petiolaris, H. argophyllus, Solidago spp.), species did not differ in their effects on C. bombi counts in a post hoc Tukey's HSD test. However, C. bombi cell counts in all pollen species were significantly lower than buckwheat and the wildflower pollen mix, by at least 60%.
In addition, we collected pollen both by hand and with honeybees for three taxa (H. annuus ‘Black Oil Seed’, ‘Germany’ and ‘wild California’), allowing us to make direct within-species comparisons between collection methods. There were significant effects of collection method on C. bombi cell counts in two of the three direct comparisons but in opposite directions. Honeybee collection had higher C. bombi cell counts relative to hand collection in H. annuus ‘Black Oil Seed’ (, p < 0.001, figure 2a) but lower C. bombi counts in H. annuus ‘Germany’ (, p = 0.012, figure 2b), and collection method had no effect in H. annuus, ‘wild California’ (, p = 0.5246, figure 2c). When the 17 Asteraceae pollen taxa were grouped by collection method in an overall comparison, we found no statistically significant difference between collection methods on C. bombi counts (, p = 0.33, figure 2d).
Figure 2.
Comparison of hand- versus honeybee-collected pollen for (a) H. annuus, ‘Black Oil Seed’, (b) H. annuus, Germany, (c) H. annuus, wild California and (d) comparison pooled across all Asteraceae taxa used in the experiment (17 treatments). Asterisks (*) denote statistically significant differences between collection method. Standard errors were calculated by back-transforming least-square means plus or minus least-square mean standard errors.
4. Discussion
Pollen from a wide variety of sunflowers reduced counts of the bumblebee gut pathogen C. bombi when compared to buckwheat pollen and wildflower mixed pollen. Bees fed Solidago spp. and Helianthus spp. pollens had 80–90% lower C. bombi cells compared to those that consumed buckwheat pollen. These results provide a much wider range of options for using sunflower pollen as a food supplement for managed bumblebees. Giacomini et al. [15] found that the intensity of C. bombi infection was lower in wild-caught workers when agricultural lands had more sunflower acreage. This study indicates that multiple sunflower cultivars or wild species could be used for pollen supplements or grown in pollinator-friendly plantings to help manage bee disease.
Although a wide range of sunflower pollen taxa dramatically reduced C. bombi infection in our study, sunflower pollen has low protein concentrations compared to other types of pollen [56]. Pollen with low protein can have multiple negative effects on bees, such as reducing hypopharyngeal gland size in honeybees [57], larval weight in Bombus terrestris [58], sweat bee offspring weight [59] and immune function in honeybees [60,61]. Although we found no differences in individual bee survival when fed sunflower, buckwheat or wildflower mixed pollen, we recommend that future work should compare the benefits and costs of sunflower pollen on bee performance, including reproduction, and ascertain the proportion of sunflower pollen in the diet that maximizes medicinal benefits while minimizing nutritional stress.
In the temperate regions of North America, Helianthus spp. and Solidago spp. are common native plants [36,62]. Because Solidago is in a different tribe than Helianthus [63] but was equally effective at reducing C. bombi, it is possible that medicinal pollen is broadly widespread in the Asteraceae. Because Asteraceae are common components of many habitats and often bloom in mid- to late summer in temperate North America, this result could have important implications. By reducing parasite infections, these plant species could reduce one of the stressors affecting bumblebee populations. In Bombus terrestris, high C. bombi infection is negatively correlated with daughter queen emergence in wild colonies [30], and high infections can reduce early colony development by 40% when queens emerge from hibernation in spring [10]. Because Solidago spp. and many Helianthus spp. bloom in late summer and autumn, infected daughter queens could have an advantage if they forage on these floral resources before entering winter hibernation.
Previous studies assessing medicinal effects of sunflower floral rewards could not determine whether medicinal effects were due to pollen or nectar because they used honeybee-collected sunflower pollen or sunflower honey [15,16], both of which contain nectar and pollen. We compared the medicinal effect of hand- versus honeybee-collected pollen to ascertain whether the likely mechanism is due to a component of pollen or nectar. Surprisingly, in comparisons of hand- and honeybee-collected pollen within taxa, we found opposite results for different taxa. Within our three comparisons, we found all possible results: honeybee-collected pollen resulted in more C. bombi (figure 2a), less C. bombi (figure 2b) or no difference (figure 2c) compared to hand-collected pollen. In a larger comparison including all taxa, most of which were collected with only one of the two methods, there was no significant difference (figure 2d). Because we did not consistently find that honeybee-collected pollen (which contains nectar) reduced C. bombi counts relative to hand-collected pollen (which does not contain nectar), overall our results suggest that the main mechanism of reduced infection is due to some component of pollen rather than nectar.
Although most of our taxa had yellowish-orange pollen typical of many species in the Helianthus and Solidago [64], some of our taxa produced pollen in distinct colours of yellow (Solidago spp., and H. annuus ‘Cobalt II’), orange (Solidago spp., H. annuus ‘Cobalt II’, and H. annuus ‘China’) or white (H. annuus ‘white’). Solidago spp. and H. annuus ‘Cobalt II’ produced both yellow- and orange-coloured pollen, which were separated into two treatments (electronic supplementary material, table S1). We hypothesized that pigments might play a role in C. bombi suppression, since pigments are known to be biologically active and affect herbivores and bacteria [65,66]. For example, in Petunia hybrid flowers with white and blue petal sections, the white part of the petal was consumed more than the blue part by two generalist caterpillars, and larvae gained more weight feeding on white than blue tissue [67]. We found no support for the hypothesis that pollen colour affects C. bombi counts, suggesting pigments did not play a significant role in suppression. Yellow and orange pollen did not differ within a taxon, and H. annuus with white pollen did not differ from taxa with yellow or orange pollen in reducing C. bombi (figure 1).
Furthermore, while our results clearly demonstrate a substantive effect, the mechanism by which sunflower pollen reduces parasitism is unknown. Future research should address whether the medicinal quality of sunflower pollen is due to secondary chemistry, nutritional components or another mechanism, such as physical attachment of pollen to the parasite or the gut wall, preventing C. bombi from adhering to the gut wall [68]. Previous studies have shown that nectar secondary chemistry suppresses C. bombi [46,69] and honeybee immunity can be stimulated by the ingestion of some honeys [70]. Pollen proteins could also play a role. For example, the ragweed (Ambrosia artemisiifolia) pollen coat proteins trigger histamine production in humans as a defence response [71]. Finally, Asteraceae pollen is notable for its spines on the outer coat [72]. Given that Crithidia is a gut parasite that attaches to the hindgut wall [68], sunflower pollen could reduce parasitism by scouring the hindgut of parasite cells. Future work is needed to determine whether H. annuus and Solidago spp. pollen contain immune stimulants that induce upregulation of genes that reduce infection.
In conclusion, we found that sunflower and goldenrod pollen dramatically reduced the parasite Crithidia bombi in Bombus impatiens, compared to both a single-species pollen control and wildflower pollen mix. This study suggests that in addition to using sunflower and goldenrod to manage bee health in agroecosystems, these native North American species could be incorporated into natural ecosystems to manage C. bombi infection. Future work should address how widespread this medicinal effect is across the Asteraceae and the breadth of this medicinal effect for additional bee species and pathogens to make responsible recommendations for management practices.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank E. Palmer-Young and C. Sutherland for statistical advice, BioBest laboratories for donating bumblebee colonies, E. Dobbs and K. Bell for coordinating and conducting the DNA analysis for pollen taxa, A. Roy, K. Stinson, A. Averill, K. M. Connolly and L. Figueroa for comments and edits on the manuscript, K. Skyrme, J. Parrott and O. Ben-Shir for assisting with honeybee colonies, T. Rothchild for sewing tent covers, L. Rieseberg for seed germination protocols, K. Michaud, D. Delany, C. Grincavitch, E. Mann, C. Sergi, A. Zhao, L. Metz, T. Shaya, P. Deneen, J. Day, B. Joyce and A. Turkle for field and laboratory assistance, and Messa Farm, Laurenitis Farm and the Rattlesnake Gutter Trust of the use of their land to obtain pollen.
Data accessibility
The datasets and R scripts supporting this article have been uploaded as part of the electronic supplementary material.
Authors' contributions
L.S.A. and R.E.I. conceived of the study with extensive design contributions from G.M.L. G.M.L. conducted the study and collected the data. G.M.L. analysed the data with assistance from L.A. G.M.L. wrote the manuscript with assistance from L.S.A. and all authors provided feedback on manuscript drafts.
Competing interests
We declare we have no competing interests.
Funding
The Lotta Crabtree foundation, Big Y Grocers, The Community Foundation of Western Massachusetts, USDA-AFRI 2013-02536 and USDA/CSREES MAS000411 and MAS00497 (Hatch) provided financial support. Any opinions, findings and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the funding agencies.
References
- 1.Kremen C, Williams NM, Thorp RW. 2002. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl Acad. Sci. USA 99, 16 812–16 816. ( 10.1073/pnas.262413599) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallai N, Salles J-M, Settele J, Vaissière BE. 2009. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 68, 810–821. ( 10.1016/j.ecolecon.2008.06.014) [DOI] [Google Scholar]
- 3.Biesmeijer JC, et al. 2006. Parallel declines in pollinators and insect-pollinated plants in Britain and the Netherlands. Science 313, 351–354. ( 10.1126/science.1127863) [DOI] [PubMed] [Google Scholar]
- 4.Ollerton J, Winfree R, Tarrant S. 2011. How many flowering plants are pollinated by animals? Oikos 120, 321–326. ( 10.1111/j.1600-0706.2010.18644.x) [DOI] [Google Scholar]
- 5.Colla SR, Packer L. 2008. Evidence for decline in eastern North American bumblebees (Hymenoptera: Apidae), with special focus on Bombus affinis Cresson. Biodivers. Conserv. 17, 1379–1391. ( 10.1007/s10531-008-9340-5) [DOI] [Google Scholar]
- 6.Potts SG, Biesmeijer JC, Kremen C, Neumann P, Schweiger O, Kunin WE. 2010. Global pollinator declines: trends, impacts and drivers. Trends Ecol. Evol. 25, 345–353. ( 10.1016/j.tree.2010.01.007) [DOI] [PubMed] [Google Scholar]
- 7.Cameron SA, Lozier JD, Strange JP, Koch JB, Cordes N, Solter LF, Griswold TL. 2011. Patterns of widespread decline in North American bumble bees. Proc. Natl Acad. Sci. USA 108, 662–667. ( 10.1073/pnas.1014743108) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goulson D, Nicholls E, Botías C, Rotheray EL. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347, 1255957 ( 10.1126/science.1255957) [DOI] [PubMed] [Google Scholar]
- 9.Vanbergen AJ. 2013. Threats to an ecosystem service: pressures on pollinators. Front. Ecol. Environ. 11, 251–259. ( 10.1890/120126) [DOI] [Google Scholar]
- 10.Brown MJ, Schmid-Hempel R, Schmid-Hempel P. 2003. Strong context-dependent virulence in a host–parasite system: reconciling genetic evidence with theory. J. Anim. Ecol. 72, 994–1002. ( 10.1046/j.1365-2656.2003.00770.x) [DOI] [Google Scholar]
- 11.Fauser A, Sandrock C, Neumann P, Sadd BM. 2017. Neonicotinoids override a parasite exposure impact on hibernation success of a key bumblebee pollinator: pesticides, parasites and bumblebee hibernation. Ecol. Entomol. 42, 306–314. ( 10.1111/een.12385) [DOI] [Google Scholar]
- 12.Nicolson SW. 2011. Bee food: the chemistry and nutritional value of nectar, pollen and mixtures of the two. Afr. Zool. 46, 197–204. ( 10.1080/15627020.2011.11407495) [DOI] [Google Scholar]
- 13.Carvell C, Meek WR, Pywell RF, Goulson D, Nowakowski M. 2007. Comparing the efficacy of agri-environment schemes to enhance bumble bee abundance and diversity on arable field margins. J. Appl. Ecol. 44, 29–40. ( 10.1111/j.1365-2664.2006.01249.x) [DOI] [Google Scholar]
- 14.Baracchi D, Brown MJF, Chittka L. 2015. Behavioural evidence for self-medication in bumblebees? F1000Res. 4, 73 ( 10.12688/f1000research.6262.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giacomini JJ, Leslie J, Tarpy DR, Palmer-Young EC, Irwin RE, Adler LS. 2018. Medicinal value of sunflower pollen against bee pathogens. Sci. Rep. 8, 14394 ( 10.1038/s41598-018-32681-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gherman BI, Denner A, Bobiş O, Dezmirean DS, Mărghitş LA, Schlüns H, Moritz RFA, Erler S. 2014. Pathogen-associated self-medication behavior in the honeybee Apis mellifera. Behav. Ecol. Sociobiol. 68, 1777–1784. ( 10.1007/s00265-014-1786-8) [DOI] [Google Scholar]
- 17.Praz CJ, Muller A, Dorn S. 2008. Specialized bees fail to develop on non-host pollen: do plants chemically protect their pollen? Ecology 89, 795–804. ( 10.1890/07-0751.1) [DOI] [PubMed] [Google Scholar]
- 18.Spear DM, Silverman S, Forrest JR, McPeek MA. 2016. Asteraceae pollen provisions protect Osmia mason bees (Hymenoptera: Megachilidae) from brood parasitism. Am. Nat. 187, 797–803. ( 10.1086/686241) [DOI] [PubMed] [Google Scholar]
- 19.Wood TJ, Roberts SPM. 2018. Constrained patterns of pollen use in Nearctic Andrena (Hymenoptera: Andrenidae) compared with their Palaearctic counterparts. Biol. J. Linn. Soc. 124, 732–746. ( 10.1093/biolinnean/bly080) [DOI] [Google Scholar]
- 20.Roulston TH, Cane JH. 2000. Pollen nutritional content and digestibility for animals. Plant Syst. Evol. 222, 187–209. ( 10.1007/BF00984102) [DOI] [Google Scholar]
- 21.Thorp RW. 1979. Structural, behavioral, and physiological adaptations of bees (Apoidea) for collecting pollen. Ann. Mo. Bot. Gard. 66, 788–812. ( 10.2307/2398919) [DOI] [Google Scholar]
- 22.Mărgăoan R, Mărghitaş LA, Dezmirean D, Mihai CM, Bobiş O. 2010. Bee collected pollen–general aspects and chemical composition. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 67, 254–259. [Google Scholar]
- 23.Standifer LN, McCaughey WF, Dixon SE, Gilliam M, Loper GM. 1980. Biochemistry and microbiology of pollen collected by honey bees (Apis mellifera L.) from almond, Prunus dulcis. II. Protein, amino acids and enzymes. Apidologie 11, 163–171. ( 10.1051/apido:19800206) [DOI] [Google Scholar]
- 24.Wilson EO. 1971. The insect societies. Cambridge, MA: Harvard University Press. [Google Scholar]
- 25.Cnaani J, Schmid-Hempel R, Schmidt JO. 2002. Colony development, larval development and worker reproduction in Bombus impatiens Cresson. Insectes Sociaux 49, 164–170. ( 10.1007/s00040-002-8297-8) [DOI] [Google Scholar]
- 26.Williams PH, Thorp RW, Richardson LL, Colla SR. 2014. Bumble bees of North America: an identification guide. Princeton, NJ: Princeton University Press. [Google Scholar]
- 27.Erler S, Popp M, Wolf S, Lattorff HMG. 2012. Sex, horizontal transmission, and multiple hosts prevent local adaptation of Crithidia bombi, a parasite of bumblebees (Bombus spp.). Ecol. Evol. 2, 930–940. ( 10.1002/ece3.250) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murray TE, Coffey MF, Kehoe E, Horgan FG. 2013. Pathogen prevalence in commercially reared bumble bees and evidence of spillover in conspecific populations. Biol. Conserv. 159, 269–276. ( 10.1016/j.biocon.2012.10.021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cordes N, Huang W-F, Strange JP, Cameron SA, Griswold TL, Lozier JD, Solter LF. 2012. Interspecific geographic distribution and variation of the pathogens Nosema bombi and Crithidia species in United States bumble bee populations. J. Invertebr. Pathol. 109, 209–216. ( 10.1016/j.jip.2011.11.005) [DOI] [PubMed] [Google Scholar]
- 30.Goulson D, O'Connor S, Park KJ. 2017. The impacts of predators and parasites on wild bumblebee colonies. Ecol. Entomol. 42, 168–181. ( 10.1111/een.12482) [DOI] [Google Scholar]
- 31.Gillespie S. 2010. Factors affecting parasite prevalence among wild bumblebees. Ecol. Entomol. 35, 737–747. ( 10.1111/j.1365-2311.2010.01234.x) [DOI] [Google Scholar]
- 32.Durrer S, Schmid-Hempel P. 1994. Shared use of flowers leads to horizontal pathogen transmission. Proc. R. Soc. Lond. B 258, 299–302. ( 10.1098/rspb.1994.0176) [DOI] [Google Scholar]
- 33.Imhoof B, Schmid-Hempel P. 1999. Colony success of the bumble bee, Bombus terrestris, in relation to infections by two protozoan parasites, Crithidia bombi and Nosema bombi . Insectes Sociaux 46, 233–238. ( 10.1007/s000400050139) [DOI] [Google Scholar]
- 34.Brown MJF, Loosli R, Schmid-Hempel P. 2000. Condition-dependent expression of virulence in a trypanosome infecting bumblebees. Oikos 91, 421–427. ( 10.1034/j.1600-0706.2000.910302.x) [DOI] [Google Scholar]
- 35.Otterstatter MC, Gegear RJ, Colla SR, Thomson JD. 2005. Effects of parasitic mites and protozoa on the flower constancy and foraging rate of bumble bees. Behav. Ecol. Sociobiol. 58, 383–389. ( 10.1007/s00265-005-0945-3) [DOI] [Google Scholar]
- 36.Reagon M, Snow AA. 2006. Cultivated Helianthus annuus (Asteraceae) volunteers as a genetic ‘bridge’ to weedy sunflower populations in North America. Am. J. Bot. 93, 127–133. ( 10.3732/ajb.93.1.127) [DOI] [Google Scholar]
- 37.USDA. 2016. Acreage report. Washington, DC: National Agricultural Statistics Service, Agricultural Statistics Board, US Department of Agriculture. [Google Scholar]
- 38.Wittkop B, Snowdon RJ, Friedt W. 2009. Status and perspectives of breeding for enhanced yield and quality of oilseed crops for Europe. Euphytica 170, 131–140. ( 10.1007/s10681-009-9940-5) [DOI] [Google Scholar]
- 39.Yang K, Wu D, Ye X, Liu D, Chen J, Sun P. 2013. Characterization of chemical composition of bee pollen in China. J. Agric. Food Chem. 61, 708–718. ( 10.1021/jf304056b) [DOI] [PubMed] [Google Scholar]
- 40.Human H, Nicolson SW, Strauss K, Pirk CWW, Dietemann V. 2007. Influence of pollen quality on ovarian development in honeybee workers (Apis mellifera scutellata). J. Insect Physiol. 53, 649–655. ( 10.1016/j.jinsphys.2007.04.002) [DOI] [PubMed] [Google Scholar]
- 41.Mallinger RE, Prasifka JR. 2017. Bee visitation rates to cultivated sunflowers increase with the amount and accessibility of nectar sugars. J. Appl. Entomol. 141, 561–573. ( 10.1111/jen.12375) [DOI] [Google Scholar]
- 42.Bell KL, Fowler J, Burgess KS, Dobbs EK, Gruenewald D, Lawley B, Morozumi C, Brosi BJ. 2017. Applying pollen DNA metabarcoding to the study of plant–pollinator interactions. Appl. Plant Sci. 5, 1600124 ( 10.3732/apps.1600124) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mateo R, Bosch-Reig F. 1997. Sugar profiles of Spanish unifloral honeys. Food Chem. 60, 33–41. ( 10.1016/S0308-8146(96)00297-X) [DOI] [PubMed] [Google Scholar]
- 44.Neff JL, Simpson BB. 1990. The roles of phenology and reward structure in the pollination biology of wild sunflower (Helianthus annuus L., Asteraceae). Isr. J. Bot. 39, 197–216. [Google Scholar]
- 45.Todd FE, Bretherick O. 1942. The composition of pollens. J. Econ. Entomol. 35, 312–317. ( 10.1093/jee/35.3.312) [DOI] [Google Scholar]
- 46.Richardson LL, Adler LS, Leonard AS, Andicoechea J, Regan KH, Anthony WE, Manson JS, Irwin RE. 2015. Secondary metabolites in floral nectar reduce parasite infections in bumble bees. Proc. R. Soc. B 282, 20142471 ( 10.1098/rspb.2014.2471) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Otterstatter MC, Thomson JD. 2006. Within-host dynamics of an intestinal pathogen of bumble bees. Parasitology 133, 749 ( 10.1017/S003118200600120X) [DOI] [PubMed] [Google Scholar]
- 48.Harder LD. 1982. Measurement and estimation of functional proboscis length in bumblebees (Hymenoptera: Apidae). Can. J. Zool. 60, 1073–1079. ( 10.1139/z82-148) [DOI] [Google Scholar]
- 49.Malfi RL, Roulston TH. 2014. Patterns of parasite infection in bumble bees (Bombus spp.) of Northern Virginia. Ecol. Entomol. 39, 17–29. ( 10.1111/een.12069) [DOI] [Google Scholar]
- 50.R Core Team. 2017. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 51.Bates D, Maechler M, Bolker B, Walker S. 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. ( 10.18637/jss.v067.i01) [DOI] [Google Scholar]
- 52.Lenth RV. 2016. Least-square means: the R package lsmeans. J. Stat. Softw. 69, 1–33. ( 10.18637/jss.v069.i01) [DOI] [Google Scholar]
- 53.Kassambara A, Kosinki M.. 2018. survminer: drawing survival curves using ‘ggplot2’. See https://CRAN.R-project.org/package=survminer.
- 54.Wickham H. 2009. Ggplot2: elegant graphics for data analysis. New York, NY: Springer. [Google Scholar]
- 55.Wilke CO. 2016. Cowplot: streamlined plot theme and plot annotations for ‘ggplot2’. R package.
- 56.Nicolson SW, Human H. 2013. Chemical composition of the ‘low quality’ pollen of sunflower (Helianthus annuus, Asteraceae). Apidologie 44, 144–152. ( 10.1007/s13592-012-0166-5) [DOI] [Google Scholar]
- 57.Pernal SF, Currie RW. 2000. Pollen quality of fresh and 1-year-old single pollen diets for worker honey bees (Apis mellifera L.). Apidologie 31, 387–409. ( 10.1051/apido:2000130) [DOI] [Google Scholar]
- 58.Tasei J-N, Aupinel P. 2008. Nutritive value of 15 single pollens and pollen mixes tested on larvae produced by bumblebee workers (Bombus terrestris, Hymenoptera: Apidae). Apidologie 39, 397–409. ( 10.1051/apido:2008017) [DOI] [Google Scholar]
- 59.Roulston TH, Cane JH. 2002. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 16, 49–65. ( 10.1023/A:1016048526475) [DOI] [Google Scholar]
- 60.Rasmont P, et al. 2005. Analysis of pollen and nectar of Arbutus unedo as a food source for Bombus terrestris (Hymenoptera: Apidae). J. Econ. Entomol. 98, 656–663. ( 10.1603/0022-0493-98.3.656) [DOI] [PubMed] [Google Scholar]
- 61.Brunner FS, Schmid-Hempel P, Barribeau SM. 2014. Protein-poor diet reduces host-specific immune gene expression in Bombus terrestris. Proc. R. Soc. B 281, 20140128 ( 10.1098/rspb.2014.0128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Werner PA, Gross RS, Bradbury IK. 1980. The biology of Canadian weeds: 45. Solidago canadensis L. Can. J. Plant Sci. 60, 1393–1409. ( 10.4141/cjps80-194) [DOI] [Google Scholar]
- 63.Bremer K. 1987. Tribal interrelationships of the Asteraceae. Cladistics 3, 210–253. ( 10.1111/j.1096-0031.1987.tb00509.x) [DOI] [PubMed] [Google Scholar]
- 64.Reiter R. 1947. The coloration of anther and corbicular pollen. Ohio J. Sci. 47, 137–152. [Google Scholar]
- 65.Gronquist M, Bezzerides A, Attygalle A, Meinwald J, Eisner M, Eisner T. 2001. Attractive and defensive functions of the ultraviolet pigments of a flower (Hypericum calycinum). Proc. Natl Acad. Sci. USA 98, 13 745–13 750. ( 10.1073/pnas.231471698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kagithoju S, Godishala V, Pabba SK, Kurra H, Nanna RS. 2012. Anti bacterial activity of flower extract of Pongamia pinnata Linn. an elite medicinal plant. Int. J. Pharm. Pharm. Sci 4, 130–132. [Google Scholar]
- 67.Johnson ET, Berhow MA, Dowd PF. 2008. Colored and white sectors from star-patterned petunia flowers display differential resistance to corn earworm and cabbage looper larvae. J. Chem. Ecol. 34, 757–765. ( 10.1007/s10886-008-9444-0) [DOI] [PubMed] [Google Scholar]
- 68.Gorbunov PS. 1996. Peculiarities of life cycle in flagellate Crithidia bombi (Protozoa, Trypanosomatidae). Zool. Zhurnal, 803–810.
- 69.Thorburn LP, Adler LS, Irwin RE, Palmer-Young EC. 2015. Variable effects of nicotine, anabasine, and their interactions on parasitized bumble bees. F1000Res. 4, 880 ( 10.12688/f1000research.6870.2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mao W, Schuler MA, Berenbaum MR. 2013. Honey constituents up-regulate detoxification and immunity genes in the western honey bee (Apis mellifera). Proc. Natl Acad. Sci. USA 110, 8842–8846. ( 10.1073/pnas.1303884110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munshi AH. 2000. Gene expression in allergenic pollen. Aerobiologia 16, 331–334. ( 10.1023/A:1026515820294) [DOI] [Google Scholar]
- 72.Blackmore S, Wortley AH, Skvarla JJ, Robinson H. 2009. Evolution of pollen in the compositae. In Systematics, evolution and biogeography of compositae, pp. 101–130. Vienna, Austria: IAPT. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and R scripts supporting this article have been uploaded as part of the electronic supplementary material.