Abstract
Second‐generation antipsychotics (SGAs) are recommended for maintenance treatment in schizophrenia. However, comparative long‐term effectiveness among SGAs is unclear. Here we provide a systematic review and meta‐analysis of randomized trials lasting ≥⃒6 months comparing SGAs head‐to‐head in schizophrenia and related disorders. The primary outcome was all‐cause discontinuation. Secondary outcomes included efficacy and tolerability, i.e., psychopathology, inefficacy‐related and intolerability‐related discontinuation, relapse, hospitalization, remission, functioning, quality of life, and adverse events. Pooled risk ratio and standardized mean difference were calculated using random‐effects models. Across 59 studies (N=45,787), lasting 47.4±32.1 weeks (range 24‐186), no consistent superiority of any SGA emerged across efficacy and tolerability outcomes. Regarding all‐cause discontinuation, clozapine, olanzapine and risperidone were significantly (p<0.05) superior to several other SGAs, while quetiapine was inferior to several other SGAs. As to psychopathology, clozapine and olanzapine were superior to several other SGAs, while quetiapine and ziprasidone were inferior to several other SGAs. Data for other efficacy outcomes were sparse. Regarding intolerability‐related discontinuation, risperidone was superior and clozapine was inferior to several other SGAs. Concerning weight gain, olanzapine was worse than all other compared non‐clozapine SGAs, and risperidone was significantly worse than several other SGAs. As to prolactin increase, risperidone and amisulpride were significantly worse than several other SGAs. Regarding parkinsonism, olanzapine was superior to risperidone, without significant differences pertaining to akathisia. Concerning sedation and somnolence, clozapine and quetiapine were significantly worse than some other SGAs. In summary, different long‐term SGA efficacy and tolerability patterns emerged. The long‐term risk‐benefit profiles of specific SGAs need to be tailored to individual patients to optimize maintenance treatment outcomes.
Keywords: Second‐generation antipsychotics, maintenance treatment, randomized controlled trials, treatment discontinuation, efficacy, tolerability, clozapine, olanzapine, risperidone
Schizophrenia is a mental disorder whose course is generally characterized by repeated relapses as well as a worsening of psychopathology and social functioning, thus requiring maintenance treatment1, 2, 3. Antipsychotics are efficacious for relapse prevention in chronic and first‐episode patients4, 5, reducing relapse risk by 2‐6‐fold versus no antipsychotic treatment2, 4, 5, 6.
A previous meta‐analysis by our group, comparing second‐generation antipsychotics (SGAs) with first‐generation antipsychotics (FGAs), found that the former as a class were superior to the latter regarding relapse prevention, all‐cause discontinuation and other relapse‐related outcomes3.
Despite the importance of long‐term treatment in schizophrenia, in which the magnitude of benefits and risks of medications may be different from acute phase treatment, no comprehensive meta‐analysis of the comparative long‐term effectiveness, efficacy and safety among oral SGAs currently exists7.
Although one meta‐analysis targeted maintenance trials that compared antipsychotics with placebo2, indirect comparisons using placebo as the common comparator are not conclusive8. Further, a multiple treatment meta‐analysis, which includes indirect comparisons, is not necessarily ideal, especially when the number of trials comparing antipsychotics directly is limited and when homogeneity of these trials cannot be assured9.
Knowledge about the comparative effectiveness, efficacy and tolerability of SGAs in the long‐term treatment of schizophrenia is important7. Specifically, differences in side effect risk9, 10, 11, some of which may increase with time, need to be weighed against potential differences in long‐term effectiveness and efficacy.
Here we report the results of the first comprehensive meta‐analysis of head‐to‐head randomized controlled trials comparing two or more SGAs in the long‐term treatment of schizophrenia, aiming to assess the comparative effectiveness, efficacy and safety of these medications.
METHODS
The meta‐analysis was performed following PRISMA guidelines12.
Search and inclusion criteria
We conducted an electronic search without language restrictions using MEDLINE/PubMed, the Cochrane library, ISI Web of Science, PsycINFO, CINAHL and the US National Institutes of Health clinical trials registry (http://www.clinicaltrials.gov). The following search terms were used: antipsychotic(s); neuroleptic(s); individual names of SGAs; schizophrenia; random, randomly, randomized; and maintenance, relapse, discontinuation or long‐term. The last search was done on October 29, 2018. The electronic search was supplemented by a hand search of reference lists of relevant studies and reviews. Authors and companies were contacted to provide missing information and unpublished data.
We included randomized, head‐to‐head comparisons of oral SGAs in adults with schizophrenia or schizoaffective disorder which reported on treatment discontinuation, whether randomization occurred during the acute or maintenance phase. As we aimed to focus on the comparative long‐term effectiveness of SGAs, we only included head‐to‐head studies lasting ≥⃒6 months.
We excluded studies with >20% of non‐schizophrenia/schizoaffective disorder patients. As long‐acting injectable formulation enhances the adherence and therefore has a significant impact on long‐term outcome13, 14, we excluded studies on long‐acting antipsychotics.
The search, selection of the literature, and data extraction were conducted independently by ≥⃒2 reviewers (KH, MN, TK, CC). Disagreements were resolved by consensus.
Outcomes
The primary outcome was all‐cause discontinuation at study endpoint.
Secondary outcomes included: a) psychopathology score change, measured by the Positive and Negative Syndrome Scale (PANSS), the Brief Psychiatric Rating Scale (BPRS) or the Clinical Global Impression ‐ Severity (CGI‐S) score (mixed models or last‐observation‐carried‐forward was prioritized over observed cases analysis); b) inefficacy‐related discontinuation (as reported by the original study authors); c) intolerability‐related discontinuation (as reported by the original study authors); d) relapse (as reported by the original study authors); e) hospitalization; f) remission (as reported by the original study authors); g) functioning score; h) quality of life (QOL); and i) adverse events.
Adverse events included: weight gain (as change from baseline or proportion of patients with clinically significant increase); prolactin increase (as change from baseline or proportion of patients with hyperprolactinemia); neuromotor adverse effects, including parkinsonism assessed with the Simpson‐Angus Rating Scale or use of anticholinergics, akathisia and dyskinesia; and sedation and/or somnolence.
Data analysis
SGAs were compared individually for each outcome. We applied a “once‐randomized‐analyzed” intent‐to‐treat (ITT) endpoint analysis. In studies that followed patients even after they were switched off the originally allocated medication during the study period, we analyzed the primary outcome based only on the first medication but, for secondary outcomes, we extracted and analyzed the data as reported in the ITT sample.
Pooled risk ratio (RR) and standardized mean difference (SMD) with 95% confidence intervals (CIs) were calculated using random‐effects models15. RR values <1 indicate superiority of the first SGA for negative outcomes (such as all‐cause discontinuation, relapse, inefficacy‐related and intolerability‐related discontinuation), while RR values >1 indicate superiority for the only positive outcome, remission. For simplicity we adjusted effect sizes, so that SMDs <0 indicate superiority of the first SGA, independent of whether a lower value (e.g., psychopathology) or higher value (e.g., functioning, QOL) is a positive outcome.
Number‐needed‐to‐treat (NNT) was calculated when categorical outcome differences were significant. Heterogeneity was only inspected when ≥⃒2 studies were analyzed, using the chi‐square test (p<0.1 indicating significant heterogeneity)16 and the I2 statistic (I2≥⃒50% indicating significant heterogeneity)17. For study quality assessment, we used the Jadad scale18, that provides a sum score for sensitivity analyses.
In addition, a priori‐defined subgroup analyses of the primary outcome were conducted (where ≥⃒2 studies existed), seeking to identify potential moderators, methodological biases, and whether findings extended to clinically relevant sub‐populations or treatment groups. Subgroup analyses included: a) randomization time point (acute vs. maintenance phase); b) sponsorship (medication‐specific sponsor vs. academia); c) study quality (high vs. low Jadad score)18; d) concealment (open or single‐blinded vs. double‐blinded); e) location (international/USA/Europe/Asia); f) dosing (fixed vs. flexible), and g) first episode vs. chronically ill.
Comprehensive Meta‐Analysis, version 3 (Biostat, NJ, USA) was used for all two‐tailed analyses, with alpha=0.05, without adjustments for multiple comparisons. Publication bias was assessed with the funnel plot, Egger's regression test19 and the “trim and fill” method20 for the primary outcome, whenever ≥⃒3 studies were analyzed.
RESULTS
Search and study characteristics
A total of 8,611 references were identified (Figure 1). After removing 152 duplicates, we excluded 7,823 of the remaining 8,459 references based on title/abstract inspection. Of 113 references subjected to full‐text inspection, 54 articles were dropped because of: inappropriate participants (N=17), review/editorial (N=11), no usable data (N=10), inappropriate medication (N=6), short‐term study (N=4), no/inadequate randomization (N=3), and meeting abstracts of already included studies (N=3).
Figure 1.
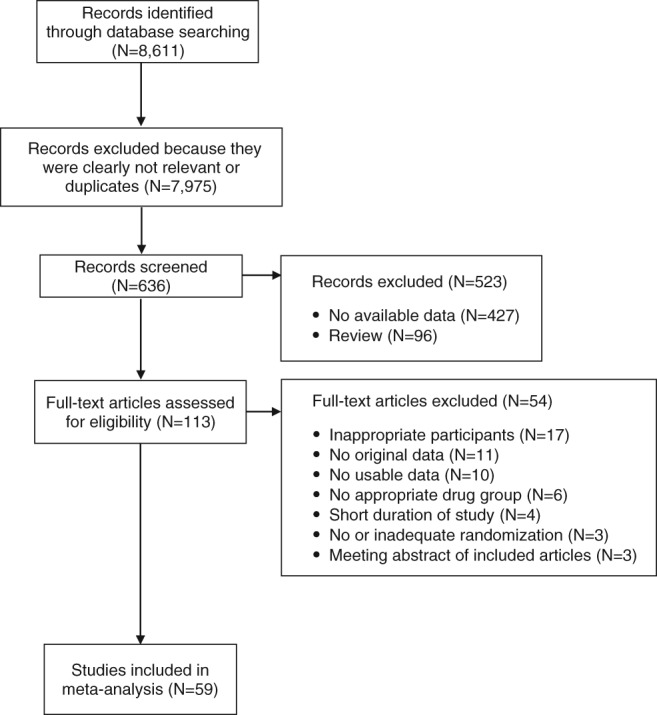
PRISMA flow chart
Altogether, we included 63 reports21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80, 81, 82, 83 (59 randomized studies) with 45,787 participants (median: 255 participants/study, range: from 12 to 18,154) (Table 1). The mean age of the population was 37.6±7.0 years; 62.1±13.3% were male and 61.1±28.8% were white. The mean study duration was 47.4±32.1 weeks (range: 24‐186).
Table 1.
Characteristics of included studies
| Study | Country | Blinding status | N. patients | Randomization time point | Duration (weeks) | First episode/chronically ill | Mean age | % male | Comparison | Dose (mean, mg/day) | Jadad score |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Addington et al21 | International | DB | 139 | Maintenance | 44 | Chronically ill | 34.6 | 65.5 | RIS vs. ZIP | 8; 114 | 3 |
| Alvarez et al22 | Spain | DB | 50 | Acute | 24 | Chronically ill | 38.4 | 70.0 | OLZ vs. ZIP | 15; 107.4 | 3 |
| Alvarez et al23;Ciudad et al24 | Spain | OL | 235 | Maintenance | 48 | Chronically ill | 36.5 | 72.3 | OLZ vs. RIS | 12.2; 4.9 | 2 |
| Breier et al25 | International | DB | 548 | Acute | 28 | Chronically ill | 39.2 | 64.2 | OLZ vs. ZIP | 15.27; 115.96 | 3 |
| Chan et al26 | Taiwan | RB | 60 | Acute | 24 | Chronically ill | 45.4 | 35.0 | OLZ vs. RIS | 4.1; 12.6 | 3 |
| Chrzanowski et al27 | International | OL | 214 | Acute | 52 | Chronically ill | 41.5 | 54.0 | APZ vs. OLZ | 22; 14.2 | 2 |
| Citrome et al28 | International | DB | 629 | Maintenance | 52 | Chronically ill | 41.7 | 69.0 | LUR vs. RIS | 84.7; 4.3 | 4 |
| Crespo‐Facorro et al29 | Spain | OL | 202 | Acute | 52 | First episode | 32.0 | 53.5 | APZ vs. QTP vs. ZIP | 11.6; 311.4; 61.0 | 3 |
| Crespo‐Facorro et al30 | Spain | OL | 174 | Acute | 156 | First episode | 27.3 | 62.1 | OLZ vs. RIS | 12.9; 3.4 | 1 |
| de Arce Cordón et al31;Gaebel et al32 | International | OL | 711 | Maintenance | 104 | Chronically ill | 41.6 | 57.8 | APZ vs. QTP | 15.1; 413.4 | 2 |
| Deberdt et al33 | USA | DB | 133 |
Maintenance (enriched design) |
26 | Chronically ill | 44.0 | NR | OLZ vs. QTP | 16.9; 439.7 | 3 |
| Durgam et al34 | International | DB | 120 | Acute | 26 | Chronically ill | 39.6 | 59.2 | ASN vs. OLZ |
Fixed dose: 5 or 10; 15 |
4 |
| Fleischhacker et al35 | International | DB | 488 | Acute | 46 | Chronically ill | 36.6 | 56.8 | APZ vs. OLZ | 23.0; 15.4 | 4 |
| Kahn et al36 | International | OL | 498 | Acute | 52 | First episode | 26.0 | 60.0 |
AMI vs. OLZ vs. QTP vs. ZIP |
450.8; 12.6; 498.6; 107.2 |
3 |
| Kane et al37 | International | DB | 566 | Acute | 28 | Chronically ill | 37.8 | 67.8 | APZ vs. OLZ | 19.3; 16.7 | 3 |
| Keefe et al38 | International | DB | 414 | Acute | 52 | Chronically ill | 39.1 | 71.3 | OLZ vs. RIS | 12.3; 5.2 | 3 |
| Kern et al39 | USA | OL | 255 | Acute | 2 6 | Chronically ill | 40.0 | 64.5 | APZ vs. OLZ | NR | 2 |
| Kinon et al40 | USA | DB | 346 | Acute | 24 | Chronically ill | 41.1 | 65.9 | OLZ vs. QTP | 15.6; 455.8 | 4 |
| Kinon et al41 | USA | DB | 394 | Acute | 24 | Chronically ill | 41.6 | 62.9 | OLZ vs. ZIP | Fixed dose:10 or 15 or 20;80 or 120 or 160 | 3 |
| Kishi et al42 | Japan | RB | 44 | Acute | 24 | Chronically ill | 39.5 | 40.9 | APZ vs. BLO | 11.5; 10.3 | 4 |
| Kumar et al43 | India | DB | 71 | Maintenance | 48 | Chronically ill | 40.7 | 50.7 | OLZ vs. RIS | 14.4; 5.8 | 3 |
| Lecrubier et al44 | France | DB | 244 | Maintenance | 26 | Chronically ill | 37.4 | 68.6 | AMI vs. OLZ |
Fixed dose; 150; 5 or 20 |
3 |
| Lieberman et al45 | USA | DB | 1,460 | Acute | 78 | Chronically ill | 40.6 | 72.3 |
OLZ vs. QTP vs. RIS vs. ZIP |
20.1; 543.4; 3.9;112.8 | 3 |
| Liu et al46 | China | OL | 80 | Acute | 52 | First episode | 29.5 | 0.00 | QTP vs. RIS | 420; 3.4 | 3 |
| Loebel et al47;NCT0078969848 | International | DB | 327 | Maintenance | 52 | Chronically ill | 37.6 | 66.8 | LUR vs. QTP | NR | 4 |
| McEvoy et al49 | USA | OL | 99 | Acute | 26 | Chronically ill | 39.7 | 81.0 | CLO vs. OLZ vs. QTPvs. RIS | 332.1; 23.4; 642.9;4.8 | 2 |
| McEvoy et al50 | USA | DB | 400 | Acute | 52 | First episode | 24.5 | 73.0 | OLZ vs. QTP vs. RIS | 11.7; 506; 2.4 | 3 |
| McQuade et al51 | International | DB | 317 | Acute | 26 | Chronically ill | 38.4 | 72.0 | APZ vs. OLZ | 25.1; 16.5 | 3 |
| Meltzer et al52 | International | RB | 980 | Acute | 104 | Chronically ill | 37.1 | 61.4 | CLO vs. OLZ | 274.2; 16.6 | 2 |
| Meltzer et al53 | USA | DB | 40 | Acute | 26 | Chronically ill | 36.8 | 67.5 | CLO vs. OLZ | 564; 33.6 | 4 |
| Mortimer et al54 | International | DB | 377 | Acute | 24 | Chronically ill | 37.8 | 65.0 | AMI vs. OLZ | 504; 13 | 5 |
| Naber et al55 | Germany | DB | 114 | Acute | 26 | Chronically ill | 34.0 | 61.0 | CLO vs. OLZ | 209; 16.2 | 3 |
| Naber et al56;NCT0060075657 | International | OL | 798 | Acute | 52 | Chronically ill | 39.7 | 58.2 | QTP vs. RIS | NR | 3 |
| Németh et al58 | International | DB | 461 | Maintenance | 26 | Chronically ill | 40.5 | 57.4 | CAR vs. RIS |
Fixed dose: 3 or 4 or 5 or 6; 3 or 4 or 6 |
5 |
| Noordsy et al59 | USA | DB | 107 | Maintenance | 24 | Chronically ill | 42.0 | 82.2 | OLZ vs. RIS |
Range: 2.5‐30; 1‐10 |
1 |
| Parabiaghi et al60 | Italy | OL | 300 | NR | 52 | Chronically ill | 42.7 | 58.0 | APZ vs. OLZ | 19.7; 13.7 | 3 |
| Purdon et al61 | Canada | DB | 65 | Maintenance | 54 | Chronically ill | 28.9 | 70.6 | OLZ vs. RIS | 11.00; 6.00 | 4 |
| Ritchie et al62 | Australia | OL | 66 | Acute | 186 | Chronically ill | 69.5 | 28.8 | OLZ vs. RIS | NR | 2 |
| Sanz‐Fuentenebroet al63 | Spain | OL | 30 | Acute | 52 | First episode | 24.5 | 70.0 | CLO vs. RIS | 220.45; 5.43 | 2 |
| Schnell et al64 | Germany | DB | 30 | NR | 52 | Chronically ill | 29.0 | 86.7 | CLO vs. ZIP | 225; 200 | 3 |
| Schoemaker et al65 | International | DB | 440 | Maintenance | 96 | Chronically ill | 36.9 | 55.5 | ASN vs. OLZ | 13.4; 13.4 | 3 |
| Schooler et al66 | USA | DB | 107 | Acute | 29 | Chronically ill | 41.9 | 79.4 | CLO vs. RIS | 456.7; 6.8 | 4 |
| Sechter et al67 | International | DB | 310 | Acute | 26 | Chronically ill | 38.4 | 55.0 | AMI vs. RIS | 683; 6.92 | 3 |
| Schreiner et al68 | International | OL | 459 | Acute | 26 | Chronically ill | 38.2 | 58.0 | OLZ vs. PAL | 11.6; 6.9 | 3 |
| Simpson et al69 | USA | DB | 126 | Maintenance | 26 | Chronically ill | NR | NR | OLZ vs. ZIP | 12.6; 135.2 | 2 |
| Strom et al70 | International | OL | 18,154 | Acute | 52 | Chronically ill | 41.1 | 55.0 | OLZ vs. ZIP | NR | 2 |
| Stroup et al71 | USA | DB | 444 | Acute | 26 | Chronically ill | 40.8 | 69.0 |
OLZ vs. QTP vs. RIS vs. ZIP |
20.5; 565.2; 4.1; 115.9 | 3 |
| Stroup et al72 | USA | DB | 115 | Acute | 78 | Chronically ill | 40.8 | 77.0 | OLZ vs. QTP vs. RIS | 20.7; 586.1; 3.7 | 3 |
| Thomas et al73 | International | OL | 9,809 | Acute |
Mean: 564.0; 489.6 days |
Chronically ill | 38.3 | 55.3 | RIS vs. SER |
Range: 2‐8; 12‐20 |
3 |
| Tran et al74 | International | DB | 339 | Acute | 28 | Chronically ill | 36.2 | 64.9 | OLZ vs. RIS | 17.2; 7.2 | 3 |
| Tunis et al75 | USA | OL | 450 | Acute | 52 | Chronically ill | 43.0 | 63.0 | OLZ vs. RIS | 13.49; 4.95 | 2 |
| Wani et al76 | India | OL | 62 |
Maintenance (enriched design) |
24 | Chronically ill | 29.8 | 62.9 | APZ vs. OLZ | NR | 1 |
| Zhang et al77 | China | OL | 254 | Acute | 52 | First episode | 26.4 | 61.0 | APZ vs. PAL vs. ZIP | NR | 2 |
| NCT0014549678 | International | DB | 468 | Maintenance | 26 | Chronically ill | 42.9 | 73.9 | ASN vs. OLZ | NR | 3 |
| NCT0020610279 | USA | OL | 1,098 | Maintenance | 104 | NR | NR | 58.8 | QTP vs. RIS |
Range: 200‐800; 2‐8 |
3 |
| NCT0021283680 | International | DB | 481 | Maintenance | 26 | Chronically ill | 40.5 | 68.2 | ASN vs. OLZ | NR | 2 |
| NCT0023637981 | International | DB | 59 | Maintenance | 24 | Chronically ill | 39.7 | NR | OLZ vs. RIS |
Range: 5‐20; 2‐6 |
3 |
| NCT0057328782 | USA | RB | 14 | Acute | 24 | First episode | 22.4 | 57.1 | CLO vs. RIS |
Range: 12.5‐100; 0.5‐5.0 |
1 |
| NCT0080210083 | USA | RB | 12 | Acute | 28 | Chronically ill | 29.0 | 61.9 | APZ vs. OLZ | NR | 2 |
AMI – amisulpride, APZ – aripiprazole, ASN – asenapine, BLO – blonanserin, CAR – cariprazine, CLO – clozapine, LUR – lurasidone, OLZ – olanzapine, PAL – paliperidone, QTP – quetiapine, RIS – risperidone, SER – sertindole, ZIP – ziprasidone, DB – double‐blind, OL – open label, RB – rater‐blinded, NR – not reported
Forty‐six studies included multiple‐episode patients, eight included exclusively first‐episode patients, four included exclusively treatment‐resistant patients (all clozapine studies), and one did not report the number of episodes of included patients79. Thirty‐four studies were double‐blind, 20 were open‐label, and five had masked raters. Forty studies were sponsored by pharmaceutical companies, 18 were publicly funded, and funding was uncertain in one study77.
The number of studies with each individual SGA were: 43 for olanzapine, 27 for risperidone, 15 for quetiapine, 12 for ziprasi‐done, 12 for aripiprazole, eight for clozapine, four for amisulpride, four for asenapine, two for lurasidone, two for paliperidone, one for blonanserin, one for cariprazine, and one for sertindole.
Thirty‐nine studies (66.1%) randomized patients in the acute phase, eighteen (30.5%) in the maintenance phase, while the randomization time point was uncertain for two studies (3.4%)60, 64. Two studies33, 76 utilized an enriched design, in that patients stabilized on drug A were randomized to continued treatment or switch to drug B. Two studies70, 75 had a “naturalistic” follow‐up design, in that switches off the originally assigned drugs were allowed.
Eleven studies reported on relapse, and six on remission. The definition of relapse varied, with only two studies using the same criteria28, 47. Three8, 31, 37 out of six studies reporting on remission used Andreasen et al's criteria84.
Primary outcome measure: all‐cause discontinuation
Across 59 studies, the pooled effect sizes of individual SGA pairs concerning all‐cause discontinuation are shown in Figure 2.
Figure 2.
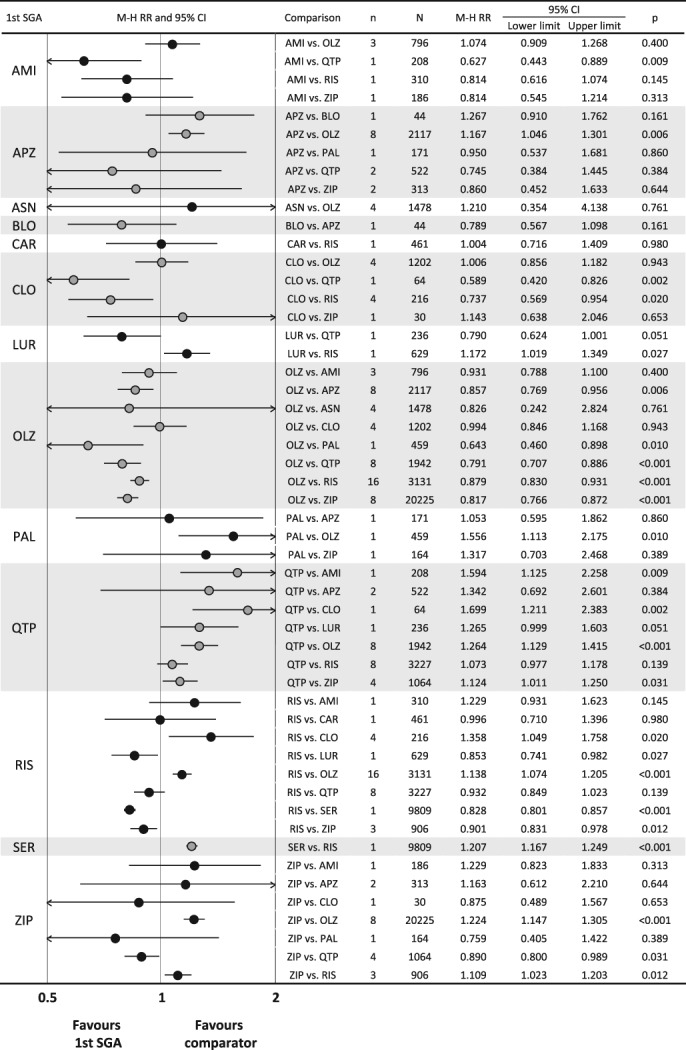
Results of comparisons of all‐cause discontinuation in meta‐analysis of second‐generation antipsychotics (SGAs). The first drug is the one written on the left side of the graph, and the comparator is written in the row of comparison. AMI – amisulpride, APZ – aripiprazole, ASN – asenapine, BLO – blonanserin, CAR – cariprazine, CLO – clozapine, LUR – lurasidone, OLZ – olanzapine, PAL – paliperidone, QTP – quetiapine, RIS – risperidone, SER – sertindole, ZIP – ziprasidone, M‐H RR – Mantel‐Haenszel risk ratio.
Clozapine had a significantly lower all‐cause discontinuation as compared with quetiapine (one study, N=64, RR=0.59, 95% CI: 0.42‐0.83, p=0.002) and risperidone (four studies, N=216, RR=0.74, 95% CI: 0.57‐0.95, p=0.020, I2=5.1%). Olanzapine had a significantly lower all‐cause discontinuation as compared with paliperidone (one study, N=459, RR=0.64, 95% CI: 0.46‐0.90, p=0.010), quetiapine (eight studies, N=1,942, RR=0.79, 95% CI: 0.71‐0.89, p<0.001, I2=55.8%), risperidone (16 studies, N=3,131, RR=0.88, 95% CI: 0.83‐0.93, p<0.001, I2=0.0%), and ziprasidone (eight studies, N=20,225, RR=0.82, 95% CI: 0.77‐0.87, p<0.001, I2=37.0%). Risperidone had a significantly lower all‐cause discontinuation as compared with sertindole (one study, N=9,809, RR=0.83, 95% CI: 0.80‐0.86, p<0.001) and ziprasidone (three studies, N=906, RR=0.90, 95% CI: 0.83‐0.98, p=0.012, I2=0.0%).
Other significant differences included the following: significantly lower all‐cause discontinuation for amisulpride vs. quetiapine (one study, N=208, RR=0.63, 95% CI: 0.44‐0.89, p=0.009); significantly higher all‐cause discontinuation for aripiprazole vs. olanzapine (eight studies, N=2,117, RR=1.17, 95% CI: 1.05‐1.30, p=0.006, I2=28.8%); significantly higher all‐cause discontinuation for lurasidone vs. risperidone (one study, N=629, RR=1.17, 95% CI: 1.02‐1.35, p=0.027); and significantly higher all‐cause discontinuation for quetiapine vs. ziprasidone (four studies, N=1,064, RR=1.12, 95% CI: 1.01‐1.25, p=0.031, I2=47.0%).
Secondary outcomes
Across 23 SGA comparisons concerning psychopathology, based on 32 studies, the following nine significant differences emerged: aripiprazole was superior to quetiapine and ziprasidone; clozapine was superior to quetiapine and risperidone; lurasidone was superior to quetiapine; olanzapine was superior to paliperidone and risperidone; and paliperidone was superior to aripiprazole and ziprasidone (Figure 3).
Figure 3.
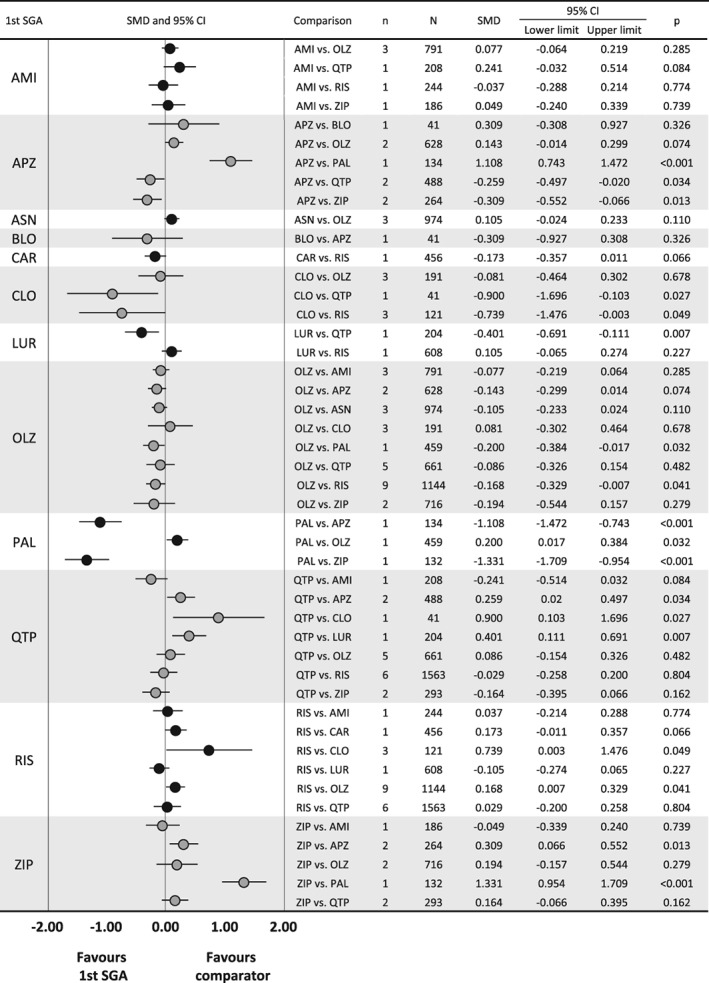
Results of comparisons of psychopathology scores in meta‐analysis of second‐generation antipsychotics (SGAs). The first drug is the one written on the left side of the graph, and the comparator is written in the row of comparison. AMI – amisulpride, APZ – aripiprazole, ASN – asenapine, BLO – blonanserin, CAR – cariprazine, CLO – clozapine, LUR – lurasidone, OLZ – olanzapine, PAL – paliperidone, QTP – quetiapine, RIS – risperidone, SER – sertindole, ZIP – ziprasidone, SMD – standardized mean difference.
Across 26 comparisons concerning intolerability‐related discontinuation, based on 50 studies, the following significant differences emerged: quetiapine was superior to amisulpride; risperidone was superior to clozapine, quetiapine and sertindole; and ziprasidone was superior to clozapine (Figure 4).
Figure 4.
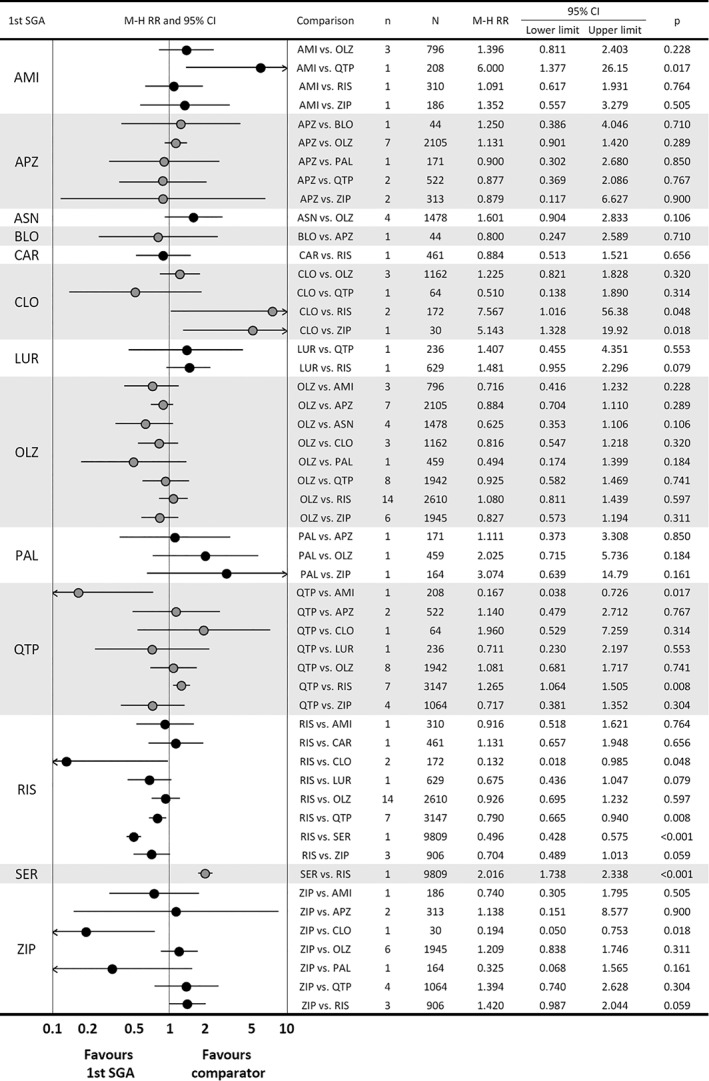
Results of comparisons of intolerability‐related discontinuation in meta‐analysis of second‐generation antipsychotics (SGAs). The first drug is the one written on the left side of the graph, and the comparator is written in the row of comparison. AMI – amisulpride, APZ – aripiprazole, ASN – asenapine, BLO – blonanserin, CAR – cariprazine, CLO – clozapine, LUR – lurasidone, OLZ – olanzapine, PAL – paliperidone, QTP – quetiapine, RIS – risperidone, SER – sertindole, ZIP – ziprasidone, M‐H RR – Mantel‐Haenszel risk ratio.
Across 20 comparisons concerning inefficacy‐related discontinuation, based on 47 studies, the following significant differences emerged: aripiprazole was superior to quetiapine; clozapine was superior to risperidone; lurasidone was superior to quetiapine; and olanzapine was superior to aripiprazole, quetiapine and ziprasidone (Figure 5).
Figure 5.
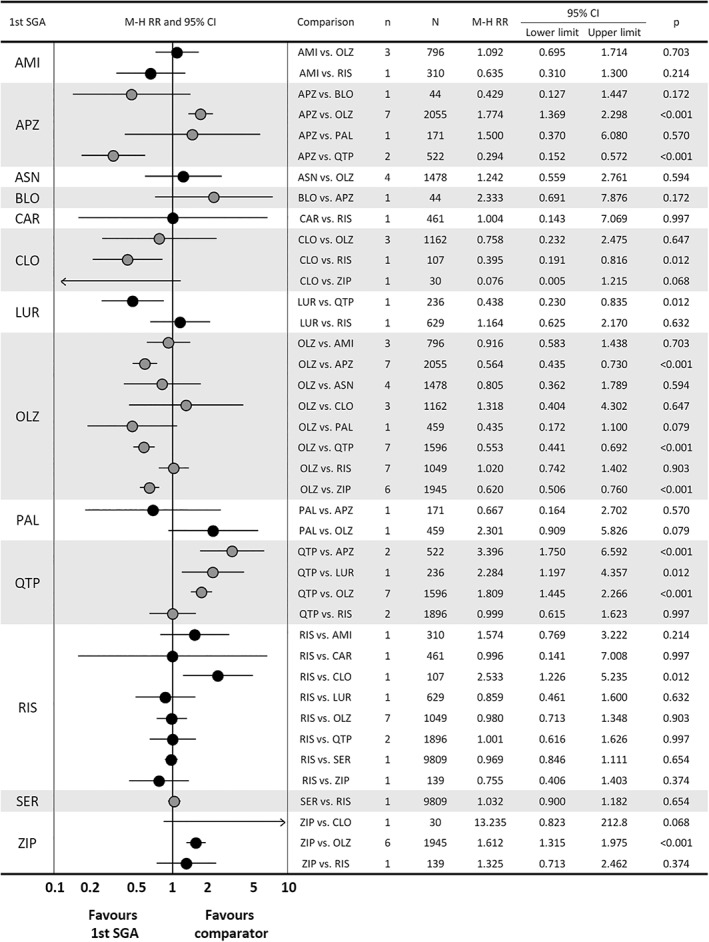
Results of comparisons of inefficacy‐related discontinuation in meta‐analysis of second‐generation antipsychotics (SGAs). The first drug is the one written on the left side of the graph, and the comparator is written in the row of comparison. AMI – amisulpride, APZ – aripiprazole, ASN – asenapine, BLO – blonanserin, CAR – cariprazine, CLO – clozapine, LUR – lurasidone, OLZ – olanzapine, PAL – paliperidone, QTP – quetiapine, RIS – risperidone, SER – sertindole, ZIP – ziprasidone, M‐H RR – Mantel‐Haenszel risk ratio.
Across 11 comparisons concerning relapse, only one significant difference emerged: the superiority of olanzapine over risperidone. Across 13 comparisons concerning hospitalization, clozapine was superior to olanzapine, and lurasidone and risperidone were superior to quetiapine. Across six comparisons concerning remission, lurasidone was superior to quetiapine, and quetiapine was superior to risperidone. Across 12 comparisons concerning functioning, aripiprazole was superior to quetiapine, cariprazine was superior to risperidone, and clozapine was superior to olanzapine. Across 11 comparisons concerning QOL, there were no significant SGA‐pair differences.
Twenty‐five comparisons based on 46 studies were meta‐analyzed for weight gain. Amisulpride, aripiprazole, quetiapine, risperidone, paliperidone and ziprasidone were superior to olanzapine; amisulpride, cariprazine, lurasidone and ziprasidone were superior to risperidone; paliperidone was superior to aripiprazole; and ziprasidone was superior to paliperidone and quetiapine (Table 2).
Table 2.
Results of meta‐analysis for adverse events
| Outcome | Comparison | n | N | RR/SMD | 95% CI | p | I 2 (%) | |
|---|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | |||||||
| Akathisia | ASN vs. OLZ | 1 | 89 | –0.21 | –2.00 | 1.58 | 0.818 | – |
| CAR vs. RIS | 1 | 460 | 0.15 | –0.18 | 0.49 | 0.361 | – | |
| CLO vs. OLZ | 1 | 58 | 0.44 | –1.26 | 2.14 | 0.614 | – | |
| CLO vs. QTP | 1 | 54 | –0.97 | –2.03 | 0.08 | 0.071 | – | |
| CLO vs. RIS | 1 | 54 | 0.30 | –1.41 | 2.00 | 0.735 | – | |
| LUR vs. RIS | 1 | 608 | 0.13 | –0.04 | 0.30 | 0.131 | – | |
| OLZ vs. QTP | 2 | 201 | –0.46 | –1.66 | 0.75 | 0.459 | 51.2 | |
| OLZ vs. RIS | 3 | 548 | –0.08 | –0.32 | 0.17 | 0.552 | 17.2 | |
| OLZ vs. ZIP | 2 | 725 | –0.11 | –0.28 | 0.05 | 0.184 | 0.0 | |
| QTP vs. RIS | 3 | 1277 | 0.16 | –0.56 | 0.89 | 0.657 | 65.4 | |
| QTP vs. ZIP | 1 | 190 | 0.26 | –0.42 | 0.93 | 0.458 | – | |
| RIS vs. ZIP | 1 | 193 | –0.17 | –0.97 | 0.64 | 0.683 | – | |
| Dyskinesia | AMI vs. OLZ | 1 | 356 | –0.11 | –0.32 | 0.09 | 0.281 | – |
| AMI vs. RIS | 1 | 310 | 0.02 | –0.21 | 0.24 | 0.886 | – | |
| ASN vs. OLZ | 1 | 89 | –1.46 | –3.25 | 0.33 | 0.109 | – | |
| CLO vs. OLZ | 2 | 88 | –0.21 | –0.71 | 0.29 | 0.416 | 0.0 | |
| CLO vs. QTP | 1 | 44 | 0.47 | –0.76 | 1.69 | 0.456 | – | |
| CLO vs. RIS | 1 | 45 | 1.01 | –0.61 | 2.64 | 0.222 | – | |
| OLZ vs. QTP | 3 | 234 | –0.35 | –0.76 | 0.07 | 0.099 | 0.0 | |
| OLZ vs. RIS | 7 | 698 | –0.02 | –0.19 | 0.15 | 0.790 | 0.0 | |
| OLZ vs. ZIP | 2 | 701 | –0.03 | –0.19 | 0.13 | 0.726 | 0.0 | |
| QTP vs. RIS | 4 | 1,301 | 0.23 | –0.28 | 0.74 | 0.375 | 58.8 | |
| QTP vs. ZIP | 1 | 165 | 0.52 | 0.05 | 0.99 | 0.030 | – | |
| RIS vs. ZIP | 1 | 156 | 0.10 | –0.44 | 0.65 | 0.709 | – | |
| Parkinsonism | AMI vs. OLZ | 2 | 562 | 0.26 | –0.34 | 0.86 | 0.399 | 77.6 |
| AMI vs. QTP | 1 | 179 | 0.30 | –0.18 | 0.79 | 0.219 | – | |
| AMI vs. RIS | 1 | 310 | 0.07 | –0.15 | 0.29 | 0.539 | – | |
| AMI vs. ZIP | 1 | 162 | 0.03 | –0.43 | 0.50 | 0.887 | – | |
| APZ vs. BLO | 1 | 44 | –0.41 | –1.74 | 0.92 | 0.546 | – | |
| APZ vs. OLZ | 3 | 1,483 | 0.06 | –0.27 | 0.38 | 0.737 | 76.5 | |
| APZ vs. QTP | 2 | 497 | –0.10 | –0.45 | 0.25 | 0.585 | 26.6 | |
| APZ vs. ZIP | 1 | 124 | –0.07 | –0.57 | 0.43 | 0.776 | – | |
| ASN vs. OLZ | 2 | 529 | 0.08 | –0.90 | 1.06 | 0.867 | 16.0 | |
| CAR vs. RIS | 1 | 460 | –0.23 | –0.61 | 0.15 | 0.233 | – | |
| CLO vs. OLZ | 3 | 201 | 0.13 | –0.18 | 0.45 | 0.402 | 0.0 | |
| CLO vs. QTP | 1 | 53 | –0.75 | –1.90 | 0.40 | 0.200 | – | |
| CLO vs. RIS | 1 | 54 | 0.30 | –1.41 | 2.00 | 0.735 | – | |
| LUR vs. RIS | 1 | 621 | –0.19 | –0.46 | 0.08 | 0.169 | – | |
| OLZ vs. QTP | 5 | 1,126 | –0.08 | –0.51 | 0.36 | 0.725 | 51.7 | |
| OLZ vs. RIS | 9 | 1,934 | –0.28 | –0.44 | –0.12 | 0.001 | 28.3 | |
| OLZ vs. ZIP | 5 | 1,808 | –0.10 | –0.23 | 0.03 | 0.129 | 0.0 | |
| QTP vs. RIS | 4 | 1,953 | –0.26 | –0.60 | 0.08 | 0.133 | 60.5 | |
| QTP vs. ZIP | 4 | 971 | –0.19 | –0.55 | 0.18 | 0.323 | 44.1 | |
| RIS vs. ZIP | 2 | 725 | 0.40 | –0.23 | 1.03 | 0.214 | 66.6 | |
| Body weight gain | AMI vs. OLZ | 3 | 742 | –0.40 | –0.54 | –0.25 | <0.001 | 0.0 |
| AMI vs. QTP | 1 | 127 | –0.06 | –0.41 | 0.29 | 0.749 | – | |
| AMI vs. RIS | 1 | 195 | –0.46 | –0.83 | –0.10 | 0.013 | – | |
| AMI vs. ZIP | 1 | 115 | 0.36 | –0.02 | 0.74 | 0.066 | – | |
| APZ vs. OLZ | 5 | 1,413 | –0.63 | –0.81 | –0.44 | <0.001 | 31.7 | |
| APZ vs. PAL | 1 | 134 | 0.37 | 0.03 | 0.71 | 0.034 | – | |
| APZ vs. QTP | 2 | 501 | –0.06 | –0.47 | 0.35 | 0.774 | 53.5 | |
| APZ vs. ZIP | 2 | 264 | 0.63 | –0.07 | 1.32 | 0.077 | 82.3 | |
| APZ vs. BLO | 1 | 44 | 0.09 | –0.50 | 0.68 | 0.770 | – | |
| ASN vs. OLZ | 4 | 1,447 | –0.39 | –0.86 | 0.08 | 0.107 | 88.0 | |
| CAR vs. RIS | 1 | 431 | –0.29 | –0.48 | –0.10 | 0.003 | – | |
| CLO vs. OLZ | 4 | 1,167 | –0.33 | –0.80 | 0.13 | 0.161 | 83.0 | |
| CLO vs. QTP | 1 | 54 | 0.02 | –0.61 | 0.64 | 0.957 | – | |
| CLO vs. RIS | 3 | 96 | –0.32 | –0.78 | 0.14 | 0.172 | 0.0 | |
| LUR vs. QTP | 1 | 111 | –0.13 | –0.54 | 0.28 | 0.526 | – | |
| LUR vs. RIS | 1 | 621 | –0.48 | –0.65 | –0.31 | <0.001 | – | |
| OLZ vs. PAL | 1 | 449 | 0.49 | 0.31 | 0.68 | <0.001 | – | |
| OLZ vs. QTP | 8 | 1,592 | 0.42 | 0.21 | 0.62 | <0.001 | 69.1 | |
| OLZ vs. RIS | 11 | 1,646 | 0.37 | 0.19 | 0.55 | <0.001 | 58.5 | |
| OLZ vs. ZIP | 6 | 1,509 | 0.74 | 0.62 | 0.85 | <0.001 | 9.6 | |
| PAL vs. ZIP | 1 | 132 | 0.62 | 0.27 | 0.97 | 0.001 | – | |
| QTP vs. RIS | 8 | 2,813 | 0.01 | –0.06 | 0.09 | 0.701 | 0.0 | |
| QTP vs. ZIP | 4 | 871 | 0.24 | 0.10 | 0.38 | 0.001 | 0.0 | |
| RIS vs. SER | 1 | 9,809 | –0.61 | –2.37 | 1.16 | 0.501 | – | |
| RIS vs. ZIP | 3 | 800 | 0.22 | 0.07 | 0.37 | 0.003 | 0.0 | |
| Prolactin increase | AMI vs. OLZ | 1 | 105 | 0.63 | 0.24 | 1.03 | 0.002 | – |
| AMI vs. QTP | 1 | 84 | 0.62 | 0.18 | 1.07 | 0.006 | – | |
| AMI vs. ZIP | 1 | 71 | 1.05 | 0.53 | 1.57 | <0.001 | – | |
| APZ vs. OLZ | 4 | 1,686 | –1.09 | –1.63 | –0.54 | <0.001 | 84.4 | |
| APZ vs. QTP | 1 | 382 | –0.23 | –1.83 | 1.38 | 0.783 | – | |
| ASN vs. OLZ | 1 | 89 | 0.07 | –0.47 | 0.61 | 0.804 | – | |
| CLO vs. OLZ | 1 | 55 | –0.29 | –0.87 | 0.30 | 0.333 | – | |
| CLO vs. QTP | 1 | 52 | 0.39 | –0.24 | 1.02 | 0.229 | – | |
| CLO vs. RIS | 1 | 50 | –1.62 | –2.36 | –0.88 | <0.001 | – | |
| LUR vs. RIS | 1 | 554 | –0.56 | –0.74 | –0.38 | <0.001 | – | |
| OLZ vs. QTP | 6 | 996 | 0.13 | 0.01 | 0.26 | 0.040 | 0.0 | |
| OLZ vs. RIS | 7 | 1,225 | –1.05 | –1.23 | –0.87 | <0.001 | 40.7 | |
| OLZ vs. ZIP | 5 | 1,510 | 0.06 | –0.16 | 0.27 | 0.596 | 73.1 | |
| QTP vs. RIS | 8 | 2,131 | –1.24 | –1.59 | –0.90 | <0.001 | 84.9 | |
| QTP vs. ZIP | 3 | 659 | 0.03 | –0.41 | 0.47 | 0.890 | 82.9 | |
| RIS vs. SER | 1 | 9,809 | 0.00 | –0.88 | 0.88 | 1.000 | – | |
| RIS vs. ZIP | 2 | 596 | 0.93 | 0.75 | 1.10 | <0.001 | 0.0 | |
| Sedation and/or somnolence | AMI vs. OLZ | 1 | 377 | 0.99 | 0.46 | 2.16 | 0.989 | – |
| AMI vs. RIS | 1 | 310 | 0.69 | 0.29 | 1.65 | 0.407 | – | |
| APZ vs. BLO | 1 | 44 | 0.50 | 0.05 | 5.12 | 0.559 | – | |
| APZ vs. OLZ | 5 | 1,802 | 0.64 | 0.38 | 1.09 | 0.099 | 68.0 | |
| APZ vs. QTP | 1 | 119 | 1.39 | 0.60 | 3.24 | 0.442 | – | |
| APZ vs. ZIP | 1 | 124 | 1.34 | 0.60 | 3.00 | 0.479 | – | |
| ASN vs. OLZ | 3 | 1,038 | 0.89 | 0.66 | 1.22 | 0.477 | 0.0 | |
| CAR vs. RIS | 1 | 460 | 0.69 | 0.30 | 1.59 | 0.385 | – | |
| CLO vs. OLZ | 1 | 956 | 1.86 | 1.54 | 2.23 | <0.001 | – | |
| CLO vs. RIS | 1 | 14 | 5.00 | 0.77 | 32.57 | 0.092 | – | |
| LUR vs. RIS | 1 | 621 | 0.76 | 0.52 | 1.12 | 0.166 | – | |
| OLZ vs. PAL | 1 | 459 | 2.85 | 1.29 | 6.31 | 0.010 | – | |
| OLZ vs. QTP | 4 | 1,220 | 0.95 | 0.83 | 1.10 | 0.531 | 0.0 | |
| OLZ vs. RIS | 7 | 1,656 | 1.14 | 0.99 | 1.32 | 0.064 | 0.0 | |
| OLZ vs. ZIP | 2 | 766 | 1.78 | 0.84 | 3.75 | 0.130 | 79.5 | |
| QTP vs. RIS | 6 | 3,095 | 1.46 | 1.09 | 1.96 | 0.010 | 78.1 | |
| QTP vs. ZIP | 3 | 861 | 1.49 | 0.89 | 2.48 | 0.129 | 56.7 | |
| RIS vs. ZIP | 3 | 906 | 1.35 | 0.94 | 1.95 | 0.104 | 41.4 | |
Significant (p<0.05) results are in bold prints. RR – risk ratio, SMD – standardized mean difference, AMI – amisulpride, APZ – aripiprazole, ASN – asenapine, BLO – blonanserin, CAR – cariprazine, CLO – clozapine, LUR – lurasidone, OLZ – olanzapine, PAL – paliperidone, QTP – quetiapine, RIS – risperidone, SER – sertindole, ZIP – ziprasidone. Effect sizes for sedation and/or somnolence are expressed in RR, others in SMD. SMD <0 and RR<1 indicate superiority of the first medication.
Prolactin increase was meta‐analyzed in 16 comparisons based on 21 studies. Clozapine, lurasidone, olanzapine, quetiapine and ziprasidone were superior to risperidone; aripiprazole and quetiapine were superior to olanzapine; olanzapine, quetiapine and ziprasidone were superior to amisulpride (Table 2).
Parkinsonism was meta‐analyzed in 20 comparisons based on 28 studies: olanzapine was superior to risperidone. Dyskinesia was meta‐analyzed in 11 comparisons based on 13 studies: ziprasidone was superior to quetiapine. Akathisia was meta‐analyzed in 11 comparisons based on 9 studies: no significant differences emerged. Sedation and/or somnolence were meta‐analyzed in 17 comparisons based on 27 studies: olanzapine and paliperidone were superior to clozapine, and risperidone was superior to quetiapine.
Subgroup analyses for primary outcome
In subgroup analyses, the significance of the primary results was altered in 49/267 (18.4%) analyses, but most subgroups were very small both in number of studies and patients. Comparative effectiveness patterns were mostly consistent in high‐quality studies and double‐blind trials.
Regarding industry sponsorship, results showing a specific drug's inferiority were neutralized when three of 43 medication‐specific manufacturer‐sponsored studies were included. In contrast, one outcome showing superiority of olanzapine was neutralized when one manufacturer‐funded study was included.
Regarding blinding, some results changed when we restricted the analyses to open label or blinded studies. Restricting the analyses to only blinded studies, 5/39 results that showed statistical significance became non‐significant. Restricting the analyses to only open label studies, 1/39 non‐significant results became statistically significant.
None of the other potential effect‐moderators addressed in subgroup analyses revealed a clear pattern of effect. There were no subgroup analyses in which the direction of the results was reversed.
Publication bias
Publication bias for all‐cause discontinuation was assessed by funnel plot. In nine of eleven comparisons with ≥⃒3 studies, the funnel plot was asymmetrical. Subsequently, we applied the trim‐and‐fill method to adjust for potential publication bias, and found that the effect sizes were similar after adjustment, and that the significance for RRs did not change, except for two comparisons. Quetiapine was not different in observed values but became inferior to risperidone in adjusted values (original RR=1.07, 95% CI: 0.98‐1.18; adjusted RR=1.11, 95% CI: 1.00‐1.24). Quetiapine was significantly inferior in observed values, but became not different from ziprasidone in adjusted values (original RR=1.12, 95% CI: 1.01‐1.25; adjusted RR=1.08, 95% CI: 0.98‐1.19).
DISCUSSION
In this first comprehensive meta‐analysis of comparative effectiveness, efficacy and tolerability of SGAs in the long‐term treatment of schizophrenia, including 59 studies and 45,787 participants, no consistent superiority of any single antipsychotic across multiple outcome domains was observed.
Regarding all‐cause discontinuation, clozapine, olanzapine and risperidone were superior to several other SGAs, whereas quetiapine was inferior to several other SGAs. Regarding psychopathology, clozapine and olanzapine were superior to several other SGAs, while again quetiapine as well as ziprasidone were inferior to several other SGAs. Regarding functioning, QOL and remission, data were sparse.
Regarding intolerability‐related discontinuation, risperidone was superior and clozapine was inferior to several other SGAs. However, it should be kept in mind that discontinuation due to adverse events often includes inefficacy‐related adverse events in modern trials and, therefore, this outcome does not purely reflect tolerability.
When broken down into individual adverse events, superiority/inferiority patterns became clearer in some domains. For example, olanzapine was associated with more body weight gain than all other non‐clozapine SGAs, whereas ziprasidone was less so than other SGAs; and amisulpride and risperidone raised serum prolactin level more than other SGAs. Furthermore, sedation and/or somnolence were more common during long‐term treatment with clozapine and quetiapine.
We focused on head‐to‐head comparisons for the current meta‐analysis. The relative lack of direct head‐to‐head maintenance comparisons may raise interest in conducting a network meta‐analysis. However, while such methodology using indirect comparisons can create rankings, the very lack of so many comparisons and the heterogeneity of the studies conducted in different populations and over several decades are likely to introduce relevant biases that are not present in meta‐analyses of direct head‐to‐head trials9.
In fact, comparing our results with those from Zhao et al85, who conducted a network meta‐analysis of relapse prevention studies in stable patients with schizophrenia that also included first‐generation and long‐acting injectable antipsychotics, some differences emerge. For example, for relapse prevention, the only significant result involving an SGA was olanzapine's superiority over chlorpromazine and haloperidol, whereas we found olanzapine to be superior to risperidone (although based on one trial only). Furthermore, regarding all‐cause discontinuation, we observed a significant superiority of olanzapine over aripiprazole, paliperidone, quetiapine, risperidone and ziprasidone in direct comparisons, while Zhao et al, including indirect comparisons, found olanzapine only superior to aripiprazole. Thus, we believe that restricting the meta‐analysis exclusively to randomized head‐to‐head comparisons yields more precise results.
What are the implications of our findings for the choice of SGA in the long‐term treatment of schizophrenia? First, we must consider the magnitude of the effect sizes for all‐cause discontinuation. Since these ranged from medium to large, we believe that they are clinically meaningful, especially during the important maintenance treatment phase2, 7, 86, 87. The results regarding psychopathology roughly matched the findings for all‐cause discontinuation, in that clozapine and olanzapine were superior to several other SGAs, whereas quetiapine seemed inferior, this time together with ziprasidone. However, the findings of divergent adverse effect outcomes, with particular disadvantages for clozapine, olanzapine and risperidone, highlight the fact that it is crucial to not view efficacy and effectiveness in isolation of tolerability. For example, clozapine and olanzapine are among the medications with some of the most problematic adverse effects, including weight gain and metabolic abnormalities10, 88 as well as, in the case of clozapine, blood dyscrasias89. Given such inconsistent results in the different outcome categories, the importance of a balanced medication choice based on each patient's own situation should be emphasized.
Regarding the comparative effectiveness of clozapine and olanzapine, we found similar results in the maintenance treatment of schizophrenia. Even in studies targeting treatment‐refractory patients, the effect sizes were similar. Since a network meta‐analysis of short‐term trials in refractory patients did not find superiority of clozapine vs. olanzapine, risperidone and ziprasidone90, which may have been driven by use of suboptimal clozapine doses or inclusion of non‐refractory patients, further high‐quality, short‐ and long‐term, head‐to‐head trials of clozapine vs. other SGAs are needed.
Several limitations of this study need to be considered. Most comparisons relied on relatively few head‐to‐head trials. As many as 139 of all 250 comparisons were based on one study only, but we only meta‐analyzed outcomes for which at least two head‐to‐head trials provided data. The number of patients per trial was also often small, and dose equivalencies used across studies might not have been balanced or consistent. Furthermore, the limited number of studies reduced the power of our exploratory subgroup analyses. Additionally, only six and eleven studies reported remission and relapse as an outcome, respectively. However, since psychopathology, treatment response and functioning can worsen with repeated relapse87, 91, information on comparative remission and relapse risk with individual antipsychotics is important.
The randomization point in the included studies differed, i.e., some studies randomized patients during the acute phase, and others during the maintenance phase. Moreover, some studies included exclusively treatment‐refractory patients, whereas some others included exclusively first‐episode patients. Relapse and remission definitions varied across studies. Moreover, two of the included studies had an enriched design, and two allowed switches after randomization, which could have affected the results. Such heterogeneity of the study design as well as patient populations introduces biases. However, we assessed the impact of patient and study design characteristics as potential moderators by conducting subgroup analyses.
Finally, although the effectiveness of long‐acting injectable antipsychotics (LAIs) in the long‐term treatment of schizophrenia is clearly important92, we excluded LAI studies, as this aspect has already been comprehensively meta‐analyzed13, 14, 93. Including LAIs in this meta‐analysis, which are not available for all SGAs, would have further increased the heterogeneity of samples and methods, the complexity of the analyses and the interpretation of the results.
In conclusion, results from this meta‐analysis suggest that there are some significant differences in the effectiveness, efficacy and tolerability among SGAs in the long‐term treatment of schizophrenia. Clozapine, olanzapine and risperidone seem to be superior to several other SGAs regarding all‐cause discontinuation, while quetiapine seems to be inferior. Regarding psychopathology scores, clozapine and olanzapine seem to be superior to several other SGAs, while quetiapine and ziprasidone seem to be less effective. Regarding discontinuation due to adverse events, only risperidone was superior and clozapine was inferior to several other SGAs.
Due to the limited number of head‐to‐head trials, the comparative effectiveness of some SGAs is unclear, and results need to be interpreted cautiously whenever they were based on few trials. Thus, a sufficiently larger database involving many SGAs and including detailed effectiveness and tolerability outcomes is desirable to further guide the evidence‐based long‐term treatment of patients with schizophrenia. In particular, identifying predictors of beneficial outcomes with specific antipsychotics would further enhance the ability to personalize treatments.
ACKNOWLEDGEMENTS
This work was supported by The Zucker Hillside Hospital Advanced Center for Intervention and Services Research for the Study of Schizophrenia grant (MH090590) from the US National Institute of Mental Health. The sponsor had no influence on the design, data acquisition, data analysis, data interpretation or writing of the report. The authors are grateful to A. Seidman and O. Uzoma for help with the literature search and data abstraction. T. Kishimoto and K. Hagi contributed equally to this work.
REFERENCES
- 1. Kahn RS, Sommer IE, Murray RM et al. Schizophrenia. Nat Rev Dis Primers 2015;1:15067. [DOI] [PubMed] [Google Scholar]
- 2. Leucht S, Tardy M, Komossa K et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta‐analysis. Lancet 2012;379:2063‐71. [DOI] [PubMed] [Google Scholar]
- 3. Kishimoto T, Agarwal V, Kishi T et al. Relapse prevention in schizophrenia: a systematic review and meta‐analysis of second‐generation antipsychotics versus first‐generation antipsychotics. Mol Psychiatry 2013;18:53‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robinson D, Woerner MG, Alvir JM et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry 1999;56:241‐7. [DOI] [PubMed] [Google Scholar]
- 5. Leucht S, Barnes TR, Kissling W et al. Relapse prevention in schizophrenia with new‐generation antipsychotics: a systematic review and exploratory meta‐analysis of randomized, controlled trials. Am J Psychiatry 2003;160:1209‐22. [DOI] [PubMed] [Google Scholar]
- 6. Leucht S, Arbter D, Engel RR et al. How effective are second‐generation antipsychotic drugs? A meta‐analysis of placebo‐controlled trials. Mol Psychiatry 2009;14:429‐47. [DOI] [PubMed] [Google Scholar]
- 7. Glick ID, Correll CU, Altamura AC et al. Mid‐term and long‐term efficacy and effectiveness of antipsychotic medications for schizophrenia: a data‐driven, personalized clinical approach. J Clin Psychiatry 2011;72:1616‐27. [DOI] [PubMed] [Google Scholar]
- 8. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Chichester: Wiley, 2011. [Google Scholar]
- 9. Correll CU, De Hert M. Antipsychotics for acute schizophrenia: making choices. Lancet 2013;382:919‐20. [DOI] [PubMed] [Google Scholar]
- 10. De Hert M, Detraux J, van Winkel R et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol 2012;8:114‐26. [DOI] [PubMed] [Google Scholar]
- 11. Leucht S, Cipriani A, Spineli L et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple‐treatments meta‐analysis. Lancet 2013;382:951‐62. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kishimoto T, Nitta M, Borenstein M et al. Long‐acting injectable vs. oral antipsychotics in schizophrenia: a systematic review and meta‐analysis of mirror‐image studies. J Clin Psychiatry 2013;74:957‐65. [DOI] [PubMed] [Google Scholar]
- 14. Kishimoto T, Hagi K, Nitta M et al. Effectiveness of long‐acting injectable vs oral antipsychotics in patients with schizophrenia: a meta‐analysis of prospective and retrospective cohort studies. Schizophr Bull 2018;44:603‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
- 16. Dickersin K, Berlin JA. Meta‐analysis: state‐of‐the‐science. Epidemiol Rev 1992;14:154‐76. [DOI] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jadad AR, Moore RA, Carroll D et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
- 19. Egger M, Davey Smith G, Schneider M et al. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duval S, Tweedie R. A Nonparametric “trim and fill” method of accounting for publication bias in meta‐analysis. J Am Statist Assoc 2000;95:89‐98. [Google Scholar]
- 21. Addington DE, Labelle A, Kulkarni J et al. A comparison of ziprasidone and risperidone in the long‐term treatment of schizophrenia: a 44‐week, double‐blind, continuation study. Can J Psychiatry 2009;54:46‐54. [DOI] [PubMed] [Google Scholar]
- 22. Alvarez E, Bernardo M, Gutiérrez‐Casares JR et al. Ziprasidone versus olanzapine in the weight gain associated with the treatment of schizophrenia: a six‐month double‐blind randomized parallel group study. Eur J Psychiatry 2012;26:248‐59. [Google Scholar]
- 23. Alvarez E, Ciudad A, Olivares JM et al. A randomized, 1‐year follow‐up study of olanzapine and risperidone in the treatment of negative symptoms in outpatients with schizophrenia. J Clin Psychopharmacol 2006;26:238‐49. [DOI] [PubMed] [Google Scholar]
- 24. Ciudad A, Olivares JM, Bousono M et al. Improvement in social functioning in outpatients with schizophrenia with prominent negative symptoms treated with olanzapine or risperidone in a 1 year randomized, open‐label trial. Prog Neuropsychopharmacol Biol Psychiatry 2006;30:1515‐22. [DOI] [PubMed] [Google Scholar]
- 25. Breier A, Berg PH, Thakore JH et al. Olanzapine versus ziprasidone: results of a 28‐week double‐blind study in patients with schizophrenia. Am J Psychiatry 2005;162:1879‐87. [DOI] [PubMed] [Google Scholar]
- 26. Chan HY, Chiang SC, Chang CJ et al. A randomized controlled trial of risperidone and olanzapine for schizophrenic patients with neuroleptic‐induced tardive dyskinesia. J Clin Psychiatry 2010;71:1226‐33. [DOI] [PubMed] [Google Scholar]
- 27. Chrzanowski WK, Marcus RN, Torbeyns A et al. Effectiveness of long‐term aripiprazole therapy in patients with acutely relapsing or chronic, stable schizophrenia: a 52‐week, open‐label comparison with olanzapine. Psychopharmacology 2006;189:259‐66. [DOI] [PubMed] [Google Scholar]
- 28. Citrome L, Cucchiaro J, Sarma K et al. Long‐term safety and tolerability of lurasidone in schizophrenia: a 12‐month, double‐blind, active‐controlled study. Int Clin Psychopharmacol 2012;27:165‐76. [DOI] [PubMed] [Google Scholar]
- 29. Crespo‐Facorro B, Ortiz‐Garcia de la Foz V, Mata I et al. Treatment of first‐episode non‐affective psychosis: a randomized comparison of aripiprazole, quetiapine and ziprasidone over 1 year. Psychopharmacology 2014;231:357‐66. [DOI] [PubMed] [Google Scholar]
- 30. Crespo‐Facorro B, Pérez‐Iglesias R, Mata I et al. Long‐term (3‐year) effectiveness of haloperidol, risperidone and olanzapine: results of a randomized, flexible‐dose, open‐label comparison in first‐episode nonaffective psychosis. Psychopharmacology 2012;219:225‐33. [DOI] [PubMed] [Google Scholar]
- 31. de Arce Cordon R, Eding E, Marques‐Teixeira J et al. Descriptive analyses of the aripiprazole arm in the risperidone long‐acting injectable versus quetiapine relapse prevention trial (ConstaTRE). Eur Arch Psychiatry Clin Neurosci 2012;262:139‐49. [DOI] [PubMed] [Google Scholar]
- 32. Gaebel W, Schreiner A, Bergmans P et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long‐acting injectable vs quetiapine: results of a long‐term, open‐label, randomized clinical trial. Neuropsychopharmacology 2010;35:2367‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deberdt W, Lipkovich I, Heinloth AN et al. Double‐blind, randomized trial comparing efficacy and safety of continuing olanzapine versus switching to quetiapine in overweight or obese patients with schizophrenia or schizoaffective disorder. Ther Clin Risk Manag 2008;4:713‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Durgam S, Landbloom RP, Mackle M et al. Exploring the long‐term safety of asenapine in adults with schizophrenia in a double‐blind, fixed‐dose, extension study. Neuropsychiatr Dis Treat 2017;13:2021‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fleischhacker WW, McQuade RD, Marcus RN et al. A double‐blind, randomized comparative study of aripiprazole and olanzapine in patients with schizophrenia. Biol Psychiatry 2009;65:510‐7. [DOI] [PubMed] [Google Scholar]
- 36. Kahn R, Fleischhacker WW, Boter H et al. Effectiveness of antipsychotic drugs in first‐episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet 2008;371:1085‐97. [DOI] [PubMed] [Google Scholar]
- 37. Kane JM, Osuntokun O, Kryzhanovskaya LA et al. A 28‐week, randomized, double‐blind study of olanzapine versus aripiprazole in the treatment of schizophrenia. J Clin Psychiatry 2009;70:572‐81. [DOI] [PubMed] [Google Scholar]
- 38. Keefe RS, Young CA, Rock SL et al. One‐year double‐blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophrenia. Schizophr Res 2006;81:1‐15. [DOI] [PubMed] [Google Scholar]
- 39. Kern RS, Green MF, Cornblatt BA et al. The neurocognitive effects of aripiprazole: an open‐label comparison with olanzapine. Psychopharmacology 2006;187:312‐20. [DOI] [PubMed] [Google Scholar]
- 40. Kinon BJ, Noordsy DL, Liu‐Seifert H et al. Randomized, double‐blind 6‐month comparison of olanzapine and quetiapine in patients with schizophrenia or schizoaffective disorder with prominent negative symptoms and poor functioning. J Clin Psychopharmacol 2006;26: 453‐61. [DOI] [PubMed] [Google Scholar]
- 41. Kinon BJ, Lipkovich I, Edwards SB et al. A 24‐week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptoms. J Clin Psychopharmacol 2006;26:157‐62. [DOI] [PubMed] [Google Scholar]
- 42. Kishi T, Matsuda Y, Matsunaga S et al. A randomized trial of aripiprazole vs blonanserin for the treatment of acute schizophrenia and related disorders. Neuropsychiatr Dis Treat 2016;12:3041‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kumar PNS, Anish PK, Rajmohan V. Olanzapine has better efficacy compared to risperidone for treatment of negative symptoms in schizophrenia. Indian J Psychiatry 2016;58:311‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lecrubier Y, Quintin P, Bouhassira M et al. The treatment of negative symptoms and deficit states of chronic schizophrenia: olanzapine compared to amisulpride and placebo in a 6‐month double‐blind controlled clinical trial. Acta Psychiatr Scand 2006;114:319‐27. [DOI] [PubMed] [Google Scholar]
- 45. Lieberman JA, Stroup TS, McEvoy JP et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005;353:1209‐23. [DOI] [PubMed] [Google Scholar]
- 46. Liu J, Sun J, Shen X et al. Randomized controlled trial comparing changes in serum prolactin and weight among female patients with first‐episode schizophrenia over 12 months of treatment with risperidone or quetiapine. Shanghai Arch Psychiatry 2014;26:88‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Loebel A, Cucchiaro J, Xu J et al. Effectiveness of lurasidone vs. quetiapine XR for relapse prevention in schizophrenia: a 12‐month, double‐blind, noninferiority study. Schizophr Res 2013;147:95‐102. [DOI] [PubMed] [Google Scholar]
- 48. A phase 3 randomized , double‐blind, active comparator‐controlled clinical trial to study the safety and efficacy of lurasidone in subjects with schizophrenia (PEARL 3 Extension Study). Clinical Trials.gov identifier NCT00789698.
- 49. McEvoy JP, Lieberman JA, Stroup TS et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry 2006;163:600‐10. [DOI] [PubMed] [Google Scholar]
- 50. McEvoy JP, Lieberman JA, Perkins DO et al. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: a randomized, double‐blind 52‐week comparison. Am J Psychiatry 2007;164:1050‐60. [DOI] [PubMed] [Google Scholar]
- 51. McQuade RD, Stock E, Marcus R et al. A comparison of weight change during treatment with olanzapine or aripiprazole: results from a randomized, double‐blind study. J Clin Psychiatry 2004;65(Suppl. 18):47‐56. [PubMed] [Google Scholar]
- 52. Meltzer HY, Alphs L, Green AI et al. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003;60:82‐91. [DOI] [PubMed] [Google Scholar]
- 53. Meltzer HY, Bobo WV, Roy A et al. A randomized, double‐blind comparison of clozapine and high‐dose olanzapine in treatment‐resistant patients with schizophrenia. J Clin Psychiatry 2008;69:274‐85. [DOI] [PubMed] [Google Scholar]
- 54. Mortimer A, Martin S, Loo H et al. A double‐blind, randomized comparative trial of amisulpride versus olanzapine for 6 months in the treatment of schizophrenia. Int Clin Psychopharmacol 2004;19:63‐9. [DOI] [PubMed] [Google Scholar]
- 55. Naber D, Riedel M, Klimke A et al. Randomized double blind comparison of olanzapine vs. clozapine on subjective well‐being and clinical outcome in patients with schizophrenia. Acta Psychiatr Scand 2005;111:106‐15. [DOI] [PubMed] [Google Scholar]
- 56. Naber D, Peuskens J, Schwarzmann N et al. Subjective well‐being in schizophrenia: a randomised controlled open‐label 12‐month non‐inferiority study comparing quetiapine XR with risperidone (RECOVER). Eur Neuropsychopharmacol 2013;23:1257‐69. [DOI] [PubMed] [Google Scholar]
- 57. A one‐year randomized , prospective, parallel, open comparison of subjective well‐being in schizophrenic out‐patients treated with quetiapine XR (SEROQUEL XR™) or oral risperidone at flexible dose in a naturalistic setting. Clinical Trials.gov identifier NCT00600756.
- 58. Németh G, Laszlovszky I, Czobor P et al. Cariprazine versus risperidone monotherapy for treatment of predominant negative symptoms in patients with schizophrenia: a randomised, double‐blind, controlled trial. Lancet 2017;389:1103‐13. [DOI] [PubMed] [Google Scholar]
- 59. Noordsy DL, Glynn SM, Sugar CA et al. Risperidone versus olanzapine among patients with schizophrenia participating in supported employment: eighteen‐month outcomes. J Psychiatr Res 2017;95:299‐307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Parabiaghi A, Tettamanti M, D'Avanzo B et al. Metabolic syndrome and drug discontinuation in schizophrenia: a randomized trial comparing aripiprazole, olanzapine and haloperidol. Acta Psychiatr Scand 2016;133:63‐75. [DOI] [PubMed] [Google Scholar]
- 61. Purdon SE, Jones BD, Stip E et al. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for Research in Schizophrenia. Arch Gen Psychiatry 2000;57:249‐58. [DOI] [PubMed] [Google Scholar]
- 62. Ritchie CW, Harrigan S, Mastwyk M et al. Predictors of adherence to atypical antipsychotics (risperidone or olanzapine) in older patients with schizophrenia: an open study of 31/2 years duration. Int J Geriatr Psychiatry 2010;25:411‐8. [DOI] [PubMed] [Google Scholar]
- 63. Sanz‐Fuentenebro J, Taboada D, Palomo T et al. Randomized trial of clozapine vs. risperidone in treatment‐naive first‐episode schizophrenia: results after one year. Schizophr Res 2013;149:156‐61. [DOI] [PubMed] [Google Scholar]
- 64. Schnell T, Koethe D, Krasnianski A et al. Ziprasidone versus clozapine in the treatment of dually diagnosed (DD) patients with schizophrenia and cannabis use disorders: a randomized study. Am J Addict 2014;23:308‐12. [DOI] [PubMed] [Google Scholar]
- 65. Schoemaker J, Stet L, Vrijland P et al. Long‐term efficacy and safety of asenapine or olanzapine in patients with schizophrenia or schizoaffective disorder: an extension study. Pharmacopsychiatry 2012;45:196‐203. [DOI] [PubMed] [Google Scholar]
- 66. Schooler NR, Marder SR, Chengappa KNR et al. Clozapine and risperidone in moderately refractory schizophrenia: a 6‐month randomized double‐blind comparison. J Clin Psychiatry 2016;77:628‐34. [DOI] [PubMed] [Google Scholar]
- 67. Sechter D, Peuskens J, Fleurot O et al. Amisulpride vs. risperidone in chronic schizophrenia: results of a 6‐month double‐blind study. Neuropsychopharmacology 2002;27:1071‐81. [DOI] [PubMed] [Google Scholar]
- 68. Schreiner A, Niehaus D, Shuriquie NA et al. Metabolic effects of paliperidone extended release versus oral olanzapine in patients with schizophrenia: a prospective, randomized, controlled trial. J Clin Psychopharmacol 2012;32:449‐57. [DOI] [PubMed] [Google Scholar]
- 69. Simpson GM, Weiden P, Pigott T et al. Six‐month, blinded, multicenter continuation study of ziprasidone versus olanzapine in schizophrenia. Am J Psychiatry 2005;162:1535‐8. [DOI] [PubMed] [Google Scholar]
- 70. Strom BL, Eng SM, Faich G et al. Comparative mortality associated with ziprasidone and olanzapine in real‐world use among 18,154 patients with schizophrenia: the Ziprasidone Observational Study of Cardiac Outcomes (ZODIAC). Am J Psychiatry 2011;168:193‐201. [DOI] [PubMed] [Google Scholar]
- 71. Stroup TS, Lieberman JA, McEvoy JP et al. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. Am J Psychiatry 2006;163:611‐22. [DOI] [PubMed] [Google Scholar]
- 72. Stroup TS, Lieberman JA, McEvoy JP et al. Effectiveness of olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia after discontinuing perphenazine: a CATIE study. Am J Psychiatry 2007;164:415‐27. [DOI] [PubMed] [Google Scholar]
- 73. Thomas SH, Drici MD, Hall GC et al. Safety of sertindole versus risperidone in schizophrenia: principal results of the sertindole cohort prospective study (SCoP). Acta Psychiatr Scand 2010;122:345‐55. [DOI] [PubMed] [Google Scholar]
- 74. Tran PV, Hamilton SH, Kuntz AJ et al. Double‐blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. J Clin Psychopharmacol 1997;17:407‐18. [DOI] [PubMed] [Google Scholar]
- 75. Tunis SL, Faries DE, Stensland MD et al. An examination of factors affecting persistence with initial antipsychotic treatment in patients with schizophrenia. Curr Med Res Opin 2007;23:97‐104. [DOI] [PubMed] [Google Scholar]
- 76. Wani RA, Dar MA, Chandel RK et al. Effects of switching from olanzapine to aripiprazole on the metabolic profiles of patients with schizophrenia and metabolic syndrome: a double‐blind, randomized, open‐label study. Neuropsychiatr Dis Treat 2015;11:685‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zhang Y, Dai G. Efficacy and metabolic influence of paliperidone ER, aripiprazole and ziprasidone to patients with first‐episode schizophrenia through 52 weeks follow‐up in China. Hum Psychopharmacol 2012;27:605‐14. [DOI] [PubMed] [Google Scholar]
- 78. A multicenter , double‐blind, flexible‐dose, 6‐month trial comparing the efficacy and safety of asenapine with olanzapine in stable subjects with predominant, persistent negative symptoms of schizophrenia. Clinical Trials.gov identifier NCT00145496.
- 79. A multicenter , open label, flexible‐dose, parallel‐group evaluation of the cataractogenic potential of quetiapine fumarate (Seroquel) and risperidone (Risperdal) in the long term treatment of participants with schizophrenia or schizoaffective disorder. Clinical Trials.gov identifier NCT00206102.
- 80.Efficacy and safety of asenapine compared with olanzapine in patients with persistent negative symptoms of schizophrenia (25543) (P05817). Clinical Trials.gov identifier NCT00212836.
- 81. A six‐month , double‐blind, randomized, international, multicenter trial to evaluate the glucoregulatory effects of risperidone and olanzapine in subjects with schizophrenia or schizoaffective disorder. Clinical Trials.gov identifier NCT00236379.
- 82. Clozapine vs . risperidone for people with first episode schizophrenia and co‐occurring substance use disorder. Clinical Trials.gov identifier NCT00573287.
- 83. Comparison of Optimal Antipsychotic Treatments for Adults With Schizophrenia (COATS) . Clinical Trials.gov identifier NCT00802100.
- 84. Andreasen NC, Carpenter WT Jr, Kane JM et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry 2005;162:441‐9. [DOI] [PubMed] [Google Scholar]
- 85. Zhao YJ, Lin L, Teng M et al. Long‐term antipsychotic treatment in schizophrenia: systematic review and network meta‐analysis of randomised controlled trials. BJPsych Open 2016;2:59‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry 2010;79:1115‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci 2014;16:505‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nielsen J, Skadhede S, Correll CU. Antipsychotics associated with the development of type 2 diabetes in antipsychotic‐naive schizophrenia patients. Neuropsychopharmacology 2010;35:1997‐2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Nielsen J, Correll CU, Manu P et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry 2013;74:603‐13. [DOI] [PubMed] [Google Scholar]
- 90. Samara MT, Dold M, Gianatsi M et al. Efficacy, acceptability, and tolerability of antipsychotics in treatment‐resistant schizophrenia: a network meta‐analysis. JAMA Psychiatry 2016;73:199‐210. [DOI] [PubMed] [Google Scholar]
- 91. Emsley R, Nuamah I, Hough D et al. Treatment response after relapse in a placebo‐controlled maintenance trial in schizophrenia. Schizophr Res 2012;138:29‐34. [DOI] [PubMed] [Google Scholar]
- 92. Correll CU, Rubio JM, Kane JM. What is the risk‐benefit ratio of long‐term antipsychotic treatment in people with schizophrenia? World Psychiatry 2018;17:149‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kishimoto T, Robenzadeh A, Leucht C et al. Long‐acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta‐analysis of randomized trials. Schizophr Bull 2014;40:192‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]


