Abstract
DNA damage response (DDR) and selective autophagy both can be activated by reactive oxygen/nitrogen species (ROS/RNS), and both are of paramount importance in cancer development. The selective autophagy receptor and ubiquitin (Ub) sensor p62 plays a key role in their crosstalk. ROS production has been well documented in latent infection of oncogenic viruses including Epstein-Barr Virus (EBV). However, p62-mediated selective autophagy and its interplay with DDR have not been investigated in these settings. In this study, we provide evidence that considerable levels of p62-mediated selective autophagy are spontaneously induced, and correlate with ROS-Keap1-NRF2 pathway activity, in virus-transformed cells. Inhibition of autophagy results in p62 accumulation in the nucleus, and promotes ROS-induced DNA damage and cell death, as well as downregulates the DNA repair proteins CHK1 and RAD51. In contrast, MG132-mediated proteasome inhibition, which induces rigorous autophagy, promotes p62 degradation but accumulation of the DNA repair proteins CHK1 and RAD51. However, pretreatment with an autophagy inhibitor offsets the effects of MG132 on CHK1 and RAD51 levels. These findings imply that p62 accumulation in the nucleus in response to autophagy inhibition promotes proteasome-mediated CHK1 and RAD51 protein instability. This claim is further supported by the findings that transient expression of a p62 mutant, which is constitutively localized in the nucleus, in B cell lines with low endogenous p62 levels recaptures the effects of autophagy inhibition on CHK1 and RAD51 protein stability. These results indicate that proteasomal degradation of RAD51 and CHK1 is dependent on p62 accumulation in the nucleus. However, small hairpin RNA (shRNA)-mediated p62 depletion in EBV-transformed lymphoblastic cell lines (LCLs) had no apparent effects on the protein levels of CHK1 and RAD51, likely due to the constitutive localization of p62 in the cytoplasm and incomplete knockdown is insufficient to manifest its nuclear effects on these proteins. Rather, shRNA-mediated p62 depletion in EBV-transformed LCLs results in significant increases of endogenous RNF168-γH2AX damage foci and chromatin ubiquitination, indicative of activation of RNF168-mediated DNA repair mechanisms. Our results have unveiled a pivotal role for p62-mediated selective autophagy that governs DDR in the setting of oncogenic virus latent infection, and provide a novel insight into virus-mediated oncogenesis.
Author summary
Reactive oxygen/nitrogen species (ROS/RNS) can induce both DNA damage response (DDR) and selective autophagy, which play crucial roles in cancer development. The selective autophagy receptor and ubiquitin (Ub) sensor p62 links their crosstalk. However, p62-mediated selective autophagy and its interplay with DDR have not been investigated in latent infection of oncogenic viruses including Epstein-Barr Virus (EBV). In this study, we provide evidence that p62-mediated selective autophagy is constitutively induced in virus-transformed cells, and that its inhibition exacerbates ROS-induced DNA damage, and promotes proteasomal degradation of CHK1 and RAD51 in a manner depending on p62 accumulation in the nucleus. However, rigorous autophagy induction results in accumulation of DNA repair proteins CHK1 and RAD51, and p62 degradation. Further, transient expression of a constitutive nucleus-localizing mutant of p62 recaptures the effects of autophagy inhibition on CHK1 and RAD51 protein stability. These findings support the claim that p62 accumulation in the nucleus in response to autophagy inhibition promotes proteasome-mediated CHK1 and RAD51 protein instability. However, small hairpin RNA (shRNA)-mediated p62 depletion did not affect CHK1 and RAD51 protein levels; rather, shRNA-mediated p62 depletion activates RNF168-dependent DNA repair mechanisms. Our results have unveiled a pivotal role for p62-mediated selective autophagy in regulation of DDR by overriding traditional DDR mechanisms in the setting of oncogenic virus latent infection, and provide a novel insight into the etiology of viral cancers.
Introduction
p62 (also named EBIAP, ZIP3, SQSTM1/Sequestosome-1), a human homolog of mouse ZIPs (Zeta PKC-interacting proteins), is well known as a selective autophagy receptor and a ubiquitn sensor, which controls myraid cellular processes, including redox homeostasis, DNA damage response (DDR), cancer development, aging, inflammation and immunity, osteoclastogenesis, and obesity, with or without the involvement of autophagy [1–3].
Autophagy, with either non-selective (random) or selective fashion, is a unique intracellular process that engulfs damaged and even functional cellular constituents and delivers them to lysosomes for digestion and recycling in the cytosol under diverse stresses, such as nutrient deprivation, viral replication, cancer hypoxia, genotoxic stress, and replicative crisis. Autophagy is thereby a crucial cellular machinery conserved from yeast to higher eukaryotes that maintains organ metabolism, genome stability, and cell survival, and functions as either tumor suppressor at early stage or promotor at late stage [4–6]. Distinct from non-selective autophagy, selective autophagy sort specific substrates to lysosomes, and is mediated by an increasing pool of receptors, including p62, NBR1, TAX1BP1, NDP52, OPTN, TRIMs, and TOLLIP [3, 7–10].
Reactive oxygen/nitrogen species (ROS and RNS), the major cause of endogenous DNA damage, can be produced in chronic viral infections, in which viral replication is generally absent [11]. They can directly modify DNA and generate different levels of lesions, including double strand breaks (DSBs) [12, 13]. Eukaryotic organisms have developed sophisticated strategies to repair DNA damage to ensure genomic integrity, with homologous recombination (HR) and nonhomologous end joining (NHEJ) being two non-redundant repair mechanisms for DSBs [14]. Unrepaired DSBs, however, incite chronic inflammation, resulting in genomic instability that promotes malignant transformation under certain conditions [15].
ROS/RNS also induce p62 expression through the Keap1-NRF2 pathway, licensing the induction of p62-mediated selective autophagy [16]. Mounting evidence indicates that DDR and selective autophagy closely crosstalk in response to oxidative stress, in which p62 plays a key role [17]. While p62 inhibits DNA damage repair, p62-mediated selective autophagy promotes DNA repair by targeting ubiquitinated substrates including p62 itself for degradation in cancer cells [18, 19], which usually harbor deficient traditional DNA repair mechanisms and heavily rely on autophagy as an alternative repair mechanism for survival [20, 21]. In this sense, p62-mediated selective autophagy, which is activated upon DNA damage caused by various stresses such as conventional chemotherapeutic agents, allows these cancer cells to escape DNA damage-induced cell death [22, 23].
ROS/RNS overproduction, deregulation of host DDR machinery, and chronic inflammation, are the most common features of viral persistent infections, and together with non-selective autophagy, have also been documented in latency of herpesviruses including Epstein-Barr Virus (EBV) [24–32]. Moreover, we and others have provided overwhelming evidence supporting that EBV latent infection reprograms the host ubiquitin machinery for its own benefits [33–35], including the employment of linear ubiquitin chain assembly complex (LUBAC)-mediated ubiquitination to modulate LMP1 signal transduction [36]. However, as the major selective autophagy receptor and a ubiquitin sensor, p62 and its relationship with EBV latency and oncogenesis have never been investigated. In our recent publication, our findings have implied a role for the p62-autophagy interplay in ROS-elicited DDR in EBV latency [37].
In this study, we aimed to investigate the potential role of p62-mediated selective autophagy in regulating DDR in EBV latent infection. Our results show that p62-mediated selective autophagy is constitutively induced in virus-transformed cells, and correlates with ROS-Keap1-NRF2 pathway activity, and that a well-balanced basal level of p62-mediated selective autophagy is essential for maintaining genomic stability in this setting.
Results
p62-mediated selective autophagy is constitutively induced in viral latency and correlates with ROS-Keap1-NRF2 pathway activity
Our recent findings have shown that treatment of EBV+ cells with the calcium ionophore ionomycin, which raises the intracellular level of calcium (Ca2+) essential for ROS production, elevates the protein levels of both p62 and LC3b-II (the smaller cleavage product of LC3b, which generally represents a marker of autophagosomal activity in mammalian cells), and induces DNA damage; autophagy deficiency also elevates p62 protein levels [37]. Since both p62 and LC3b are targeted by autophagy for degradation, their turnover represents the autophagic flux (autophagic degradation activity) [38, 39]. These results have implied that the p62-autophagy interplay may be involved in oxidative stress in EBV latent infection.
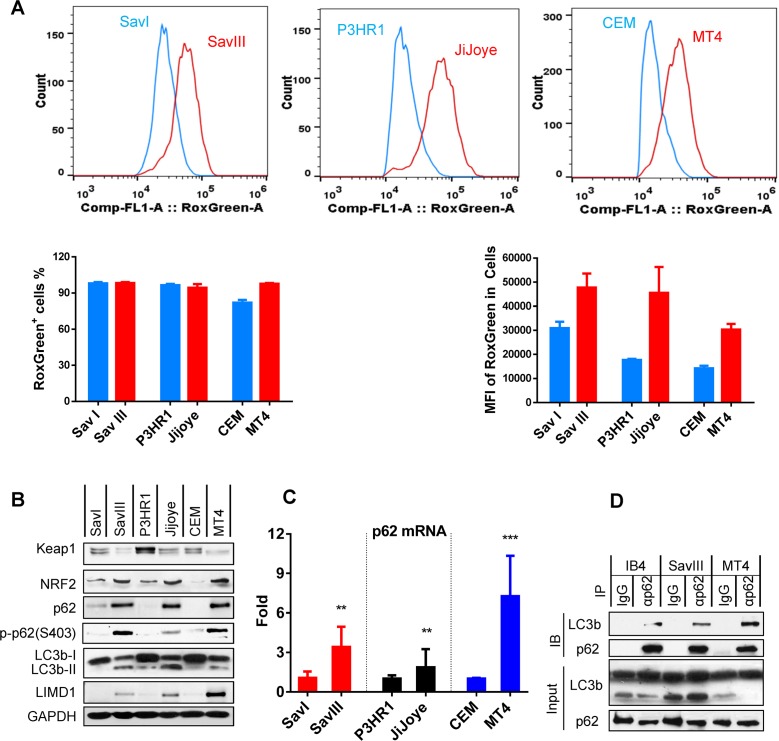
We thus sought to evaluate the correlation between intracellular ROS, and p62 and autophagy levels in EBV latency programs. Results show that, although nearly 100% cells of each tested virus-associated cancer cell line produce ROS, their levels, as indicated by mean of fluorescence intensity (MFI), are consistently higher in SavIII, JiJoye, and MT4, compared to SavI, P3HR1, and CEM, respectively (Fig 1A). Correspondingly, the cell lines with higher ROS production remarkably express higher p62 consistently at both protein and mRNA levels (Fig 1B and 1C). Furthermore, the basal levels of p62-mediated selective autophagy activity, as indicated by both the cleaved LC3b product LC3b-II and phosphorylation of p62(Ser403), correspond to the endogenous ROS levels, and are readily detectable by immunoblotting in EBV type III latency and human T-cell leukemia virus-1 (HTLV1)-transformed MT4 cell line (Fig 1B). Phosphorylation of p62(Ser403), which promotes p62-Ub binding, is crucial for activation of p62-selective autophagy [40]. Furthermore, interaction between selective autophagy receptors and Ub-like proteins (UBLs), such as LC3b, is the molecular basis for selective autophagy [41, 42]. Our IP results show that endogenous p62 and LC3b interact in virus-transformed cells (Fig 1D). These results indicate that a basal level of p62-mediated selective autophagy is constitutively induced and correlates with the endogenous ROS level in viral latency.
Fig 1. p62-mediated selective autophagy is endogenously induced in viral latency and correlates with ROS-Keap1-NRF2 pathway activity.
A. Endogenous ROS production in viral latency was measured by flow cytometry with the CellRox Green reagent (Invitrogen). Three independent repeats were conducted, and representative results are shown. Results are the mean ± standard error (SE) of duplicates for each sample. MFI = mean fluorescence intensity. B. The correlation of endogenous p62 protein levels and autophagy activity with the Keap1-NRF2 pathway activity in viral latency was evaluated by immunoblotting with indicated antibodies. C. The correlation of p62 expression with viral latency was evaluated at the transcription level by qPCR. The mRNA levels in SavI, P3HR1, and CEM were set to 1, and compared with the paired SavIII, JiJoye, and MT4 cell lines, respectively. D. Interaction of endogenous p62 with LC3b in virus-transformed cells was evaluated by IP. Cell lysates (1 mg each) were pre-cleared with mouse IgG (Sigma) before subjected to IP with IgG or anti-p62 clone D-3 (Santa Cruz). immunoprecipitants and inputs (5% of cell lysates) were probed with indicated antibodies.
The antioxidant transcription factor NRF2 is spontaneously degraded by the ubiquitin (Ub) E3 ligase complex Keap1/Cul3/RBX1 under normoxia; ROS/oxidative stress triggers autophagic degradation of Keap1, resulting in the accumulation and activation of NRF2, which then induces p62 expression [43, 44]. Thus, we evaluated the Keap1-NRF2 pathway activities, indicated by Keap1 and NRF2 expression levels, in these cell lines. As indicated in Fig 1B, the endogenous p62 levels positively correlate with the Keap-NRF2 pathway activities, strongly suggesting that the Keap1-NRF2 pathway induces p62 expression at least in part in viral latency. In support of our findings, Keap1-NRF2 pathway is also activated in KSHV latency [45, 46].
Together, these results indicate that p62-mediated selective autophagy is constitutively induced in oncovirus latency, and correlates with the endogenous ROS-Keap1-NRF2 pathway activity.
ROS that correlate with Keap1-NRF2 pathway activity contribute to p62 expression and activation of p62-mediated autophagy in viral latency
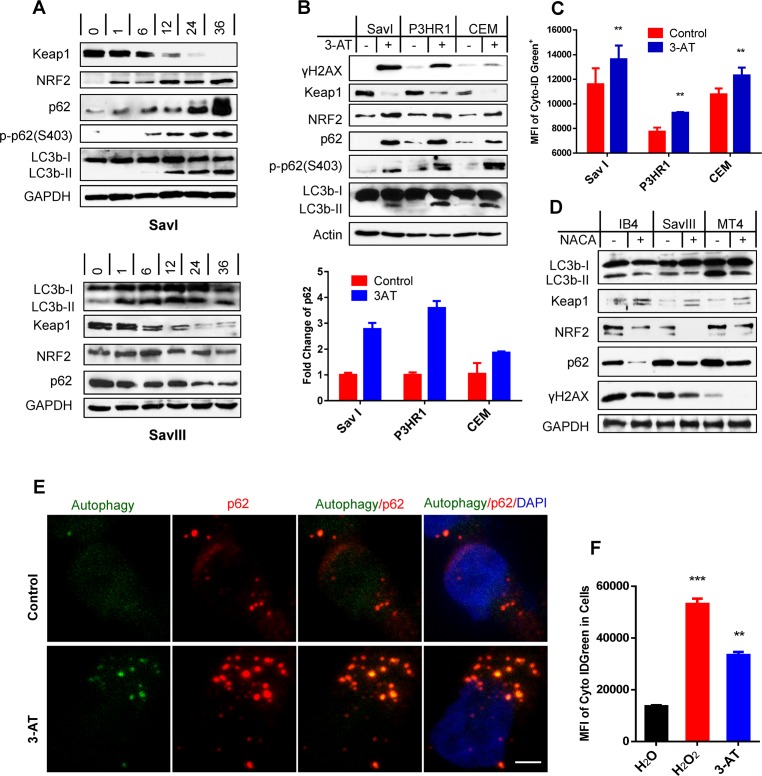
We next aimed to verify the induction of p62 by the ROS-Keap1-NRF2 pathway in viral latency. To this end, we first treated SavI and SavIII cells with the clinical topoisomerase II inhibitor doxorubicin (Doxo), which generates the highest level of mitochondrial ROS causing DSBs [47]. Results show that Doxo augments the activities of the Keap1-NRF2 pathway in both cell lines in a time-dependent manner. Interestingly, p62 protein levels and p-p62(S403) are increased by Doxo treatment in SavI cells, but p62 protein levels are decreased in SavIII cells. Moreover, LC3b-II is increased in both cell lines but decreased at late stage in SavIII cells (Fig 2A). These results are consistent with the notion that considerable levels of ROS are required for induction of p62 and mediated autophagy, but excess ROS and selective autophagy result in autophagic degradation of p62 and LC3b in that both are targets of p62-mediated selective autophagy [38, 39].
Fig 2. ROS correlate with Keap1-NRF2 pathway activity and contribute to p62 expression and activation of p62-mediated autophagy in viral latency.
A. SavI and SavIII cells, which were derived from the same patient, were treated with 2 µM of the topoisomerase II inhibitor doxorubicin HCl (Doxo) (UBPBio) for different time periods. B-C. SavI, P3HR1, and CEM were treated with 20 mM of the H2O2 catalase 3-amino-1,2,4-triazole (3-AT) (Fisher Scientific) or vehicle control for 48 h. Cells were then subjected to IB, qPCR, and flow cytometry analyses. D. IB4, LCL45, and MT4 were treated with 3 mM of the antioxidant N-acetylcysteine amide (NACA) (Sigma) or vehicle control for 30 h. The treated cells were then subjected to analyses for p62, autophagy, Keap1-NRF2 pathway, and ROS production. E. IB4 cells were treated with 20 mM 3-AT (Fisher Scientific) or vehicle control for 48 h, and analyzed for p62-autophagosome colocalization by confocal microscopy. Living cells were first stained with a Cyto-ID Autophagy Detection kit (Enzo), and then fixed for staining with the mouse p62 antibody (D-3) and Alexa 555 coupled anti-mouse antibody (Invitrogen). Bar = 2 µm. F. IB4 cells were treated with 50 µM H2O2 for 30 min and then medium was replaced and continued in culture for 2 days, or treated with 20 mM 3-AT for 48 h, before subjected to analysis of autophagy flux by flow cytometry with the Cyto-ID Autophagy Detection kit. MFI = mean fluorescence intensity.
We next used 3-amino-1,2,4-triazole (3-AT) to inhibit the endogenous activities of catalase, an enzyme converting H2O2 to H2O+O2, in cell lines with lower ROS levels to elevate their endogenous ROS levels. Then, we evaluated the Keap1-NRF2 pathway activity, and p62, autophagy, and DNA damage levels. Results show that 3-AT treatment substantially elevates endogenous levels of the Keap1-NRF2 pathway activities and p62 expression at both protein and mRNA levels, and also induces p62(S403) phosphorylation, autophagy and the DNA damage hallmark γH2AX that are readily detectable (Fig 2B). Accumulation of LC3-II does not necessarily reflect an increased autophagic activity; instead it may represent its decreased clearance due to the blockage of autophagic degradation. Thus, we further measured autophagy flux, as indicated by MFI, by flow cytometry (Fig 2C). In contrast, quenching endogenous ROS with the ROS scavenger N-acetylcysteine amide (NACA) in indicated cell lines substantially dampens p62 levels and autophagy activities due to blockage of their endogenous Keap1-NRF2 pathway activities, as well as attenuates endogenous DNA damage (Fig 2D). Furthermore, confocal microscopy (Fig 2E) and flow cytometry (Fig 2F) results show that treatment of IB4 cells with 3-AT or with the traditional oxidative DNA damage inducer H2O2 remarkably increases p62 expression, autophagosomes and autophagy flux, and that most p62 foci co-localize with autophagosomal bodies in the cytoplasm.
Taken together, these results indicate that endogenous ROS, which correlate with Keap1-NRF2 pathway activity, are responsible for p62 expression and for induction of p62-mediated selective autophagy in viral latency.
Autophagy inhibition sensitizes EBV+ cells to ROS-induced DNA damage that is associated with p62 accumulation
Since we have previously shown that mild ionomycin treatments induce p62 expression and profound autophagy in lymphoblastic cell lines (LCLs), but stringent treatments promote p62 degradation due to induction of massive autophagy [37], we used ionomycin treatment here to study the p62-autohagy interplay in regulating DDR in LCLs, which serve as a system crucial for genetic and functional study of carcinogen sensitivity and DNA repair [48].
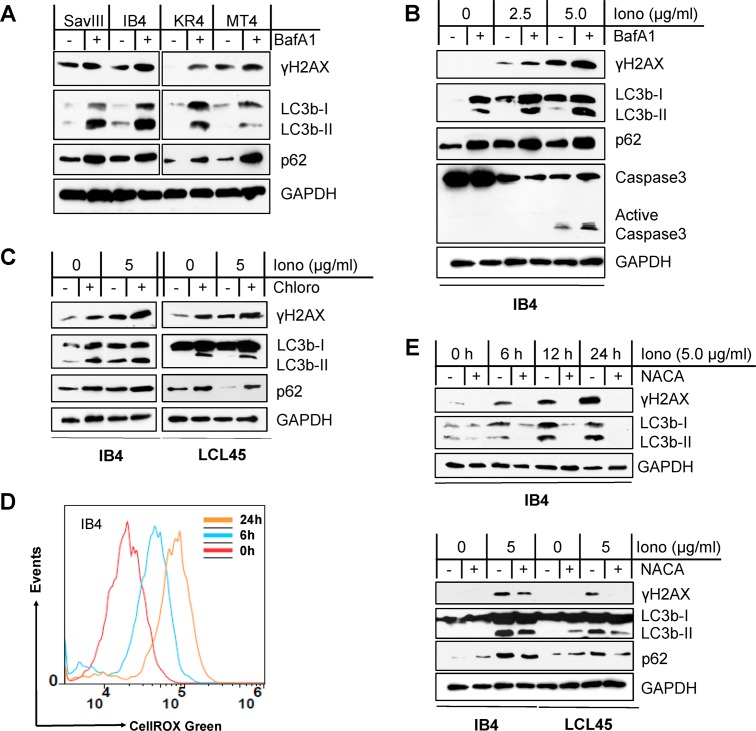
We first used the lysosome-specific inhibitor bafilomycin A1 (BafA1), which inhibits lysosomal activity that occurs after LC3 processing, to inhibit autophagy activity induced by ionomycin in LCLs. Results show that both p62 and LC3b, which are both selectively degraded by p62-mediated autophagy [38, 39], are accumulated in ionomycin-treated cells due to impaired autophagy activities. As a consequence, the levels of γH2AX are remarkably augmented (Fig 3A and 3B). To minimize the interference of potential “off-target” effects of BafA1, we performed this experiment using another lysosome-specific inhibitor chloroquine, and obtained similar results (Fig 3C). These results indicate that the autophagy-p62 interplay plays a role in DDR in EBV latency.
Fig 3. Autophagy inhibition sensitizes EBV+ cells to ROS-induced DNA damage that is associated with p62 accumulation.
A. Cell lines with higher endogenous autophagy levels were treated with 0.4 µM of the vacuolar ATPase inhibitor bafilomycin A1 (BafA1) (Sigma) or vehicle control for 24 h, and then DNA damage (γH2AX) was evaluated by immunoblotting. B-C. The LCL lines IB4 and LCL45 were treated with ionomycin (Iono) (Sigma) with indicated concentrations for 48 h plus 0.4 µM BafA1 or vehicle control for 24 h or plus 50 µM of the lysosome inhibitor chloroquine (Chloro) (MP Biomedicals) or vehicle control for 6 h. p62, autophagy, and γH2AX were analyzed by immunoblotting. D. IB4 cells were treated with 5 µg/ml Iono or vehicle control for different time periods, and ROS production was measured by flow cytometry with the CellRox Green reagent (Invitrogen). A representative result from three independent repeats is shown. E. IB4 and LCL45 cells were treated with 5 µg/ml Iono plus 3 mM NACA or vehicle control for different time periods (upper panel) or for 30 h (lower panel). p62, autophagy, and γH2AX were analyzed by immunoblotting.
We further show that ionomycin triggers profound ROS production and DNA damage in LCLs in a time-dependent manner (Fig 3D and 3E), and the ROS scavenger NACA offsets the effects of ionomycin (Fig 3E). Thus, these results indicate that ROS are responsible for ionomycin-induced autophagy and DNA damage in EBV latency.
Autophagy inhibition promotes DNA damage-induced cell death in association with p62 accumulation in the nucleus
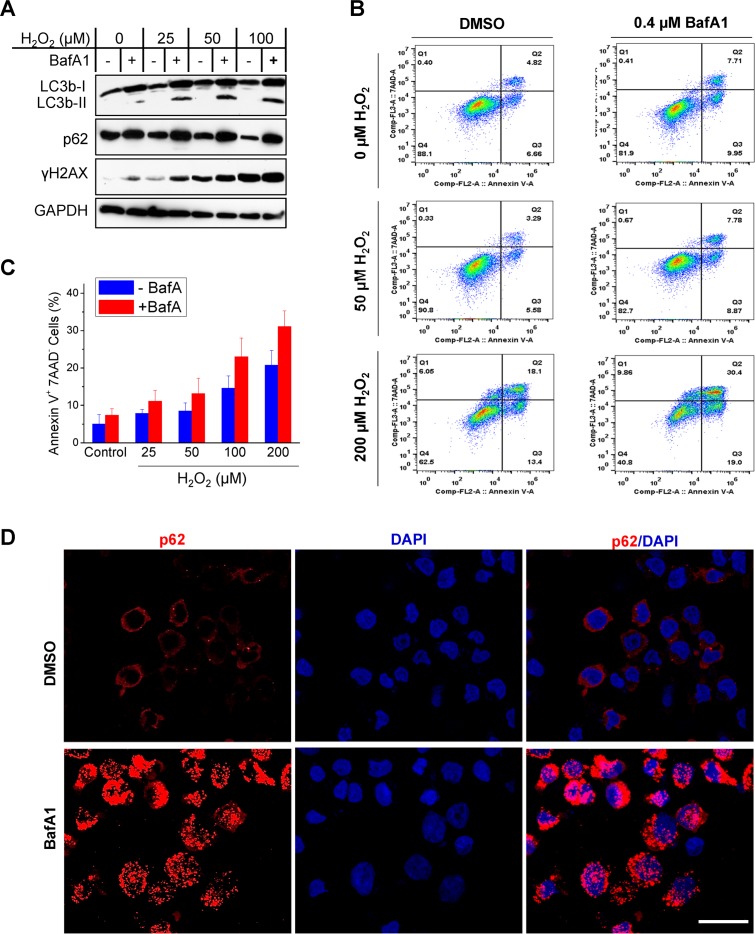
To confirm the requirement of ROS for induction of autophagy and DNA damage in EBV latency, we further used H2O2 to treat IB4 cells. Results show that H2O2 treatment induces profound DNA damage and reduces the endogenous p62 protein level in a dose-dependent manner, and blockage of autophagy activity with BafA1 potentiates the DNA damage that correlates with elevated p62 protein levels (Fig 4A).
Fig 4. Autophagy inhibition promotes cell death in association with p62 accumulation in the nucleus.
IB4 cells were treated with indicated concentrations of H2O2 in medium for 30 min. Then the medium was removed, and 0.4 µM BafA1 (Sigma) or vehicle control was added in freshly replaced medium for 48 h. A. p62, autophagy, and DNA damage were analyzed by immunoblotting. B-C. Cell death was analyzed by flow cytometry for Annexin V and 7-AAD expression. Results from a representative experiment of five independent repeats are shown (B), and statistical analysis results are expressed as mean ± standard error (SE) (C). D. p62 subcellular localization was visualized under confocal microscope. Bar = 10 µm.
Furthermore, autophagy inhibition by BafA1 promotes cell death (as indicated by 7-AAD expression and Annexin-V binding, or caspase-3 activity) induced by H2O2 (Fig 4B and 4C) or ionomycin (Fig 3B), respectively. Importantly, confocal microscopy results further show that p62 translocates from the cytoplasm to the nucleus in response to autophagy inhibition (Fig 4D).
Taken together, these results (Figs 3 and 4) indicate that autophagy inhibition exacerbates ROS-induced DNA damage by promoting p62 stabilization and nuclear translocation, further supporting that p62-mediated autophagy promotes DDR in EBV latency.
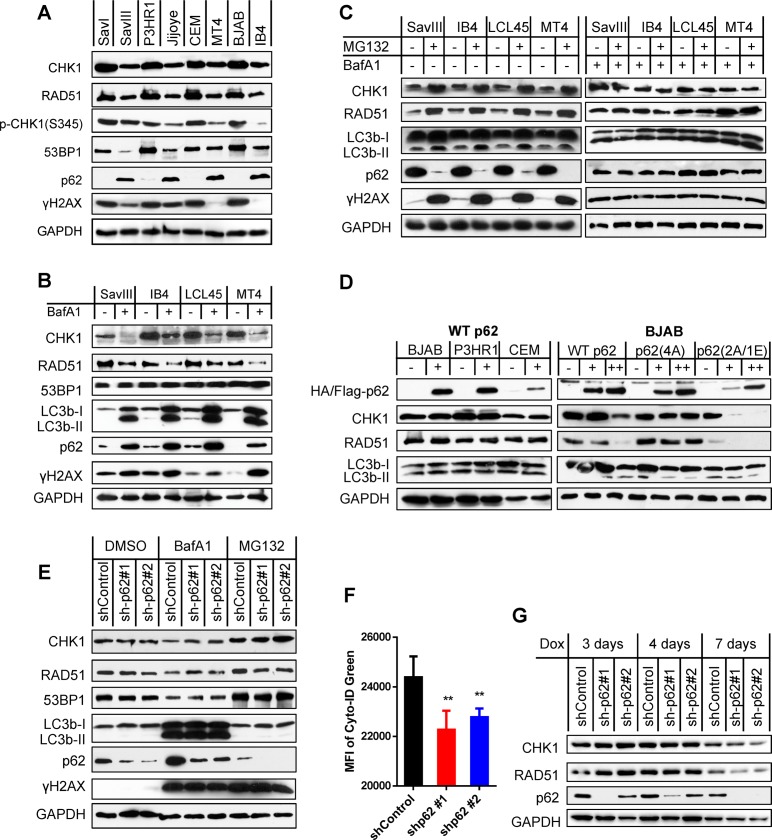
Nuclear p62 accumulation upon autophagy inhibition destabilizes HR DNA repair proteins CHK1 and RAD51 in viral latency
It has been reported that autophagy inhibition or nuclear p62 accruing from autophagy deficiency promotes proteasomal degradation of HR DNA repair proteins such as RAD51, CHK1 and FLNA [17, 49]. Our results show that SavIII, JiJoye, MT4, and IB4, which have higher endogenous p62 and autophagy levels (Fig 1B), have lower CHK1 and RAD51 protein levels as well as CHK1 activity (as indicated by phosphorylation of CHK1(S345)), compared to SavI, P3HR1, CEM, and BJAB, respectively (Fig 5A). These results suggest that the p62-autophagy interplay may also regulate proteasome-dependent stability of CHK1 and RAD51 proteins and CHK1 activity in viral latency. It has also been reported that p62 promotes NHEJ by activating the Keap1-NRF2 pathway in a feedback loop, which consequently induces expression of NHEJ-specific repair proteins such as 53BP1 [50]. However, our results show that 53BP1 reversely correlates with p62 at the protein level in viral latency (Fig 5A), indicating that NHEJ-mediated DNA repair activity is also compromised in virus-transformed cells. In addition, endogenous DNA damage (as indicated by γH2AX expression) is consistently lower in viral latency with higher p62-mediated autophagy levels, in which both HR and NHEJ pathways are deficient (Fig 5A), supporting our hypothesis that p62-mediated autophagy functions as an alternative mechanism that enables these cells to resist to DNA damage.
Fig 5. p62 accumulation upon autophagy inhibition destabilizes HR DNA repair proteins CHK1 and RAD51 in viral latency.
A. Correlation of p62 with CHK1, RAD51 and 53BP1 protein levels in viral latency were analyzed in paired cell lines. B. Cell lines with higher levels of p62 protein were treated with 0.4 µM BafA1 (Sigma) for 48 h. Indicated proteins were probed by immunoblotting. C. Cell lines with higher levels of p62 protein were treated with 10 µM of the proteasome inhibitor MG132 for 6 h (left panel), or pre-treated with 0.4 µM BafA1 before MG132. Indicated proteins were probed by immunoblotting. D. Cell lines with lower levels of p62 protein were transfected with 5 µg (+) or 10 µg (++) of HA-p62 plasmids, its mutants with Flag tag, or vector control in each electroporation (1X107 cells). Indicated proteins were analyzed by immunoblotting 48 h post-transfection. E-F. IB4 cells stably harboring p62 shRNA or shRNA control in 1 µg/ml puromycin were treated with 0.4 µM BafA1 for 48 h or 10 µM MG132 for 6 h, before subjected to immunoblotting or flow cytometry. shRNA expression was induced by 1 µg/ml doxycycline for 2 days before the drug treatments. MFI = mean fluorescence intensity. G. CHK1 and RAD51 protein stability was evaluated by immunoblotting in virus-transformed IB4 cells stably expressing control shRNA or p62 shRNA that were induced by 1 µg/ml doxycycline for different time points.
To check if autophagy has a role in regulation of the stability of these DNA repair proteins in virus-transformed cells, we inhibited endogenous autophagy activities with BafA1. Results show that autophagy inhibition did not affect 53BP1, but clearly decreases CHK1 and RAD51 protein levels that are associated with elevated endogenous p62 protein levels (Fig 5B).
To validate whether proteasome also mediates degradation of CHK1 and RAD51 in virus-transformed cells, we used the proteasome inhibitor MG132 to treat these cells that express high levels of endogenous p62. As expected, our results show that MG132 treatment remarkably increases the protein levels of CHK1 and RAD51 (Fig 5C, left panel), confirming that CHK1 and RAD51 protein stability is controlled in a proteasome-dependent manner in virus-transformed cells. In addition to its ability to inhibit proteasomal activity, MG132 is capable of inducing autophagy in various cancer cells [51]. Our results show that MG132 also increases the LC3b cleavage product LC3b-II in virus-transformed cells, and dramatically reduces p62 protein levels (Fig 5C, left panel).
To further validate the role of autophagy-p62 interplay in proteasomal degradation of CHK1 and RAD51, we pre-treated the cells with BafA1 to inhibit autophagy before inhibition of proteasome activity. Results show that MG132 failed to cause CHK1 and RAD51 accumulation after p62 restoration by autophagy inhibition (Fig 5C, right panel). These observations are in line with the previous report showing that CHK1 and RAD51 accumulation is attributable to p62 depletion resulting from robust autophagy induction [18].
Together, our results indicate that the CHK1 and RAD51 protein levels reversely correlate with the p62 levels in viral latency, implying a role of the autophagy-p62 interplay in negative regulation of their proteasome-mediated stability. However, the accumulation of CHK1 and RAD51 and decrease of p62 after MG132 treatment did not mitigate DNA damage; instead, DNA damage is strikingly increased (Fig 5C). This observation can be explained by the fact that proteasome function is required for DNA damage repair [52].
Next, we sought to evaluate whether transient expression of p62 regulates DNA repair protein stability. To this end, we transfected the expression plasmids harboring HA-p62 or pcDNA4 vector control into BJAB, P3HR1, and CEM cells, which express low levels of endogenous p62. Then, we analyzed CHK1, RAD51, and 53BP1. Surprisingly, results show that exogenic expression of p62 did not affect the protein levels of CHK1 and RAD51, and did not induce LC3b cleavage either (Fig 5D, left panel). By checking total p62 expression, we found that transfection of p62 expression plasmids did not evidently increase p62 levels, likely due to relatively low transfection efficiency of B cells. To address this issue, we transfected these cells with more p62 plasmids, and results show that a greater p62 expression is able to downregulate these proteins (Fig 5D, right panel). We further employed two p62 mutants, with p62(4A) constitutively localizing in the cytoplasm and p62(2A/1E) mainly localizing in the nucleus, since a recent report shows that nuclear accumulation of p62 responding to autophagy inhibition is required for its degradation of CHK1 and RAD51 [18]. As expected, the nucleus-localizing mutant p62(2A/1E), but not the cytoplasm-localizing mutant p62(4A), remarkably decreases CHK1 and RAD51 protein levels (Fig 5D, right panel). Together with Fig 5B and 5C, these results suggest that p62-mediated destabilization of DNA repair proteins is dependent on its accruing from autophagy inhibition, which we show results in p62 nuclear translocation (Fig 4D).
To further confirm the conditional role of p62 in downregulation of the DNA repair proteins, we depleted p62 expression in IB4 cells using shRNA-mediated gene knockdown. Results show that p62 depletion reduces autophagy activity, as shown by decreased autophagy flux (Fig 5F), consistent with the notion that p62 is required for endogenous autophagy induction. However, the protein levels of CHK1, RAD51, and 53BP1 have no consistent and apparent changes in p62-depleted cells (Fig 5E and 5G). Additional inhibition of the residual autophagy activities, or treatment with MG132, which induces autophagy (Fig 5C), also did not cause apparent difference on their levels in p62-depleted cells versus control cells (Fig 5E). Considering that the majority of p62 is spontaneously located in the cytoplasm of virus-transformed cells (Fig 4D), shRNA-mediated depletion might have minor effect on nuclear p62. Thus, these results are indeed consistent with our findings and a recent report that nuclear localization of p62 is required for destabilization of DNA repair proteins [18].
Collectively, these results demonstrate that p62 accumulation in the nucleus in response to autophagy inhibition promotes proteasomal degradation of RAD51 and CHK1 in virus-transformed cells. These results also indicate that a fine balance of p62 and autophagy levels is required to confine endogenous p62 in the cytoplasm of virus-transformed cells. Further study is required to determine how autophagy inhibition causes p62 accumulation in the nucleus.
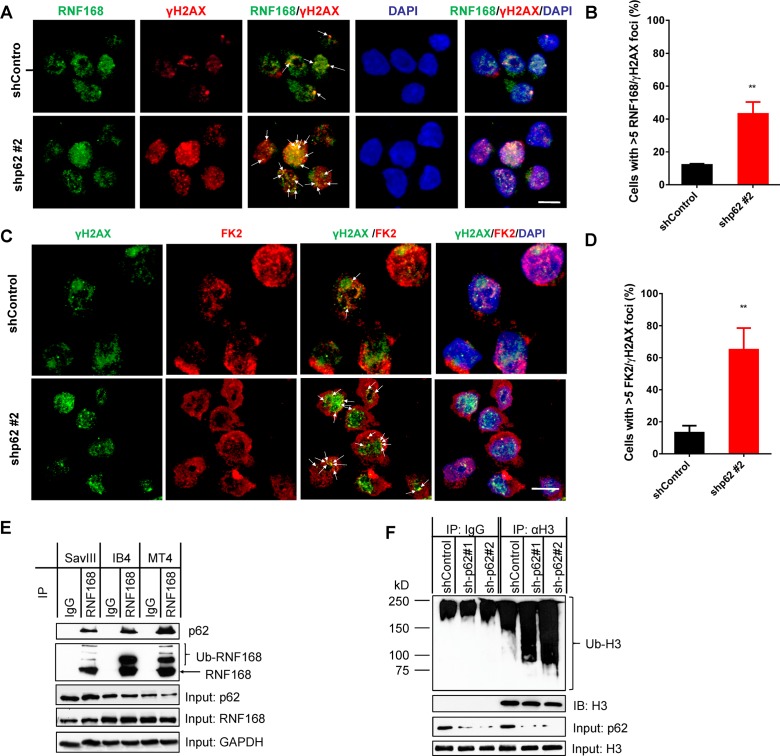
p62 depletion by RNA interference promotes RNF168-mediated chromatin ubiquitination and DNA repair in viral latency
It has been shown that p62 inhibits both HR and NHEJ [18], through its physical interaction with RNF168, which mediates histone ubiquitination that is prelude to activation of both HR and NHEJ DSB repair mechanisms [19].
Consistently, our confocal microscopy results show that RNF168 co-localizes with endogenous γH2AX DNA damage foci in IB4 cells. More importantly, shRNA-mediated p62 depletion significantly increases RNF168-γH2AX foci (Fig 6A and 6B). Further, using the anti-ubiquitinated proteins antibody FK2, we show that shRNA-mediated p62 depletion significantly increases ubiquitination and FK2-γH2AX foci in the nucleus (Fig 6C and 6D). We further validated the interaction of endogenous p62 with RNF168 by immunoprecipitation (IP) in virus-transformed cells treated with BafA1 (Fig 6E), and the increased histone H3 ubiquitination due to p62 depletion in cells treated with ionomycin (Fig 6F). Our IP assays failed to detect definite p62-RNF168 interaction and H3 ubiquitination in these cells without treatments. In conclusion, these results indicate that p62 depletion promotes chromatin ubiquitination and RNF168-mediated DNA repair mechanisms.
Fig 6. p62 depletion by RNA interference promotes RNF168-mediated chromatin ubiquitination and DNA repair in viral latency.
IB4 cells stably expressing control or p62 shRNA were maintained with 1 µg/ml puromycin, and shRNA expression was induced by 1 µg/ml doxycycline for 3 days before subjected to confocal microscopy analysis for: A. RNF168, γH2AX and their colocalization in the nucleus with a rabbit RNF168 antibody (Millipore) and an Alexa 488 coupled anti-rabbit antibody (Invitrogen), and a mouse γH2AX(S139) antibody (BioLegend) and an Alexa 555 coupled anti-mouse antibody (BioLegend); C. chromatin ubiquitination, γH2AX and their colocalization in the nucleus with the mouse ubiquitin antibody clone FK2 (Millipore) and the Alexa 555 coupled anti-mouse antibody, and a rabbit γH2AX(S139) antibody (Cell Signaling Technol.) and the Alexa 488 coupled anti-rabbit antibody. Bar = 6 µm. B and D. Quantification of RNF168/γH2AX foci or FK2/γH2AX foci in A and C, respectively. E. Interaction between RNF168 and p62 was assessed by immunoprecipitation. The indicated cell lines were treated with 0.4 µM BafA1 for 48h, and then cell lysates (0.5 mg total proteins for each) were subjected to immunoprecipitation with a rabbit RNF168 antibody (Proteintech Group Inc.), and immunoprecipitated proteins were analyzed by immunoblotting with the p62 antibody and the sheep RNF168 antibody (Invitrogen). F. Histone H3 ubiquitination was assessed in p62-depleted IB4 cells treated with 2.5 µg /ml ionomycin for 48 h. Cell lysates (0.5 mg total proteins for each) from IB4 cells stably expressing control or p62 shRNA were subjected to denatured immunoprecipitation with a rabbit H3 antibody (Invitrogen), and immunoprecipitated H3 was probed with the FK2 antibody and the mouse H3 antibody (1G1) (Santa Cruz).
Discussion
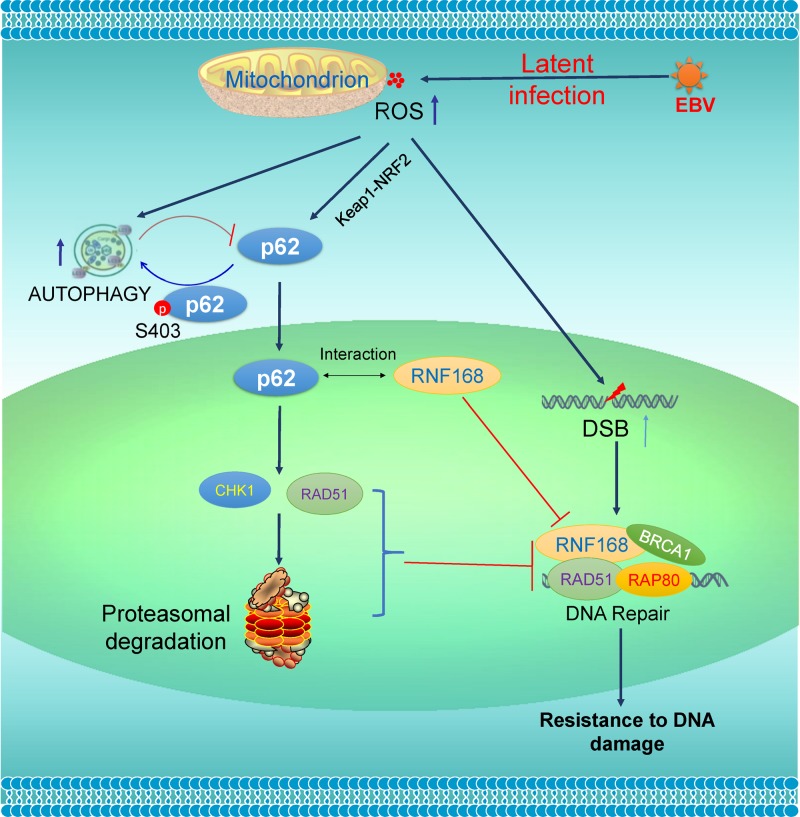
In this study, we provide several lines of evidence that support a crucial role for p62-mediated autophagy in regulation of DDR in oncogenic virus latent infection. First, p62 is upregulated by ROS that correlates with the activity of the Keap1-NRF2 pathway, and considerable levels of p62-mediated selective autophagy are constitutively induced in this setting. Second, inhibition of autophagy in virus-transformed cells exacerbates ROS-induced DNA damage, and destabilizes the DNA repair proteins RAD51 and CHK1 in a manner depending on p62 accumulation in the nucleus; in contrast, excess autophagy induction promotes accumulation of the DNA repair proteins CHK1 and RAD51 that is associated with p62 degradation. Third, shRNA-mediated p62 depletion promotes RNF168-mediated chromatin ubiquitination and DNA repair in EBV latency. These findings have defined a crucial role for p62-mediated autophagy in regulation of DDR in viral latency (Fig 7).
Fig 7. A diagram showing the interaction of p62-mediated autophagy with DDR in EBV latency.
EBV latent infection produces ROS, which further induce p62 expression through the Keap1-NRF2 pathway and activate p62-mediated selective autophagy. ROS also cause DNA damage, including double strand breaks (DSBs). p62 accumulated in the nucleus due to autophagy inhibition inhibits DSB repair through promoting proteasome-mediated degradation of CHK1 and RAD51 and interacting with RNF168. A moderate level of endogenous p62-mediated autophagy in virus-transformed cells endows them with resistance to DNA damage. Loss of the p62-autophagy balance by exogenic stresses that inhibit autophagy or inducing ROS will exacerbate endogenous DNA damage.
The p62-autophagy interplay is well balanced and controlled in diverse contexts, with cancer and aging being two representative systems [4–6, 53]. Loss of this balance by exogenic or endogenous stresses may result in different impacts on DDR. Pharmaceutical inhibition of autophagy, or spontaneous autophagy deficiency during aging, chronic inflammation, or neurodegeneration, leads to p62 accumulation, consequently attenuating DNA repair that accounts for the etiology of age-related disorders [54, 55]. In contrast, substantial enhancement of basal levels of autophagy in cancer cells by anticancer chemotherapeutic drugs or by radiation therapy promotes p62 degradation, and consequently confers these cells resistance to DNA damage-induced cell death [23, 56, 57]. Consistent with our findings, oncogenic viruses, including EBV, are known to inhibit ROS and autophagy at the early stage of lytic infection for optimal replication and oncogenic transformation [58–60], but induce ROS-mediated autophagy in their latency to suppress replication and support oncogenic survival [26, 27, 32, 38, 61–67].
Moreover, our results indicate that the basal levels of p62-mediated autophagy are distinctly regulated in different EBV latency programs. ROS are produced separately by the EBV products LMP1, EBNA1/2, and EBERs, amongst which LMP1 induces predominant ROS [68–72]. In consistent, our results show that EBV type III latency produces a greater level of ROS compared with type I latency (Fig 1A). Thus, cells with type III latency express higher endogenous levels of p62-mediated autophagy (Fig 1B), which are required to overcome the higher risk of DNA damage in response to endogenous higher oxidative stress and replication stress to support their aggressive proliferation, and growth and survival demands. As such, a higher level of p62-mediated autophagy also confers these cells greater resistance to DNA damage in response to drug treatments. The type III latency cell line P3HR1, which was derived from the parental JiJoye but lacks LMP1 expression, resembles type I latency cell lines in ROS production, and expression of p62 and autophagy (Fig 1), and DNA repair proteins (Fig 5), further supporting that LMP1 contributes the majority to these events.
Surprisingly, our results consistently show that, in contrast to p62 elimination by massive autophagy, p62 depletion by shRNA potentiates, but not alleviates, DNA damage. There are a few possibilities to explain this paradox. First, p62 depletion impairs p62-mediated selective autophagy that is resident in the cytoplasm (Fig 2E) and required for maintaining the ability of the cell to resist to DNA damage (Fig 5E and 5F); second, other p62-mediated, autophagy-independent, DNA damage-protecting functions are abrogated after shRNA-mediated p62 depletion and consequently DNA damage is accelerated, given the fact that p62 is a multifunctional protein [3]. Investigation of these potential p62-mediated but autophagy-independent functions in viral latency and oncogenesis is underway. In fact, p62 depletion resulting in worsen DNA damage is coincident with its role as a tumor promoter, which is induced by Ras that accounts for at least 25% of human cancers [73]. p62 overexpression in hepatocellular carcinoma (HCC) predicts poor prognosis [74].
Based on the observations from us and other relevant studies, we propose that p62 plays a dichotomous role in DDR, depending on the presence or absence of autophagy that determines the p62 protein level and subcellular localization. Higher levels of nuclear p62 resulting from defective autophagy inhibit DNA repair and therefore perturb genomic instability that facilitates tumor initiation [75]. In line with our findings, it has been reported that p62 ablation decreases tumorigenesis in mouse models with defective autophagy [73]. A recent report has also shown that autophagy resulting from telomere shortening during replicative crisis protects genomic stability, and acts as a suppressor of tumor initiation [76]. In contrast, considerable levels of p62 in cancer cells promote DNA repair by mediating selective autophagy activation in the cytoplasm, and consequently confer these cancer cells resistance to DNA damage. p62 is upregulated at considerable levels in different cancer cells, including breast and prostate cancers, where it is required for induction of selective autophagy to support cancer cell metabolism and survival [74, 77, 78].
Regarding the mechanisms underneath deregulation of DDR by the p62-autophagy interplay, our results indicate that autophagy inhibition promotes proteasomal degradation of RAD51 and CHK1 in a manner depending on p62 accumulation in the nucleus (Fig 5), and that p62 depletion promotes RNF168-mediated DDR (Fig 6). Our results show that the majority of endogenous p62 is tethered to autophagosomes in the cytoplasm (Fig 2E), but inhibition of DNA repair requires p62 in the nucleus [18]. Consistently, we show that autophagy inhibition promotes p62 nuclear translocation (Fig 4D), although the mechanism remains to be disclosed. Thus, our results define a new role for p62-mediated autophagy in preventing DNA damage by confining p62 in the cytoplasm. In conclusion, p62-mediated selective autophagy not only confers invulnerability to DNA damage, but also at least partially contributes to the deficiency of traditional DDR mechanisms, in virus-transformed cells. In this regard, it is to our understanding that an oncogenic virus gains a dual benefit by invoking p62-mediated autophagy: one facet of p62-mediated autophagy endows its host cell with ability to resist DNA damage to support cell survival; the other facet hijacks the traditional DDR mechanisms in the host cell to facilitate genomic instability that promotes accumulation of oncogenic mutations.
Although p62 was reported to promote NHEJ by inducing 53BP1 expression through the Keap1-NRF2 pathway that requests p62 S349 phosphorylation [50, 79], our results show that p62 reversely correlates with 53BP1 in viral latency (Fig 5A), and that p62 accumulation failed to regulate 53BP1 levels (Fig 5B). Thus, p62 does not regulate 53BP1 in our system, and how 53BP1 is downregulated in virus-transformed cells is worthy of further examination. Rather, p62 interacts with RNF168 in response to autophagy inhibition, and consequently inhibits RNF168-mediated chromatin ubiquitination in viral latency (Fig 6). In this regard, our findings are consistent with a recent study [18], which has shown that p62 can impede both HR and NHEJ through its interaction with RNF168, given that RNF168-mediated histone ubiquitination is prerequisite for activation of all DSB repair mechanisms [19]. Although these dsDNA repair pathways are compromised in EBV latency, we realize that they still have considerable levels of activity, which render successful CRISPR-mediated genome editing in these cells [80–83].
Viruses have evolved diverse strategies to hijack host traditional DDR machinery during their chronic infections to perturb genomic integrity, including their ability to deregulate the p62-autophagy balance, which we believe only makes partial contribution. In fact, our group has recently shown that traditional DDR mechanisms are also deficient in aging T cells in chronic HCV infection [84, 85], at least partially attributable to endogenous p62 accruing from deficient autophagy in these cells. p62 also inhibits DDR through other mechanisms that have not been fully elucidated. For example, nuclear p62 interacts with and inhibits PML nuclear bodies, which are involved in DNA repair [86, 87]. Moreover, other autophagy mechanisms, such as chaperone-mediated autophagy [88], also participate in DDR, by regulating stability of DDR-related proteins such as HP1α and CHK1 [89, 90], and by regulating p62-dependent or -independent cellular functions [91]. It is of great interest to investigate these potential mechanisms and their coupled cellular mechanisms in virus-mediated oncogenesis.
Endogenous ROS/RNS trigger signal cascades that activate both DDR and autophagy programs. Unrepaired damaged DNA can serve as a major source of genomic instability particularly in cancer cells where traditional DDR and cell death pathways are compromised. Thus, cancer cells heavily rely on autophagy, not only to replenish their deficient DNA repair mechanisms, but also to corroborate their higher metabolic demand than normal cells do. Therefore, cancer cells are more vulnerable to autophagy inhibition, providing viable opportunities for therapeutic strategy by targeting autophagy, in particular in combination with another cellular mechanism that is specifically coupled with autophagy in a given cancer context for improving clinical efficacy and specificity [3, 20, 92].
Materials and methods
Cell lines
SavI, SavIII, P3HR1 and JiJoye are human B cell lines derived from EBV-positive Burkitt’s lymphoma (BL) patients. P3HR1 was derived from JiJoye but does not express LMP1 due to lacking the entire EBNA2 ORF in the viral genome [93]. BJAB is an EBV-negative BL line. The lymphoblastic cell line (LCL) IB4 was derived from umbilical cord B-lymphocytes latently infected with EBV in vitro. KR4 is a LCL with gamma irradiation resistance [94]. LCL45 is a newly established LCL by in vitro transforming primary B cells of a healthy adult peripheral blood with the EBV strain B95.8. CEM is a HTLV1-negative, EBV-negative T cell line derived from acute leukemia, and MT4 is a HTLV1-transformed CD4+ T cell line derived from umbilical cord blood lymphocytes. B and T cell lines are cultured with RPMI1640 medium plus 10% FBS and antibiotics. All cell culture supplies were purchased from Life Technologies.
Antibodies and reagents
p62 (D-3), LIMD1 (H-4), and histone H3 (1G1) mouse monoclonal antibodies were from Santa Cruz for immunoprecipitation or immunoblotting. p62-Alexa Fluor 488 mouse antibody for flow cytometry was from Millipore or R&D Systems. Phospho-p62(S403), NRF2 (D1Z9C) mouse monoclonal antibody, and HRP-coupled secondary antibodies were from Cell Signaling Technologies. RNF168 rabbit polyclonal antibody and the FK2 mouse monoclonal antibody that recognizes K29-, K48-, and K63-linked polyubiquitin chains and monoubiquitin conjugation but not free ubiquitin for immunofluorescence were from Millipore. The RNF168 rabbit polyclonal antibody for IP was from Proteintech Group Inc. RNF168 sheep polyclonal, and LC3b and histone H3 rabbit polyclonal antibodies were from Invitrogen. RAD51 rabbit polyclonal and CHK1 (G-4) mouse monoclonal antibodies were from Abcam and Santa Cruz, respectively. The γH2AX(S139) mouse and rabbit antibodies were from BioLegend and Cell Signaling Technologies, respectively. Mouse HA (clone HA-7) and Flag (clone M2) antibodies were from Sigma. Secondary antibodies coupled with FITC, Alexa Fluor, APC, PE, Cy5, or PerCP and human anti-CD19-PE were from BioLegend, BD Biosciences, Invitrogen, or eBioscience.
HA-p62 cloned in pcDNA4 was a gift from Yu-Ying He [95], and Flag-p62 mutants were gifts from Dr. Ying Zhao [18]. Flag-p62(R186A/K187A/K264A/R265A) (designated as p62(4A)) cannot localize in the nucleus and Flag-p62(K7A/D69A/I314E) (designated as p62(2A/1E)) mainly localizes in the nucleus [18]. CellRox Green, MG132, chloroquine, and doxorubicin HCl, were purchased from Invitrogen, EMD Millipore, MP Biomedicals, and UBPBio, respectively. Ionomycin calcium salt, N-acetylcysteine amide, bafilomycin A1, doxycycline, and mouse and rabbit IgG were purchased from Sigma. H2O2 was from Santa Cruz.
Transfection and selection of stable transfectants
A set of p62 shRNA cloned in pTRIPz/Puro comprising three individual p62 shRNA and a scramble control plasmids were purchased from Dharmacon. We selected two of them for this study and the targeting sequences on the human p62/SQSTM1 gene are: shRNA#1: 5’-TCTCTTTAATGTAGATTCG-3’ and shRNA#2: 5’- TCAGGAAATTCACACTCGG-3’. Lentiviral packing, preparation, infection, and selection of stable cells by puromycin (0.5 µg/ml) were performed as detailed in our previous publication [37]. shRNA expression was induced by 1 µg/ml doxycycline for 3 days, and then cells (expressing red fluorescence) were subjected to a second selection by FACS on a FACS/Aria Fusion Cell Sorter (BD Biosciences). Stable transfectants were maintained in complete medium plus 1.0 µg/ml puromycin.
For transfection of BJAB, P3HR1, and CEM, GenePulser XCell (Bio-Rad) was used with optimal programs. These representative cell lines were chosen in that they are easier to be transfected with this technique, compared with other B cell lines.
Confocal microscopy
Cells were fixed in 2% paraformaldehyde (PFA) for 20 min, permeabilized with 0.3% Triton X-100 in phosphate-buffered saline (PBS) for 10 min, blocked with 5% bovine serum albumin (BSA) in PBS for 1 h, and then incubated with indicated primary antibodies at 4°C overnight. Cells were washed with PBS with 0.1% Tween-20 for three times, and then incubated with corresponding secondary antibodies coupled with FITC, Alexa Fluor, APC, PE, Cy5, or PerCP, at room temperature for 1 h. Cells were then washed and mounted with DAPI Fluoromount-G (SouthernBiotech, Birmingham, AL). Images were acquired with a confocal laser-scanning inverted microscope (Leica Confocal, Model TCS sp8, Germany).
Immunoprecipitation and immunoblotting
To assess endogenous p62-RNF168 interaction, 1X107 cells for each sample were lysed with NP40 lysis buffer (150 mM NaCl, 1% NP-40, 50 mM Tris-pH 8.0, plus protease inhibitors), and cell lysates were subjected to immunoprecipitation (IP) with 1.5 µg anti-RNF168 for overnight, and then incubated with 40 µl Protein A/G beads (Santa Cruz) for 1 h. After three washes, proteins on beads were denatured in 1% SDS before subjected to immunoblotting (IB). IB was carried out with indicated antibodies and signals were detected with an enhanced chemiluminescence (ECL) kit following the manufacturer’s protocol (Amersham Pharmacia Biotech). For H3 ubiquitination assay, endogenous H3 was pulled down with an H3 antibody in denaturing IP, as detailed in our publication [96].
RNA Extraction and real-time quantitative PCR
Total RNA was isolated from tested cells using an RNeasy Mini kit according to the manufacturer's protocols (Qiagen). The eluted RNA was subjected to reverse transcriptase reactions, which were performed with the use of GoScript RT kit following the manufacturer's instructions (Promega).
Quantitative real-time PCR (qPCR) was performed with the use of SYBR Green (Applied Biosystems), on a CFX96 Real-time PCR Detection System (Bio-Rad). All reactions were run in triplicates. Mean cycle threshold (Ct) values were normalized to 18s rRNA, yielding a normalized Ct (ΔCt). ΔΔCt value was calculated by subtracting respective control from the ΔCt, and expression level was then calculated by 2 raised to the power of respective -ΔΔCt value. The averages of 2^(-ΔΔCt) in the control samples were set to 1 or 100%. Results are the average ± standard error (SE) of triplicates for each sample. Primers for real-time qPCR are as follows: p62: F: 5'-CAGGCGCACTACCGCGATG-3' and R: 5'-ACACAAGTCGTAGTCTGGGCAGAC-3'. Keap1: F: 5’-CCATGGGCGAGAAGTGTGTCC-3’; R: 5'-ACAGGTTGAAGAACTCCTCTTGCTTG-3’. 18s rRNA: F: 5’-GGCCCTGTAATTGGAATGAGTC-3’ and R: 5’-CCAAGATCCAACTACGAGCTT-3’.
Flow cytometry
Samples were fixed with 2% PFA for 20 min at RT, then wash with flow buffer (eBioscience). Samples were then incubated with PE-conjugated anti-human CD19 antibody (eBioscience) or isotype controls for 20 min at RT, then wash with flow buffer, followed by incubation with p62-Alexa Fluor 488 antibody for 60 min at RT. Samples were then washed with flow buffer, and analyzed with BD C6 plus flow cytometer.
For intracellular ROS measurement, 1X106 cells in 500 µl medium per well were seeded in 24-well plates, and cultured overnight. 1 µl CellROX Green Reagent (Invitrogen) was added to each well and incubated for 30 min. Cells were then washed 3 times with PBS, and fixed with 2% PFA for 20min at RT, followed by extensive washes and then incubated with PE-conjugated anti-human CD19 antibody (eBioscience) for 20min at RT, before subjected to flow cytometry.
Apoptosis assays
Apoptosis was quantified using flow cytometry as detailed in our previous publication [37], for Annex V binding (BD Biosciences) and 7-Aminoactinomycin D (7-AAD) expression (eBioscience). Caspase 3 activity was evaluated by Western blotting.
Statistical analysis
Unpaired, two-tailed student t tests were executed using GraphPad Prism (version 6) to determine the differences between two data sets obtained from three independent experiments. p<0.05 (*) and p<0.01 (**), and p<0.001 (***) were considered significant. Data are expressed as mean ± standard error (SE) of duplicate or triplicate samples, and representative results from at least three independent repeats with similar results are shown.
Acknowledgments
We thank Drs. Yu-Ying He and Ying Zhao for the p62 expression plasmids. This study was conducted with resources and the use of facilities at the James H. Quillen Veterans Affairs Medical Center. The contents in this publication are solely the responsibility of the authors and does not necessarily represent the official views of the NIH, the Department of Veterans Affairs, or the United States Government.
Data Availability
All relevant data are within the manuscript.
Funding Statement
This study was supported by the NIDCR of NIH under Award Number R15DE027314 to Shunbin Ning, and in part by the NIH grant C06RR0306551. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Moscat J, Karin M, Diaz-Meco MT. p62 in Cancer: Signaling Adaptor Beyond Autophagy. Cell. 2016;167(3):606–9. 10.1016/j.cell.2016.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bitto A, Lerner CA, Nacarelli T, Crowe E, Torres C, Sell C. p62/SQSTM1 at the interface of aging, autophagy, and disease. AGE. 2014;36(3):9626 10.1007/s11357-014-9626-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ning S, Wang L. The Multifunctional Protein p62 and Its Mechanistic Roles in Cancers. Current Cancer Drug Targets. 2019;19:1–11. 10.2174/1568009618666181016164920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilde L, Tanson K, Curry J, Martinez-Outschoorn U. Autophagy in cancer: a complex relationship. Biochemical Journal. 2018;475(11):1939–54. 10.1042/BCJ20170847 [DOI] [PubMed] [Google Scholar]
- 5.Singh SS, Vats S, Chia AY-Q, Tan TZ, Deng S, Ong MS, et al. Dual role of autophagy in hallmarks of cancer. Oncogene. 2018;37(9):1142–58. 10.1038/s41388-017-0046-6 [DOI] [PubMed] [Google Scholar]
- 6.Rybstein MD, Bravo-San Pedro JM, Kroemer G, Galluzzi L. The autophagic network and cancer. Nature Cell Biology. 2018;20(3):243–51. 10.1038/s41556-018-0042-2 [DOI] [PubMed] [Google Scholar]
- 7.Farre J-C, Subramani S. Mechanistic insights into selective autophagy pathways: lessons from yeast. Nat Rev Mol Cell Biol. 2016;17(9):537–52. 10.1038/nrm.2016.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaffagnini G, Martens S. Mechanisms of Selective Autophagy. Journal of Molecular Biology. 2016;428(9Part A):1714–24. 10.1016/j.jmb.2016.02.004 PubMed PMID: PMC4871809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sparrer KMJ, Gack MU. TRIM proteins: New players in virus-induced autophagy. PLOS Pathogens. 2018;14(2):e1006787 10.1371/journal.ppat.1006787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murakawa T, Yamaguchi O, Hashimoto A, Hikoso S, Takeda T, Oka T, et al. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nature Communications. 2015;6:7527 10.1038/ncomms8527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Medicine and Cellular Longevity. 2016;2016:44 10.1155/2016/1245049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sallmyr A, Fan J, Rassool FV. Genomic instability in myeloid malignancies: Increased reactive oxygen species (ROS), DNA double strand breaks (DSBs) and error-prone repair. Cancer Letters. 2008;270(1):1–9. 10.1016/j.canlet.2008.03.036 [DOI] [PubMed] [Google Scholar]
- 13.Vilenchik MM, Knudson AG. Endogenous DNA double-strand breaks: Production, fidelity of repair, and induction of cancer. Proceedings of the National Academy of Sciences. 2003;100(22):12871–6. 10.1073/pnas.2135498100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang HHY, Pannunzio NR, Adachi N, Lieber MR. Non-homologous DNA end joining and alternative pathways to double-strand break repair. Nat Rev Mol Cell Biol. 2017;18(8):495–506. 10.1038/nrm.2017.48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tubbs A, Nussenzweig A. Endogenous DNA Damage as a Source of Genomic Instability in Cancer. Cell. 2017;168(4):644–56. 10.1016/j.cell.2017.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain A, Lamark T, Sjøttem E, Bowitz Larsen K, Atesoh Awuh J, Øvervatn A, et al. p62/SQSTM1 Is a Target Gene for Transcription Factor NRF2 and Creates a Positive Feedback Loop by Inducing Antioxidant Response Element-driven Gene Transcription. Journal of Biological Chemistry. 2010;285(29):22576–91. 10.1074/jbc.M110.118976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewitt G, Carroll B, Sarallah R, Correia-Melo C, Ogrodnik M, Nelson G, et al. SQSTM1/p62 mediates crosstalk between autophagy and the UPS in DNA repair. Autophagy. 2016;12(10):1917–30. 10.1080/15548627.2016.1210368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Zhang N, Zhang L, Li R, Fu W, Ma K, et al. Autophagy Regulates Chromatin Ubiquitination in DNA Damage Response through Elimination of SQSTM1/p62. Molecular Cell. 2016;63(1):34–48. 10.1016/j.molcel.2016.05.027 [DOI] [PubMed] [Google Scholar]
- 19.Schwertman P, Bekker-Jensen S, Mailand N. Regulation of DNA double-strand break repair by ubiquitin and ubiquitin-like modifiers. Nat Rev Mol Cell Biol. 2016;17(6):379–94. 10.1038/nrm.2016.58 [DOI] [PubMed] [Google Scholar]
- 20.Santana-Codina N, Mancias JD, Kimmelman AC. The Role of Autophagy in Cancer. Annual Review of Cancer Biology. 2017;1(1):19–39. 10.1146/annurev-cancerbio-041816-122338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Connor Mark J. Targeting the DNA Damage Response in Cancer. Molecular Cell. 2015;60(4):547–60. 10.1016/j.molcel.2015.10.040 [DOI] [PubMed] [Google Scholar]
- 22.Mathew R, White E. Autophagy, Stress, and Cancer Metabolism: What Doesn't Kill You Makes You Stronger. Cold Spring Harbor Symposia on Quantitative Biology. 2011;76:389–96. 10.1101/sqb.2012.76.011015 [DOI] [PubMed] [Google Scholar]
- 23.Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, et al. Autophagy and chemotherapy resistance: a promising therapeutic target for cancer treatment. Cell Death & Disease. 2013;4(10):e838 10.1038/cddis.2013.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee DY, Sugden B. The latent membrane protein 1 oncogene modifies B-cell physiology by regulating autophagy. Oncogene. 2008;27(20):2833–42. 10.1038/sj.onc.1210946 [DOI] [PubMed] [Google Scholar]
- 25.Ditzel M, Broemer M, Tenev T, Bolduc C, Lee TV, Rigbolt KTG, et al. Inactivation of effector caspases through nondegradative polyubiquitylation. Mol Cell. 2008;32(4):540–53. 10.1016/j.molcel.2008.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cavignac Y, Esclatine A. Herpesviruses and Autophagy: Catch Me If You Can! Viruses. 2010;2(1):314 10.3390/v2010314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pratt ZL, Sugden B. How human tumor viruses make use of autophagy. Cells. 2012;1(3):617–30. 10.3390/cells1030617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hau P, Tsao S. Epstein–Barr Virus Hijacks DNA Damage Response Transducers to Orchestrate Its Life Cycle. Viruses. 2017;9(11):341 10.3390/v9110341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Whitehurst CB, Vaziri C, Shackelford J, Pagano JS. Epstein-Barr Virus BPLF1 Deubiquitinates PCNA and Attenuates Polymerase η Recruitment to DNA Damage Sites. Journal of Virology. 2012;86(15):8097–106. 10.1128/JVI.00588-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hafez A, Luftig M. Characterization of the EBV-Induced Persistent DNA Damage Response. Viruses. 2017;9(12):366 10.3390/v9120366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Leo A, Colavita F, Ciccosanti F, Fimia GM, Lieberman PM, Mattia E. Inhibition of autophagy in EBV-positive Burkitt’s lymphoma cells enhances EBV lytic genes expression and replication. Cell Death &Amp; Disease. 2015;6:e1876 https://www.nature.com/articles/cddis2015156#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pujals A, Favre L, Pioche-Durieu C, Robert A, Meurice G, Le Gentil M, et al. Constitutive autophagy contributes to resistance to TP53-mediated apoptosis in Epstein-Barr virus-positive latency III B-cell lymphoproliferations. Autophagy. 2015;11(12):2275–87. 10.1080/15548627.2015.1115939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masucci MG. Epstein-Barr virus oncogenesis and the ubiquitin-proteasome system. Oncogene. 2004;23(11):2107–15. 10.1038/sj.onc.1207372 [DOI] [PubMed] [Google Scholar]
- 34.Dantuma NP, Masucci MG. The ubiquitin/proteasome system in Epstein-Barr virus latency and associated malignancies. Sem Cancer Biol. 2003;13(1):69–76. DOI: 10.1016/S1044-579X(02)00101-3 [DOI] [PubMed] [Google Scholar]
- 35.Shackelford J, Pagano J. Role of the ubiquitin system and tumor viruses in AIDS-related cancer. BMC Biochem. 2007;8(Suppl 1):S8 10.1186/1471-2091-8-S1-S8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L, Wang Y, Zhao J, Ren J, Hall KH, Moorman JP, et al. LUBAC modulates LMP1 activation of NFκB and IRF7. Journal of Virology. 2017;91(4):e1138–16. 10.1128/JVI.01138-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, Riggs K, Kohne C, Yohanon JU, Foxler DE, Sharp TV, et al. LIMD1 Is Induced by and Required for LMP1 Signaling, and Protects EBV-Transformed Cells from DNA Damage-Induced Cell Death. Oncotarget. 2018;9(5):6282–97. 10.18632/oncotarget.23676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lussignol M, Esclatine A. Herpesvirus and Autophagy: “All Right, Everybody Be Cool, This Is a Robbery!”. Viruses. 2017;9(12):372 10.3390/v9120372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barth S, Glick D, Macleod KF. Autophagy: assays and artifacts. The Journal of pathology. 2010;221(2):117–24. 10.1002/path.2694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matsumoto G, Wada K, Okuno M, Kurosawa M, Nukina N. Serine 403 phosphorylation of p62/SQSTM1 regulates selective autophagic clearance of ubiquitinated proteins. Mol Cell. 2011;44 10.1016/j.molcel.2011.07.039 [DOI] [PubMed] [Google Scholar]
- 41.Rogov V, Dotsch V, Johansen T, Kirkin V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol Cell. 2014;53 10.1016/j.molcel.2013.12.014 [DOI] [PubMed] [Google Scholar]
- 42.Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taguchi K, Fujikawa N, Komatsu M, Ishii T, Unno M, Akaike T, et al. Keap1 degradation by autophagy for the maintenance of redox homeostasis. Proceedings of the National Academy of Sciences. 2012;109(34):13561–6. 10.1073/pnas.1121572109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang DD, Lo S-C, Cross JV, Templeton DJ, Hannink M. Keap1 Is a Redox-Regulated Substrate Adaptor Protein for a Cul3-Dependent Ubiquitin Ligase Complex. Molecular and Cellular Biology. 2004;24(24):10941–53. 10.1128/MCB.24.24.10941-10953.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar B, Roy A, Asha K, Sharma-Walia N, Ansari MA, Chandran B. HACE1, an E3 ubiquitin-protein ligase, Mitigates KSHV Infection Induced Oxidative Stress by Promoting Nrf2 Activity. Journal of Virology. 2019:JVI.01812-18. 10.1128/jvi.01812-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gjyshi O, Flaherty S, Veettil MV, Johnson KE, Chandran B, Bottero V. KSHV Induces Nrf2 Activation in Latently Infected Endothelial Cells through SQSTM1 Phosphorylation and Interaction with Polyubiquitinated Keap1. Journal of Virology. 2015;89(4):2268–86. 10.1128/JVI.02742-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurz EU, Douglas P, Lees-Miller SP. Doxorubicin Activates ATM-dependent Phosphorylation of Multiple Downstream Targets in Part through the Generation of Reactive Oxygen Species. Journal of Biological Chemistry. 2004;279(51):53272–81. 10.1074/jbc.M406879200 [DOI] [PubMed] [Google Scholar]
- 48.Hussain T, Mulherkar R. Lymphoblastoid Cell lines: a Continuous in Vitro Source of Cells to Study Carcinogen Sensitivity and DNA Repair. International Journal of Molecular and Cellular Medicine. 2012;1(2):75–87. [PMC free article] [PubMed] [Google Scholar]
- 49.Liu EY, Xu N, O’Prey J, Lao LY, Joshi S, Long JS, et al. Loss of autophagy causes a synthetic lethal deficiency in DNA repair. Proceedings of the National Academy of Sciences. 2015;112(3):773–8. 10.1073/pnas.1409563112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim SB, Pandita RK, Eskiocak U, Ly P, Kaisani A, Kumar R, et al. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(43):E2949–E55. Epub 10/08. 10.1073/pnas.1207718109 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ding W-X, Ni H-M, Gao W, Yoshimori T, Stolz DB, Ron D, et al. Linking of Autophagy to Ubiquitin-Proteasome System Is Important for the Regulation of Endoplasmic Reticulum Stress and Cell Viability. The American Journal of Pathology. 2007;171(2):513–24. 10.2353/ajpath.2007.070188 PubMed PMID: PMC1934546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacquemont C, Taniguchi T. Proteasome Function Is Required for DNA Damage Response and Fanconi Anemia Pathway Activation. Cancer Research. 2007;67(15):7395–405. 10.1158/0008-5472.CAN-07-1015 [DOI] [PubMed] [Google Scholar]
- 53.Martinez-Lopez N, Athonvarangkul D, Singh R. Autophagy and aging. Advances in experimental medicine and biology. 2015;847:73–87. 10.1007/978-1-4939-2404-2_3 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matsuzawa-Ishimoto Y, Hwang S, Cadwell K. Autophagy and Inflammation. Annual Review of Immunology. 2018;36(1):73–101. 10.1146/annurev-immunol-042617-053253 . [DOI] [PubMed] [Google Scholar]
- 55.Hansen M, Rubinsztein DC, Walker DW. Autophagy as a promoter of longevity: insights from model organisms. Nature Reviews Molecular Cell Biology. 2018;19(9):579–93. 10.1038/s41580-018-0033-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Marinković M, Šprung M, Buljubašić M, Novak I. Autophagy Modulation in Cancer: Current Knowledge on Action and Therapy. Oxidative Medicine and Cellular Longevity. 2018;2018:18 10.1155/2018/8023821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bouwman P, Jonkers J. The effects of deregulated DNA damage signalling on cancer chemotherapy response and resistance. Nat Rev Cancer. 2012;12(9):587–98. 10.1038/nrc3342 [DOI] [PubMed] [Google Scholar]
- 58.Cirone M. EBV and KSHV Infection Dysregulates Autophagy to Optimize Viral Replication, Prevent Immune Recognition and Promote Tumorigenesis. Viruses. 2018;10:599 10.3390/v10110599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Granato M, Santarelli R, Farina A, Gonnella R, Lotti LV, Faggioni A, et al. Epstein-Barr Virus Blocks the Autophagic Flux and Appropriates the Autophagic Machinery To Enhance Viral Replication. Journal of Virology. 2014;88(21):12715–26. 10.1128/JVI.02199-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gilardini Montani MS, Santarelli R, Granato M, Gonnella R, Torrisi MR, Faggioni A, et al. EBV reduces autophagy, intracellular ROS and mitochondria to impair monocyte survival and differentiation. Autophagy. 2018:1–16. 10.1080/15548627.2018.1536530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paul P, Münz C. Autophagy and Mammalian Viruses. Advances in Virus Research. 2016;95:149–95. 10.1016/bs.aivir.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 62.Silva LM, Jung JU. Modulation of the autophagy pathway by human tumor viruses. Semin Cancer Biol. 2013;23(5):323–8. 10.1016/j.semcancer.2013.05.005 ; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taylor G, Mautner J, Munz C. Autophagy in herpesvirus immune control and immune escape. Herpesviridae. 2011;2(1):2 10.1186/2042-4280-2-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams LR, Taylor GS. Autophagy and immunity–insights from human herpesviruses. Frontiers in Immunology. 2012;3:170 10.3389/fimmu.2012.00170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Leo A, Colavita F, Ciccosanti F, Fimia GM, Lieberman PM, Mattia E. Inhibition of autophagy in EBV-positive Burkitt's lymphoma cells enhances EBV lytic genes expression and replication. Cell death & disease. 2015;6(9):e1876–e. 10.1038/cddis.2015.156 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhattacharjee S, Bose P, Patel K, Roy SG, Gain C, Gowda H, et al. Transcriptional and epigenetic modulation of autophagy promotes EBV oncoprotein EBNA3C induced B-cell survival. Cell Death & Disease. 2018;9(6):605 10.1038/s41419-018-0668-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McFadden K, Hafez AY, Kishton R, Messinger JE, Nikitin PA, Rathmell JC, et al. Metabolic stress is a barrier to Epstein–Barr virus-mediated B-cell immortalization. Proceedings of the National Academy of Sciences. 2016;113(6):E782–E90. 10.1073/pnas.1517141113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gruhne B, Sompallae R, Masucci MG. Three Epstein-Barr virus latency proteins independently promote genomic instability by inducing DNA damage, inhibiting DNA repair and inactivating cell cycle checkpoints. Oncogene. 2009;28(45):3997–4008. 10.1038/onc.2009.258 [DOI] [PubMed] [Google Scholar]
- 69.Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, et al. Reactive oxygen signaling and MAPK activation distinguish Epstein–Barr Virus (EBV)-positive versus EBV-negative Burkitt's lymphoma. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(1):175–9. 10.1073/pnas.0408381102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gruhne B, Sompallae R, Marescotti D, Kamranvar SA, Gastaldello S, Masucci MG. The Epstein-Barr virus nuclear antigen-1 promotes genomic instability via induction of reactive oxygen species. Proceedings of the National Academy of Sciences. 2009;106(7):2313–8. 10.1073/pnas.0810619106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raab-Traub N. Epstein-Barr virus in the pathogenesis of NPC. Seminars in Cancer Biology. 2002;12(6):431–41. [DOI] [PubMed] [Google Scholar]
- 72.Kim SM, Hur DY, Hong SW, Kim JH. EBV-encoded EBNA1 regulates cell viability by modulating miR34a-NOX2-ROS signaling in gastric cancer cells. Biochem Biophys Res Commun. 2017;494(3–4):550–5. 10.1016/j.bbrc.2017.10.095 . [DOI] [PubMed] [Google Scholar]
- 73.Duran A, Linares JF, Galvez AS, Wikenheiser K, Flores JM, Diaz-Meco MT, et al. The Signaling Adaptor p62 Is an Important NF-κB Mediator in Tumorigenesis. Cancer Cell. 2008;13(4):343–54. 10.1016/j.ccr.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 74.Umemura A, He F, Taniguchi K, Nakagawa H, Yamachika S, Font-Burgada J, et al. p62, Upregulated during Preneoplasia, Induces Hepatocellular Carcinogenesis by Maintaining Survival of Stressed HCC-Initiating Cells. Cancer Cell. 2016;29(6):935–48. 10.1016/j.ccell.2016.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang J, Peng H, Xu Y, Xie X, Hu R. SQSTM1/p62 (sequestosome 1) senses cellular ubiquitin stress through E2-mediated ubiquitination. Autophagy. 2018;14(6):1072–3. 10.1080/15548627.2017.1332566 ;. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nassour J, Radford R, Correia A, Fusté JM, Schoell B, Jauch A, et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019. 10.1038/s41586-019-0885-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson HGR, Harris JW, Wold BJ, Lin F, Brody JP. p62 overexpression in breast tumors and regulation by prostate-derived Ets factor in breast cancer cells. Oncogene. 2003;22(15):2322–33. 10.1038/sj.onc.1206325 [DOI] [PubMed] [Google Scholar]
- 78.Roodman GD, Hiruma Y, Kurihara N. p62: A Potential Target for Blocking Microenvironmental Support of Myeloma. Clinical Lymphoma and Myeloma. 2009;9:S25–S6. 10.3816/CLM.2009.s.004 [DOI] [Google Scholar]
- 79.Ichimura Y, Waguri S, Sou YS, Kageyama S, Hasegawa J, Ishimura R, et al. Phosphorylation of p62 activates the Keap1-Nrf2 pathway during selective autophagy. Mol Cell. 2013;51 10.1016/j.molcel.2013.08.003 [DOI] [PubMed] [Google Scholar]
- 80.Jiang S, Wang LW, Walsh MJ, Trudeau SJ, Gerdt C, Zhao B, et al. CRISPR/Cas9-Mediated Genome Editing in Epstein-Barr Virus-Transformed Lymphoblastoid B-Cell Lines. Current Protocols in Molecular Biology. 2018;121(1):31.12.1–31.12.23. 10.1002/cpmb.51 [DOI] [PubMed] [Google Scholar]
- 81.van Diemen FR, Lebbink RJ. CRISPR/Cas9, a powerful tool to target human herpesviruses. Cellular Microbiology. 2017;19(2):e12694 10.1111/cmi.12694 [DOI] [PubMed] [Google Scholar]
- 82.Chen Y-C, Sheng J, Trang P, Liu F. Potential Application of the CRISPR/Cas9 System against Herpesvirus Infections. Viruses. 2018;10(6):291 10.3390/v10060291 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jiang S, Wang LW, Walsh MJ, Trudeau SJ, Gerdt C, Zhao B, et al. CRISPR/Cas9-Mediated Genome Editing in Epstein-Barr Virus-Transformed Lymphoblastoid B-Cell Lines. Curr Protoc Mol Biol. 2018;121:31 12 1–31 12 23. 10.1002/cpmb.51 . [DOI] [PubMed] [Google Scholar]
- 84.Zhao J, Dang X, Lam N, Cao D, Wu X, Zheng M, et al. Insufficiency of DNA repair enzyme ATM promotes naïve CD4 T cell loss in chronic hepatitis C virus infection. Cell Discovery. 2018;4(16): 10.1038/s41421-018-0015-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nguyen LN, Zhao J, Cao D, Dang X, Wang L, Lian J, et al. Inhibition of TRF2 accelerates telomere attrition and DNA damage in naïve CD4 T cells during HCV infection. Cell Death and Diseases. 2018;9:900 10.1038/s41419-018-0897-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pankiv S, Lamark T, Bruun J-A, Øvervatn A, Bjørkøy G, Johansen T. Nucleocytoplasmic Shuttling of p62/SQSTM1 and Its Role in Recruitment of Nuclear Polyubiquitinated Proteins to Promyelocytic Leukemia Bodies. Journal of Biological Chemistry. 2010;285(8):5941–53. 10.1074/jbc.M109.039925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dellaire G, Bazett-Jones DP. PML nuclear bodies: dynamic sensors of DNA damage and cellular stress. BioEssays. 2004;26(9):963–77. 10.1002/bies.20089 [DOI] [PubMed] [Google Scholar]
- 88.Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nature Reviews Molecular Cell Biology. 2018;19(6):365–81. 10.1038/s41580-018-0001-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chen S, Wang C, Sun L, Wang D-L, Chen L, Huang Z, et al. RAD6 Promotes Homologous Recombination Repair by Activating the Autophagy-Mediated Degradation of Heterochromatin Protein HP1. Molecular and Cellular Biology. 2015;35(2):406–16. 10.1128/MCB.01044-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park C, Suh Y, Cuervo AM. Regulated degradation of Chk1 by chaperone-mediated autophagy in response to DNA damage. Nature Communications. 2015;6:6823 10.1038/ncomms7823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Eliopoulos AG, Havaki S, Gorgoulis VG. DNA Damage Response and Autophagy: A Meaningful Partnership. Frontiers in Genetics. 2016;7:204 PubMed PMID: PMC5116470. 10.3389/fgene.2016.00204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Islam MA, Sooro MA, Zhang P. Autophagic Regulation of p62 is Critical for Cancer Therapy. International Journal of Molecular Sciences. 2018;19(5):1405 10.3390/ijms19051405 PubMed PMID: PMC5983640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hinuma Y, Konn M, Yamaguchi J, Wudarski DJ, Blakeslee JR, Grace JT. Immunofluorescence and Herpes-Type Virus Particles in the P3HR-1 Burkitt Lymphoma Cell Line. Journal of virology. 1967;1(5):1045–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kozbor D, Lagarde AE, Roder JC. Human hybridomas constructed with antigen-specific Epstein-Barr virus-transformed cell lines. Proc Natl Acad Sci U S A. 1982;79(21):6651–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qiang L, Zhao B, Ming M, Wang N, He T-C, Hwang S, et al. Regulation of cell proliferation and migration by p62 through stabilization of Twist1. Proceedings of the National Academy of Sciences. 2014;111(25):9241–6. 10.1073/pnas.1322913111 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 96.Ning S, Campos AD, Darnay B, Bentz G, Pagano JS. TRAF6 and the three C-terminal lysine sites on IRF7 are required for its ubiquitination-mediated activation by the Tumor Necrosis Factor Receptor family member Latent Membrane Protein 1. MolCellBiol. 2008;28(20):6536–46. 10.1128/MCB.00785-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript.