Abstract
Anthrozoological neuroscience, which we propose as the use of neuroscience techniques to study human-animal interaction, may help to elucidate mechanisms underlying the associated psychological, physiological, and other purported health effects. This preliminary study investigates the neural response to animal photographs in pet owners and non-pet owners, and both attraction and attachment to companion animals as modulators of human perception of companion animal photographs. Thirty male participants, 15 “Pet Owners” (PO) and 15 “Non-Pet Owners” (NPO), viewed photographs of companion animals during functional MR1 (fMRI) scans at 3 T and provided ratings of attraction to the animal species represented in the photographs. Fourteen subjects additionally submitted and viewed personal pet photographs during fMRI scans, and completed the Lexington Attachment to Pets Scale (LAPS). PO exhibited greater activation than NPO during the viewing of animal photographs in areas of the insula, and frontal and occipital cortices. Moreover, ratings of attraction to animals correlated positively with neural activation in the cingulate gyrus, precentral gyrus, inferior parietal lobule, and superior temporal gyrus during the viewing of representative photographs. For subjects with household pets, scores on the LAPS correlated positively with neural activation during the viewing of owned pet photographs in the precuneus, cuneus, and superior parietal lobule. Our preliminary findings suggest that human perception of companion animals involve the visual attention network, which may be modulated at the neural level by subjective experiences of attraction or attachment to animals. Our understanding of human-animal interactions through anthrozoological neuroscience may better direct therapeutic applications, such as animal-assisted therapy.
Keywords: Anthrozoological neuroscience, Companion animals, fMRI, Human-animal interaction, Pet ownership
1. Introduction
Studies of human-animal interaction are new to the realm of neuroscience. Therapeutic effects of human-animal interaction have long been proposed in both home and clinical settings (Fine, 2010; Siegel, 1993; Carson, 2006; Friedmann and Son, 2009). Over the past few years, animal-assisted therapy (AAT) as a form of complementary medicine has grown in popularity, further attesting to its value for patients with disabilities that require such service (e.g., for individuals with visual or hearing impairments, epilepsy, autism, or post-traumatic stress disorder), emotional support (e.g., for individuals with mood disorders, anxiety), and therapy (e.g., for hospitalized or nursing home patients) (Banks, 2002; Bass et al., 2009; Guerino et al., 2015; Marx et al., 2010; Berget and Braastad, 2011; Hunt and Chizkov, 2014; Rabbitt et al., 2014; Wisdom et al., 2009; O’Haire et al., 2015; Yount et al., 2013).
Anthrozoological neuroscience, which we propose as a subdomain of social neuroscience, (Van Overwalle, 2009) is the application of neuroscience techniques (e.g., neuroimaging) to anthrozoology (the study of human-animal interactions). The neural representations of human-animal interaction (HAI), which encompass the visual perception of animals investigated in this study, may provide insights regarding the psychological, physiological, and long-term health effects of pet ownership or AAT.
Pet ownership, especially that of dogs, has long been proposed to support psychological health (Beals, 2009; Virues-Ortega and Buela-Casal, 2006; Raina et al., 1999; Carmack, 1991; Angulo, 1999; Siegel et al., 1999). Prospective studies implementing AAT provided more evidence of the beneficial effects from HAI, such as enhanced mood in hospitalized children (Kaminski et al., 2002), improved perceived quality of life in rehabilitation patients (Lust et al., 2007), and decreased dysphoria and anxiety in HIV/AIDS-diagnosed men (Pepper TD, 2000). HAI may alleviate negative moods even among healthy participants (Honda and Yamazaki, 2006). Studies of the physiological effects of HAI found that cardiovascular reactivity was different between the petting of one's own pet versus an unknown pet (Baun et al., 1984; Allen et al., 2002).
Perhaps the most compelling research of the HAI phenomenon involved biochemical changes. A single session of animal-assisted activity increased IgA antibody concentrations and reduced cortisol levels (Barker et al., 2005; Charnetski et al., 2004; Odendaal, 2000; Odendaal and Meintjes, 2003). Furthermore, positive interaction with a dog increased levels of β-endorphin, oxytocin, prolactin, β-phenylethylamine, and dopamine, as well as decreased levels of the stress hormone cortisol (Odendaal and Meintjes, 2003). These studies are beginning to elucidate the mechanisms underpinning the effects of HAI which are typically viewed as phenomena (Beetz et al., 2012).
The first two investigations into anthrozoological neuroscience used neuroimaging studies to evaluate brain responses to animals. The first study used positron emission tomography (PET) and found that a familiar pet's presence led to “relaxing” effects, which included lower heart rate variability and psychological stress, as well as deactivation in the middle frontal lobe, putamen and thalamus (Sugawara et al., 2012). The only prior functional MRI (fMRI) study explored human perception of suffering humans versus suffering animals (Franklin et al., 2013). Overlapping regions of activation in the insula and cingulate gyrus were elicited by both species and ascribed to the common empathic factor. However, suffering dogs caused greater activation in parietal and inferior frontal regions compared to suffering humans; these differences were attributed to the different semantic relevance and salience. Overall, these studies demonstrate that the emotional responses elicited by companion animals are dynamic, and may lead to both positive and negative psychological effects (Honda and Yamazaki, 2006; Somervill et al., 2009; Turner et al., 2003).
To extend these earlier investigations, the primary goal of our study was to evaluate neural correlates in the perception of companion animals, and compare “Pet Owner” versus “Non-Pet Owner” responses. Additionally, we explore correlations between pet ownership-associated neural activation and both their attraction and attachment to companion animals. If identification with pet ownership, attraction, and attachment are found to moderate the neural correlates in perception of companion animals, it would follow that such factors may also moderate the therapeutic potential of HAI as presented in the anthrozoological literature.
2. Materials and methods
2.1. Participants
The study was approved by the University of Hawaii Institutional Review Board (the Cooperative Committee on Human Studies). Thirty right-handed, non-smoking, male participants (18–70 years old) were recruited from the local community or by word-of-mouth. Recruitment targeted 15 self-identified “Pet Owners” (POs) and 15 “Non-Pet Owners” (NPOs). All subjects provided written informed consent prior to participation. Exclusion criteria included any confounding neurological or chronic psychiatric disorder, severe medical illness that might lead to significantly abnormal laboratory results that could affect the fMRI BOLD signals (e.g., hematocrit < 34%), and contraindications for MR studies including ferromagnetic implants or severe claustrophobia. Women were also excluded from the study to avoid sex-specific variations on emotional responses to the visual stimuli.
The participants completed detailed medical history questionnaires during interviews with trained research staff, and were screened by a physician prior to scanning to ensure they fulfilled the study criteria and had no contraindication for the MR scans. All participants were asked to consider their own history of pet ownership and their attitude towards companion animals in order to direct self-assignment to PO or NPO groups, regardless of current ownership status.
2.2. Questionnaires
Several structured questionnaires were administered to collect demographic information and pet ownership history. Additionally, the participants rated conceptual attraction to six companion animal species groups (dog, cat, fish, small mammal, bird, reptile/amphibian) on a 10-point Likert scale (1–no attraction; 10–strong attraction), which was averaged for a mean Attraction score. Following the fMRI scan, all subjects were presented two pseudorandomly selected photographs from each experimental animal block presented, and asked to write any thoughts/memories that were evoked while viewing the images (see Fig. 1).
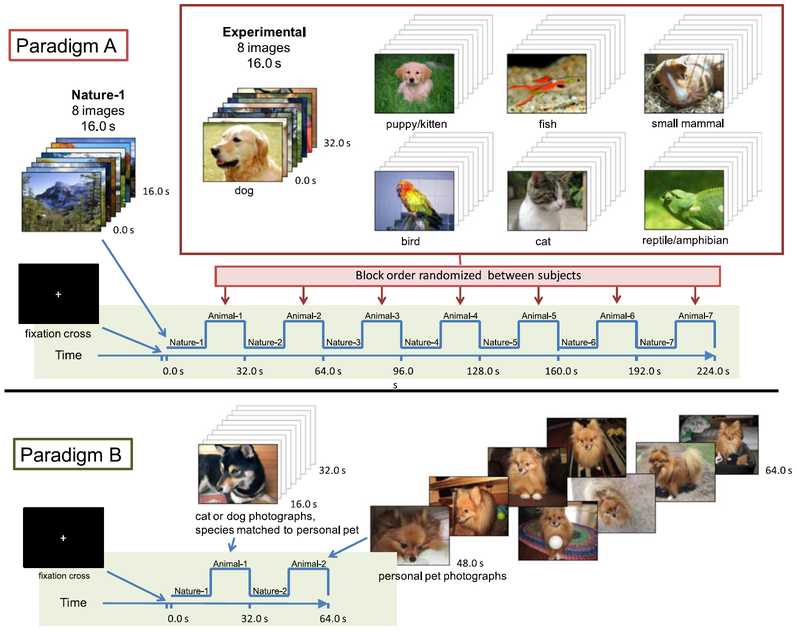
Fig. 1.
(A) Paradigm A included seven control epochs (nature) and seven experimental epochs (dog, puppy/kitten, fish, small mammal, bird, cat, reptile/amphibian). The order of nature epochs and animal epochs was pseudo-randomized amongst subjects. (B) Paradigm B, if applicable, comprised two control epochs and two experimental epochs. The second experimental epoch comprised personal pet photographs submitted by subjects, and the first experimental epoch comprised unfamiliar animals of matched species. For both paradigms, epochs comprised eight images displayed 2.0 s each, for total epoch durations of 16.0 s.
The participants currently living with a pet were asked to submit eight colored photographs of the animal for input into the fMRI paradigm, and to complete a questionnaire to profile the specific owner-pet relationship. All participants also completed the Lexington Attachment to Pets Scale (LAPS), a 23-item Likert-style questionnaire with the maximum score of 69 indicating strongest attachment to a target pet (Johnson et al., 1992).
2.3. fMRI - Image acquisition
Functional MRI was performed using a 3 T Siemens TIM Trio scanner and a 12-channel phase-array head coil. A single-shot echo-planar imaging (EPI) pulse sequence was used (gradient echo, echo time (TE)/repetition time (TR)=30/2000 ms, FOV = 22 cm, 28 slices, 5 mm thickness, 70° flip angle, 64 × 64 matrix). Registration of functional scans into a stereotactic spaceinvolved alignment with a high-resolution structural scan (magnetization-prepared gradient-echo or MP-RAGE; sagittal, TR/TR/inversion time = 2200/4.91/1000 ms, 208 × 256 × 144 resolution).
2.4. fMRI – Activation tasks
A block design was used for the presentation of all images in the fMRI paradigms (see Fig. 1). A black screen with a centered fixation cross preceded each paradigm and was displayed for 2.0 s.
Paradigm A (224.0 s duration, 112 TR periods, see Fig.1A) was presented to all subjects and included seven control and seven experimental epochs for the seven animal groups of interest: dog, puppy/kitten, cat, fish, small mammal (mouse, rabbit, or guinea pig), bird, and reptile/amphibian. Each epoch comprised eight images, each displayed for 2.0 s, for a total duration of 16.0 s. Epochs of companion animal photos and epochs of nature landscapes alternated without breaks. Within this alternating block design (i.e., Animal 1–Nature 1–Animal 2–Nature 2, etc.), the order that the animal epochs and nature epochs were presented was pseudo-randomized across subjects. All photos were 800 × 600 pixels (“landscape” orientation).
If applicable (for individuals living with an animal), Paradigm B (64.0 s duration, 32 TR periods, see Fig. 1B) comprised two control epochs of nature landscapes and two experimental epochs, which alternated without breaks. The second experimental epoch was created by concatenating eight pet photographs submitted by each subject. The first experimental epoch comprised eight animal photographs of matched species. These photographs depicted the animal in natural poses, such as sitting or standing. Each of the eight images in an epoch was displayed for 2.0 s, for a total epoch duration of 16.0 s. All photos were 800 × 600 pixels (“landscape” orientation).
2.5. Image analyses and statistics
FMR1B Software Library (FSL 4.1; FMR1B Analysis Group, UK) was used to analyze the fMRI data. Head motion was corrected using FMRIB Linear Image Registration Tool (MCFLIRT) software, and non-brain tissues were removed using FSL's Brain Extraction Tool (BET). A 6-mm Gaussian filter was applied for spatial smoothing, and data were detrended and high-pass filtered (32 s). Registration of functional data to high-resolution T1-weighted MP-RAGE structural data was performed using FLIRT with a linear full search and 12 degrees-of-freedom (DOF). The high-resolution data were then registered to the Montreal Neurological Institute (MNI) 152 template with a 12 DOF linear full search.
The FMRIB Local Analysis of Mixed Effects procedure in the general linear model was used to analyze PO versus NPO response. The FMRIB procedure was also used to assess the correlation between demeaned Attraction score and neural response during the viewing of representative photographs. The Z-statistic images were threshold using Z > 1.96 and a cluster p threshold of 0.05 (cluster-extent correction due to multiple comparisons). For illustrative purposes, a post-hoc regression of subject-level data was conducted for the mean contrast of parameter estimate (COPE) values, extracted at the MNI coordinates of the local maxima in the correlation with Attraction score.
The FMRIB procedure was used to analyze subjective response during the viewing of personal pet photographs. The second experimental epoch in Paradigm B (block of interest) was parsed out by applying a custom model at the first-level of analysis. In the subsequent group mean analysis, the demeaned LAPS score was applied as a covariate. The Z-statistic images were thresholded using Z > 1.96 and a duster p threshold of 0.05 (cluster-extent correction due to multiple comparisons).
3. Results
3.1. Demographics (Table 1)
Table 1.
Subject characteristics between Pet Owner (PO) and Non-Pet Owner (NPO) groups.
| NPO (N = 15) | PO (N = 15) |
Chi Square, Mann- Whitney U, or t-test |
|
|---|---|---|---|
| Age (Mean ± S.D.) | 36.1 ± 17.6 | 32.4 ± 13.9 | t(28) = 0.65, p > 0.250 |
| Race (n) | X2=93, p=0.025 | ||
| Asian or Pacific Islander | 7 | 2 | |
| African American | 1 | 0 | |
| Hispanic | 2 | 0 | |
| Native American | 0 | 0 | |
| Caucasian | 5 | 13 | |
| Highest Level of Education (n) | X2=1.5, p > 0.250 | ||
| Less than high school | 0 | 0 | |
| High school/GED | 0 | 1 | |
| Attended college | 5 | 5 | |
| 2-year college degree | 2 | 3 | |
| 4-year college degree | 3 | 2 | |
| Post-graduate degree | 5 | 4 | |
| Marital Status (n) | X2=1.1, p > 0.250 | ||
| Single | 11 | 11 | |
| Married | 3 | 4 | |
| Separated/Divorced | 1 | 0 | |
| Widowed | 0 | 0 | |
| Household Income (n) | X2=3.7, p > 0.250 | ||
| Less than $25,000 | 2 | 4 | |
| $25,000–$49,999 | 6 | 2 | |
| $50,000–$99,000 | 2 | 3 | |
| $100,000 + | 3 | 5 | |
| Unwilling to disclose | 2 | 1 | |
| Close emotional bonds (n) | X2=4.9, p > 0.250 | ||
| 0–4 | 7 | 5 | |
| 5–9 | 6 | 7 | |
| 10+ | 2 | 3 | |
| Attraction to animal species, median ratinga | |||
| Bird | 5 | 3 | 72 |
| Cat | 3 | 7 | 55.5* |
| Dog | 7 | 9 | 24*** |
| Fish | 4 | 3 | 100.5 |
| Small mammal | 3 | 4 | 94.5 |
| Reptile/amphibian | 2 | 3 | 79.5 |
| Mean ± S.E. | 4.2 ± 0.3 | 5.3 ± 0.3 | t(28) = 2.20, p = 0.037* |
Median rating for “general attraction” to animal species; 1 = no attraction; 10 = strong attraction.
p < 0.05, significant difference between the two groups.
p < 0.001, significant difference between the two groups.
Fifteen POs (mean age 32.4 years, SD = 13.9) were compared to fifteen NPOs (mean age = 36.1 years, SD = 17.6). Distribution of race varied between the two groups, with more Asian or Pacific Islanders in the NPO group and more Caucasians in the PO group (X2=9.3, p=0.025), despite the similar age, marital status, education, household income, and size of social network. POs rated attraction to dogs (U=24.00, p < 0.001) and cats (U=55.50, p=0.018) higher than NPOs, and presented higher mean Attraction to companion animals, t(28)=2.20, p=0.037.
In the post-scan survey, POs reported a greater number of thoughts as evoked by the pseudo-randomly selected companion animal photographs of Paradigm A than NPOs (U=64.50, p=0.047).
Fourteen subjects (13 POs and 1 NPO) submitted photographs of a household pet for presentation in Paradigm B. Submissions included 10 household dogs and 4 cats. The average length of ownership for each pet was 3.42 ± 1.2 years. Median LAPS score was 44 ± 12.74 (range 28–63). Seven subjects indicated companionship as their primary motivation for pet ownership, four subjects stated that someone else in the household wanted the pet, and two cited miscellaneous reasons. Of the fourteen subjects, four claimed to have a primary role in pet care, three denied a primary/shared role, and seven reported to have shared pet responsibilities equally with another household member. All pets were reported to spend at least partial time indoors.
3.2. fMRI data
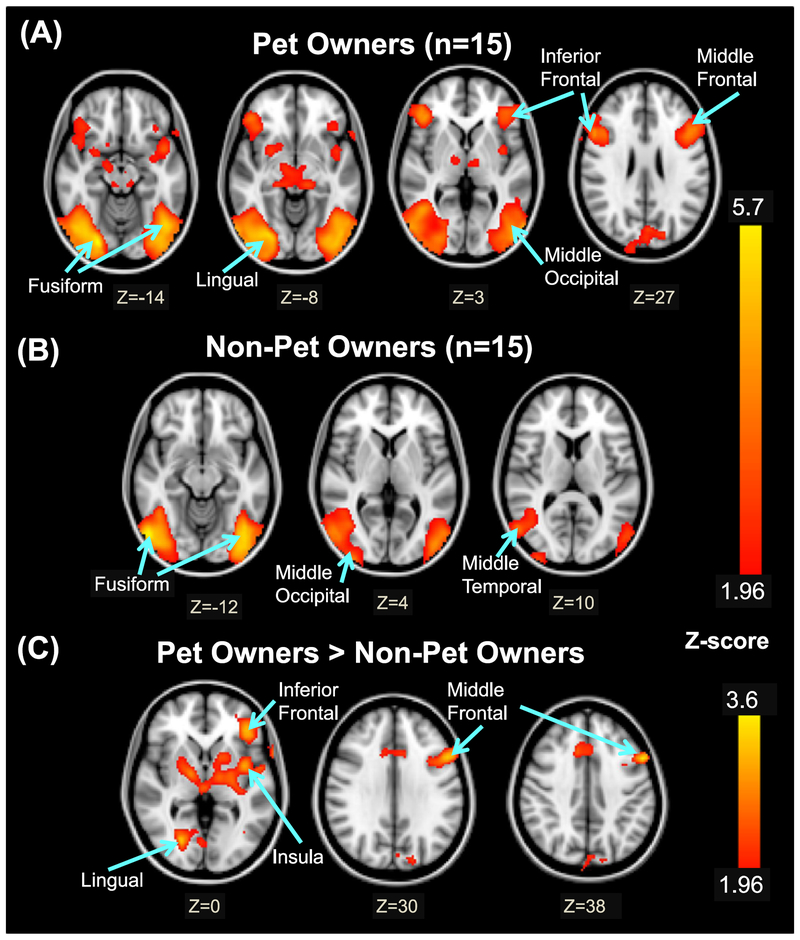
Table 2 and Fig. 2 showed that during Paradigm A viewing of companion animals, brain regions activated in POs included the left middle frontal gyrus (BA 8), bilateral inferior frontal gyri (BA 9), bilateral fusiform gyri (BA 19, 37), left middle occipital gyrus (BA 19), right lingual gyrus, and right cerebellum. Fewer brain regions were activated in NPOs; these included the right middle temporal gyrus (BA 19), bilateral fusiform gyri (BA 19), right middle occipital gyrus, and bilateral cerebellum. Consequently, POs exhibited greater activation than NPOs in the left middle frontal and inferior frontal gyri (BA 8, 9), left insula, lingual gyrus, and bilateral cerebellum at a Z-threshold of 1.96. The reverse comparison (NPO > PO) did not show group differences in any brain region.
Table 2.
Brain regions showing greater activation in Pet Owners compared to Non-Pet Owners, and group average responses, during Paradigm A – viewing of companion animal photographs (N = 30).
| MNI Coordinates |
Cluster Size (Voxels) | Cluster (corrected)-p | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Brain Region | BA | Side | X | Y | Z | Z score | |||
| Pet Owner (PO, n=15) | |||||||||
| Middle Frontal Gyrus | 8 | L | −48 | 16 | 30 | 4.23 | 3760 | 0.0394 | |
| Inferior Frontal Gyrus | L | −42 | 38 | 0 | 3.53 | 3760 | 0.0394 | ||
| Inferior Frontal Gyrus | 9 | R | 54 | 16 | 24 | 4.15 | 8242 | 0.0005 | |
| Temporal Fusiform Gyrus | 37 | L | −46 | −68 | −14 | 5.08 | 19109 | < 0.0001 | |
| Middle Occipital Gyrus | 19 | L | −54 | −82 | 6 | 4.93 | 19109 | < 0.0001 | |
| Occipital Fusiform Gyrus | 19 | R | 26 | −86 | −14 | 5.79 | 19109 | < 0.0001 | |
| Occipital Lingual Gyrus | R | 30 | −78 | −8 | 5.11 | 19109 | < 0.0001 | ||
| Cerebellum Posterior Declive | R | 48 | −70 | −18 | 5.38 | 19109 | < 0.0001 | ||
| Non-Pet Owner (NPO, n=15) | |||||||||
| Middle Temporal Gyrus | 37 | R | 58 | −68 | 10 | 3.56 | 6103 | 0.0036 | |
| Temporal Fusiform Gyrus | 19 | R | 54 | −76 | −12 | 5.54 | 6103 | 0.0036 | |
| Temporal Fusiform Gyrus | L | −42 | −58 | −18 | 3.95 | 6103 | 0.0036 | ||
| Middle Occipital Gyrus | R | 38 | −100 | 4 | 3.94 | 6103 | 0.0036 | ||
| Occipital Fusiform Gyrus | L | −42 | −76 | −12 | 5.12 | 4662 | 0.0150 | ||
| Cerebellum Anterior Culmen | R | 42 | −56 | −24 | 5.49 | 6103 | 0.0036 | ||
| Cerebellum Anterior Culmen | L | −40 | −42 | −26 | 3.11 | 4662 | 0.0150 | ||
| Cerebellum Posterior Tuber | R | 56 | −60 | −32 | 2.36 | 6103 | 0.0036 | ||
| Pet Owner > Non-Pet Owner | |||||||||
| Middle Frontal Gyrus | 8 | L | −54 | 14 | 38 | 3.46 | 8923 | 0.0003 | |
| Middle Frontal Gyrus | 9 | L | −52 | 16 | 30 | 3.24 | 8923 | 0.0003 | |
| Inferior Frontal Gyrus | L | −40 | 34 | 0 | 3.13 | 8923 | 0.0003 | ||
| Occipital Lingual Gyrus | R | 24 | −74 | 0 | 3.2 | 7710 | 0.0008 | ||
| Insula | L | −38 | 0 | 0 | 3.02 | 8923 | 0.0003 | ||
| Cerebellum Anterior Lobe | L | −26 | −52 | −30 | 3.09 | 7710 | 0.0008 | ||
| Cerebellum Anterior Culmen | R | 24 | −60 | −22 | 3.53 | 7710 | 0.0008 | ||
| Cerebellum Posterior Uvula | L | −32 | −76 | −28 | 3.04 | 7710 | 0.0008 | ||
| Cerebellum Posterior Declive | R | 30 | −84 | −18 | 3.33 | 7710 | 0.0008 | ||
| Non-Pet Owner > Pet Owner No areas of different activation | |||||||||
fMRI data processing was performed using the FEAT Version 5.98 procedure, part of FSL. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 1.96 and a (corrected) cluster significance threshold of p < 0.05.
Fig. 2.
Z-Score Maps Showing Activated Brain Regions in each group, and group differences in brain activations during the viewing of Animal Photographs. (A) Brain regions activated in the Pet Owners. (B) Brain regions activated in Non-Pet Owners. (C) Brain regions with greater activation in Pet Owners than Non-Pet Owners. Bottom numbers represent Z-coordinates of the axial slice. Activated areas are shown with Z> 1.96 and a duster significance threshold of p-(corrected) < 0.05. (See Table 2 for MN1 Coordinates of the cluster maxima, cluster size and significance for these activated brain regions).
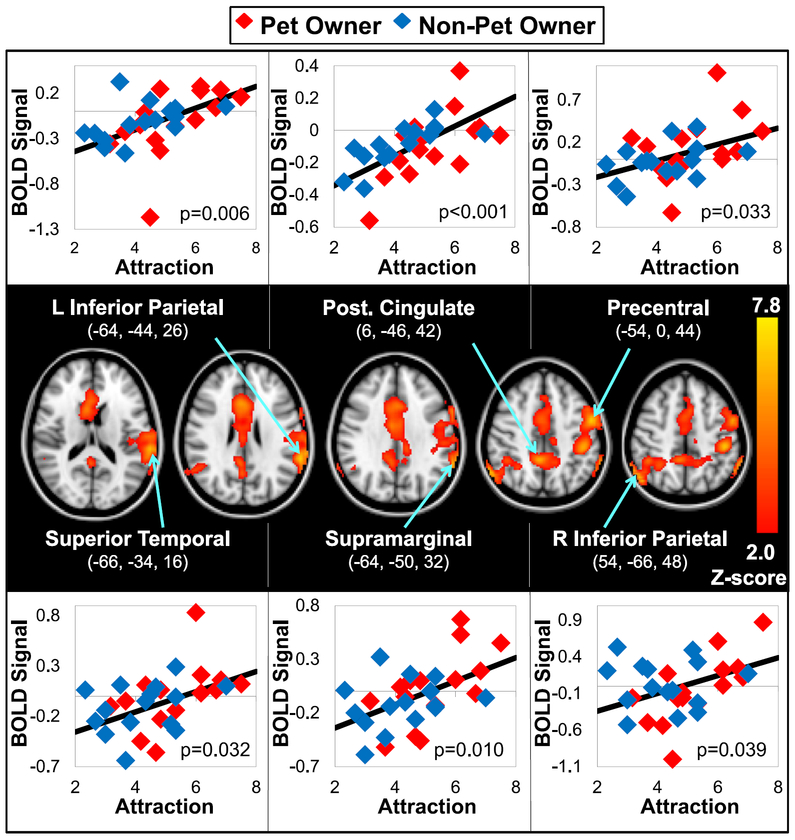
Attraction Score predicted the BOLD response during the viewing of Paradigm A in the left precentral gyrus, bilateral inferior parietal lobule (BA 39, 40), left supramarginal gyrus, left superior temporal gyrus, and right cingulate gyrus (BA 31) at a Z-threshold of 1.96 (Table 3, Fig. 3).
Table 3.
Attraction Score Predicted the BOLD Response in Selected Brain Regions During Viewing of Paradigm A Companion Animal Photographs (N = 30).
| MNI Coordinates |
Cluster Size (Voxels) | Cluster (corrected)-p | ||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | BA | Side | X | Y | Z | Z score | ||
| Frontal Precentral Gyrus | L | −54 | 0 | 44 | 4.27 | 7645 | 0.0009 | |
| Inferior Parietal Lobule | 39 | R | 54 | −66 | 48 | 4.02 | 9538 | 0.0002 |
| Inferior Parietal Lobule | 40 | L | −64 | −44 | 26 | 4.3 | 7645 | 0.0009 |
| Parietal Supramarginal Gyrus | L | −64 | −50 | 32 | 3.91 | 7645 | 0.0009 | |
| Superior Temporal Gyrus | L | −66 | −34 | 16 | 4.05 | 7645 | 0.0009 | |
| Posterior Cingulate Gyrus | 31 | R | 6 | −46 | 42 | 3.94 | 9538 | 0.0002 |
fMRI data processing was performed using the FEAT Version 5.98 procedure, part of FSL. Z (Caussianised T/F) statistic images were thresholded using clusters determined by Z > 1.96 and a (corrected) cluster significance threshold of p < 0.05.
Fig. 3.
Stronger Attraction rating, regardless of Pet Ownership status, predicted stronger BOLD Response during the viewing of Paradigm A animal photographs. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 1.96 and a cluster significance threshold of p-(corrected) < 0.05. Mean Attraction ratings are averaged Likert ratings of “general attraction” to six companion animal species groups (1=no attraction; 10=strong attraction). Scatterplots above and below these Z-maps at specific brain regions show the corresponding subject-level data; BOLD responses are shown as contrast of parameter estimate (COPE) values. Results of the post-hoc regressions are indicated next to the plots (no group or interaction effects were found). See Table 3 for details regarding cluster size and significance.
Post-hoc regression analyses were performed from BOLD signals extracted at the local maxima (MNI coordinates in Table 3) of the brain regions that correlated with the Attraction scores in both groups (p-values: 0.039 to < 0.001, Fig. 3). In all she regions, the correlation between mean COPE values and Attraction ratings were not different between the two groups.
In the subgroup of participants who provided their personal pet photographs, LAPS scores also correlated with regional brain activation in bilateral superior parietal lobule (BA 7), precuneus (BA 7), and cuneus (BA 19) (Table 4, Fig. 4).
Table 4.
Brain regions showing correlation between Lexington Attachment to Pets Scale and brain activation during Paradigm B – viewing of owned pet photographs (N = 14).
| MNI Coordinates |
Cluster Size (Voxels) | Cluster (corrected)-p | ||||||
|---|---|---|---|---|---|---|---|---|
| Brain Region | BA | Side | X | Y | Z | Z score | ||
| Superior Parietal Lobule | L | −26 | −64 | 56 | 5.98 | 6655 | 0.004 | |
| Superior Parietal Lobule | 7 | R | 28 | −58 | 50 | 4.95 | 6655 | 0.004 |
| Precuneus | 7 | R | 28 | −54 | 48 | 4.99 | 6655 | 0.004 |
| Occipital Cuneus | 19 | R | 6 | −80 | 44 | 4.35 | 6655 | 0.004 |
fMRI data processing was performed using the FEAT Version 5.98, part of FSL. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 1.96 and a (corrected) cluster significance threshold of p < 0.05.
Fig. 4.
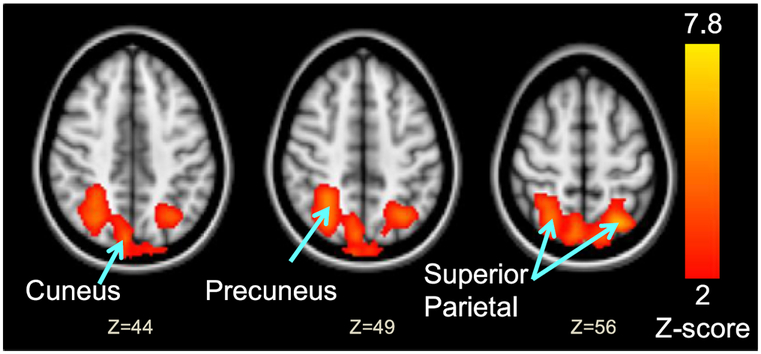
Lexington Attachment to Pets Scale (LAPS) scores correlated with brain activation during the viewing of Paradigm B personal pet photographs. N=14. Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 1.96 and a cluster significance threshold of p < 0.05 (corrected). Bottom numbers represent Z-coordinates of axial slice. See Table 4 for the MNI coordinates of the cluster maxima, cluster size and significance of these brain regions.
4. Discussion
To our knowledge, this is the first study to investigate brain regions involved in human perception of companion animals and demonstrate that POs had greater brain activation than NPOs, especially in brain regions involved in attention. In addition, regardless of pet ownership, those with higher scores of Attraction to animals had greater neural responses while viewing companion animal images, suggesting that salience of the animal photographs to the viewer increases with attraction. Furthermore, greater attachment to their owned pets was associated with greater neural activation in the parietal and occipital regions during the viewing of personally-submitted cat or dog photographs. These findings, although preliminary, support the notion that these brain regions are germane to HAI and might have implications for interpersonal interactions.
4.1. Identification with pet ownership
During the viewing of companion animal photographs, the PO group had greater brain activation than NPO group in frontal, insula and cerebellar regions, which are brain regions involved in the dorsal attention network (Tomasi et al., 2004·, 2007). These findings indicate that the PO group had greater attentional modulation of top-down processing (Corradi-Dell’Acqua et al., 2015; Vuilleumier, 2005) compared to the NPO group, due in part to the greater salience of animal photographs to the PO viewer.
The greater number of thoughts or memories evoked by the companion animal photographs in PO than in NPO subjects also suggests group differences at the cognitive level. While some subjects reported the association of animal photographs with previous pets or the pets of friends, others freely associated to a number of other themes (e.g., the Discovery Channel). Such diverse prior experiences and pre-existing attitudes might have been projected during the perception of novel companion animals by the participants, which would lead to the greater magnitude and extent of streams of consciousness (Haynes and Rees, 2005) during the viewing of the animal photogrpahs in POs than NPOs.
While subjects were unequivocal in their self-designation as a “pet owner” or “non-pet owner”, pet ownership might be represented more accurately by a parametric measure that accounts for both pet ownership history and attitudes toward animals. Future research should examine such component elements in order to determine which are directly associated with the group differences found in the present study. Nevertheless, this dichotomous categorization of the PO versus NPO groups was effective in demonstrating group differences in both their recall of associated memories and in their brain activation patterns during the perception of companion animals.
One of the brain regions that showed greater activation in POs than NPOs was the insula, which has a role not only in attention, but also in the processing of both positive and negative emotions (Franklin et al., 2013; Saxbe et al., 2013), and interoception (Nguyen et al., 2016). This greater insula activation suggests that the companion animal photographs were interpreted with greater salience, perhaps evoked by different body-based emotional experiences (i.e., interoception), in the PO compared to NPO group. Future research should evaluate possible group differences between POs versus NPOs in somatosensory and interoceptive processing of animal stimuli. Group membership may predict interindividual variability toward body sensation versus intellectual abstraction approaches (Saxbe et al., 2013) in the emotion processing of companion animal photographs.
4.2. Animal attraction
The subjects’ ratings of attraction to companion animals predicted their BOLD response during the viewing of representative animal photographs in a number of higher-level cortical and subcortical areas for visual processing (Pessoa et al., 2002; Leveroni et al., 2000) and visual attention (Tomasi et al., 2004). These correlated brain regions were independent of pet ownership status. While preliminary, these positive correlations suggest that the neural response during the viewing of companion animal photographs in these brain regions was associated with attraction to the depicted stimuli.
However, the most significant correlation with the Attraction score was found in the posterior cingulate, a region that has been associated with cognitive (Leech and Sharp, 2014), executive (Pearson et al., 2011), and affective processes (Gobbini et al., 2004; Leibenluft et al., 2004), as well as visual attention (Tomasi et al., 2004, 2007). Although the posterior cingulate has not been identified as a brain region involved in interpersonal attractiveness (Winston et al., 2007; Ueno et al., 2014), it was consistently involved in visual attention (Tomasi et al., 2004, 2007), recognition, (Leveroni et al., 2000) and familiarity (Gobbini et al., 2004; Natu and O’Toole, 2011). Attraction may be modulated by familiarity via the structural mere exposure effect, which describes the enhanced hedonic appreciation of novel stimuli that conform to a robust representation of a particular prototype, acquired over day-to-day exposure (Zizak and Reber, 2004). Therefore, the subjects might have rated greater attraction and had greater activation in the cingulate gyrus to the novel animal photographs if they had more familiarity with companion animals. Furthermore, attraction may lead to selective attention (Yagi et al., 2009), with greater salience of the image to the viewer and greater activation in the attention network, including the posterior cingulate gyrus (Tomasi et al., 2004, 2007), during the perception of companion animal photographs.
4.3. Pet attachment
Greater brain activation in the parietal and occipital cortices during the viewing of personal pet photographs was found in participants with more attachment to their own pets. Since these brains regions were involved with increasing attention (Tomasi et al., 2007, 2006) and attentional load (Chang et al., 2004), the personally owned pet photographs likely were more salient to the viewer. These brain regions also may be involved in the perception of an object of attachment, although few fMRI studies have evaluated the neural correlates of attachment. Consistent with our findings, greater activation was found in the occipital cortex (Leibenluft et al., 2004; Nitschke et al., 2004) and precuneus (Leibenluft et al., 2004) of mothers viewing pictures of their own infants or children, compared to those of control children. Moreover, compared to typically developing controls, children with reactive attachment disorder showed only smaller visual cortices on the whole brain analysis, which further implicated the visual cortex's role in attachment (Shimada et al., 2015).
The range of attachment scores represented by the participants demonstrates that all owner-pet relationships are not equal. Future anthrozoological studies should explore the evolution of attachment longitudinally over a particular owner-pet relationship, identifying experiences that precipitate attachment and determining the threshold for attachment at which AAT and pet ownership may be most effective.
4.4. Limitations
Our study has several limitations. First, the relatively small sample size combined with the short duration of paradigm exposure provided low power to identify the neural correlates with greater sensitivity. Second, the short duration of epochs led to very short exposure to the animal photographs, which prevented us from further comparing the neural responses between the different animal groups (Paradigm A) and evaluating the attachment response to the subjects’ own pet photographs (Paradigm B). Future studies showing longer duration of exposure or a repeat epoch may allow more reliable assessments of the pet owners’ attachment to his or her own pet. Since dogs and cats were rated much higher on measures of self-reported attraction, neural response to these species could be grouped separately from images of birds, reptiles, and small mammals in future studies with larger sample sizes. Third, since our subject pool was limited to men, our findings cannot be generalized to both gender groups. As gender differences have been reported for people's experiences with companion animals (Prato-Previde et al., 2006; Turner et al., 2003; Miller et al., 2009), their neural correlates should be evaluated. Finally, this study is limited to the visual component for the perception of companion animals. Future anthrozoolgical neuroscience research will benefit from multi-modal paradigms as additional sensory components, including auditory, olfactory and somatosensory, would provide more complete experiences of HAI for studying its neural correlates.
5. Conclusion
This study provided preliminary findings that may aid future research for HAI and supported past anthrozoological studies. Our three variables of interest, self-identification with pet ownership, and both attraction and attachment to companion animals, may modulate human perception of companion animal photographs. These findings have important implications for HAI applications, including animal-assisted therapy (AAT) and pet ownership, which have long been purported to impart physical and psychological health effects (Fine, 2010; Siegel 1993; Carson, 2006; Friedmann and Son, 2009). These findings also suggest that the efficacy of AAT may differ between pet owner and non-pet owner populations, and by the degrees of attraction and attachment elicited by therapy animals. Lastly, the attraction and attachment-dependent brain response to perception of companion animals suggest a subjective component to the therapeutic effects of HAI, which is likely a function of more than simple physical presence or contact with the animals. Future anthrozoological neuroscience research will help to better direct our approach in animal therapy and expand our understanding of social interactions.
Acknowledgements
This work was supported by the grants from the National Institutes of Health (U54NS56883; G12 MD007601). This study was also supported by small grant awards from the Undergraduate Research Opportunities Program, the Richard H. & Mildred D. Kosaki Foundation, and the Associated Students of the University of Hawaii.
We also thank the Honors program and the Department of Psychology at the University of Hawaii for this opportunity, and the many technical and clinical staff at the Neuroscience and MR Research Center for their invaluable assistance during the conduct of this study.
Footnotes
Mailing Address: Neuroscience and MR Research Program Department of Medicine JABSOM, University of Hawaii, 1356 Lusitana Street, 7th Floor, Honolulu, HI 96813, USA.
References
- Angulo FJ, 1999. Pet ownership among HIV-infected persons in the Multicenter AIDS Cohort Study: Health Risk or psychological Benefit? Dissertation Abstracts International: The Sciences and Engineering. 56, 5444. [Google Scholar]
- Allen K, Blascovich J, Mendes WB, 2002. Cardiovascular reactivity and the presence of pets, friends, and spouses: the truth about cats and dogs. Psychosom Med. 64 (5), 727–739. [DOI] [PubMed] [Google Scholar]
- Banks MR, Banks WR, 2002. The Effects of Animal-Assisted Therapy on Loneliness in an Elderly Population in Long-Term Care Facilities. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2002 July 1 57 (7), pp. M428–M32. [DOI] [PubMed] [Google Scholar]
- Bass MM, Duchowny CA, Llabre MM, 2009. The effect of therapeutic horseback riding on social functioning in children with autism. J. Autism Dev. Disord. 39 (9), 1261–1267. [DOI] [PubMed] [Google Scholar]
- Berget B, Braastad BO, 2011. Animal-assisted therapy with farm animals for persons with psychiatric disorders. Ann. Ist. Super Sanita. 47 (4), 384–390. [DOI] [PubMed] [Google Scholar]
- Beals EE, 2009. Emotional Benefits of Dog Ownership: Impact of the Presence of a Pet Dog on Owners' Responses to Negative Mood Induction. (Dissertation Abstracts International: The Sciences and Engineering). 70; p. 2564. [Google Scholar]
- Baun MM, Bergstrom N, Langston NF, Thoma L, 1984. Physiological effects of human/companion animal bonding. Nurs Res. 33 (3), 126–129. [PubMed] [Google Scholar]
- Barker SB, Knisely JS, McCain NL, Best AM, 2005. Measuring stress and immune response in healthcare professionals following interaction with a therapy dog: a pilot study. Psychol. Rep. 96 (3 Pt 1), 713–729. [DOI] [PubMed] [Google Scholar]
- Beetz A, Uvnas-Moberg K, Julius H, Kotrschal K, 2012. Psychosocial and psychophysiological effects of human-animal interactions: the possible role of oxytocin. Front Psychol. 3, 234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson L, 2006. The animal/human bond. Am. J. Health Educ. 37 (6), 361–365. [Google Scholar]
- Carmack BJ, 1991. The role of companion animals for persons with AIDS/HIV. Holist. Nurs. Pract 5 (2), 24–31. [DOI] [PubMed] [Google Scholar]
- Charnetski CJ, Riggers S, Brennan FX, 2004. Effect of petting a dog on immune system function. Psychol Rep. 95 (3 Pt 2), 1087–1091. [DOI] [PubMed] [Google Scholar]
- Corradi-Dell’Acqua C, Fink GR, Weidner R, 2015. Selecting category specific visual information: top-down and bottom-up control of object based attention. Conscious Cogn. 35, 330–341. [DOI] [PubMed] [Google Scholar]
- Chang L, Tomasi D, Yakupov R, et al. , 2004. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 56 (2), 259–272. [DOI] [PubMed] [Google Scholar]
- Handbook on Animal-Assisted Therapy. In: Fine AH (Ed.), Third edition Academic Press, San Diego, p. iv. [Google Scholar]
- Friedmann E, Son H 2009. The human-companion animal bond: how humans benefit. Vet. Clin. North Am. Small Anim. Pract. 39 (2), 293–326. [DOI] [PubMed] [Google Scholar]
- Franklin RG Jr, Nelson AJ, Baker M, et al. , 2013. Neural responses to perceiving suffering in humans and animals. Soc Neurosci. 8 (3), 217–227. [DOI] [PubMed] [Google Scholar]
- Guerino MR, Briel AF, Araújo M, 2015. 31. Hippotherapy as a treatment for socialization after sexual abuse an emotional stress. J. Phys. Then Sci. 27 (3), 959–962. 10.1589/jpts.27.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobbini MI, Leibenluft E, Santiago N, Haxby JV, 2004. Social and emotional attachment in the neural representation of faces. Neuroimage 22 (4), 1628–1635. [DOI] [PubMed] [Google Scholar]
- Hunt MG, Chizkov RR, 2014. Are therapy dogs like Xanax? Does animal-assisted therapy impact processes relevant to cognitive behavioral psychotherapy?. Anthrozoös 27 (3), 457–469. [Google Scholar]
- Honda A, Yamazaki K, 2006. Effects of horseback riding and contact with horses on mood change and heart rate Japanese. J. Health Psychol. 19 (1), 48–55. [Google Scholar]
- Haynes J-D, Rees G, 2005. Predicting the stream of consciousness from activity in human visual cortex. Curr. Biol. 15 (14), 1301–1307. [DOI] [PubMed] [Google Scholar]
- Johnson TP, Garrity TF, Stallones L, 1992. Psychometric evaluation of the lexington attachment to pets scale (LAPS). Anthrozoos 5 (3), 160–175. [Google Scholar]
- Kaminski M, Pellino T, Wish J, 2002. Play and pets: the physical and emotional impact of child-life and pet therapy on hospitalized children. Child. Health Care 31 (4), 321–335. [Google Scholar]
- Lust E, Ryan-Haddad A, Coover K, Snell J, 2007. Measuring clinical outcomes of animal-assisted therapy: impact on resident medication usage. Consul. Pharm. 22 (7), 580–585. [DOI] [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM, 2000. Neural systems underlying the recognition of familiar and newly learned faces. J. Neurosci. 20 (2), 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech R, Sharp DJ, 2014. The role of the posterior cingulate cortex in cognition and disease. Brain 137 (Pt 1), 12–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T, Haxby JV, 2004. Mothers’ neural activation in response to pictures of their children and other children. Biol. Psychiatry 56 (4), 225–232. [DOI] [PubMed] [Google Scholar]
- Marx MS, Cohen-Mansfield J, Regier NG, Dakheel-Ali M, Srihari A, Thein K, 2010. The impact of different dog-related stimuli on engagement of persons with Dementia. Am. J. Alzheimers Dis. Other Demen. 25 (1), 37–45. 10.1177/1533317508326976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Kennedy C, DeVoe D, Hickey M, Nelson T, Kogan L, 2009. An examination of changes in oxytocin levels in men and women before and after interaction with a bonded dog. Anthrozoos, 31–42. [Google Scholar]
- Nguyen VT, Breakspear M, Hu X, Guo CC, 2016. The integration of the internal and external milieu in the insula during dynamic emotional experiences. Neuroimage. 124, Part A, pp. 455–463. [DOI] [PubMed] [Google Scholar]
- Natu V, O’Toole AJ, 2011. The neural processing of familiar and unfamiliar faces: a review and synopsis. Br. J. Psychol. 102 (4), 726–747. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ, 2004. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage 21 (2), 583–592. [DOI] [PubMed] [Google Scholar]
- O’Haire ME, Guérin Né A, Kirkham AC, 2015. Animal-assisted intervention for trauma: a systematic literature review. Front. Psychol. 6,1121 10.3389/fpsyg.2015.01121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odendaal JS, 2000. Animal-assisted therapy - magic or medicine? J. Psychosom. Res. 49 (4), 275–280. [DOI] [PubMed] [Google Scholar]
- Odendaal JS, Meintjes RA, 2003. Neurophysiological correlates of affiliative behaviour between humans and dogs. Vet. J. 165 (3), 296–301. [DOI] [PubMed] [Google Scholar]
- Pepper TD, 2000. Effects of Brief Exposure to a Pet Therapy Dog on Affective States of HIV (positive men. Dissertation Abstracts International). 61 (2216). [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG, 2002. Attentional control of the processing of neural and emotional stimuli. Brain Res. Cogn. Brain Res. 15 (1), 31–45. [DOI] [PubMed] [Google Scholar]
- Pearson JM, Heilbronner SR, Barack DL, Hayden BY, Platt ML, 2011. Posterior cingulate cortex: adapting behavior to a changing world. Trends Cogn. Sci. 15 (4), 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prato-Previde E, Fallani G, Valsecchi P, 2006. Gender differences in owners interacting with pet dogs: an observational study. Ethology 112 (1), 64–73. [Google Scholar]
- Rabbitt SM, Kazdin AE, Hong JE, 2014. Acceptability of animal-assisted therapy: attitudes toward AAT, psychotherapy, and medication for the treatment of child disruptive behavioral problems. Anthrozoös 27 (3), 335–350. [Google Scholar]
- Raina P, Waltner-Toews D, Bonnett B, Woodward C, Abernathy T, 1999. Influence of companion animals on the physical and psychological health of older people: an analysis of a one-year longitudinal study. J. Am.Geriatr. Soc. 47 (3), 323–329. [DOI] [PubMed] [Google Scholar]
- Siegel JM, 1993. Companion animals: in sickness and in health. J. Soc. Issues 49, 157–167. [Google Scholar]
- Siegel JM, Angulo FJ, Detels R, Wesch J, Mullen A, 1999. AIDS diagnosis and depression in the multicenter AIDS Cohort Study: the ameliorating impact of pet ownership. AIDS Care 11 (2), 157–170. [DOI] [PubMed] [Google Scholar]
- Sugawara A, Masud MM, Yokoyama A, et al. , 2012. Effects of presence of a familiar pet dog on regional cerebral activity in healthy volunteers: a positron emission tomography study. Anthrozoös 25 (1), 25–34. [Google Scholar]
- Somervill JW, Swanson AM, Robertson RL, Arnett MA, MacLin OH, 2009. Handling a dog by children with attention-deficit/hyperactivity disorder: calming or exciting? North Am. J. Psychol. 11, 111–120. [Google Scholar]
- Saxbe DE, Yang XF, Borofsky LA, Immordino-Yang MH, 2013. The embodiment of emotion: language use during the feeling of social emotions predicts cortical somatosensory activity. Soc. Cogn. Affect. Neurosci. 8 (7), 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada K, Takiguchi S, Mizushima S, et al. , 2015. Reduced visual cortex grey matter volume in children and adolescents with reactive attachment disorder. Neuroimage Clin. 9, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DC, Rieger G, Gygax L, 2003. Spouses and cats and their effects on human mood. Anthrozoös 16 (3), 213–228. [Google Scholar]
- Tomasi D, Ernst T, Caparelli EC, Chang L, 2004. Practice-induced changes of brain function during visual attention: a parametric fMRI study at 4 T. Neuro-image 23 (4), 1414–1421. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T, 2007. Different activation patterns for working memory load and visual attention load. Brain Res. 9 (1), 158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Ernst T, Caparelli EG, Chang L, 2006. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 T. Hum. Brain Mapp. 27 (8), 694–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner DC, Rieger G, Gygax L, 2003. Spouses and cats and their effects on human mood. Anthrozoos 16 (3), 213–228. [Google Scholar]
- Ueno A, Ito A, Kawasaki I, et al. , 2014. Neural activity associated with enhanced facial attractiveness by cosmetics use. Neurosci. Lett. 566, 142–146. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, 2009. Social cognition and the brain: a meta-analysis. Hum. Brain Mapp. 30 (3), 829–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virues-Ortega J, Buela-Casal G, 2006. Psychophysiological effects of human-animal interaction: theoretical issues and long-term interaction effects. J. Nerv. Ment. Dis. 194 (1), 52–57. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P, 2005. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 9 (12), 585–594. [DOI] [PubMed] [Google Scholar]
- Wisdom JP, Saedi GA, Green CA, 2009. Another breed of “Service” animals: STARS study findings about pet ownership and recovery from serious mental illness. Am. J. Orthopsychiatry 79 (3), 430–436. 10.1037/a0016812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston JS, O’Doherty J, Kilner JM, Perrett DI, Dolan RJ, 2007. Brain systems for assessing facial attractiveness. Neuropsychologia 45 (1), 195–206. [DOI] [PubMed] [Google Scholar]
- Yount R, Ritchie EC, Laurent MS, Chumley P, Olmert MD, 2013. The role of service dog training in the treatment of combat-related PTSD. Psychiatr. Ann. 43, 292–295. [Google Scholar]
- Yagi Y, Ikoma S, Kikuchi T, 2009. Attentional modulation of the mere exposure effect. J. Exp. Psychol. Learn Mem. Cogn. 35 (6), 1403–1410. [DOI] [PubMed] [Google Scholar]
- Zizak DM, Reber AS, 2004. Implicit preferences: the role(s) of familiarity in the structural mere exposure effect. Conscious Cogn. 13 (2), 336–362. [DOI] [PubMed] [Google Scholar]