Abstract
HIV-infection is associated with neuroinflammation and greater psychopathological symptoms, which may be mediated by imbalances in the kynurenic pathway (KP). Two key KP enzymes that catabolize kynurenine include kynurenine-aminotransferase II (KATII), which yields antioxidative kynurenine acid [KYNA] in astrocytes, and kynurenine-3-monooxygenase (KMO), which produces neurotoxic metabolites in microglia. The relationships between polymorphisms in KMO and KATII, psychopathological symptoms, and cerebrospinal fluid (CSF) [KYNA] were evaluated in subjects with and without HIV-infection. Seventytwo HIV-positive and 72-seronegative (SN) participants were genotyped for KATII-rs1480544 and KMO-rs1053230. Although our participants were not currently diagnosed with depression or anxiety, they were assessed for psychopathological distress with Center for Epidemiologic Studies-Depression scale and Symptom Checklist-90-Revised. CSF-[KYNA] was also measured in 100 subjects (49 HIV/51 SN). HIV-participants had more psychopathological distress than SN, especially for anxiety. KATII-by-HIV interactions were found on anxiety, interpersonal sensitivity and obsessive compulsivity; KATII-C-carriers had lower scores than TT-carriers in SN but not in HIV. In contrast, the KMO-polymorphism had no influence on psychopathological symptoms in both groups. Overall, CSF-[KYNA] increased with age independently of HIV-serostatus, except KATII-TT-carriers tended to show no age-dependent variations. Therefore, the C-allele in KATII-rs1480544 appears to be protective against psychopathological distress in SN but not in HIV individuals, who had more psychopathological symptoms and likely greater neuroinflammation. The age-dependent increase in CSF-[KYNA] may reflect a compensatory response to age-related inflammation, which may be deficient in KATII-TT-carriers. Targeted treatments that decrease neuroinflammation and increase KYNA in at risk KATII-TT-carriers may reduce psychopathological symptoms in HIV.
Keywords: Psychopathological symptoms, HIV, Kynurenine aminotransferase II, Kynurenine 3-monooxygenase, Kynurenic acid
Introduction
Since the advent of highly active antiretroviral therapy (HAART) in the mid 90’s, HIV/AIDS patients are living longer and have fewer opportunistic infections. However, relative to the general population, they have a higher incidence of co-morbid psychopathologies, including depression, anxiety, and somatization (Owe-Larsson et al. 2009; Jin et al. 2010; Rezaei et al. 2013). In the United States, rates of current depression are significantly higher at 36 % in HIV patients (Asch et al. 2003; Rabkin 2008) compared to 6.8 % in the general population (CDC 2011).
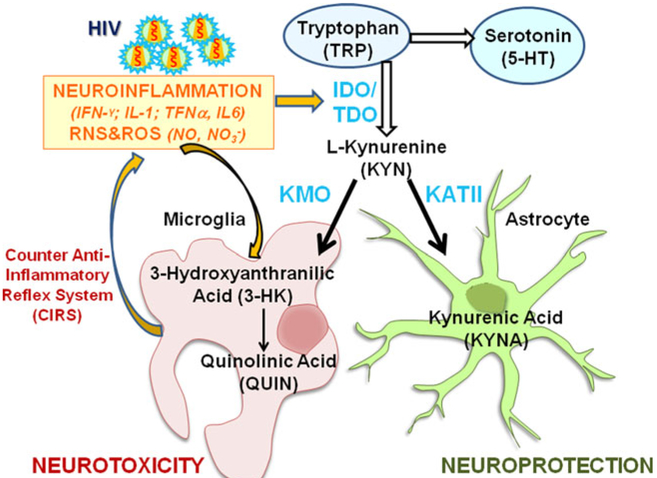
Recent studies suggest that psychopathology symptoms observed in HIV patients (Gostner et al. 2015; Routy et al. 2015), and in depressed individuals with melancholy features (Gabbay et al. 2010), history of suicide attempts (Sublette et al. 2011), anhedonia (Gabbay et al. 2012), or major depressive disorder (Myint et al. 2007) may result from imbalances in various tryptophan catabolites. Tryptophan (TRP) is the precursor for serotonin, one of the most studied markers of depression, and the first substrate of the kynurenine pathway (KP). TRP is degraded by indolamine 2,3-dioxygenase (IDO) into kynurenine (KYN), which is further catabolized into either kynurenic acid (KYNA) in astrocytes, or 3-hydroxykynurenine (3-HK), and quinolinic acid (QUIN) in microglia (Maddison and Giorgini 2015) (Fig. 1). 3-HK and QUIN have neurotoxic and oxidative effects (Maddison and Giorgini 2015) that are associated with depression and anxiety (Maes 2011; Anderson and Maes 2014) whereas KYNA has antioxidant properties (Lugo-Huitron et al. 2011) that may counteract the neurotoxicity mediated by 3-HK and QUIN (Schwarcz et al. 2012; Anderson and Maes 2014). Moreover, the catabolism of TRP is activated by pro-inflammatory cytokines (Zunszain et al. 2012; Molteni et al. 2013; Campbell et al. 2014), leading ultimately to an imbalance of KP catabolism toward the neurotoxic metabolites (Maes 2011). In depression, activation of the TRP catabolites by pro-inflammatory cytokines, such as IL-1, TNFα, IL-6 (Dantzer et al. 2008; Maes et al. 2012), is often counteracted by a down-regulation of the immune-inflammatory response with increased immunosuppressive factors, such as haptoglobin, acute phase proteins or prostaglandin production (Adib-Conquy and Cavaillon 2009; Maes et al. 2012). These factors in turn may attenuate the primary immune-inflammatory response, which has been described as the counter anti-inflammatory reflex system (CIRS) associated with depressive symptoms (Maes et al. 2012). Therefore, depression involves an intricate interplay between TRP catabolites and inflammation.
Fig. 1.
The kynurenine pathway. Tryptophan (TRP), the precursor for serotonin (5-HT) can also be catabolized into L-kynurenine (KYN) by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3- dioxygenase (TDO). In the brain, L-kynurenine is mainly converted to 3- hydroxykynurenine (3-HK) by kynurenine 3-monooxygenase (KMO) in microglial cells, to kynurenic acid (KYNA) by kynurenine aminoatransferase II (KATII) in astrocytes. 3-HK is subsequently converted to quinolinic acid (QUIN). Neuroinflammation, along with oxidative/nitrosative stress, increase IDO activity toward KYN; these processes may further contribute to psychopathological symptoms by driving the production toward neurotoxic metabolites. HIV-infection triggers neuroinflammation that may lead to activation of the TRP and KP pathway, and ultimately increase risk for depression. In turn, increased TRP catabolites may attenuate indirectly the primary immune-inflammation; this mechanism is called Compensatory Inflammation Reflex System (CIRS). RNS reactive nitrogen species, ROS reactive oxygen species, HIV human infection virus, CIRS compensatory inflammation reflex system
HIV patients have more neuroinflammation (i.e. greater cytokine production), and higher than typical KYNA levels in CSF (Heyes et al. 1992; Baran et al. 2000; Atlas et al. 2007) and in postmortem brain tissues (Baran et al. 2012; Baran and Kepplinger 2014). Since KYNA is anti-oxidative, the elevated CSF KYNA in HIV patients (Davies et al. 2010) may reflect a compensatory response similar to CIRS (Maes and Rief 2012). Moreover, treatment-naïve HIV individuals had a higher than normal serum KYN:TRP ratio that correlated with greater depressive symptoms, but after HAART, they had a decreased KYN:TRP ratio that correlated with improvements in the depressive symptoms (Martinez et al. 2014). Therefore, elevated levels of QUIN and 3-HK may lead to neurotoxic effects (Maddison and Giorgini 2015) and increased risk for psychopathological symptoms. These effects may be worsened by the neuroinflammation associated with HIV infection (Martinez et al. 2014; Cassol et al. 2015), since HIV patients with depressive symptoms had even more inflammation (i.e. elevated IFN-γ, CXCL9 and CLXCL10 in plasma) and higher KYN:TRP ratios than those without depressive symptoms (Cassol et al. 2015).
In addition to neuroinflammation, the catabolism of KYN towards neurotoxic or neuroprotective KP catabolites are modulated by polymorphisms in kynurenine-3-monooxygenase (KMO) and kynurenine aminotransferase II (KATII); hence, studying the functional polymorphisms in these genes may provide useful biomarkers in relation to psychopathological symptoms in HIV patients. Reliable biomarkers for depression and/or psychopathological symptoms may lead to identification of novel treatments of these symptoms in HIV individuals. The current study examined the relationships between psychopathological symptoms and the polymorphisms of KMO-rs1053230 and KATII-rs1480544, which are located in the two genes encoding enzymes involved in the neurotoxic and neuroprotective branches of KP (Fig. 1), as well as CSF-[KYNA] to indicate the antioxidant response, in HAART-stabled HIV individuals.
KMO directs KYN towards the neurotoxic pathway, and the C-allele at rs1053230 in KMO may be a risk factor for psychopathological symptoms, as shown in patients with bipolar type I (Lavebratt et al. 2014), or with major depression (Claes et al. 2011). Thus, we expected HIV KMO-C-carriers would have more depressive symptoms than HIV T-carriers. In contrast, KATII directs the KYN catabolism towards the antioxidant KYNA, and the C-allele at rs1480544 in KATII was more prevalent in healthy controls than in patients with bacterial meningitis, another neuroinflammatory condition with higher CSF cytokines levels (de Souza et al. 2011). Therefore, we expected participants with the KATII C-allele would have fewer psychopathological symptoms than those with the other genotypes. Lastly, polymorphisms in these two genes also may modulate CSF-[KYNA]. Although KMO-rs1053230 C-carriers with bipolar disorders type I had higher levels (Lavebratt et al. 2014), KMO-rs1053230 C-carriers with schizophrenia (Holtze et al. 2012), and KATII-rs1480544 C-carriers with bacterial meningitis (Coutinho et al. 2014), all had lower CSF-[KYNA]. Therefore, we expected the C-carriers of the KMO or KATII genes to have lower CSF-[KYNA].
Materials and Methods
Participants
All participants signed an informed consent approved by the joint Cooperative Institutional Review Boards for the University of Hawai‘i and the Queen’s Medical Center. After the initial telephone screening of >1,000 potential subjects, 144 participants [72 seronegative controls (SN) and 72 seropositive participants (HIV)] who were carefully evaluated by the team physicians to ensure they fulfilled the study criteria were included in the current study. The inclusion criteria were: 1) Men or women of any ethnicity, >18 years and able to provide informed consent; 2) Confirmed HIV serostatus with documentation from medical records for HIV+, or a negative Clearview HIV test for the SN participants; 3) HIV participants were stable on an antiretroviral regimen for >6 months and had a nadir CD4 count <500/mm3. The exclusion criteria for the current study were: 1) History of co-morbid psychiatric illness, including clinically significant depression. 2) Significantly abnormal screening laboratory tests (>2 SD) that might indicate a chronic medical condition (e.g. diabetes, severe cardiac, renal or liver disorders) that might influence the outcome measures. 3) History of moderate to severe substance use disorders, except for tobacco and cannabis use, within 2 years of the study or positive urine toxicology for methamphetamines, cocaine, and opiates. 4) An estimated intelligence quotient <80, verified by the Wechsler Test of Adult Reading. All participants were evaluated for depressive and psychopathological symptoms (see below), and provided blood samples for the genotyping. A subgroup (n = 100) also consented to lumbar punctures for the measurements of cerebrospinal fluid (CSF) [KYNA].
Psychopathology Measures
All participants completed the Center for Epidemiologic Studies-Depression Scale (CES-D, (Radloff 1977)), a 20-item questionnaire that assessed how often they had symptoms associated with depression that were experienced during the week prior to the evaluation. Item responses range from 0 (Rarely or None of the Time) to 3 (Most or Almost all the Time). A score ≥16 was used to identify those at risk for clinical depression and a physician evaluated the subject to exclude those with clinically significant depression. All participants also completed the Symptom Checklist-90-Revised (SCL-90-R®, Pearson), a 90-item test that evaluated how often a participant felt distressed by a range of symptoms within the past week. Responses ranged from 0 to 4 (0 = Not at all, 1 = A little bit, 2 = Moderately, 3 = Quite a bit, 4 = Extremely). These questions were grouped into nine subscales, with T score conversion, for the following dimensions: Depression (DEP), Anxiety (ANX), Somatization (SOM), Obsessive Compulsiveness (O-C), Hostility (HOS), Interpersonal Sensitivity (I-S), Phobic Anxiety (PHB), Paranoid Ideation (PAR), and Psychoticism (PSY). These subscales were summarized into three global indices: General Symptom Index (GSI) measured overall psychopathological distress, and was calculated by summing the scores of all subscale scores, and then dividing by the total number of responses; Positive Symptom Total (PST) provided the number of self- reported symptoms; Positive Symptom Distress Index (PSDI) measured symptom intensity. A T-score of 63 or higher on the GSI or on two of the dimensional subscales indicated possible clinical significance.
Genotyping
Genomic DNA was extracted from whole blood samples using DNeasy Blood and Tissue kits (catalog #69506, Qiagen Inc, Valencia, CA) for 144 (72 SN and 72 HIV) participants. Custom TaqMan® SNP Genotyping Assays for the KATII SNP rs1480544 and KMO SNPrs1053230 (catalog #4351379 and #4324018) and TaqMan® Universal PCR Master Mix, no AmpErase® UNG (catalog #4351379 and #4324018, Life Technologies, Grand Island, NY) for Polymerase Chain Reaction (PCR) endpoint genotyping were used. Replication and quality control filters (i.e., call rates >95 %) were used, and 5 participants (2 SN and 3 HIV) were excluded from our analysis since the call rates for both genotypes were lower than 95 %. The genotype distribution was 106 CC, 28 TC, and 5 TT for the KMO-rs1053230, and 20 TT, 65 TC, and 54 CC for KATII-rs1480544. In subsequent analyses, due to the rare TT genotype, we combined the 28 TC group with the 5 TT in one group (KMO T-carrier), and we combined the TC and the CC groups (KATII C-carriers) based on previous literature showing associations with C-allele differed from TT-carriers (de Souza et al. 2011; Coutinho et al. 2014). Genotype frequencies for the two examined variants did not differ from Hardy-Weinberg equilibrium (KATII-rs1480544: χ2 = 0.004, p = 0.95; KMO-rs1053230: χ2 = 2.98, p = 0.08).
Kynurenic Acid (KYNA) Concentration Measurement
CSF KYNA concentration was determined for 100 (51 SN and 49 HIV) participants by high performance liquid chromatography (HPLC, Surveyor, Thermo Electron) coupled with a fluorescence detector (Jasco FP-1520, Easton MD). In brief, 100 μl of CSF sample was treated with 10 μl of perchloric acid (3 M), the mixture was vortexed vigorously and then centrifuged at 13,500g for 5 min. The supernatant was transferred to HPLC inserts for analysis. Twenty-five μl of the supernatant was injected onto an Ascentis C18 column (150 × 3 mm, 2.7 μm, Supelco, St. Louis MO) with a pre-column filter (0.2 μm, Therm Electron). The analyte was eluted out using a mobile phase containing 250 mM of zinc acetate; 50 mM sodium acetate with 7 % acetonitrile at a flow rate of 300 μl/min. Fluorescence was detected using an excitation wavelength of 344 nm and an emission wavelength of 398 nm. The gain value was set at 100 and the attenuation value at 16. Data were acquired and processed using an ss420 data converter from analog to digital signal and Chromoquest software. The limit of detection was 50 pg/ml.
Statistical Analysis
Statistical analyses were performed using SAS Enterprise Guide 7.1 software. Mann-Whitney-U, Chi Square, or Fisher’s Exact tests were used to compare subject characteristics between groups. CSF-[KYNA] was Log transformed due to the skewness of the data distribution. Age was included as a covariate in all statistical models with CSF-[KYNA] and questionnaires, since CSF-[KYNA] increases with age (Kepplinger et al. 2005; Oxenkrug 2013). Multivariate general linear model (GLM) were used to evaluate the following explanatory variables on SCL-90-R® and CES-D scores: 1) the main effects of HIV serostatus, including history of depression or anxiety disorders, SSRI/SNRI treatment status, and age as covariates; 2) the main and interactive effects of HIV serostatus, KATII and KMO genotypes, with age and SSRI/SNRI treatment status as covariates; 3) the main and interactive effects of HIV status, KATII and KMO genotypes, and CSF Log[KYNA], while adjusting for age and SRRI/SNRI treatment status.
The main and interactive effects of age, HIV status, KATII and KMO genotypes on CSF Log[KYNA] were investigated also using a multivariate GLM with SSRI/SNRI treatment status as a categorical covariate. Lastly, univariate regressions were performed to examine the associations between CSF Log[KYNA] and age in SN and HIV individuals. All values are presented as means ± standard error unless otherwise stated.
Results
Participant Characteristics (Table1)
Table 1.
Demographic and clinical characteristics of the participants
| All participants (n = 144) |
Participants w/CSF KYNA (n=100) |
|||||
|---|---|---|---|---|---|---|
| SN n=72 | HIV n=72 | p-valuea | SN n=51 | HIV n=49 | p-valuea | |
| Age (years) | 47.4 ± 1.7 | 50.3 ± 1.3 | 0.21 | 46.3 ± 2.04 | 50.8 ± 1.7 | 0.11 |
| Sex, # of males (%) | 63 (88 %) | 69 (96 %) | 0.07 | 45 (88 %) | 47 (96 %) | 0.27 |
| Education (years) | 14.5 ± 0.3 | 14.7 ± 0.3 | 0.30 | 14.6 ± 0.5 | 14.7 ± 0.4 | 0.44 |
| Index of social positionb (range: 14–77) | 40.4 ± 2.0 | 39.3 ± 2.0 | 0.52 | 41.2 ± 2.6 | 37.5 ± 2.3 | 0.17 |
| # with past diagnosis for depression (%) | 14 (19.5 %) | 30 (42 %) | 0.004 | 9 (18 %) | 19 (39 %) | 0.02 |
| # with past diagnosis for anxiety disorders (%) | 4 (6 %) | 14 (24 %) | 0.002 | 2 (4 %) | 11 (22 %) | 0.006 |
| # on current SSRI/SNRI treatments (%) | 8 (11 %) | 23 (32 %) | 0.002 | 6 (12 %) | 19 (39 %) | 0.002 |
| Race | ||||||
| American Indian/Alaska Native | 1(1 %) | 0(0 %) | 0.14 | 1(2 %) | 0(0 %) | 0.38 |
| Asian | 18(25 %) | 11(15 %) | 11(22 %) | 8(16 %) | ||
| Black or African American | 1(1 %) | 7(10 %) | 1(2 %) | 4(8 %) | ||
| More than one race | 9(13 %) | 11(15 %) | 4(8 %) | 8(16 %) | ||
| Native Hawaiian/Pacific Islander | 5(7 %) | 3(4 %) | 4(8 %) | 2(4 %) | ||
| White | 38(53 %) | 40(56 %) | 30(58 %) | 27(56 %) | ||
| # on HAART (%) | – | 63 (87.5 %) | – | – | 87.8 % | – |
| HIV duration (months) | – | 160.8 ± 11.3 | – | – | 154.2 ± 13.2 | – |
| CD4 (#/mm3) | – | 502.1 ± 29.2 | – | – | 481.7 ± 34.3 | – |
| Nadir CD4 (#/mm3) | – | 182.3 ± 17.1 | – | – | 181.7 ± 19.3 | – |
| # with detectable viral load (>75 copy/mL), % | – | 14 (19.4 %) | – | – | 11 (22.4 %) | – |
| Log viral load (log copy/mL) | – | 3.1 ± 0.24 | – | – | 3.0 ± 0.25 | – |
| CSF protein (mg/dL) | 41.9 ± 2.0 | 45.9 ± 2.1 | 0.14 | 40.5 ± 1.9 | 45.8 ± 2.5 | 0.17 |
| CSF glucose (mg/dL) | 62.0 ± 0.7 | 64.8 ± 1.5 | 0.44 | 62.8 ± 0.8 | 64.9 ± 1.8 | 0.73 |
| # KATII rs1480544 C-carrier (%)c | 65 (90 %) | 58 (81 %) | 0.14 | 45 (88 %) | 36 (74 %) | 0.09 |
| # KMO rs1053230 CC (%)c | 58 (80 %) | 52 (72 %) | 0.30 | 42 (82 %) | 32 (65 %) | 0.07 |
Presented are means and standard errors of demographic data for participants overall, and for the sub-cohort of participants with CSF-[KYNA] measurements
Mann-Whitney, ChiSq, or Fisher’s exact p value
Higher scores indicate lower social position
Distribution of the alleles in the entire cohort fulfilled the criteria for Hardy-Weinberg equilibrium for KATII SNP rs1480544 (p = 0.95), and the KMO SNP rs1053230 (p=0.08)
Across all participants, as well as the subgroup with CSF-[KYNA] measurements, SN and HIV participants had similar age, sex proportion, education, race distribution, and Hollingshead Index of Social Position score (ISP), but the SN group tended to have more KATII C-carriers than the HIV group. As reported previously (Manji and Miller 2004), HIV individuals had non-significantly higher total CSF protein levels, but similar glucose concentrations, compared to SN participants. 87.5 % HIV participants in this cohort were on HAART therapy and 80.6 % had undetectable viral loads. Although we excluded subjects with clinically significant depression or anxiety disorders, more HIV participants than SN had a past history of or treated depression and anxiety disorders.
Psychopathological Distress in HIV Participants
Although the participants were not found to be clinically depressed at the time of the study, more HIV individuals had a history of clinical depression (39–40 %) and anxiety disorders (22–24 %) than SN controls (Table 1). Hence, more HIV participants (32 %) than SN controls (11 %) were prescribed selective serotonin re-uptake inhibitors/selective serotonin and norepinephrine reuptake inhibitors (SSRI/SNRI) treatments (p=0.002, χ2=9.25, Table 1). We found that SSRI/SNRI treatments had a strong effect on most of the psychopathological subscales (1-way ANCOVA: p = 0.01 to p<0.0001) while history of depression or anxiety disorders had no effects. Hence, the SSRI/SNRI treatment was further included as a categorical covariate in all statistical models.
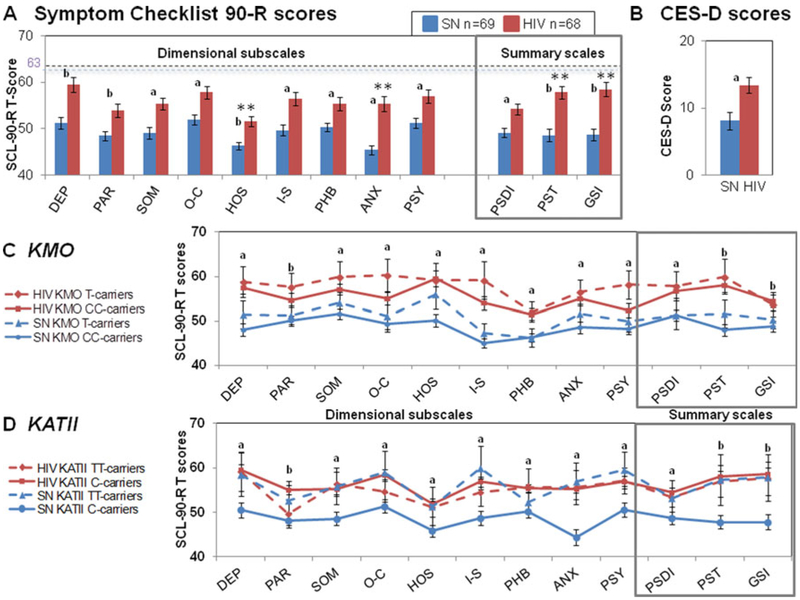
HIV participants had more psychopathological symptoms (SCL-90-R®) than SN individuals with the most significant group difference in anxiety (ANX) and hostility (HOS) T-scores, and in both global severity index (GSI) and positive symptom total (PST) summary scores (Table 2). Using an univariate GLM, HIV individuals had the largest group differences in anxiety T-scores (ANX, +17 %) while the others T-scores were 8–13 % higher in HIV compared to SN (Fig. 2a). HIV individuals also had higher scores on the three SCL-90-R® summary scales (Fig. 2a, box); however, HIV individuals had 15–16% higher scores on global severity index (GSI) and positive symptom total (PST) but only 9 % higher positive symptom distress index (PSDI) compared to SN.
Table 2.
HIV serostatus, history of psychopathological disorders, and SSRI/SNRI treatments on psychopathological symptoms
| Mean ± stand errors |
p-values[F-value, df] | ||
|---|---|---|---|
| SN (n=72) | HIV (n = 72) | HIV serostatus effect | |
| Raw score (0–60) | |||
| CES-D | 8.14 ± 1.11 | 12.9 ± 1.21 | 0.15 [2.09,1] |
| SCL-90-R®, dimensional subscale T-scores | |||
| DEP | 51.15 ± 1.29 | 58.93 ± 1.57 | 0.02 [5.75,1] |
| PAR | 48.62 ± 1.00 | 53.75 ± 1.43 | 0.03 [4.83,1] |
| SOM | 49.13 ± 1.20 | 55.14 ± 1.29 | 0.02 [5.44,1] |
| O-C | 52.03 ± 1.07 | 57.37 ± 1.26 | 0.04 [4.32,1] |
| HOS | 46.45 ± 0.87 | 51.42 ± 1.11 | 0.001 [10.74,1] |
| I-S | 49.93 ± 1.19 | 55.92 ± 1.42 | 0.05 [3.84,1] |
| PHB | 50.41 ± 0.84 | 55.04 ± 1.37 | 0.09 [2.87,1] |
| ANX | 45.39 ± 0.93 | 54.82 ± 1.55 | 0.0003 [13.9,1] |
| PSY | 51.38 ± 1.11 | 56.72 ± 1.37 | 0.05 [3.76,1] |
| SCL-90-R®, summary scale T-scores | |||
| PSDI | 49.03 ± 1.01 | 53.76 ± 1.22 | 0.06 [3.65,1] |
| PST | 48.69 ± 1.31 | 57.21 ± 1.37 | 0.002 [9.81,1] |
| GSI | 48.70 ± 1.25 | 57.92 ± 1.52 | 0.001 [11.14,1] |
Presented are Multivariate General Linear Model p-, F-values, and degree of freedom ([F-value, df]) with the psychopathological symptoms scores for the Center for Epidemiologic Studies Depression scale (CES-D) and Symptom Checklist-90-R (SCL-90-R®) scores as dependent variables, and HIV serostatus, diagnosis (history of depression or anxiety disorders), and SSRI/SNRI treatments as explanatory variable while adjusting for age
Symptom Checklist 90-R (SCL-90-R) T-scores: DEP depression, PAR paranoid ideation, SOM somatization, O-C obsessive-compulsiveness, HOS hostility, I-S interpersonal sensitivity, PHB phobic anxiety, ANX anxiety, PSY psychoticism, PSDI positive symptom distress index, PST positive symptom total, GSI global severity index
Fig. 2.
Genetic variations in KMO and KATII, Psychopathological symptoms in HIV participants. Means and standard errors for (a) Symptom Checklist 90-R (SCL-90-R®) dimensional T-scores and summary scores, and (b) Center for Epidemiologic Studies Depression scale (CES-D) were reported in SN (blue bars) and HIV (red bars) participants. SN and HIV participants divided by (c) KMO or (d) KATII genotype have different levels of psychopathological symptoms for all SCL-90-R® T-scores. DEP depression, PAR paranoid ideation, SOM somatization, O-C obsessive-compulsiveness, HOS hostility, I-S interpersonal sensitivity, PHB phobic anxiety, ANX anxiety, PSY psychoticism, PSDI positive distress index, PST positive symptom total, GSI global severity index. **p-values ≤ 0.05 using the General linear model. ap ≤ 0.05, Post hoc analysis. bp ≤ 0.0001, Post hoc analysis
Moreover, HIV individuals had higher scores on Center for Epidemiologic Studies-Depression scale [CES-D, (Radloff 1977)] (+62 %; Fig. 2b), and were 7.5 times more likely than SN participants to have CES-D scores ≥16 (p=0.02; Fig. 2b), which is considered high risk for clinical depression (Lewinsohn et al. 1997). Those individuals were further evaluated clinically.
Genetic Variations in the KP-Related Genes (KMO and KATII) and Psychopathological Distress
CES-D and SCL-90-R® T-scores for all dimensional and summary scales showed no significant KMO-by-HIV interactions and main effect of the KMO genotypes (Fig. 2c, Table 3).
Table 3.
HIV serostatus and KATII or KMO genotypes on psychopathological symptoms
| Multivariate general linear model, p-values[F-value, df] | ||||||
|---|---|---|---|---|---|---|
| HIV | KMO | KATII | HIV × KMO | HIV × KATII | KMO × KATII | |
| Raw score (0–60) | ||||||
| CES-D | 0.07 [3.18,1] | 0.38 [0.76, 1] | 0.69 [0.16, 1] | 0.83 [0.05, 1] | 0.75 [0.10, 1] | 0.19 [1.66, 1] |
| SCL-90-R®, dimensional subscale T-scores | ||||||
| DEP | 0.36 [0.85, 1] | 0.81 [0.06, 1] | 0.52 [0.42, 1] | 0.27 [1.23, 1] | 0.23 [1.48, 1] | 0.44 [0.61, 1] |
| PAR | 0.27 [1.23, 1] | 0.28 [1.19, 1] | 0.74 [0.11, 1] | 0.27 [1.22, 1] | 0.09 [2.98, 1] | 0.66 [0.19, 1] |
| SOM | 0.25 [1.33, 1] | 0.97 [0.00, 1] | 0.45 [0.58, 1] | 0.87 [0.03, 1] | 0.34 [0.92, 1] | 0.26 [1.26, 1] |
| O-C | 0.53 [0.40, 1] | 0.81 [0.06, 1] | 0.94 [0.00, 1] | 0.78 [0.08, 1] | 0.04 [4.37,1] | 0.27 [1.22, 1] |
| HOS | 0.23 [1.47, 1] | 0.31 [1.05, 1] | 0.12 [2.47, 1] | 0.96 [0.00, 1] | 0.15 [2.10, 1] | 0.28 [1.18, 1] |
| I-S | 0.45 [0.58, 1] | 0.37 [0.80, 1] | 0.25 [1.31, 1] | 0.39 [0.74, 1] | 0.02 [5.29, 1] | 0.68 [0.17, 1] |
| PHB | 0.15 [3.50, 1] | 0.63 [0.24, 1] | 0.83 [0.05, 1] | 0.55 [0.37, 1] | 0.87 [0.03, 1] | 0.66 [0.20, 1] |
| ANX | 0.09 [2.94, 1] | 0.25 [1.35, 1] | 0.06 [3.62, 1] | 0.51 [0.43, 1] | 0.02 [4.86, 1] | 0.88 [0.02, 1] |
| PSY | 0.40 [0.72, 1] | 0.99 [0.00, 1] | 0.21 [1.59, 11] | 0.75 [0.10, 1] | 0.13 [2.28, 1] | 0.70 [0.14, 1] |
| SCL-90-R®, summary scale T-scores | ||||||
| PSDI | 0.33 [0.97, 1] | 0.93 [0.01, 1] | 0.68 [0.17, 1] | 0.22 [0.64, 1] | 0.33 [0.97, 1] | 0.67 [0.18, 1] |
| PST | 0.18 [1.84, 1] | 0.67 [0.18, 1] | 0.30 [1.08, 1] | 0.14 [0.70, 1] | 0.09 [2.99, 1] | 0.63 [0.23, 1] |
| GSI | 0.11 [2.52, 1] | 0.74 [0.11, 1] | 0.38 [0.77, 1] | 0.87 [0.03, 1] | 0.09 [2.77, 1] | 0.40 [0.70, 1] |
Presented are Multivariate General Linear Model p-, F-values, and degree of freedom ([F-value, df]) with the psychopathological symptoms scores for the Center for Epidemiologic Studies Depression scale (CES-D) and Symptom Checklist-90-R (SCL-90-R®) scores as dependent variables, and HIV serostatus, KATII or KMO genotypes as explanatory variable while adjusting for age and SSRI/SNRI treatment as a categorical term. p-values in bold font indicate significance or trends for significance
Symptom Checklist 90-R (SCL-90-R) T-scores: DEP depression, PAR paranoid ideation, SOM somatization, O-C obsessive-compulsiveness, HOS hostility, I-S interpersonal sensitivity, PHB phobic anxiety, ANX anxiety, PSY psychoticism, PSDI positive symptom distress index, PST positive symptom total, GSI global severity index
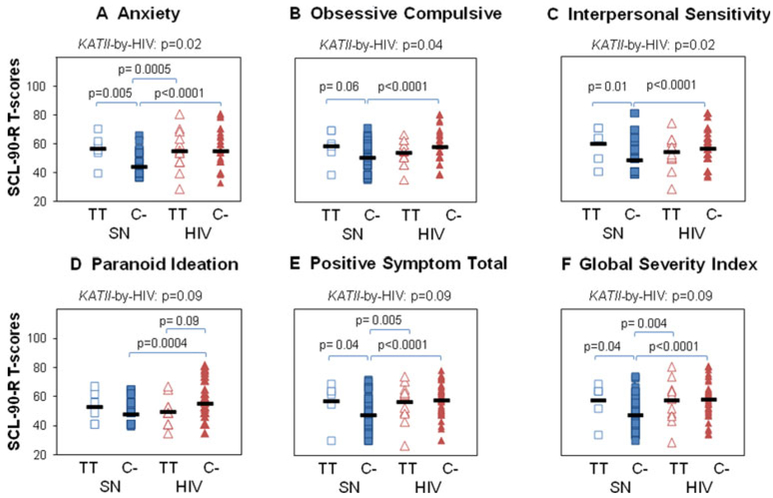
HIV serostatus-by-KATII interactions were found on ANX, O-C, I-S T-scores and trends for these interactions were also observed for PAR T-scores and the PST and GSI summary scores (Fig. 2d, Table 3). Across these six subdomain T-scores, the SN-KATII C-carriers had lower scores than SN-KATII-TT-carriers whereas HIV C-carriers had similar scores as HIV TT-carriers (Fig. 3a–f). We further examined potential KATII-by-KMO interactions on CES-D and SCL-90-R® scores (Table 3). This analysis was performed independently of the HIV serostatus because no interactions were observed between HIV and either KATII or KMO genotypes. No KATII-by-KMO interactions were found on any of the subscales of interest (Table 3).
Fig. 3.
HIV-by-KATII interactions on SCL-90-R® T-scores. Significant HIV-by-KATII interactions were illustrated for each SCL-90-R® subscale and post-hoc analysis reported. SCL-90-R®: (a) anxiety (ANX), (b) obsessive compulsiveness (O-C), (c) interpersonal sensitivity (I-S), (d) paranoid ideation (PAR), e) positive symptom total (PST), (f) global severity index (GSI) T-scores. KATII genotypes: C-carriers [C-] or TT-carriers [TT]. P-values for KATII-by-HIV serostatus interactions obtained with the multivariate GLM, are presented along with the post hoc univariate p-values
CSF Log[KYNA], HIV Serostatus, and KATII or KMO Genotypes on Psychopathological Symptoms
CSF Log[KYNA] was non-significantly higher in HIV individuals (630 ± 0.006 pg/ml) than SN participants (557 ± 0.005 pg/ml). However, univariate regression analysis showed that CSF Log[KYNA] was predicted by HIV duration (r=0.301, p = 0.04, F(2, 48) = 4.47) and by CD4 counts (r = −0.315, p = 0.03, F(2, 48) = 5.32). Moreover, regardless of HIV-serostatus, individuals on SSRI/SNRI medications (n = 25, [KYNA] = 691.5 ± 0.007 pg/ml) tended to have higher CSF Log[KYNA] than non-medicated individuals (n=75, [KYNA] = 560 ± 0.003 pg/ml), [p(SSRI/SNRI) = 0.06, F(1, 100) = 3.7].
Additionally, CSF Log[KYNA] did not differ between genotype groups for either KMO (CC, N= 70, 583 ± 0.004 pg/ ml; T-carriers, N= 25, 616 ± 0.01 pg/ml) or KATII (TT, N=18, 559 ± 0.006 pg/ml, C-carriers, N= 77, 599 ±0.005), and no HIV-by-KMO, HIV-by-KATII, nor KATII-by-KMO interactions were observed on CSF Log[KYNA].
Furthermore, on the multivariate GLM, when psychopathological symptoms scores (CES-D and SCL-90-R®) were used as dependent variables, and HIV serostatus, KATII or KMO genotypes, and CSF Log[KYNA] were included as explanatory variables, while adjusting for age and SSRI/SNRI treatment as a categorical term, no main effect of HIV-serotatus, KATII or KMO genotypes were found. In addition, no HIV-by-CSF Log[KYNA], KATII-by-CSF Log[KYNA], or KMO-by-CSF Log[KYNA] interactions on CES-D and SCL-90-R® scores were observed (Table 4). The univariate multiple regression model also showed no correlations between CSF Log[KYNA] and any psychopathological distress scores (Wilk’s lambda: p = 0.85, F(13, 100)=0.59).
Table 4.
CSF Log[KYNA], HIV serostatus, and KATII or KMO genotypes on psychopathological symptoms
| Multivariate general linear model, p-values[F-value, df] | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CSF Log[KYNA] | HIV | KMO | KATII | CSF Log[KYNA] × KMO | CSF Log[KYNA] × KATII | CSF Log[KYNA] × HIV | HIV × KMO | HIV × KATII | KMO × KATII | |
| Raw score (0–60) | ||||||||||
| CES-D | 0.72 [0.13,1] | 0.07 [3.39, 1] | 0.71 [0.14, 1] | 0.68 [0.17, 1] | 0.74 [0.11, 1] | 0.68 [0.17, 1] | 0.08 [3.12, 1] | 0.49 [0.48, 1] | 0.99 [0.00, 1] | 0.37 [0.81, 1] |
| SCL-90-R®, dimensional subscale T-scores | ||||||||||
| DEP | 0.09 [2.98, 1] | 0.59 [0.30, 1] | 0.10 [2.73, 1] | 0.21 [1.60, 1] | 0.08 [3.12, 1] | 0.22 [1.53, 1] | 0.55 [0.37, 1] | 0.09 [2.97, 1] | 0.61 [0.26, 1] | 0.91 [0.01, 1] |
| PAR | 0.34 [0.93, 1] | 0.17 [1.92, 1] | 0.54 [0.37, 1] | 0.20 [1.65, 1] | 0.47 [0.51, 1] | 0. 21 [1.59, 1] | 0.17 [1.93, 1] | 0.61 [0.26, 1] | 0.18 [1.85, 1] | 0.63 [0.24, 1] |
| SOM | 0.75 [0.10, 1] | 0.66 [0.20, 1] | 0.37 [0.80, 1] | 0.93 [0.01,1] | 0.40 [0.72, 1] | 0.92 [0.01, 1] | 0.70 [0.14, 1] | 0.39 [0.75, 1] | 0.76 [0.09, 1] | 0.37 [0.81, 1] |
| O-C | 0.32 [0.99, 1] | 0.53 [0.40, 1] | 0.07 [3.28, 1] | 0.42 [0.65, 1] | 0.07 [3.34, 1] | 0. 14 [0.69, 1] | 0.53 [0.39, 1] | 0.90 [0.02, 1] | 0.38 [0.78, 1] | 0.53 [0.04, 1] |
| HOS | 0.21 [1.57, 1] | 0.21 [1.56, 1] | 0.87 [0.03, 1] | 0.97 [0.33, 1] | 0.79 [0.07, 1] | 0.35 [0.87, 1] | 0.27 [1.24, 1] | 0.67 [0.18, 1] | 0.42 [0.65, 1] | 0.22 [1.50, 1] |
| I-S | 0.61 [0.26, 1] | 0.36 [0.86, 1] | 0.42 [0.66, 1] | 0.53 [0.39, 1] | 0.35 [0.90, 1] | 0.57 [0.32, 1] | 0.35 [0.90, 1] | 0.81 [0.06, 1] | 0.14 [2.19, 1] | 0.98 [0.00, 1] |
| PHB | 0.79 [0.08, 1] | 0.01 [6.69, 1] | 0.01 [6.74, 1] | 0.90 [0.02, 1] | 0.01 [6.86, 1] | 0.88 [0.02, 1] | 0.02 [6.0, 1] | 0.78 [0.08, 1] | 0.61 [0.27, 1] | 0.66 [0.19, 1] |
| ANX | 0.90 [0.02, 1] | 0.26 [1.26, 1] | 0.50 [0.46, 1] | 0.93 [0.01, 1] | 0.43 [0.64, 1] | 0.84 [0.04, 1] | 0.29 [1.13, 1] | 0.84 [0.04, 1] | 0.17 [1.93, 1] | 0.96 [0.00, 1] |
| PSY | 0.94 [0.1, 1] | 0.37 [0.74, 1] | 0.75 [0.10, 1] | 0.44 [0.60, 11] | 0.74 [0.11, 1] | 0.39 [0.75, 1] | 0.38 [0.78, 1] | 0.69 [0.17, 1] | 0.20 [1.2, 1] | 0.99 [0.00, 1] |
| SCL-90-R®, summary scale T-scores | ||||||||||
| PSDI | 0.39 [0.76, 1] | 0.18 [1.82, 1] | 0.20 [1.67, 1] | 0.47 [0.53, 1] | 0.18 [1.80, 1] | 0.49 [0.48, 1] | 0.20 [1.69, 1] | 0.33 [0.95, 1] | 0.75 [0.10, 1] | 0.83 [0.04, 1] |
| PST | 0.42 [0.66, 1] | 0.57 [0.33, 1] | 0.16 [2.0, 1] | 0.63 [0.23, 1] | 0.15 [2.15, 1] | 0.65 [0.20, 1] | 0.58 [0.31, 1] | 0.23 [1.53, 1] | 0.31 [1.04, 1] | 0.91 [0.01, 1] |
| GSI | 0.39 [0.75, 1] | 0.21 [1.59, 1] | 0.07 [3.3, 1] | 0.56 [0.34, 1] | 0.07 [3.49, 1] | 0.58 [0.31, 1] | 0.23 [1.44, 1] | 0.38 [0.77, 1] | 0.50 [0.46, 1] | 0.66 [0.20, 1] |
Presented are Multivariate General Linear Model p-, F-values, and degree of freedom ([F-value, df]) with the psychopathological symptoms scores for the Center for Epidemiologic Studies Depression scale (CES-D) and Symptom Checklist-90-R (SCL-90-R®) scores as dependent variables, and CSF Log[KYNA], HIV serostatus, and KATII or KMO genotypes as explanatory variable while adjusting for age and SSRI/SNRI treatment as a categorical term. Bolded numbers indicate significance or trends for significance
Symptom Checklist 90-R (SCL-90-R) T-scores: DEP depression, PAR paranoid ideation, SOM somatization, O-C obsessive-compulsiveness, HOS hostility, I-S interpersonal sensitivity, PHB phobic anxiety, ANX anxiety, PSY psychoticism, PSDI positive symptom distress index, PST positive symptom total, GSI global severity index
Age, Genetic Variations and CSF Log[KYNA]
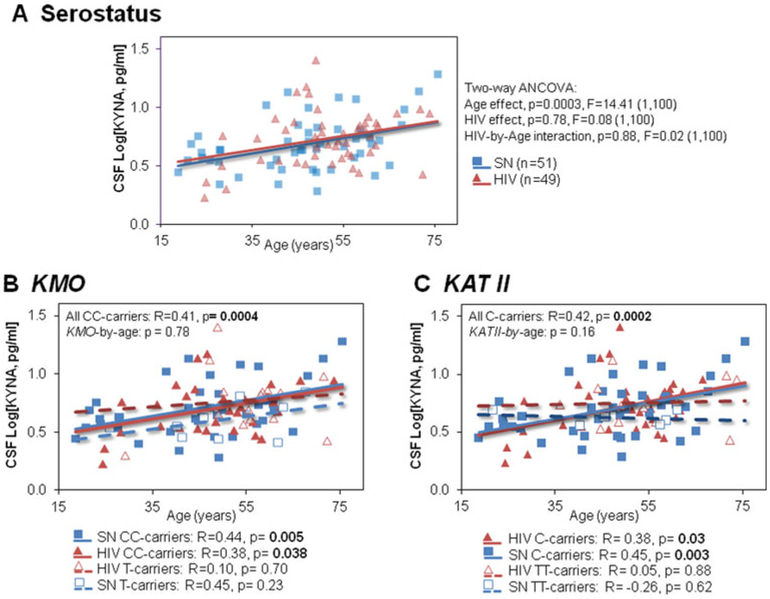
Consistent with earlier studies (Kepplinger et al. 2005; Oxenkrug 2013), CSF Log[KYNA] showed age-dependent increases across all subjects (Fig. 4a). On the multivariate analyses, we evaluated the main and interactive effects of the HIV serostatus, KATII or KMO genotypes, and age, with CSF Log[KYNA] as an explanatory variable, while covarying for SSRI/SNRI treatment status. We found only a trend for the main effect of age (p= 0.07, F(1, 100)= 3.36), but no main effects of HIV serostatus, KATII or KMO genotypes and no interactive effects (Age-by-KMO, Age-by-KATII; Age-by-HIV, and KMO-by-KATII, p-interaction> 0.2). However, post hoc analysis showed that only the KMO CC-carriers showed significant age-dependent increases in CSF Log[KYNA] independent of HIV-serostatus (Fig. 4b). Similarly, across all KATII genotype groups, only the C-carriers had higher CSF Log[KYNA] with older age, also independent of HIV-serostatus (Fig. 4c). No age-by-HIV serostatus nor age-by-HIV serostatus-by-genotype interactions were found on CSF Log[KYNA].
Fig. 4.
CSF-[KYNA] increases with age depending on genotypes. (a) Correlations of CSF Log[KYNA] with age in SN (n = 51) and HIV (n=49) for all participants; no age-by-serostatus interaction was observed on CSF logKYNA (p-value, F–value, degree of freedom and sample size) (b) Independent of HIV serostatus, KMO CC-homozygotes (solid lines) had higher CSF Log[KYNA] with older age, contrast to T-carriers (dashed lines), but no genotype-by-age interaction nor genotype-by-age-by-HIV serostatus interaction was observed on CSF Log[KYNA]. (c) Similarly, older age in KATII C-carriers (solid lines) correlated with higher CSF Log[KYNA], contrast to TT-carriers (dashed lines). No KATII-by-age-by-HIV serostatus interaction was observed on CSF Log[KYNA]
Discussion
This is the first study that investigated the effects of KMO and KATII genotypes for genes which encode two of the major KP enzymes on psychopathological symptoms and CSF-[KYNA] levels in HIV patients. First, we replicated previous findings showing that HIV individuals had more psychopathological symptoms compared to SN controls (Owe-Larsson et al. 2009; Jin et al. 2010; Rezaei et al. 2013). Second, consistent with our hypothesis, the C-allele at the KATII-rs1480544 appeared to have a protective effect against psychopathological distress, since the SN C-carriers had fewer psychopathological symptoms than the SN TT-carriers. However, HIV participants did not benefit from this protective effect that might have been masked by the greater effects from HIV-associated neuroinflammation. Third, the KMO-rs1053230 polymorphisms had no significant impact on psychopathological symptoms in HIV or SN individuals. Finally, CSF-[KYNA] increases in an age-dependent manner primarily in the KATII C-carriers and the KMO CC-carriers independent of HIV-serostatus, which suggests that modulation of KP metabolite levels by these genotypes may contribute to an antioxidant response in brain aging.
Consistent with previous studies (Owe-Larsson et al. 2009; Jin et al. 2010; Rezaei et al. 2013), our HIV participants had more psychopathological distress (+16 % GSI) across a wide range of symptoms (+15 % PST), but with moderate intensity as indicated by PSDI scores (+9 %) compared to SN controls. However, none of them had significant clinical depression and/or anxiety disorders at the time of the study. Furthermore, across all symptom subscales, only T-scores on anxiety symptoms were markedly elevated (+17 %) in the HIV individuals. These findings contrast with those previously described in HIV patients not treated with HAART, who had elevated scores (>20 %) in many more subscales, including the interpersonal sensitivity, depression or psychoticism subscales (Blanch et al. 2001; Jin et al. 2010). The less severe psychological symptoms in our HIV participants may be due to the fact that they were stable on HAART-medications.
In our HIV patients, those with greater psychopathological symptoms also had lower CD4+ counts (Lu et al. 2009; Margalho et al. 2011), and tended to have higher CSF-[KYNA], especially in those with longer duration of HIV diagnosis and lower CD4+ counts. Taken together, these findings suggest that the higher CSF-[KYNA] might be a response to the greater psychopathological distress, which might be mediated by the disease severity (i.e. lower CD4+ count and duration of HIV diagnosis). However, we found no direct correlation between CSF-[KYNA] and any of the psychopathological symptom subscales across the subject groups, which might be due to the small sample size. Furthermore, the non-significantly elevated CSF-[KYNA] in our HIV patients compared to those in the SN controls might be due to the subclinical depressive symptoms in our HIV cohort.
Although our HIV patients were not clinically depressed during the study, those maintained on SSRI/SNRI had even more psychopathological distress symptoms, and tended to have higher CSF-[KYNA] than non-medicated participants. Altogether, these findings suggest that these SSRI/SNRI treated HIV individuals may not be adequately treated for their psychological symptoms.
In SN controls, the KATII-rs1480544 C-carriers had less psychopathological distress than TT-carriers, especially for anxiety, suggesting the C-allele might be protective against psychopathological symptoms. We hypothesized that this C-allelic neuroprotection was mediated by higher activity of the KATII enzyme (Baran et al. 2012; Baran and Kepplinger 2014), which catalyzes the production of the antioxidant KYNA. The KATII-rs1480544 C-allele might increase the mRNA expression and production of KATII protein (Coutinho et al. 2014), although our SN subjects did not show KATII genotype group difference in CSF-[KYNA]. Furthermore, the KATII-C-allele protective effect was not found in our HIV cohort. This might be due to the higher levels of pro-inflammatory cytokines (Hong and Banks 2015), such as IFN-γ, IL1 and IL6, and elevated oxidative metabolites in HIV infected individuals (Panee et al. 2015). All of these molecules may increase the KYN catabolism and lead to elevated KP metabolites in both neurotoxic and neuroprotective branches (Maes 2011). Therefore, this enhanced KYN catabolism might have masked the protective effect of the C-allele against psychopathological symptoms in these HIV participants.
Contrary to our hypothesis that C-carriers of KMO, the gene for the enzyme that catalyzes the neurotoxic branch, would have more psychopathological symptoms, neither the HIV nor SN subjects showed such associations in the current study. These findings contrast with the greater psychotic symptoms in KMO-rs105323-C-carriers with bipolar disorders (Lavebratt et al. 2014) or the higher prevalence KMO-rs105323-C-carriers in patients with depression (Claes et al. 2011). This discrepancy may be due to the relatively asymptomatic subjects in our study, since the group T-scores were all in the subclinical range (T< 63). Therefore, future studies in HIV patients with more severe psychiatric symptoms should be evaluated in relation to this KMO genotype, especially since several KMO inhibitors have been developed to minimize the neurotoxic metabolites (Maddison and Giorgini 2015).
Regardless of HIV-serostatus, CSF-[KYNA] increased with age, which is consistent with findings in schizophrenic patients (Erhardt et al. 2001), patients with chronic pain (Swartz et al. 1990) and those with acute headaches (Kepplinger et al. 2005). The age-dependent increase in CSF-[KYNA] might represent both brain-derived KYNA, as well as additional KYNA from the plasma, since the blood brain barrier (BBB) permeability increases with age (Elahy et al. 2015), which might also allow the entry of KYNA from the plasma to the CSF. Furthermore, KATII-rs1480544 C-carriers, but not carriers of the other genotypes, showed age-related increases of CSF-[KYNA] regardless of HIV serostatus. Since the KATII-rs1480544 C-allele might protect against psychopathological distress through an increase of KATII activity toward the neuroprotective KYNA metabolite, the age-dependent increase of CSF-[KYNA] suggests an even stronger compensatory antioxidant response (i.e. CIRS) in the older individuals. Although only the KMO-rs1053230 CC-carriers, and not the T-carriers, showed significant age-dependent increase in CSF-[KYNA], no genotype-by age interaction was found, which is consistent with the findings in patients with bipolar type 1 (Lavebratt et al. 2014). The lack of KMO genotype or genotype-by-age effect on CSF-[KYNA] is consistent with our findings of similar psychopathological symptoms across the KMO genotype groups.
This study has several limitations. First, the sample size in the subgroups with TT genotype for KATII and T-carriers for KMO was relatively small; future studies with larger sample sizes and broader racial diversity are needed to validate these group differences in psychological symptoms and CSF-[KYNA]. Second, our study population did not have significant clinical depression and/or anxiety disorders at the time of the study. Future studies should also include clinically depressed individuals, who might have more alterations in KP-related phenotypes, and include more prodromal markers, such as fatigue which may predict psychopathological distress (Dabaghzadeh et al. 2013). Third, although imbalance in KYN metabolism is implicated in the development of psychopathological symptoms in HIV individuals (Davies et al. 2010; Baran et al. 2012; Cassol et al. 2015), it can be found in multiple disorders including stroke (Bensimon et al. 2014), diabetes (da Silva Dias et al. 2015), cardiovascular disorders (Nikkheslat et al. 2015), Alzheimer’s Disease (Leonard 2007), Huntington’s Disease (Mazarei and Leavitt 2015), and cancer (Hufner et al. 2015). Therefore, our results regarding how KMO and KATII might influence KYN metabolism and the psychopathological distress might not be specific to HIV infection. Fourth, only one KP metabolite, KYNA, was investigated in relation to the KATII and KMO polymorphisms. Although CSF [KYNA] may better reflect [KYNA] in the brain than plasma [KYNA], and that the latter does not correlate with CSF [KYNA] (Kepplinger et al. 2005), future studies should evaluate systematically all KP metabolites in the CSF. Assessing KP metabolite ratios, as indicators of the enzyme activities may further elucidate the molecular mechanisms underlying the psychopathological phenotypes, especially during aging. Lastly, since KP catabolism may be modulated by neuroinflammation (Campbell et al. 2014), future studies should account for the relationships between inflammatory cytokines (i.e. IFN-γ, IL-1β), CSF-[KYNA] and other metabolites in the KP on psychopathological symptoms of HIV patients.
In summary, we showed that the severity of psychopathological symptoms is modulated differently in HIV and SN controls by the polymorphism in KATII-rs1480544, but not by the polymorphism of KMO-rs105323. Since these gene products may direct the catabolism of KYN, which is also influenced by neuroinflammation, targeted treatments in individuals at risk for psychopathological symptoms (e.g., KATII T-carriers), by decreasing neuroinflammation and increasing KYNA, might reduce depressive symptoms in HIV. For example, KYNA analogs that are already being investigated for the treatment of migraine (Knyihar-Csillik et al. 2008), seizures (Demeter et al. 2012), and cognitive deficits in HIV (Baran and Kepplinger 2014), may also be useful in the treatment of psychopathological symptoms, and ultimately improved the quality of life in HIV patients.
Acknowledgments
We thank all the research participants for their contribution to the study, the community providers who refer the participants, and the clinical research staff of the University of Hawaii Neuroscience and MR Research Program at the Queen’s Medical Center, who recruited and evaluated these participants. We also thank Rosanne Harrigan, Ed.D. for her invaluable guidance and instructions related to this Masters of Science in Clinical Research (MSCR) thesis project, and for reading the manuscript, and Caroline Jiang, M.S., for statistical analysis assistance.
Funding This study was supported by NIH grants: R01-MH61427, K24-DA016170, R01-DA035659, U54-NS56883, P30-CA071789, R25-RR019321.
Footnotes
Compliance with Ethical Standards All procedures performed in studies involving our human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Conflict of Interest The authors declare no conflicts of interest related to this work.
References
- Adib-Conquy M, Cavaillon JM (2009) Compensatory anti-inflammatory response syndrome. Thromb Haemost 101:36–47 [PubMed] [Google Scholar]
- Anderson G, Maes M (2014) Oxidative/nitrosative stress and immunoinflammatory pathways in depression: treatment implications. Curr Pharm Des 20:3812–3847 [DOI] [PubMed] [Google Scholar]
- Asch SM, Kilbourne AM, Gifford AL, Burnam MA, Turner B, Shapiro MF, Bozzette SA (2003) Underdiagnosis of depression in HIV: who are we missing? J Gen Intern Med 18:450–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas A, Gisslen M, Nordin C, Lindstrom L, Schwieler L (2007) Acute psychotic symptoms in HIV-1 infected patients are associated with increased levels of kynurenic acid in cerebrospinal fluid. Brain Behav Immun 21:86–91 [DOI] [PubMed] [Google Scholar]
- Baran H, Kepplinger B (2014) D-Cycloserine lowers kynurenic acid formation–new mechanism of action. Eur Neuropsychopharmacol 24:639–644 [DOI] [PubMed] [Google Scholar]
- Baran H, Hainfellner JA, Kepplinger B, Mazal PR, Schmid H, Budka H (2000) Kynurenic acid metabolism in the brain of HIV-1 infected patients. J Neural Transm 107:1127–1138 [DOI] [PubMed] [Google Scholar]
- Baran H, Hainfellner JA, Kepplinger B (2012) Kynurenic acid metabolism in various types of brain pathology in HIV-1 infected patients. Int J Tryptophan Res 5:49–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensimon K, Herrmann N, Swardfager W, Yi H, Black SE, Gao FQ, Snaiderman A, Lanctot KL (2014) Kynurenine and depressive symptoms in a poststroke population. Neuropsychiatr Dis Treat 10:1827–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanch J, Martinez E, Rousaud A, Blanco JL, Garcia-Viejo MA, Peri JM, Mallolas J, De Lazzari E, De Pablo J, Gatell JM (2001) Preliminary data of a prospective study on neuropsychiatric side effects after initiation of efavirenz. J Acquir Immune Defic Syndr 27:336–343 [DOI] [PubMed] [Google Scholar]
- Campbell BM, Charych E, Lee AW, Moller T (2014) Kynurenines in CNS disease: regulation by inflammatory cytokines. Front Neurosci 8:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassol E, Misra V, Morgello S, Kirk GD, Mehta SH, Gabuzda D (2015) Altered monoamine and acylcarnitine metabolites in HIV-positive and HIV-negative subjects with depression. J Acquir Immune Defic Syndr 69:18–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC (2011) Mental illness surveillance among adults in the United States. Morbidity and mortality weekly report [Google Scholar]
- Claes S, Myint AM, Domschke K, Del-Favero J, Entrich K, Engelborghs S, De Deyn P, Mueller N, Baune B, Rothermundt M (2011) The kynurenine pathway in major depression: haplotype analysis of three related functional candidate genes. Psychiatry Res 188:355–360 [DOI] [PubMed] [Google Scholar]
- Coutinho LG, Christen S, Bellac CL, Fontes FL, Souza FR, Grandgirard D, Leib SL, Agnez-Lima LF (2014) The kynurenine pathway is involved in bacterial meningitis. J Neuroinflammation 11:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Dias IC, Carabelli B, Ishii DK, de Morais H, de Carvalho MC, Rizzo de Souza LE, Zanata SM, Brandao ML, Cunha TM, Ferraz AC, Cunha JM, Zanoveli JM (2015) Indoleamine-2,3-dioxygenase/kynurenine pathway as a potential pharmacological target to treat depression associated with diabetes. Mol Neurobiol. doi: 10.1007/s12035-015-9617-0 [DOI] [PubMed] [Google Scholar]
- Dabaghzadeh F, Khalili H, Ghaeli P, Alimadadi A (2013) Sleep quality and its correlates in HIV positive patients who are candidates for initiation of antiretroviral therapy. Iran J Psychiatry 8:160–164 [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW (2008) From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 9:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies NW, Guillemin G, Brew BJ (2010) Tryptophan, neurodegeneration and HIV-associated neurocognitive disorder. Int J Tryptophan Res 3:121–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza FR, Fontes FL, da Silva TA, Coutinho LG, Leib SL, Agnez-Lima LF (2011) Association of kynurenine aminotransferase II gene C401T polymorphism with immune response in patients with meningitis. BMC Med Genet 12:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demeter I, Nagy K, Gellert L, Vecsei L, Fulop F, Toldi J (2012) A novel kynurenic acid analog (SZR104) inhibits pentylenetetrazole-induced epileptiform seizures. An electrophysiological study: special issue related to kynurenine. J Neural Transm 119:151–154 [DOI] [PubMed] [Google Scholar]
- Elahy M, Jackaman C, Mamo JC, Lam V, Dhaliwal SS, Giles C, Nelson D, Takechi R (2015) Blood–brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing I& A 12:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Blennow K, Nordin C, Skogh E, Lindstrom LH, Engberg G (2001) Kynurenic acid levels are elevated in the cerebrospinal fluid of patients with schizophrenia. Neurosci Lett 313:96–98 [DOI] [PubMed] [Google Scholar]
- Gabbay V, Klein RG, Katz Y, Mendoza S, Guttman LE, Alonso CM, Babb JS, Hirsch GS, Liebes L (2010) The possible role of the kynurenine pathway in adolescent depression with melancholic features. J Child Psychol Psychiatry 51:935–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Ely BA, Babb J, Liebes L (2012) The possible role of the kynurenine pathway in anhedonia in adolescents. J Neural Transm 119:253–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostner JM, Becker K, Kurz K, Fuchs D (2015) Disturbed amino acid metabolism in HIV: association with neuropsychiatric symptoms. Front Psychiatry 6:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyes MP, Brew BJ, Saito K, Quearry BJ, Price RW, Lee K, Bhalla RB, Der M, Markey SP (1992) Inter-relationships between quinolinic acid, neuroactive kynurenines, neopterin and beta 2-microglobulin in cerebrospinal fluid and serum of HIV-1-infected patients. J Neuroimmunol 40:71–80 [DOI] [PubMed] [Google Scholar]
- Holtze M, Saetre P, Engberg G, Schwieler L, Werge T, Andreassen OA, Hall H, Terenius L, Agartz I, Jonsson EG, Schalling M, Erhardt S (2012) Kynurenine 3-monooxygenase polymorphisms: relevance for kynurenic acid synthesis in patients with schizophrenia and healthy controls. J Psychiatry Neurosci 37:53–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Banks WA (2015) Role of the immune system in HIV-associated neuroinflammation and neurocognitive implications. Brain Behav Immun 45:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufner K, Oberguggenberger A, Kohl C, Geisler S, Gamper E, Meraner V, Egeter J, Hubalek M, Beer B, Fuchs D, Sperner-Unterweger B (2015) Levels in neurotransmitter precursor amino acids correlate with mental health in patients with breast cancer. Psychoneuroendocrinology 60:28–38 [DOI] [PubMed] [Google Scholar]
- International HapMap C (2003) The international HapMap project. Nature 426:789–796 [DOI] [PubMed] [Google Scholar]
- Jin C, Zhao G, Zhang F, Feng L, Wu N (2010) The psychological status of HIV-positive people and their psychosocial experiences in eastern China. HIV Med 11:253–259 [DOI] [PubMed] [Google Scholar]
- Kepplinger B, Baran H, Kainz A, Ferraz-Leite H, Newcombe J, Kalina P (2005) Age-related increase of kynurenic acid in human cerebrospinal fluid - IgG and beta2-microglobulin changes. Neurosignals 14:126–135 [DOI] [PubMed] [Google Scholar]
- Knyihar-Csillik E, Mihaly A, Krisztin-Peva B, Robotka H, Szatmari I, Fulop F, Toldi J, Csillik B, Vecsei L (2008) The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci Res 61:429–432 [DOI] [PubMed] [Google Scholar]
- Lavebratt C, Olsson S, Backlund L, Frisen L, Sellgren C, Priebe L, Nikamo P, Traskman-Bendz L, Cichon S, Vawter MP, Osby U, Engberg G, Landen M, Erhardt S, Schalling M (2014) The KMO allele encoding Arg452 is associated with psychotic features in bipolar disorder type 1, and with increased CSF KYNA level and reduced KMO expression. Mol Psychiatry 19:334–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard BE (2007) Inflammation, depression and dementia: are they connected? Neurochem Res 32:1749–1756 [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Seeley JR, Roberts RE, Allen NB (1997) Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychol Aging 12:277–287 [DOI] [PubMed] [Google Scholar]
- Lu SH, Tang XP, Deng XL, Chen WL, Hu RX (2009) Relationship between psychological distress and T lymphocyte in HIV/AIDS patients. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi = Zhonghua Shiyan He Linchuang Bingduxue Zazhi = Chin J Exp Clin Virol 23:23–25 [PubMed] [Google Scholar]
- Lugo-Huitron R, Blanco-Ayala T, Ugalde-Muniz P, Carrillo-Mora P, Pedraza-Chaverri J, Silva-Adaya D, Maldonado PD, Torres I, Pinzon E, Ortiz-Islas E, Lopez T, Garcia E, Pineda B, Torres-Ramos M, Santamaria A, La Cruz VP (2011) On the antioxidant properties of kynurenic acid: free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol Teratol 33:538–547 [DOI] [PubMed] [Google Scholar]
- Maddison DC, Giorgini F (2015) The kynurenine pathway and neurodegenerative disease. Semin Cell Dev Biol 40:134–141 [DOI] [PubMed] [Google Scholar]
- Maes M (2011) Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog Neuropsychopharmacol Biol Psychiatry 35:664–675 [DOI] [PubMed] [Google Scholar]
- Maes M, Rief W (2012) Diagnostic classifications in depression and somatization should include biomarkers, such as disorders in the tryptophan catabolite (TRYCAT) pathway. Psychiatry Res 196:243–249 [DOI] [PubMed] [Google Scholar]
- Maes M, Berk M, Goehler L, Song C, Anderson G, Galecki P, Leonard B (2012) Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med 10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manji H, Miller R (2004) The neurology of HIV infection. J Neurol Neurosurg Psychiatry 75(Suppl 1):i29–i35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margalho R, Pereira M, Ouakinin S, Canavarro MC (2011) Adherence to HAART, quality of life and psychopathological symptoms among HIV/AIDS infected patients. Acta Medica Port 24(Suppl 2):539–548 [PubMed] [Google Scholar]
- Martinez P, Tsai AC, Muzoora C, Kembabazi A, Weiser SD, Huang Y, Haberer JE, Martin JN, Bangsberg DR, Hunt PW (2014) Reversal of the Kynurenine pathway of tryptophan catabolism may improve depression in ART-treated HIV-infected Ugandans. J Acquir Immune Defic Syndr 65:456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazarei G, Leavitt BR (2015) Indoleamine 2,3 dioxygenase as a potential therapeutic target in Huntington’s disease. J Huntingtons Dis 4:109–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molteni R, Macchi F, Zecchillo C, Dell’agli M, Colombo E, Calabrese F, Guidotti G, Racagni G, Riva MA (2013) Modulation of the inflammatory response in rats chronically treated with the antidepressant agomelatine. Eur Neuropsychopharmacol 23:1645–1655 [DOI] [PubMed] [Google Scholar]
- Myint AM, Kim YK, Verkerk R, Scharpe S, Steinbusch H, Leonard B (2007) Kynurenine pathway in major depression: evidence of impaired neuroprotection. J Affect Disord 98:143–151 [DOI] [PubMed] [Google Scholar]
- Nikkheslat N, Zunszain PA, Horowitz MA, Barbosa IG, Parker JA, Myint AM, Schwarz MJ, Tylee AT, Carvalho LA, Pariante CM (2015) Insufficient glucocorticoid signaling and elevated inflammation in coronary heart disease patients with comorbid depression. Brain Behav Immun 48:8–18 [DOI] [PubMed] [Google Scholar]
- Owe-Larsson B, Sall L, Salamon E, Allgulander C (2009) HIV infection and psychiatric illness. Afr J Psychiatry (Johannesbg) 12:115–128 [DOI] [PubMed] [Google Scholar]
- Oxenkrug G (2013) Serotonin-kynurenine hypothesis of depression: historical overview and recent developments. Curr Drug Targets 14:514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panee J, Pang X, Munsaka S, Berry MJ, Chang L (2015) Independent and co-morbid HIV infection and Meth use disorders on oxidative stress markers in the cerebrospinal fluid and depressive symptoms. J Neuroimmune Pharmacol Off J Soc NeuroImmune Pharmacol 10:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG (2008) HIV and depression: 2008 review and update. Curr HIV/AIDS Rep 5:163–171 [DOI] [PubMed] [Google Scholar]
- Radloff LS (1977) The CES-D scale: A self report depression scale for research in the general population. Appl Psychol Meas 1:385–401 [Google Scholar]
- Rezaei S, Taramian S, Kafie SM (2013) Psychopathological dimensions in substance abusers with and without HIV/AIDS and healthy matched group. Addict Health 5:115–125 [PMC free article] [PubMed] [Google Scholar]
- Routy JP, Mehraj V, Vyboh K, Cao W, Kema I, Jenabian MA (2015) Clinical relevance of kynurenine pathway in HIV/AIDS: an immune checkpoint at the crossroads of metabolism and inflammation. AIDS Rev 17:96–106 [PubMed] [Google Scholar]
- Schwarcz R, Bruno JP, Muchowski PJ, Wu HQ (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat Rev Neurosci 13:465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sublette ME, Galfalvy HC, Fuchs D, Lapidus M, Grunebaum MF, Oquendo MA, Mann JJ, Postolache TT (2011) Plasma kynurenine levels are elevated in suicide attempters with major depressive disorder. Brain Behav Immun 25:1272–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swartz KJ, Matson WR, MacGarvey U, Ryan EA, Beal MF (1990) Measurement of kynurenic acid in mammalian brain extracts and cerebrospinal fluid by high-performance liquid chromatography with fluorometric and coulometric electrode array detection. Anal Biochem 185:363–376 [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, Thuret S, Price J, Pariante CM (2012) Interleukin-1beta: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology 37:939–949 [DOI] [PMC free article] [PubMed] [Google Scholar]