Abstract
Liquid biopsies collect and analyze tumor components in body fluids, and there is an increasing interest in the investigation of liquid biopsies as a surrogate for tumor tissue in the management of both primary and secondary brain tumors. Herein we critically review available literature on spinal fluid and plasma circulating tumor cells (CTCs) and cell-free tumor (ctDNA) for diagnosis and monitoring of leptomeningeal and parenchymal brain metastases. We discuss technical issues and propose several potential applications of liquid biopsies in different clinical settings (ie, for initial diagnosis, for assessment during treatment, and for guidance of treatment decisions). Last, ongoing clinical studies on CNS metastases that include liquid biopsies are summarized, and recommendations for future clinical studies are provided.
Keywords: circulating tumor cells, clinical implications, CNS metastases, ctDNA, liquid biopsy
Molecular characterization of tumors is fundamental to modern clinical oncology practice. While advanced imaging techniques can provide a wealth of valuable information, diagnosis of malignancy has historically relied on direct microscopic examination of surgically biopsied tissues and molecular testing of these surgical specimens. Due to anatomic considerations, malignancies of the central nervous system (CNS) may not be amenable to surgical biopsy, and especially repeated biopsy. However, in the era of targeted therapies and molecularly driven clinical decision making, this information has never been more essential: In parenchymal brain metastases, temporally and spatially distinct malignancies from a single patient demonstrate clonal evolution.1 Molecular assessment of tumor tissues to tailor therapy at diagnosis and throughout treatment is therefore indispensable. Recent advances in genomic sequencing from cell-free fluid samples (“liquid biopsies”) present a potential solution when a conventional tissue biopsy is not feasible.2 Liquid biopsies collect and analyze tumor components in body fluids, including circulating tumor cells (CTCs), cell-free tumor DNA (ctDNA), RNAs (ctRNA), and exosomes.
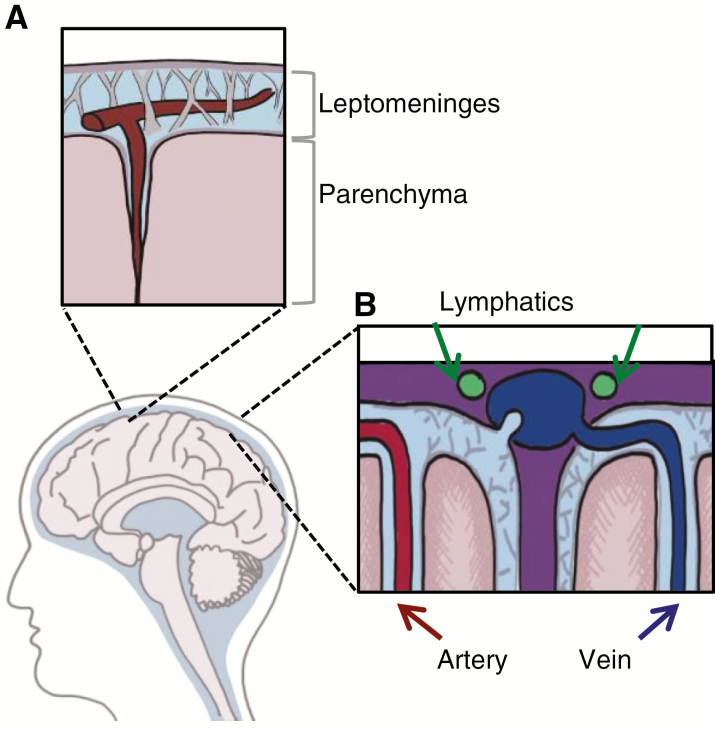
The CNS encompasses 2 distinct anatomic compartments: the densely cellular parenchyma and the cerebrospinal fluid (CSF)–filled leptomeningeal space (Fig. 1A). Entry into each of these compartments is governed by distinct barrier systems: the blood–brain barrier (parenchyma) and the blood–CSF barrier (leptomeninges). A priori, this anatomic sequestration seems to limit the use of CSF-based liquid biopsy to tumors that interface directly with the CSF. However, these 2 compartments may not be as anatomically isolated as was once thought. Recently, perivascular (Virchow–Robin) spaces that communicate with CSF have been found to extend deep into the brain parenchyma.3 In addition, newly identified lymphatic vessels serving the leptomeningeal compartment4 have been discovered to drain into cervical lymph nodes. Together, these systems cooperate to provide an alternative means of communication between the leptomeningeal and parenchymal spaces and the systemic circulation (Fig. 1B).
Fig. 1.
Anatomic compartments in the central nervous system. (A) The CSF-containing leptomeninges comprise the pia and arachnoid and enter into perivascular spaces surrounding cortical vessels, the Virchow–Robin spaces. (B) Newly discovered lymphatic vessels along the dural sinus drain the CSF-filled leptomeninges.
The principal biological fluids relevant for the study of CNS malignancies include serum and CSF. Although blood collection may be more straightforward, CSF offers a number of advantages: Quiescent CSF is paucicellular and possesses a low background level of cell-free DNA. In addition, the low protein and lipid content, and minimal cellularity of this fluid translate to more straightforward processing and increased signal-to-noise ratio.5 Moreover, CNS tumor–derived ctDNA is poorly detectable in plasma6,7 and CNS tumor–derived CTCs are found at much lower concentrations in peripheral blood than in CSF.8
In this review, the Response Assessment in Neuro-Oncology (RANO) Leptomeningeal Metastasis and the RANO Brain Metastasis Working Groups have critically reviewed the literature on CSF and plasma CTCs and ctDNA for diagnosis and monitoring of CNS metastases (Fig. 2A), and propose potential applications in future clinical studies.
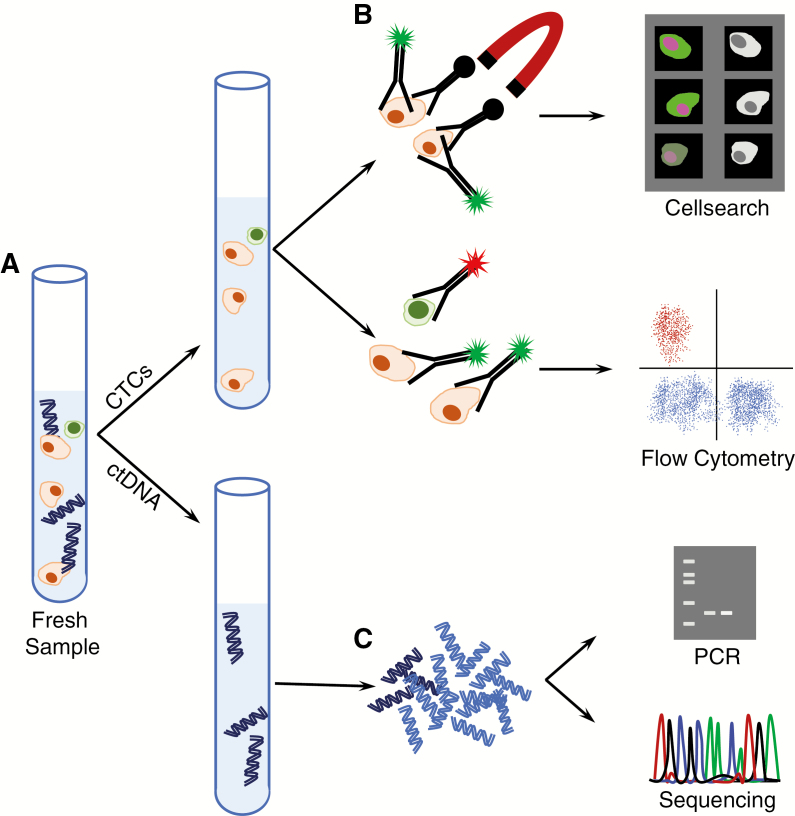
Fig. 2.
Liquid biopsies. (A) Cerebrospinal fluid contains both cellular and acellular material. (B) Centrifugation isolates cells for circulating tumor cell (CTC) analyses. Antibodies against cancer cell surface markers conjugated with ferromagnetic particles enable isolation of cancer cells. These cells are further detected with fluorescently conjugated antibodies as part of the CellSearch system. Alternatively, cells may be stained with fluorescently conjugated antibodies against a variety of cell surface markers and enumerated using flow cytometry. (C) Acellular material contains extracellular DNA (ctDNA). After isolation by ultracentrifugation, and library preparation, this DNA can be amplified and subjected to analysis of a single locus (PCR), or entire exomes, genes, or genomes.
Circulating Tumor Cells
General Concepts on CSF Cytology in Leptomeningeal Metastases
The identification of malignant cells in the CSF represents an historical standard for the diagnosis of leptomeningeal metastases (LM). In the absence of tumor cells in the CSF, the diagnosis may also be based on neurological symptoms and typical contrast enhancement of the leptomeninges on MRI of brain or spine.9–11 As a diagnostic technique, CSF cytology suffers from sensitivity problems, with a sensitivity of 44–67% at first lumbar puncture, increasing to 84–91% upon repeated sampling.8,12–19 Furthermore, CSF cytology results are not always conclusive: The presence of so-called suspicious or atypical cells may influence the sensitivity and specificity rates.20 In the last decade, new assays to detect and quantify CTCs have been developed. These include the Veridex CellSearch assay and immunoflow cytometry methods.14–19,21,22
Veridex CellSearch Assay
The Veridex CellSearch assay is FDA approved and was originally developed for detection of CTCs in blood. Epithelial tumor cells are immunomagnetically enriched by addition of anti-EpCAM (epithelial cell adhesion molecule) ferrofluid (Fig. 2B).23 Subsequently, the sample is immunofluorescently stained with 4′6-diamidino-2-phenylindole (DAPI) dihydrochloride for nuclear staining; anti-CD45 allophycoocyanin to label leukocytes; and anti-cytokeratin (CK) 8, 18-phycoerythrin (PE), and anti-cytokeratin 19 phycoerythrin (CK-PE) for epithelial tumor cell staining. CTCs are defined as nucleated DAPI and CK-PE positive cells lacking CD45 expression. Several adaptations of the technique have been proposed for the detection and quantification of tumor cells in the CSF.15,17,18,21,24 The CellSearch technology can also be used to detect melanoma cells in the CSF by using staining for proteins expressed by melanoma cells such as high-molecular-weight melanoma-associated antigen (HMW-MAA)/melanoma chondroitin sulfate proteoglycan (MCSP) and CD146.24 Trained operators are employed in this system to reduce interreviewer discordant results.25–27
Immunoflow Cytometry Techniques
An additional method to detect CTCs in the CSF is through the use of immunoflow cytometry techniques with fluorescently labeled antibodies against membrane-bound tumor cell proteins, such as EpCAM for epithelial tumor cells and HMW-MAA/MCSP for melanoma.24,27 A fluorescence activated cell sorting system is then employed to enumerate CTCs. In these assays, immunomagnetic enrichment with anti-EpCAM (or anti-MCSP) MicroBeads prior to flow cytometry is used.17,19,28 To distinguish CTCs from leukocytes, anti-CD45 fluorescein isothiocyanate for leukocyte labeling is added. In addition to these markers, some groups use anti-CD33 to improve differentiation between monocyte/macrophages/granulocytes (CD45− CD33+ CD326+) and epithelial (tumor) cells (CD45− CD33− CD326+).28,29 Other groups use Hoechst 33258 and DRAQ5 for nuclear DNA staining.14,19
Current Research on CSF CTCs in Leptomeningeal Metastases
To date, a number of studies have employed CellSearch technology or immunoflow cytometry techniques to detect malignant cells in CSF and diagnose LM (Table 1). The available studies on CTCs in the CSF of patients with LM have reported a sensitivity for detection of CTCs substantially higher than cytology at first lumbar puncture (78–100% vs 44–67%). Specificity of CTCs has ranged between 84% and 100%. However, studies reporting the highest values have a limited sample size. A major limitation is that most of the studies were performed on either breast or lung cancers: Thus, without direct comparison, the utility of CSF-CTCs among different epithelial primaries remains unknown. Moreover, different EpCAM-based immunoflow cytometry methods have been employed across the various studies.
Table 1.
Studies on CSF circulating tumor cells (CTCs) versus CSF cytology in LM
| Study | Assay | N | Patient Population | Sensitivity CTC (95% CI) | Specificity CTC (95% CI) | Sensitivity Cytology (95% CI) | Specificity Cytology (95% CI) |
|---|---|---|---|---|---|---|---|
| Patel et al, 2011 | C | 5 | Breast cancer with confirmed LM | First pilot study on an (adapted) CellSearch technology for CSF, showing that CTCs in the CSF can be quantitatively detected and correlate with disease burden and response to chemotherapy | |||
| LeRhun et al, 2012 | C | 8 | Breast cancer with confirmed LM | Pilot study showing the identification and quantification of CTCs in CSF with an adapted CellSearch technology and its promising role to evaluate response to therapy. | |||
| Subirá et al,b 2012 | FC | 78 | Clinically suspected LM and previous diagnosis of epithelial-cell tumors | 75.5 (63.5–87.6) | 96.1 (88.8–100) | 65.3 (52.0–78.6) | 100 (100–100) |
| Nayak et al, 2013 | C | 51 | Clinical suspicion of LM/solid tumors (mainly NSCLC and breast cancer) | 100 (78.1–100) | 97.2 (85.4–99.9) | 66.7 (38.3–88.1) | Used as gold standard |
| LeRhun et al, 2013 | C | 2 | Melanoma and confirmed LM | Pilot study showing that with an adapted CellSearch method using an antibody against melanoma (HMW-MAA), melanoma cells can be detected in the CSF. | |||
| Lee et al, 2015 | C | 38 | Confirmed LM or clinical suspicion of LM/breast cancer | 80.95 (58.1–94.4) | 84.62 (54.5–97.6) | 66.67 (43.04–85.35) | Used as gold standard |
| Subirá et al,b 2015 | FC | 144 | Confirmed LM or clinical suspicion LM, epithelial cell tumors | 79.8 (NA) | 84 (NA) | 50 (NA) | 100 (NA) |
| Tu et al, 2015 | C | 18 | MRI confirmed LM/lung cancer | 77.8 (52.4–93.6) | 100 (47.8–100) | 44.4 (21.5–69.2) | Not reported |
| Acosta et al 2016 | FC | 6a | Clinical suspicion of LM, carcinoma | 100 (NA) | 100 (NA) | Not reported | Not reported |
| Milojkovic Kerklaan et al, 2016 | FC | 29 | Clinical suspicion of LM and negative or inconclusive MRI, epithelial cell tumors | 100 (75–100) | 100 (79–100) | 61.5 (32–86) | 100 (79–100) |
| Jiang et al, 2017 | C | 21 | Clinical suspicion of LM, NSCLC | 95.2 (NA) | 100 (NA) | 57.1 (NA) | Not reported |
| Lin et al, 2018 | C | 95 | Clinical suspicion of LM, lung (n = 36), breast (n = 31), miscellaneous (n = 28) | 93 (84–100) | 95 (90–100) | 29 (NA) | Not reported |
C = CellSearch Veridex; FC = flow cytometry; NA = not available; HMW-AA/MCSP = human molecular weight–melanoma associated antigen/melanoma-associated chondroitin sulfate proteoglycan; a = number of samples instead of number of patients; b = study cohorts are overlapping.
These techniques are on the verge of full clinical implementation, although patient numbers in the studies are small, and the diagnosis of LM in case of negative CSF cytology is always disputable. Overall, there are sufficient data to support adding CTC to standard workup. In general one CSF examination, including CTC analysis, is expected to be sufficient in the majority of patients with suspicion of LM. Fewer than 10% of patients will require additional lumbar puncture for diagnosis.15,19,30 Both anti-EpCAM and anti–HMW-MAA/MCSP assays do not provide 100% sensitivity, as epithelial tumor cells can lose EpCAM expression due to epithelial to mesenchymal cell transition31 and HMW-MAA/MCSP expression on melanoma cells is only 85%.27 In light of this, CTCs can be employed as tools for high-sensitivity detection, but presence/absence of malignancy is generally confirmed by formal cytology. Large-scale prospective quantification of the rate of cell surface marker loss in epithelial malignancies and melanoma is needed.
Besides a higher sensitivity of CTC analysis in CSF compared with CSF cytology, an advantage of CTC detection is that it is quantitative, whereas CSF cytology is not. Currently, there are only small patient series that performed serial lumbar punctures with quantification of CTCs in CSF during treatment.21,24 The results indicate that CTC numbers in CSF can potentially be used to measure treatment response, but additional larger studies are needed to validate these findings.
It is currently unknown whether CellSearch technology or immunoflow cytometry is the best technique to detect tumor cells in CSF. Similar sensitivity and specificity rates are gained with both methods, but no direct comparison with adequate power has been done. A drawback of the CellSearch technology is that it requires dedicated CellSearch reagents and equipment in specialized central labs with trained operators.25,26 Benefits are that CSF samples can be preserved up to 96 hours in a CellSave collection tube before measurement and CellSearch technology is FDA approved. Furthermore, a predefined tumor cell gate is used, which allows fully automatic identification and enumeration of CTCs in CSF, which could allow an easier and broader application of this technique. On the other hand, a benefit of the immunoflow cytometry assay for CTC detection is that standard flow cytometry equipment can be used. However, immunoflow cytometry methods for CTC detection in CSF are not standardized between laboratories.
Beyond diagnosis of LM, new CTC detection techniques offer the opportunity to isolate single CTCs to enable single tumor cell analyses (tumor DNA, RNA, and protein). For example, Cordone et al32,33 showed the presence of syndecan-1 and MUC-1 overexpression and the putative stem cell markers CD15, CD24, CD44, and CD133 on CTCs in the CSF of breast cancer patients with LM, possibly related to tumor invasiveness. Two groups performed genomic sequencing of isolated breast cancer cells in the CSF of LM patients showing mutations identical to the primary breast cancer as well as new mutations suggesting clonal diversity.33,34 A recent study8 performed on cells isolated from CSF of non–small cell lung cancer (NSCLC) patients with epidermal growth factor receptor (EGFR) mutations or anaplastic lymphoma kinase rearrangements and LM has shown that the genetic profiles of CTCs were highly concordant with the molecular alterations present in the primary tumor (89.5%), and some clinically relevant resistance mutations (EGFR T790M, methionine amplifications, Erb-B2 receptor tyrosine kinase 2 [ERBB2] amplifications) were uncovered.
Cell-Free DNA
Techniques
Cell-free tumor DNA (ctDNA) is typically collected from biological fluids after removal of cells with a low-speed centrifugation, followed by removal of cell debris and particulate matter with high-speed centrifugation. DNA is then extracted using commercially available silica-column based kits prior to library preparation and subsequent sequencing (Fig. 2C). Technically successful and clinically useful analyses require detection of mutations at low allelic frequency. For this reason, although plasma may contain higher concentration of cell-free DNA, this is typically composed of majority normal genomic DNA, constituting a high background signal and a technical challenge. In contrast, DNA extracted from CSF is enriched in ctDNA, with a relative absence of genomic DNA. Thus, it is possible to call somatic mutations in CSF in the face of lower sequence coverage. In practical terms, CSF can be collected and stored on ice for up to 3 hours prior to initial removal of cellular material and long-term storage at −80°C. Subsequent ultracentrifugation, DNA extraction, library preparation, and sequencing can then be undertaken in batches. Sequencing approaches have ranged from digital PCR and massively parallel targeted exome or amplicon sequencing to whole exome sequencing, depending on the clinical question.
Current Research on CSF ctDNA in CNS Metastases
Published studies on ctDNA in CSF of CNS metastases are listed in Table 2.
Table 2.
Studies on cell-free DNA sequencing in plasma or CSF of CNS metastases
| Study | Site of CNS Malignancy | n | Primary | Biological Fluid Sampled | Sequencing Method | CNS Malignancy Mutation Detection Rate |
|---|---|---|---|---|---|---|
| Swinkels et al, 2000 | LM | 2 | Lung adenocarcinoma | CSF | Mutant- allele- specific amplification (PCR) | KRAS mutation detectable in CSF 2/2 (100%) |
| De Mattos et al, 2015 | P | 12 | 6 breast cancer, 2 lung cancer, 4 glioblastoma | CSF plasma | Targeted sequencing | CNS disease only: 58% CSF, 0% plasma; CNS and non-CNS disease: 60% CSF, 55.5% plasma |
| Momtaz et al, 2016 | P, LM | 11 | Patients with BRAF-mutated malignancies | CSF | Targeted sequencing | BRAF mutations detected in CSF of 6/11 (54%) |
| Pentsova et al, 2016 | P, LM | 41 | 11 lung cancer, 11 breast cancer, 6 melanoma, 1 bladder cancer, 2 gastrointestinal, 2 ovarian, 1 neuroendocrine, 2 thyroid, 2 prostate, 2 renal, 1 sarcoma | CSF | Targeted sequencing | Mutations detectable in CSF of 20/32 (63%) patients with parenchymal mets 3/4 (75%) patients with LM |
| Marchio et al, 2017 | LM | 2 | Lung adenocarcinoma | CSF plasma | Targeted sequencing | KRAS mutations detectable in CSF 2/2 (100%) |
| Siravegna et al, 2017 | P | 1 | HER 2 + breast CSF adenocarcinoma | CSF plasma | Digital droplet PCR whole exome sequencing | ERBB2 CNYC TP53 PIK3CA |
| Fan et al, 2018 | LM | 11 | EGFR-mutated NSCLC | CSF | Targeted sequencing | EGFR mutations detectable in CSF 11/11 (100%); mutations were not concordant in 1/11 (9%) |
| Li et al, 2018 | LM | 42 | EGFR-mutated NSCLC | CSF | Targeted sequencing | EGFR mutations detectable in CSF of 92% (n = 28) |
| Huang et al, 2018 | LM | 1 | CUP adenocarcinoma | CSF | Targeted sequencing | HER2 and MPL amplification PIK3CA, CDKN2A and P53 mutations |
Abbreviations: P = parenchyma; LM = leptomeninges; PIK3CA = phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha; HER2 = human epidermal growth factor receptor 2; MPL = myeloproliferative leukemia; CDKN2A = cyclin-dependent kinase inhibitor 2A.
In the case of parenchymal brain metastases (BM), targeted sequencing of ctDNA from CSF may be more sensitive than plasma to detect known targetable mutations.6,35 Large-scale genomic characterization demonstrates that BM harbor clinically actionable mutations not found in matched primary tumors in more than 50% of cases.1 Investigations are ongoing to determine whether clinically actionable alterations are shared by CSF and parenchymal BM. In one study DNA from plasma, CSF, parenchymal tumor samples, and germline DNA from 12 patients (6 breast cancer BM, 2 lung cancer BM, 4 glioblastomas) were subjected to targeted sequencing.6 Putative clinically actionable drivers in the brain tumors (such as EGFR, PTEN, ESR1, IDH1, FGFR2, and ERBB2) were more frequently detected in CSF ctDNA than in plasma. Consistent with these findings, Pentsova et al36 detected clinically actionable mutations in the CSF of 20/32 (63%) patients with parenchymal brain metastases, while no mutations were found in 9 patients without CNS involvement by cancer.
Unlike parenchymal brain metastases, LM inhabit the anatomic compartment containing CSF: Sampling CSF directly samples the relevant space. While sequencing of cellular material from CSF yields both normal and cancer cell DNA, sequencing of acellular material yields cancer cell DNA.36 Lacking the anatomic constraints present in parenchymal malignancies, liquid biopsy of the CSF in leptomeningeal metastasis appears highly promising. In the case of BRAF-mutated malignancies, ctDNA was isolated and sequenced in 3/3 patients with radiographic evidence of LM, but in 2/5 patients with only parenchymal BM.35 In the case of leptomeningeal metastasis from solid tumor, ctDNA was isolated and sequenced from 2/2 patients with cytology-proven leptomeningeal metastasis, superior to analogous analyses from plasma.37 In addition, K-ras mutations were detected in the CSF of 2/2 patients with cytology-negative LM.38 Cell-free tumor DNA was successfully isolated and sequenced in 100% (n = 11)39 and 92% (n = 28)40 of patients with LM from EGFR-mutant NSCLC. This was similarly successful in 75% (n = 4) of mixed population of LM from different primary solid tumors.36 These successful exome sequencing efforts have led to further advances, including quantification of SHP1P2 promoter methylation from CSF-derived ctDNA, again demonstrating superior sensitivity and specificity compared with traditional cytology in patients with LM.41 Formal studies are currently under way to leverage ctDNA technology to quantitatively describe tumor burden in the leptomeningeal space.
Technical Issues
Although these studies are promising, larger studies are needed to validate whether ctDNA sequencing can reliably capture the clinically relevant genes found in a patient’s CNS cancer. Many studies employ digital PCR or targeted sequencing of a limited number of genes, and may not represent the full spectrum of clinically relevant oncogenic drivers. Moreover, copy-number changes and certain fusions (eg, anaplastic lymphoma kinase) are technically challenging and may be overlooked by standard “off the shelf” whole exome sequencing approaches. Finally, reliable detection of subclonal resistance mutations in the blood or CSF of patients harboring CNS disease has not yet been adequately addressed. As technologies and analytic capabilities improve, expanding the number of genes and improving the sensitivity to detect mutations and copy-number changes may improve the sensitivity of liquid biopsies in patients with CNS tumors.42,43
We foresee that the majority of centers will not have the technical capacity to carry out these analyses in-house and samples will necessarily be transported. Issues of sample handling, storage, and shipment must therefore be addressed. Comparable to the current situation in tissue sequencing, there is little consensus as to how these genomic data will be shared with clinicians treating the patients. In the research setting, genomic analyses from solid tumors are shared as part of cooperative alliances, or in the setting of public databases (eg, The Cancer Genome Atlas). However, datasets acquired from liquid biopsies from CNS malignancies are available only once published on an individual basis. The rarity of CNS malignancies demands a cooperative consensus approach to sharing ctDNA data in a de-identified and accessible manner. While specifying the organization and constraints of such an arrangement is beyond the scope of this review, we do suggest that making use of currently available pre-existing structures, such as the cBioPortal,44 will allow for wide dissemination of this information and rapidly increase the rate of discovery.
CTCs versus ctDNA
Thus far, there are a lack of studies comparing the detection rate and the clinical usefulness of CTCs and ctDNA in both plasma and CSF of patients with CNS metastases. Several open issues need to be clarified.45,46 It is unclear which biomarker is the most accurate to capture the genetic profile and represent the spatial and temporal heterogeneity of tumors, but it is likely that CTCs and ctDNA provide complementary information. For example, single sample of ctDNA may not provide complete information on heterogeneity of tumor cells in terms of mutational status. Conversely, the analysis of the differential phenotypes of CTCs could identify the mutational status of specific subpopulations at the single cell level. Together, these data will allow for understanding of genomic and transcriptional changes over time under treatment pressure.45
Clinical Applications of Liquid Biopsies
Liquid biopsies may be useful for initial diagnosis, for assessment during treatment, and for guidance of therapeutic decisions (Table 3).
Table 3.
Potential clinical applications of liquid biopsy in the management of CNS metastases
| • Diagnosis of LM when CSF cytology is negative or inconclusive |
| • Diagnosis of brain metastasis from unknown primary tumor or multiple lesions |
| • Quantification of residual tumor following surgical resection |
| • Differential diagnosis between pseudoprogression/radionecrosis and tumor progression |
| • Early indication of tumor response following cytotoxic or targeted agents |
| • Early diagnosis of tumor relapse |
| • Prediction of resistance to targeted agents |
| • Monitoring of treatment of resistance mutations with specific targeted agents |
| • Evaluation of prognosis (based on number of cells and molecular features) |
| • Screening in patients at high risk for brain or leptomeningeal metastases. |
Several applications at the time of cancer diagnosis are attractive: CTC detection shows promise as an additional tool for diagnosing leptomeningeal disease when CSF cytology is negative or inconclusive. A frequent dilemma in neuro-oncology arises in cases of patients presenting with a surgically inaccessible solitary enhancing mass lesion on brain MRI, which can be diagnosed as either metastasis from unknown primary or malignant glioma.47 In the future, liquid biopsies (ctDNA) might represent a non-invasive tool for differential diagnosis in such patients. However, feasibility of this approach in clinical practice may prove challenging. Sequencing by ctDNA of CSF could also be informative in patients with multiple BM, who rarely undergo a biopsy, and may harbor different mutations in comparison to the primary tumor.
Quantitative ctDNA and/or quantification of CTCs from parenchymal and LM from CSF and plasma could allow in both BM and LM a more precise quantification of tumor burden at baseline for prognostic purposes and for stratification of patients in clinical trials. In this regard, several studies in breast cancer have shown correlations of CTCs in the peripheral blood with tumor burden and prognosis.48–52 The number of CTCs at baseline and subsequent determinations were reported to correlate with progression-free survival (PFS) and overall survival (OS) in patients with metastatic breast cancer.53 Moreover, the correlation with OS was most significant when CTCs were measured at cancer baseline compared with other stages of disease. As with breast cancer, plasma CTCs in prostate54,55 and colorectal54 cancer have been utilized with similar cutoff values. Quantitative analysis of ctDNA in plasma may also have diagnostic and prognostic implications. Several studies have demonstrated a high concordance between the mutational profile of candidate genes in matched tumor and plasma DNA samples in patients with breast cancer, colorectal cancer, or NSCLC.56–60 In metastatic breast cancer, increasing ctDNA levels have been associated with inferior survival.61,62 Beyond the potential to estimate tumor burden non-invasively, the concordance between mutations present in plasma ctDNA and tumor tissue samples is increasingly important for diagnosis and targeting of specific molecular subtypes of solid tumors.
Assessment of residual tumor following surgical resection of a parenchymal brain metastasis is another potential application of liquid biopsies. In this scenario, blood and CSF samples are collected before and after surgery, at the same timepoints, correlating with MRI, to account for dynamic alterations in the inflammation and blood–brain barrier. Collection times must be chosen with care, as mechanical tumor spill in the CSF may occur within 2 or 3 weeks postoperatively. These additional analyses may help to better interpret MRI findings in the perioperative period. The use of plasma ctDNA to evaluate residual disease following surgery has been already reported in 2 prospective colorectal cancer studies. In one, a significant and progressive decrease in plasma ctDNA levels in postoperative days was reported.63 In the second, patients with detectable plasma postoperative ctDNA demonstrated a 10-fold risk of recurrence compared with patients with undetectable ctDNA.60 In the case of breast cancer, plasma ctDNA detection predicted relapse in early breast cancer following surgery alone56 or neoadjuvant chemotherapy.57
Similarly, liquid biopsies might be useful to evaluate response in parenchymal brain metastases after local treatments such as radiosurgery or fractionated stereotactic radiotherapy. Such information may help clinicians to distinguish pseudoprogression or radionecrosis from true tumor progression on standard MRI in both clinical trials and daily practice.64,65 Prospective studies evaluating the changes of CTCs and/or ctDNA following such treatments are needed, in combination with standard and advanced neuroimaging.
In a related fashion, liquid biopsies may be employed to monitor brain and LM following systemic and/or intrathecal treatments, and to detect response and progression earlier than MRI and CSF cytology. For instance, O6-methylguanine-DNA methyltransferase promoter methylation in serum or plasma has been shown to predict response to alkylating agents, such as temozolomide in glioblastoma64,66,67 or dacarbazine in metastatic colorectal cancer.67 A recent paper68 has reported that ctDNA in CSF reflected the clinical course in a patient with BRAF-mutated melanoma LM undergoing treatment with dabrafenib and trametinib. The mutant ctDNA fraction gradually decreased from 53% at the time of diagnosis to 0 at the time of clinical improvement, and mutant ctDNA was again detected in CSF at high levels concomitantly with neurological deterioration.
The utility of plasma ctDNA to monitor response to targeted agents and emergence of mechanisms of resistance has been demonstrated for patients with advanced NSCLC or metastatic colorectal cancer harboring EGFR mutations and undergoing treatment with EGFR inhibitors69–71 or antibody-mediated EGFR blockade.72,73 In patients with NSCLC, a reduction in the levels of plasma ctDNA harboring EGFR mutations was observed in 96% of patients after the first treatment cycle, providing an early indication of response to treatment, while the emergence of the resistance mutation EGFR T790M was observed in ctDNA before clinical disease progression.70 In patients with metastatic colorectal cancer, acquired mechanisms of resistance (KRAS mutation, MET amplification) were identified in blood ctDNA of about one third of patients.72,73
Liquid biopsies could serve to identify drug-resistance mechanisms in patients whose primary tumor responded to targeted agents but then relapsed in the CNS. In 4 out of 12 patients with progressive CNS disease during treatment with inhibitors of oncogenic mutations, Pentsova et al identified drug-resistance mutations in the CSF that were not present in the tissue of the primary tumor before treatment.36 Three of 4 patients with an EGFR-mutated NSCLC receiving first- or second-generation EGFR inhibitors developed a T790M mutation (2 patients) or a KRAS G12A mutation (1 patient), both common causes of EGFR tyrosine kinase inhibitor (TKI) resistance in NSCLC.36 In a fourth patient with BRAF V600E mutant melanoma, the acquired resistance mutation NRAS G12R was found.74,75 Jiang et al8 detected the EGFR resistance gene T790M in extracranial lesions in 7 of 9 patients. In contrast, this was detected in the CSF of only 1 of 14 patients with advanced NSCLC with EGFR mutations and LM. This low percentage of T790M mutation in the CSF may be related to an incomplete penetration of the TKIs into CSF and/or a spatiotemporal heterogeneous distribution of T790M.76–78
Recommendations for Clinical Studies
Prospective studies to validate the clinical utility of liquid biopsies (both CTCs and ctDNA) in CNS metastases are required. Unlike primary brain tumors, the genomic landscapes of both extracranial and intracranial disease are clinically relevant in CNS metastasis. Thus, studies should analyze tumor genomic sequences obtained simultaneously from plasma and CSF, and compare these with those of the primary tumor and/or extracranial metastases. Of utmost importance will be the correlations between liquid biopsies and intracranial and extracranial disease burden.78 In particular liquid biopsies of plasma could better define activity of systemic disease, thus improving stratification for trials focused on CNS metastases.
An advantage of liquid biopsies of plasma and CSF is the possibility of repeated sampling, capturing cancer’s evolutionary dynamics. Clinically, this will improve monitoring under treatment, and evaluation of response and progression: These tools could allow a more precise definition of both intracranial and extracranial PFS. To meet this objective, additional studies are needed, comparing circulating biomarkers with neuroimaging findings and CSF cytology at different timepoints. With regard to parenchymal metastases without overt leptomeningeal involvement, factors potentially influencing the sensitivity of CSF liquid biopsy must be clarified, such as tumor location, size/volume, and proximity to the subarachnoid space.
Prospective longitudinal studies should correlate liquid biopsy results with survival. In the case of CTCs, small series suggest that decreased CTC numbers in CSF during LM treatment correlate with treatment response.21,24 Similarly, large prospective studies are needed to determine the prognostic value of CTC enumeration in CSF at diagnosis. An additional, essential question to be addressed includes that of site of CSF sample. In modern practice, CSF may be sampled from the ventricles (Ommaya), cisterna magna, or lumbar cistern. The relative characteristics of liquid biopsies obtained from these cites of CSF sampling and their relationship to the radiographic site(s) of disease should be formally addressed.
With respect to ctDNA, 2 randomized trials of first-generation TKIs for NSCLC investigated the clinical utility of plasma ctDNA analysis (secondary endpoint) as a surrogate for EGFR testing of tissue. The first study79 demonstrated comparable predictive value of blood and tissue molecular biomarkers for PFS and OS prediction. The second study80 revealed that baseline EGFR-mutation positive patients, who became EGFR negative in plasma ctDNA at the end of induction therapy, had a longer PFS and OS than those who remained EGFR-mutation positive.
Phase 0 and I trials of CNS metastases should include liquid biomarker discovery to define cutoff values for both CTC and ctDNA to allow for further validation in phase II and III trials. Several observational studies and phases II–III trials are ongoing in CNS metastases to validate liquid biopsy as a surrogate response marker (Table 4).
Table 4.
Ongoing clinical studies on CNS metastases including liquid biopsy
| Study Number | Patient Population | Type of Study | Fluid Sample | Technique | Primary Outcome | Secondary Outcomes |
|---|---|---|---|---|---|---|
| NSCLC | ||||||
| NCT02607605 | 10 patients with advanced lung cancer with LM | Observational Prospective | CSF | Cell-free DNA (cfDNA) using QIAamp Circulating Nucleic Acid kit (Qiagen) | Positive rate between the cfDNA and cytological examination of CSF [time frame: 2 y] | The relationship between the number of cfDNA and OS [time frame: 2 y] |
| NCT02803619 | 60 patients with EGFRm+ NSCLC and LM | Observational Prospective | CSF | Not reported | OS after the diagnosis of leptomeningeal metastasis in NSCLC patients [time frame: 1 y] |
--- |
| NCT03029065 | 50 patients with BM or LM from NSCLC | Observational Prospective | CSF plasma | cfDNA using next- generation sequencing technique | Investigate whether the cfDNA can be used for concomitant diagnosis to improve the treatment efficacy and prognosis of patients with brain (meningeal) metastasis by monitoring tumor-related genetic mutations in cfDNA in the plasma and CSF [time frame: 6 mo] | --- |
| NCT03257735 | 50 patients with BM from NSCLC | Observational Prospective | CSF plasma tumor tissue | cfDNA using next- generation sequencing technique | To compare the gene mutation in CSF, blood, and tumor tissue at baseline and after 2 months of treatment [time frame: 2 mo] |
To compare the gene mutation status of CSF, blood, and tumor tissue after the first session and at the time of tumor progression [time frame: 6 mo] |
| NCT03257124 | 80 patients with EGFR T790M mutated NSCLC and BM and/ or LM who failed tyrosine kinase Inhibitors | Phase II trial experimental arm: AZD9291 (160 mg per oral daily; 1 cycle of 28 days) in BM or LM cohort in T790M positive | CSF plasma tumor tissue | Not reported | OS in BM and LM cohorts, respectively | - Whole body disease control rate - Time to brain progression - PFS - Adverse events (CTCAE v4.0) - Exploratory analysis of EGFR mutation/T790M in tissue, plasma, and CSF |
| Breast cancer | ||||||
| NCT03252912 | 51 patients with LM from breast cancer | Observational Prospective | Plasma | CTCs using CellSearch technique | Sensitivity of the CellSearch technique on CSF samples in comparison with the conventional cytology on 1‒3 CSF samples [time frame: through study completion, an average of 2 y] | — |
| NCT01645839 | 144 patients with LM from breast cancer | Phase III trial • Arm A: Standard systemic treatment without intrathecal liposomal ARA-C • Arm B: Standard systemic treatment with intrathecal liposomal ARA-C |
CSF | CTCs using Veridex technique | Neurological PFS | - Clinical PFS (Montreal Cognitive Assessment Scale [MOCA] score) - Cytological PFS - Radiological PFS - OS - Tolerability of liposomal ARA-C - Research and quantification of tumor cells in the CSF for the diagnosis and monitoring of meningeal metastasis of breast cancer |
| Miscellanea | ||||||
| NCT02071056 | 22 patients with LM from metastatic solid tumors | Observational Prospective | CSF plasma tumor tissue | Not reported | Tumor DNA detectability and cytological confirmation of leptomeningeal metastasis [time frame: 1 y] | - Comparison of circulating tumor DNA levels in CSF with levels in plasma. - Correlation of circulating tumor DNA levels and patient survival. - Circulating tumor DNA detection in CSF of patients with cytological evidence of leptomeningeal metastasis. - Circulating tumor DNA identification in the CSF of patients prior to diagnosis of leptomeningeal metastasis. - Measurement of circulating tumor DNA levels and cancer cell numbers in CSF following initiation of intrathecal chemotherapy for leptomeningeal metastasis. [time frame: 1 y] |
| NCT01713699 | 100 patients with LM from metastatic solid tumors | Observational Prospective | CSF | CTCs EpCAM + detected by CellSearch technique | To determine the sensitivity and specificity of detection of CTCs in patients with EpCAM expressing tumors compared with cytology in the CSF of patients clinically suspected for LM [time frame: 3 mo after end of study] | - To determine the relationship between the number of CTCs in CSF and the patient’s neurological condition and World Health Organization performance score - To determine the change in the CTC number between two sampling points and correlate this with the patient’s neurological condition and therapy - To determine the relationships between demographics/tumor status and CTCs number in CSF. - To determine the relationship between the CTC cells in the CSF and the CTCs in the peripheral blood - To confirm EPCAM positivity in archived primary tumor tissue and tumor cells in CSF. - To compare the predictive value of 2 CTC enumeration methods [time frame: 3 mo after end of study] |
Conclusions
Applications of liquid biopsies in CNS metastases have continued to expand. However, most published studies are retrospective and comprise small, heterogeneous patient cohorts. Thus, optimal use of the CTCs and/or ctDNA in the setting of diagnosis, monitoring, and guidance of treatment decisions has yet to be defined. Now, many ongoing clinical trials in patients with brain and LM incorporate longitudinal CSF and blood collection. An essential question is whether liquid biopsy–driven management will translate into improved patient outcomes. Ultimately, implementation of liquid biopsy approaches in clinical practice will occur only after well-designed and controlled studies are performed.
Funding
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Conflict of interest statement.
Adrienne Boire has consulted for Arix Bioscience. Patents pending application no: 62/258,044, November 20, 2015, and 62/052,966, September 19, 2014.
Priscilla K. Brastianos has received honoraria from Merck and Genentech, consulted for Lilly, Merck, and Angiochem and received research funding from Merck.
Morris D. Groves has received honoraria from Abbvie.
Eudocia Q. Lee consulted for Eli Lilly.
Nancy Lin has received research funding from Pfizer; Genentech/Roche, Kadmon, Novartis, and Array Biopharma and consulted for Seattle Genetics Shionogi, and Daichii.
Jeffrey Raizer was an employee of Astellas and received honoraria from Stock Agenus and Celldex.
Roberta Rudà has received honoraria from UCB.
Michael Weller has received honoraria for lecture from Mundipharma.
Martin J. van den Bent has received honoraria and research grants from AbbVie, Celgene, Agio, Daichi Sankyo, and BMS.
Patrick Y. Wen has received honoraria and research support from Agios, AstraZeneca, Eli Lily, Genentech/Roche, Merck, Novartis, Sanofi-Aventis, and Abbvie.
Riccardo Soffietti has received honoraria and research support from MSD, Roche, Merck Serono, Celldex Therapeutics, Novartis, PUMA, and Mundipharma.
Dieta Brandsma, Emilie Le Rhun, Manmeet Ahluwalia, Larry Junck, Michael A. Vogelbaum, and Susan Chang have nothing to disclose.
References
- 1. Brastianos PK, Carter SL, Santagata S, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14(9):531–548. [DOI] [PubMed] [Google Scholar]
- 3. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4(147):147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Connolly ID, Li Y, Gephart MH, Nagpal S. The “Liquid Biopsy”: the role of circulating DNA and RNA in central nervous system tumors. Curr Neurol Neurosci Rep. 2016;16(3):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Mattos-Arruda L, Mayor R, Ng CK, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. 2015;61(3):514–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jiang BY, Li YS, Guo WB, et al. Detection of driver and resistance mutations in leptomeningeal metastases of NSCLC by next-generation sequencing of cerebrospinal fluid circulating tumor cells. Clin Cancer Res. 2017;23(18):5480–5488. [DOI] [PubMed] [Google Scholar]
- 9. Chamberlain M, Junck L, Brandsma D, et al. Leptomeningeal metastases: a RANO proposal for response criteria. Neuro Oncol. 2017;19(4):484–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chamberlain M, Soffietti R, Raizer J, et al. Leptomeningeal metastasis: a Response Assessment in Neuro-Oncology critical review of endpoints and response criteria of published randomized clinical trials. Neuro Oncol. 2014;16(9):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Rhun E, Weller M, Brandsma D, et al. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol. 2017;28(suppl_4):iv84–iv99. [DOI] [PubMed] [Google Scholar]
- 12. Wasserstrom WR, Glass JP, Posner JB. Diagnosis and treatment of leptomeningeal metastases from solid tumors: experience with 90 patients. Cancer. 1982;49(4):759–772. [DOI] [PubMed] [Google Scholar]
- 13. van Oostenbrugge RJ, Twijnstra A. Presenting features and value of diagnostic procedures in leptomeningeal metastases. Neurology. 1999;53(2):382–385. [DOI] [PubMed] [Google Scholar]
- 14. Subirá D, Serrano C, Castañón S, et al. Role of flow cytometry immunophenotyping in the diagnosis of leptomeningeal carcinomatosis. Neuro Oncol. 2012;14(1):43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nayak L, Fleisher M, Gonzalez-Espinoza R, et al. Rare cell capture technology for the diagnosis of leptomeningeal metastasis in solid tumors. Neurology. 2013;80(17):1598–1605; discussion 1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Subirá D, Simó M, Illán J, et al. Diagnostic and prognostic significance of flow cytometry immunophenotyping in patients with leptomeningeal carcinomatosis. Clin Exp Metastasis. 2015;32(4):383–391. [DOI] [PubMed] [Google Scholar]
- 17. Lee JS, Melisko ME, Magbanua MJ, et al. Detection of cerebrospinal fluid tumor cells and its clinical relevance in leptomeningeal metastasis of breast cancer. Breast Cancer Res Treat. 2015;154(2):339–349. [DOI] [PubMed] [Google Scholar]
- 18. Tu Q, Wu X, Le Rhun E, et al. CellSearch technology applied to the detection and quantification of tumor cells in CSF of patients with lung cancer leptomeningeal metastasis. Lung Cancer. 2015;90(2):352–357. [DOI] [PubMed] [Google Scholar]
- 19. Milojkovic Kerklaan B, Pluim D, Bol M, et al. EpCAM-based flow cytometry in cerebrospinal fluid greatly improves diagnostic accuracy of leptomeningeal metastases from epithelial tumors. Neuro Oncol. 2016;18(6):855–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Glantz MJ, Cole BF, Glantz LK, et al. Cerebrospinal fluid cytology in patients with cancer: minimizing false-negative results. Cancer. 1998;82(4):733–739. [DOI] [PubMed] [Google Scholar]
- 21. Patel AS, Allen JE, Dicker DT, et al. Identification and enumeration of circulating tumor cells in the cerebrospinal fluid of breast cancer patients with central nervous system metastases. Oncotarget. 2011;2(10):752–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Le Rhun E, Massin F, Tu Q, Bonneterre J, Bittencourt Mde C, Faure GC. Development of a new method for identification and quantification in cerebrospinal fluid of malignant cells from breast carcinoma leptomeningeal metastasis. BMC Clin Pathol. 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Riethdorf S, Fritsche H, Müller V, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920–928. [DOI] [PubMed] [Google Scholar]
- 24. Le Rhun E, Tu Q, De Carvalho Bittencourt M, et al. Detection and quantification of CSF malignant cells by the CellSearch technology in patients with melanoma leptomeningeal metastasis. Med Oncol. 2013;30(2):538. [DOI] [PubMed] [Google Scholar]
- 25. Kraan J, Sleijfer S, Strijbos MH, et al. External quality assurance of circulating tumor cell enumeration using the CellSearch(®) system: a feasibility study. Cytometry B Clin Cytom. 2011;80(2):112–118. [DOI] [PubMed] [Google Scholar]
- 26. de Wit S, van Dalum G, Terstappen LW. Detection of circulating tumor cells. Scientifica (Cairo). 2014;2014:819362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Campoli MR, Chang CC, Kageshita T, Wang X, McCarthy JB, Ferrone S. Human high molecular weight-melanoma-associated antigen (HMW-MAA): a melanoma cell surface chondroitin sulfate proteoglycan (MSCP) with biological and clinical significance. Crit Rev Immunol. 2004;24(4):267–296. [DOI] [PubMed] [Google Scholar]
- 28. van Bussel MTJ, Pluim D, Bol M, Beijnen JH, Schellens JHM, Brandsma D. EpCAM-based assays for epithelial tumor cell detection in cerebrospinal fluid. J Neurooncol. 2018;137(1):1–10. [DOI] [PubMed] [Google Scholar]
- 29. Acosta M, Pereira J, Arroz M. Screening of carcinoma metastasis by flow cytometry: A study of 238 cases. Cytometry B Clin Cytom. 2016;90(3):289–294. [DOI] [PubMed] [Google Scholar]
- 30. Lin X, Fleisher M, Rosenblum M, et al. Cerebrospinal fluid circulating tumor cells: a novel tool to diagnose leptomeningeal metastases from epithelial tumors. Neuro Oncol. 2017;19(9):1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hyun KA, Koo GB, Han H, et al. Epithelial-to-mesenchymal transition leads to loss of EpCAM and different physical properties in circulating tumor cells from metastatic breast cancer. Oncotarget. 2016;7(17):24677–24687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cordone I, Masi S, Summa V, et al. Overexpression of syndecan-1, MUC-1, and putative stem cell markers in breast cancer leptomeningeal metastasis: a cerebrospinal fluid flow cytometry study. Breast Cancer Res. 2017;19(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Magbanua MJ, Melisko M, Roy R, et al. Molecular profiling of tumor cells in cerebrospinal fluid and matched primary tumors from metastatic breast cancer patients with leptomeningeal carcinomatosis. Cancer Res. 2013;73(23):7134–7143. [DOI] [PubMed] [Google Scholar]
- 34. Li X, Zhang Y, Ding J, et al. Clinical significance of detecting CSF-derived tumor cells in breast cancer patients with leptomeningeal metastasis. Oncotarget. 2018;9(2):2705–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Momtaz P, Pentsova E, Abdel-Wahab O, et al. Quantification of tumor-derived cell free DNA (cfDNA) by digital PCR (DigPCR) in cerebrospinal fluid of patients with BRAFV600 mutated malignancies. Oncotarget. 2016;7(51):85430–85436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pentsova EI, Shah RH, Tang J, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34(20):2404–2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Marchiò C, Mariani S, Bertero L, et al. Liquoral liquid biopsy in neoplastic meningitis enables molecular diagnosis and mutation tracking: a proof of concept. Neuro Oncol. 2017;19(3):451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Swinkels DW, de Kok JB, Hanselaar A, Lamers K, Boerman RH. Early detection of leptomeningeal metastasis by PCR examination of tumor-derived K-ras DNA in cerebrospinal fluid. Clin Chem. 2000;46(1):132–133. [PubMed] [Google Scholar]
- 39. Fan Y, Zhu X, Xu Y, et al. Cell-cycle and DNA-damage response pathway is involved in leptomeningeal metastasis of non-small cell lung cancer. Clin Cancer Res. 2018;24(1):209–216. [DOI] [PubMed] [Google Scholar]
- 40. Li YS, Jiang BY, Yang JJ, et al. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non-small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol. 2018;29(4):945–952. [DOI] [PubMed] [Google Scholar]
- 41. Vinayanuwattikun C, Mingmalairak S, Jittapiromsak N, et al. SHP-1 promoter 2 methylation in cerebrospinal fluid for diagnosis of leptomeningeal epithelial-derived malignancy (carcinomatous meningitis). J Neurooncol. 2016;129(3):395–403. [DOI] [PubMed] [Google Scholar]
- 42. Newman AM, Bratman SV, To J, et al. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20(5):548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shankar GM, Balaj L, Stott SL, Nahed B, Carter BS. Liquid biopsy for brain tumors. Expert Rev Mol Diagn. 2017;17(10):943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Campos CDM, Jackson JM, Witek MA, Soper SA. molecular profiling of liquid biopsy samples for precision medicine. Cancer J. 2018;24(2):93–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang J, Bettegowda C. Applications of DNA-based liquid biopsy for central nervous system neoplasms. J Mol Diagn. 2017;19(1):24–34. [DOI] [PubMed] [Google Scholar]
- 47. Campos S, Davey P, Hird A, et al. Brain metastasis from an unknown primary, or primary brain tumour? A diagnostic dilemma. Curr Oncol. 2009;16(1):62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cristofanilli M, Budd GT, Ellis MJ, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. [DOI] [PubMed] [Google Scholar]
- 49. Nagaiah G, Abraham J. Circulating tumor cells in the management of breast cancer. Clin Breast Cancer. 2010;10(3):209–216. [DOI] [PubMed] [Google Scholar]
- 50. Bidard FC, Peeters DJ, Fehm T, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol. 2014;15(4):406–414. [DOI] [PubMed] [Google Scholar]
- 51. Janni WJ, Rack B, Terstappen LW, et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res. 2016;22(10):2583–2593. [DOI] [PubMed] [Google Scholar]
- 52. Hayes DF, Cristofanilli M, Budd GT, et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin Cancer Res. 2006;12(14 Pt 1):4218–4224. [DOI] [PubMed] [Google Scholar]
- 53. Zhang L, Riethdorf S, Wu G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res. 2012;18(20):5701–5710. [DOI] [PubMed] [Google Scholar]
- 54. Aggarwal C, Meropol NJ, Punt CJ, et al. Relationship among circulating tumor cells, CEA and overall survival in patients with metastatic colorectal cancer. Ann Oncol. 2013;24(2):420–428. [DOI] [PubMed] [Google Scholar]
- 55. Amato RJ, Melnikova V, Zhang Y, et al. Epithelial cell adhesion molecule-positive circulating tumor cells as predictive biomarker in patients with prostate cancer. Urology. 2013;81(6):1303–1307. [DOI] [PubMed] [Google Scholar]
- 56. Garcia-Murillas I, Schiavon G, Weigelt B, et al. Mutation tracking in circulating tumor DNA predicts relapse in early breast cancer. Sci Transl Med. 2015;7(302):302ra133. [DOI] [PubMed] [Google Scholar]
- 57. Beaver JA, Jelovac D, Balukrishna S, et al. Detection of cancer DNA in plasma of patients with early-stage breast cancer. Clin Cancer Res. 2014;20(10):2643–2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bettegowda C, Sausen M, Leary RJ, et al. Detection of circulating tumor DNA in early- and late-stage human malignancies. Sci Transl Med. 2014;6(224):224ra224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Diehl F, Schmidt K, Choti MA, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14(9):985–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tie J, Kinde I, Wang Y, et al. Circulating tumor DNA as an early marker of therapeutic response in patients with metastatic colorectal cancer. Ann Oncol. 2015;26(8):1715–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dawson SJ, Tsui DW, Murtaza M, et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368(13):1199–1209. [DOI] [PubMed] [Google Scholar]
- 62. Stover DG, Parsons HA, Ha G, et al. Association of cell-free DNA tumor fraction and somatic copy number alterations with survival in metastatic triple-negative breast cancer. J Clin Oncol. 2018;36(6):543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schøler LV, Reinert T, Ørntoft MW, et al. Clinical implications of monitoring circulating tumor DNA in patients with colorectal cancer. Clin Cancer Res. 2017;23(18):5437–5445. [DOI] [PubMed] [Google Scholar]
- 64. Balaña C, Ramirez JL, Taron M, et al. O6-methyl-guanine-DNA methyltransferase methylation in serum and tumor DNA predicts response to 1,3-bis(2-chloroethyl)-1-nitrosourea but not to temozolamide plus cisplatin in glioblastoma multiforme. Clin Cancer Res. 2003;9(4):1461–1468. [PubMed] [Google Scholar]
- 65. Alexander BM, Brown PD, Ahluwalia MS, et al. ; Response Assessment in Neuro-Oncology (RANO) group. Clinical trial design for local therapies for brain metastases: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018;19(1):e33–e42. [DOI] [PubMed] [Google Scholar]
- 66. Fiano V, Trevisan M, Trevisan E, et al. MGMT promoter methylation in plasma of glioma patients receiving temozolomide. J Neurooncol. 2014;117(2):347–357. [DOI] [PubMed] [Google Scholar]
- 67. Barault L, Amatu A, Bleeker FE, et al. Digital PCR quantification of MGMT methylation refines prediction of clinical benefit from alkylating agents in glioblastoma and metastatic colorectal cancer. Ann Oncol. 2015;26(9):1994–1999. [DOI] [PubMed] [Google Scholar]
- 68. Li Y, Pan W, Connolly ID, et al. Tumor DNA in cerebral spinal fluid reflects clinical course in a patient with melanoma leptomeningeal brain metastases. J Neurooncol. 2016;128(1):93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Thress KS, Brant R, Carr TH, et al. EGFR mutation detection in ctDNA from NSCLC patient plasma: a cross-platform comparison of leading technologies to support the clinical development of AZD9291. Lung Cancer. 2015;90(3):509–515. [DOI] [PubMed] [Google Scholar]
- 70. Remon J, Menis J, Hasan B, et al. The APPLE trial: feasibility and activity of AZD9291 (osimertinib) treatment on positive plasma T790M in EGFR-mutant NSCLC patients. EORTC 1613. Clin Lung Cancer. 2017;18(5):583–588. [DOI] [PubMed] [Google Scholar]
- 71. Bardelli A, Corso S, Bertotti A, et al. Amplification of the MET receptor drives resistance to anti-EGFR therapies in colorectal cancer. Cancer Discov. 2013;3(6):658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: learning from lung cancer. Nat Rev Clin Oncol. 2014;11(8):473–481. [DOI] [PubMed] [Google Scholar]
- 73. Misale S, Arena S, Lamba S, et al. Blockade of EGFR and MEK intercepts heterogeneous mechanisms of acquired resistance to anti-EGFR therapies in colorectal cancer. Sci Transl Med. 2014;6(224):224ra226. [DOI] [PubMed] [Google Scholar]
- 74. Lo RS, Shi H. Detecting mechanisms of acquired BRAF inhibitor resistance in melanoma. Methods Mol Biol. 2014;1102:163–174. [DOI] [PubMed] [Google Scholar]
- 75. Shi H, Hugo W, Kong X, et al. Acquired resistance and clonal evolution in melanoma during BRAF inhibitor therapy. Cancer Discov. 2014;4(1):80–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sasaki S, Yoshioka Y, Ko R, et al. Diagnostic significance of cerebrospinal fluid EGFR mutation analysis for leptomeningeal metastasis in non-small-cell lung cancer patients harboring an active EGFR mutation following gefitinib therapy failure. Respir Investig. 2016;54(1):14–19. [DOI] [PubMed] [Google Scholar]
- 77. Togashi Y, Masago K, Masuda S, et al. Cerebrospinal fluid concentration of gefitinib and erlotinib in patients with non-small cell lung cancer. Cancer Chemother Pharmacol. 2012;70(3):399–405. [DOI] [PubMed] [Google Scholar]
- 78. Camidge DR, Lee EQ, Lin NU, et al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018;19(1):e20–e32. [DOI] [PubMed] [Google Scholar]
- 79. Karachaliou N, Mayo-de las Casas C, Queralt C, et al. ; Spanish Lung Cancer Group. Association of EGFR L858R mutation in circulating free DNA with survival in the EURTAC trial. JAMA Oncol. 2015;1(2):149–157. [DOI] [PubMed] [Google Scholar]
- 80. Mok T, Wu YL, Lee JS, et al. Detection and dynamic changes of EGFR mutations from circulating tumor DNA as a predictor of survival outcomes in NSCLC patients treated with first-line intercalated erlotinib and chemotherapy. Clin Cancer Res. 2015;21(14):3196–3203. [DOI] [PubMed] [Google Scholar]