Abstract
Background
Update 3 of the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW) recognizes amplification of epidermal growth factor receptor (EGFR) as one important aberration in diffuse gliomas (World Health Organization [WHO] grade II/III). While these recommendations endorse testing, a cost-effective, clinically relevant testing paradigm is currently lacking. Here, we use real-world clinical data to propose a financially effective diagnostic test algorithm in the context of new guidelines.
Methods
To determine the prevalence, distribution, neuroradiographic features (Visually Accessible REMBRANDT Images [VASARI]), and prognostic relevance of EGFR amplification in lower-grade gliomas, we assembled a consecutive series of diffuse gliomas. For validation we included publicly available data from The Cancer Genome Atlas. For a cost-utility analysis we compared combined EGFR and isocitrate dehydrogenase (IDH) testing, EGFR testing based on IDH results, and no EGFR testing.
Results
In n = 71 WHO grade II/III gliomas, we identified EGFR amplification in 28.2%. With one exception, all EGFR amplifications occurred in IDH-wildtype gliomas. Comparison of overall survival showed that EGFR amplification denotes a significantly more aggressive subset of tumors (P < 0.0001, log-rank). The radiologic phenotype in the EGFR-amplified tumors includes diffusion restriction (15%, P = 0.02), >5% tumor contrast enhancement (75%, P = 0.016), and mild (not avid) enhancement (P = 0.016). The proposed testing algorithm reserves EGFR fluorescence in situ hybridization (FISH) testing for IDH-wildtype cases. Implementation would result in ~37.9% cost reduction at our institution, or about $1.3–4 million nationally.
Conclusion
EGFR-amplified diffuse gliomas are “glioblastoma-like” in their behavior and may represent undersampled glioblastomas, or subsets of IDH-wildtype diffuse gliomas with inherently aggressive biology. EGFR FISH after IDH testing is a financially effective and clinically relevant test algorithm for routine clinical practice.
Keywords: glioblastoma-like glioma, VASARI, WHO grade II/III
Key Points.
EGFR-amplified diffuse gliomas are “glioblastoma-like” in their behavior.
EGFR-amplified diffuse gliomas may represent undersampled glioblastomas.
A cost-effective WHO/cIMPACT-NOW update 3 algorithm is applied for EGFR amplification in gliomas.
Importance of the Study.
EGFR amplification in IDH-wildtype diffuse gliomas identifies an aggressive, glioblastoma-like subset. New guidelines from cIMPACT-NOW have recently incorporated this diagnostic subclassification; however, to our knowledge, no practically relevant test algorithm has been proposed and assessed for financial effectiveness. Here we employed a series of n = 71 WHO grade II/III tumors from clinical practice, assessed prevalence, confirmed prognostic relevance, and correlated IDH and EGFR status with clinical and neuroradiographic VASARI features. Based on these data, and taking financial sustainability into account, we compared several testing paradigms and derived a cost-effective test algorithm that incorporates testing for EGFR amplification within the context of the current WHO classification guidelines and those recommended by cIMPACT-NOW update 3. Given that most neuropathology laboratories assess chromosome 1p/19q status by FISH, the proposed approach accounts for availability, cost-effectiveness, and prognostic relevance.
The 2016 World Health Organization (WHO) Classification of Tumors of the Central Nervous System1 defines specific glioma entities based on their molecular signature for optimized prognostication and treatment stratification. Arguably the most drastic change has been the recognition of isocitrate dehydrogenase 1 and 2 (IDH1/2) mutations as an essential component for the diagnosis of diffuse astrocytic tumors and oligodendrogliomas.1–3 Indeed, despite lacking diagnostic histological features, diffuse gliomas that harbor wildtype IDH demonstrate biological and clinical similarities to glioblastoma (GBM), WHO grade IV,4 and many, but not all, WHO grade II/III tumors may actually represent histologically undersampled WHO grade IV GBM. More recent studies suggest that the heterogeneous group of lower-grade IDH-wildtype gliomas could be further subclassified5–7; however, subclassification of IDH-wildtype glioma represents a new frontier, which awaits reliable clinical implementation. To address the lack of additional biomarkers designating a glioma as WHO grade IV beyond IDH testing, the Consortium to Inform Molecular and Practical Approaches to CNS Tumor Taxonomy (cIMPACT-NOW)8 recently issued update 3, specifically recommending diagnostic criteria for “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV.” The minimal molecular criterion was determined to be presence of either telomerase reverse transcriptase (TERT) promoter mutation, simultaneous gain of whole chromosome 7 and loss of whole chromosome 10, or amplification of epidermal growth factor receptor (EGFR).
Amplification of EGFR, a frequent aberration in GBM, has also been reported in diffuse gliomas (WHO grade II/III).3,9–11 The prognostic significance of EGFR amplification in diffuse gliomas, particularly in the context of additional relevant molecular genetic markers (IDH mutations, O6-methylguanine-DNA methyltransferase [MGMT] promoter methylation, and 1p/19q codeletion), has emerged relatively recently.12–14EGFR amplification may also predict the clinical course of patients with tumors that lack classic histological characteristics of GBM, yet display imaging features indicative of a more aggressive tumor type.14 While perhaps, based on the results of previous studies, it is generally accepted that diffuse gliomas bearing EGFR amplification can be expected to behave similar to GBM regardless of histology, to our knowledge a study specifically focusing on practically relevant testing strategies of EGFR amplification in WHO grade II/III gliomas (with or without IDH mutations) has not been performed.
Here, we examined the diagnostic and prognostic relevance of EGFR amplification, as well as the prevalence thereof, in a molecularly annotated clinical cohort of WHO grade II/III gliomas.15 We also assessed economic implications of EGFR testing by performing a cost-effectiveness analysis. Defining a cost-effective testing paradigm for clinically relevant subsets of glioma patients is a key step in the improvement of diagnostic classification and patient care.
Methods
Study Design and Ethical Approval
The study was designed as a retrospective chart review of existing clinical data. For this type of study, formal consent is not required. Appropriate institutional review board approval was obtained and all procedures were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was conducted at Massachusetts General Hospital, which includes a clinical molecular diagnostics laboratory (certified by Clinical Laboratory Improvement Amendments).
Study Population and Data Collection
These cases represent a reanalyzed subset of a previously studied cohort, which included patients with a WHO grade II/III glioma diagnosis who underwent brain tumor surgery at our hospital between January 2010 and December 2015.15 All included patients underwent molecular-genetic testing as a component of their routine clinical care. We identified patients using an internally developed laboratory information and management system (wikilims), and clinicopathological characteristics and outcomes were extracted from the electronic medical record. The primary endpoint was overall survival, measured from the date of diagnosis until the date of death; we censored patients who were alive or lost to follow-up after the last time of contact. We also extracted “pre-surgical” neuroimaging findings (see below) and the type of neurosurgical procedure (ie, biopsy vs resection).
Integrated Neuropathological Diagnosis
At least one board-certified neuropathologist rendered each primary diagnosis according to the WHO guidelines. All study cases were originally classified according to the 2007 WHO guidelines.16 As previously described, we converted these diagnoses into the 2016 CNS WHO terminology via consideration of IDH mutation as well as 1p/19q copy number status.1,15
Neuroimaging
To assess associations between EGFR amplification status and neuroimaging features, one of the authors (O.R.) reviewed all MRI data and applied the standardized preoperative qualitative and semi-quantitative imaging variables provided by the Visually Accessible REMBRANDT Images (VASARI) project.17–21 Briefly, the VASARI MRI feature set is a system designed to enable consistent description of gliomas using a set of defined visual features and controlled vocabulary (https://radiopaedia.org/articles/vasari-mri-feature-set; last accessed October 20, 2018).21
Molecular Genetic Testing
Fluorescence in situ hybridization (FISH) —. FISH followed previously established protocols.22 Briefly, to identify gene-to-copy number ratios (G:CN) on formalin-fixed paraffin embedded sections, we hybridized probes for EGFR/centromere 7 (CEP7) by employing the bacterial artificial chromosome EGFR (CTD-2113A18) in combination with a copy number control corresponding to CEP7 (Abbott-Vysis 06J54-027). We used a strict definition for defining gene amplification as a G:CN ratio of >2.2 scored in 50 tumor nuclei. Specifically, polysomy, high polysomy, or equivocal G:CN ratio (1.8–2.2) was scored as negative for amplification.23,24 For diagnostic classification according to WHO (2016) we determined the chromosome 1p/19q status as previously described.25Next-generation sequencing (NGS)—. Isolated nucleic acids from tumor specimens were analyzed using our NGS assay that employs anchored multiplex polymerase chain reaction (PCR)26 to detect single-nucleotide variants, insertions/deletions, and copy number variation in a target set of cancer-related genes. For diagnostic classification of IDH status we used NGS, rapid IDH testing,27 and IDH1 R132H–specific immunohistochemistry (IHC).15 To identify MGMT promoter methylation status we used a methylation-specific PCR. Briefly, following bisulfite treatment, PCRs was performed with primers specific to the methylated and unmethylated promoter sequences. The PCR products were analyzed using an ABI3500xL instrument (Thermo Fisher Scientific).
The Cancer Genome Atlas Data
Data from The Cancer Genome Atlas (TCGA) were retrieved from the TCGA FireBrowse website (www.firebrowse.org) or cBioPortal (www.cbioportal) (both last accessed March 29, 2018).28,29 Specifically, we selected the subset of IDH-wildtype diffuse glioma and distinguished these by EGFR copy number status; survival data were taken from the clinical data portal.
Cost Modeling
Our cost model uses current billable cost for each testing modality, set to $135 per p.R132H-specific IDH1 IHC, $360 per EGFR FISH testing, and $1800 for NGS. To model various testing scenarios, we used the following formulas: (1) performing NGS testing alone: MNGS = (NGS)*NALL; (2) performing IDH IHC alone: MIDH = (IHC)* NALL; (3) performing IDH IHC and EGFR FISH on all cases: MIDH+FISH = (IDH+FISH)* NALL; and (4) the cost of performing IDH IHC as a first pass screen, where EGFR FISH is only performed on cases that are wildtype for IDH: MIDH+FISHwt = (IDH)* NALL +(FISH)* NIDHwt. To estimate the national impact of these scenarios we extracted annual data on diffuse gliomas from the American Brain Tumor Association website (http://www.abta.org; last accessed October 20, 2018), a derivation from the Central Brain Tumor Registry of the United States.30
Statistical Analysis
For contingency testing we employed Fisher’s exact tests (association with dichotomous factors), χ2, or t-tests (comparison of means). The Kaplan–Meier method was used to estimate overall survival differences by EGFR amplification status, and we used the log-rank method to compare survival differences. Data analysis was conducted by using GraphPad Prism 5.0b, and significance was defined as P < 0.05.
Results
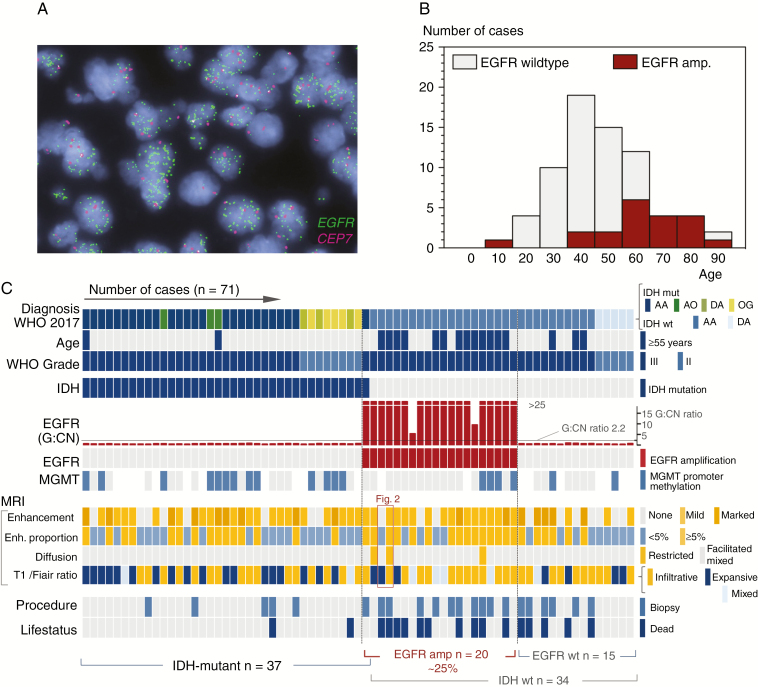
EGFR-Amplified Gliomas Typically Are IDH-Wildtype and Occur in Older Patients
Our study cohort is composed of 71 consecutive WHO grade II/III tumors. Briefly, the majority of cases (77%) consisted of 2 diagnostic groups: “anaplastic astrocytoma, IDH-wildtype” and “anaplastic astrocytoma, IDH-mutant” (n = 29 and 26, respectively), whereas all other histological subtypes represent 23% of the study cohort (n = 16/71). One third of all cases in our cohort showed EGFR amplification (28.2%; n = 20 of 71). An example of EGFR amplification by FISH is shown in Fig. 1A, and the clinical characteristics of the entire study cohort are summarized in Table 1. EGFR-amplified tumors occurred in older patients with a median age at diagnosis of 61.5 versus 41 years (P < 0.00001; Fig. 1B). Notably, EGFR-amplified tumors occurred almost exclusively in IDH-wildtype cases (n = 19 of 20 cases). For the vast majority (~95%), EGFR-amplified gliomas are IDH-wildtype and occur typically in older patients. The one exception was an anaplastic astrocytoma, IDH-mutant with multifocal clusters of EGFR amplification. The rare co-mutation of EGFR amplification with IDH mutation is reflected in larger TCGA cohorts (2/283 cases or 0.71% of cases). Comparison against other established (genetic) prognosticators indicated that EGFR amplification occurred independently of MGMT promoter methylation status (Supplementary Table 1) and EGFR amplification was mutually exclusive with codeletion of chromosomes 1p and 19q, the diagnostic hallmark of oligodendroglioma.
Fig. 1.
EGFR amplification in diffuse gliomas. (A) Dual color fluorescence in situ hybridization for EGFR in a case of anaplastic astrocytoma. The gene (EGFR, green probe signals) to copy number (chromosome enumeration probe 7, CEP7 in magenta) ratio was >25:1. (B) Age distribution of EGFR amplification indicates that only 7 of the 20 patients with EGFR-amplified gliomas were <55 years old (35%). (C) Landscape of 71 consecutive WHO grade II/III gliomas tested for EGFR amplification (columns) alongside main findings (rows). Note that IDH mutations and EGFR amplification status are, with one exception, mutually exclusive. For details on the subgroup analyses, see results; a detailed analysis of the MRI features is provided in Supplementary Table 2; the red box indicates the 2 cases shown in Fig. 2.
Table 1.
Study demographics
| Features | Cases N = 71 (%) | EGFR-Amplified N = 20 (%) | No EGFR Amplification N = 51 (%) | P-value |
|---|---|---|---|---|
| Tumor type | 0.000197* | |||
| Anaplastic astrocytoma, IDH-wildtype | 29 (40.8%) | 19 (95%) | 10 (19.6%) | 0.0002** |
| Anaplastic astrocytoma, IDH-mutant | 26 (36.6%) | 1 (5.0%) | 25 (49.0%) | |
| Anaplastic oligodendroglioma, IDH-mutant and 1p/19q codeleted | 3 (4.2%) | 0 (0%) | 3 (5.9%) | |
| Diffuse astrocytoma, IDH-wildtype | 5 (7.0%) | 0 (0%) | 5 (9.8%) | |
| Diffuse astrocytoma, IDH-mutant | 3 (4.2%) | 0 (0%) | 3 (5.9%) | |
| Oligodendroglioma IDH-mutant and 1p/19q codeleted | 5 (7.0%) | 0 (0%) | 5 (9.8%) | |
| WHO grade | ||||
| III | 58 (82%) | 20 (100%) | 38 (75%) | 0.0141* |
| II | 13 (12%) | 0 (0%) | 13 (25%) | |
| Median age in years | 46 | 61.5 | 41 | |
| Number of patients ≤55 | 53 (75%) | 7 (35%) | 46 (90%) | <0.00001* |
| Number of patients >55 | 18 (25%) | 13 (65%) | 5 (10%) | |
| MRI characteristics M | ||||
| Contrast enhancing | 37 (52%) | 14 (70%) | 23 (45%) | 0.070 |
| No enhancement | 34 (48%) | 6 (30%) | 28 (55%) | |
| MRI characteristics (IDH-wildtype) | (N = 34) | (N = 19) | (N = 15) | |
| Contrast enhancing | 24 (70.6%) | 16 (84.2%) | 8 (53.3%) | 0.068 |
| No enhancement | 10 (29.4%) | 3 (15.8%) | 7 (46.7%) | |
| MRI characteristics (WHO grade III) | (N = 58) | (N = 20) | (N = 38) | |
| Contrast enhancing | 45 (77.6%) | 17 (85%) | 28 (73.7%) | 0.509 |
| No enhancement | 13 (22.4%) | 3 (15%) | 10 (39.5%) | |
| Procedure type | (N = 20) | (N = 51) | ||
| Biopsy | 22 (31%) | 11 (55%) | 11 (22%) | 0.009 |
| Resection | 49 (69%) | 9 (45%) | 40 (78%) | |
| Procedure type (IDH-wildtype) | (N = 34) | (N = 19) | (N = 15) | |
| Biopsy | 16 (47%) | 10 (53%) | 6 (40%) | 0.51 |
| Resection | 18 (53%) | 9 (47%) | 9 (60%) |
* P-values derived from Fisher’s exact test (WHO grade, number of patients at age of diagnosis) and chi-square analysis (tumor type). ** Results from Fischer’s exact test comparing EGFR-amplified vs non-amplified anaplastic astrocytomas, IDH-wildtype vs all other groups combined. Significant difference in tumor type is caused by the high number of EGFR-amplified tumors in this subgroup. Msee also Supplementary Table 2.
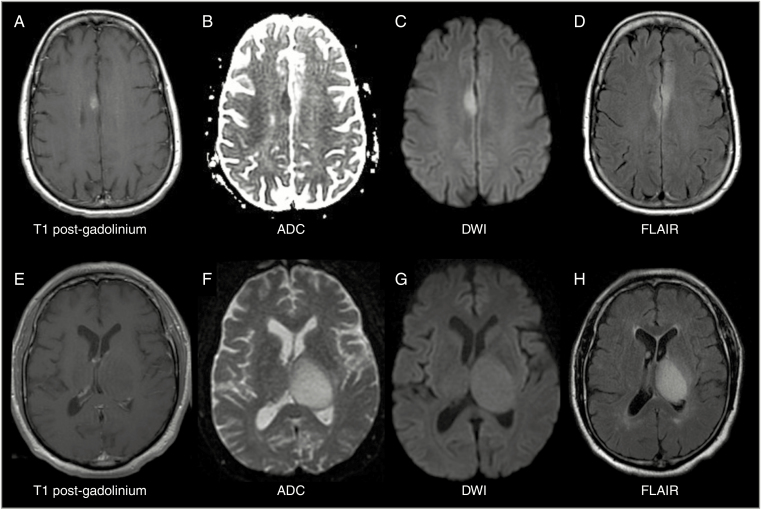
Fig. 2.
Representative spectrum of MRI neuroimaging features in EGFR-amplified, IDH-wildtype lower-grade glioma. Top row (A–D) shows example of a T1-weighted post-contrast enhancing (A), diffusion restricted (B, C), FLAIR infiltrative (D), EGFR-amplified anaplastic astrocytoma. Bottom row (E–H) shows an example of a non-enhancing (E), diffusion facilitated (F, G), and expansile (H) EGFR-amplified anaplastic astrocytoma. Abbreviations: ADC, apparent diffusion coefficient; DWI, diffusion-weighted image; FLAIR, fluid-attenuated inversion recovery.
Lesional Enhancement on MRI Is Not Specific for EGFR Amplification
The majority (85%) of the EGFR-amplified tumors demonstrated contrast enhancement on MRI at the time of diagnosis (presurgical imaging). However, a large percentage of non-amplified tumors also demonstrated evidence of contrast enhancement (70%; Fig. 1); the difference did not reach statistical significance (P = 0.24, Fisher’s exact). In the subset of n = 34 IDH-wildtype tumors, 53.3% of the 15 EGFR non-amplified tumors (n = 8/15) demonstrated contrast enhancement, whereas 84.2% in the 19 EGFR-amplified, IDH-wildtype tumors showed contrast enhancement (n = 16/19); the difference did not reach statistical significance (P = 0.068, Fisher’s exact). Additionally, in the subset of WHO grade III tumors (n = 58), contrast enhancement was present at similar frequency among EGFR non-amplified tumors (73.7%) and EGFR-amplified tumors (85.0%, P = 0.509, Fisher’s exact). Thus, while present in the majority of EGFR-amplified cases (Table 1), contrast enhancement alone on MRI cannot serve as a surrogate to identify this subset of diffuse gliomas; however, degree of enhancement may be a helpful factor. Further, recent novel multifeature MRI algorithms are able to predict EGFR expression status.31 Of note, while overall rare, diffusion restriction on MRI was seen only in EGFR-amplified, IDH-wildtype tumors and reached significance (P = 0.0199). Additionally, presence of >5% contrast enhancement, mild (not avid) enhancement, and an infiltrative/mixed pattern on T1/fluid attenuated inversion recovery ratio was significantly increased among EGFR-amplified, IDH-wildtype tumors (P = 0.0161 and P = 0.0296, respectively; see Supplementary Table 2).
EGFR Amplification Identifies a Highly Aggressive Subset of IDH-Wildtype Glioma
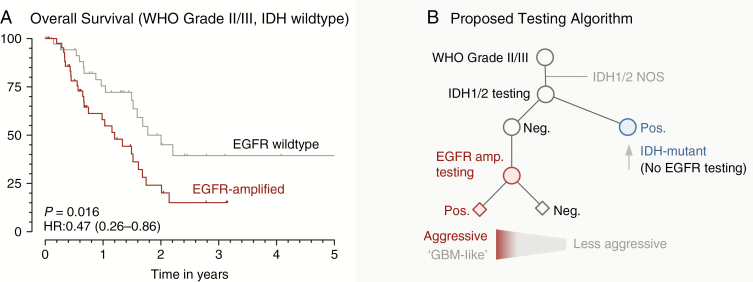
EGFR amplification is associated with significantly shorter overall survival compared with non-amplified tumors (P < 0.0001, hazard ratio [HR]: 0.47, 95% CI: 0.26–0.86; Supplementary Figure. 1A, B). The high prevalence of EGFR amplification in IDH1/2 wildtype cases (known to have a more aggressive disease course)32–34 raised the question of independent prognostic significance. To clarify this notion, we employed multivariate Cox proportional hazard regression modeling in the entire cohort of diffuse gliomas and confirmed that EGFR amplification does not carry independent prognostic significance (HR: 0.8 ± 0.5, P = 0.16) beyond the IDH mutation status (HR: 2.89 ± 1.1, P = 0.007). In other words, at least part of the prognostic relevance of EGFR amplification is attributable to the relatively good prognosis of IDH-mutant diffuse gliomas. Given that the distinct prognostic and biological properties of IDH-mutant gliomas3 have been acknowledged in the current 2016 WHO classification,1 we assessed prognostic relevance of EGFR amplification status in the subset of IDH-wildtype tumors (Supplementary Figure 1C). Patients with EGFR-amplified, IDH-wildtype gliomas had an aggressive course compared with double (EGFR, IDH) wildtype patients. Due to the number and distribution of events in these specific lower-grade glioma subgroups (P = 0.16, log-rank), we employed publicly available diffuse glioma data from the cohort of TCGA for validation (Supplementary Figure. 1D). We identified significantly shorter overall survival in the EGFR-amplified subset of IDH-wildtype (P = 0.019, log-rank) diffuse glioma patients, and we estimated the median overall survival difference by pooling our data with those of TCGA to be 0.8 years (9.6 mo). The significantly shorter overall survival of patients with EGFR amplification among those with IDH-wildtype tumors indicates clinical significance of this biomarker (P = 0.016, HR = 0.47; 95% CI: 0.28–0.86; Fig. 3E). To assess possible undersampling, we compared biopsy versus resection rates in the entire cohort versus the IDH-wildtype subset. There was a significantly higher rate of resections in the EGFR non-amplified group (P = 0.009; Table 1); however, there was no significant difference in the fraction of biopsies versus resections in the IDH-wildtype subset (P = 0.51; Table 1). Thus, the highly aggressive disease course with a median survival of ~1.2 years indicates that EGFR amplification in IDH-wildtype glioma denotes a disease course that is “glioblastoma-like.”
Fig. 3.
EGFR Amplification identifies a highly aggressive subset of IDH-wildtype glioma. (A) Kaplan–Meier survival estimates in IDH-wildtype gliomas according to their EGFR amplification status. P-value from log-rank test (for details, see supplement). (B) Proposed testing algorithm. Based on the data presented herein and the requirement to determine IDH mutation status (WHO 2106), we propose to test for EGFR amplification in the subset of IDH-wildtype glioma, WHO grade II/III. Abbreviations: amp., amplification (ie, gene copy number gain); Pos. positive (mutant, amplified); Neg. negative (not mutant, not amplified).
Economic EGFR Testing Cost Analysis Supports Testing of IDH-Wildtype Cases
A variety of testing modalities are available to molecularly characterize brain tumors. While we recognize that the selection of molecular tests is contingent upon many circumstances beyond cost (eg, availability of NGS, or case-based features), the prioritization of testing from a resource utilization standpoint presents a growing challenge for many institutions and the health care system at large. Therefore, we present a test cost comparison of several testing scenarios at our institution as well as national estimates (Table 2). The cost for universal NGS testing serves as a reference, in acknowledgment that while of high cost, this testing modality affords a more comprehensive molecular assessment, in particular for the detection of noncanonical IDH mutations in the appropriate setting.15 Alternatively, we considered universal EGFR FISH testing concurrent with IDH IHC (IDH1 p.R132H), which was associated with the next highest financial costs ($35 145; nationally $4 937 130). Additionally, we examined the cost of EGFR FISH testing only in IDH-wildtype cases ($21 825; nationally $2 024 010). Finally, we examined IDH testing alone (ie, no EGFR testing). While theoretical cost reductions associated with this lattermost strategy (~72.7%; national extrapolation: ~$2.9–3.6 million) are economically attractive, our findings presented here argue for a more judicious employment of EGFR FISH testing. The results of this analysis are summarized in Table 2.
Table 2.
Economic analysis of adding EGFR amplification testing to existing IDH testing strategies
| This Study | Cost Reduction* | National Estimates | Cost Reduction* | |
|---|---|---|---|---|
| Total # grade II/III gliomas | 71 | 9974 | ||
| Total # IDH-wildtype gliomas | 34 | 1882 | ||
| NGS (all) cost | $42 600–127 800 | $5 984 400–17 953 200 | ||
| IDHIHC + EGFRFISH (all) cost | $35 145 | $4 937 130 | ||
| IDHIHC (all) + EGFRFISH (IDH-wildtype) cost | $21 825 | $13 320 | $2 024 010 | $2 913 120 |
| IDHIHC (all) | $9585 | $25 560 | $1 346 490 | $3 590 640 |
National estimates are derived from publicly available resources (see methods). Abbreviations: IDHIHC, IDH1 p.R132H specific immunohistochemistry; EGFR FISH, fluorescence in situ hybridization for EGFR (and control probe) for gene to copy number assessment; NGS, next generation sequencing (DNA panel for full assessment of IDH1/2 including non-canonical mutations).
Proposal for a Cost-Effective EGFR Testing Algorithm
Based on the prognostic relevance, distribution of amplified cases, and our economic analysis (Table 2), we propose testing for EGFR amplification in grade II/III gliomas only when they are found to be IDH wildtype. Specifically, elimination of EGFR testing in IDH-mutant cases results in ~37.9% cost reduction. Our proposed testing scheme is based on the existing WHO (2016) classification, and at its inception takes into account WHO grade II/III and IDH genotyping. The proposed strategy would achieve 3 objectives: first, the integration of EGFR amplification status into existing classification strategies; second, the resolution of distinct biologic and prognostic categories currently encompassed within the basket of IDH-wildtype tumors; and third, the delineation of a financially responsible testing algorithm for diagnostic neuropathologists.
Discussion
Among WHO grade II/III IDH-wildtype gliomas, we report that EGFR amplification portends a significantly shorter overall survival compared with EGFR non-amplified tumors. In the heterogeneous group of IDH-wildtype tumors we find that specifically the EGFR-amplified subset is associated with older age at diagnosis and a clinical course that is similar to GBM. We find that EGFR amplification is not associated with MGMT promoter methylation status, neither can these tumors necessarily be distinguished by contrast enhancement on MRI.
Our findings support the evolving consensus on the importance of testing for EGFR amplification in histologically WHO grade II/III gliomas. Previous efforts to determine the prognostic significance of EGFR in the setting of bona fide GBM, WHO grade IV, have not established EGFR as an independent biomarker.35–37 Thus, while representing a frequent molecular aberration in GBM, EGFR testing is currently not recommended in this context. In contrast, the importance of IDH mutations in prognostication of glial tumors4 has been acknowledged by the most recent WHO classification,1 and here we propose a testing algorithm that builds on initial IDH assessment. The current official WHO guidelines—despite acknowledging that anaplastic astrocytoma, IDH wildtype, may follow a clinical course similar to that of GBM—offers no mechanisms for making this grade IV diagnosis based on molecular characteristics. Recently, however, the updated recommendations from cIMPACT-NOW have identified molecular markers in diffuse gliomas that are predictive of grade IV–like behavior, including EGFR amplification.8 These recommendations are based on previous studies which suggest that diffuse astrocytoma (IDH wildtype) may represent a dissolving diagnosis and the apparent specificity of EGFR amplification to delineate GBM-like behavior.38 Prior reports have proposed reclassification of IDH-wildtype gliomas according to various additional molecular features. In part, these studies did include EGFR amplification; however, it has mostly been examined as part of a more complete molecular signature, aiming to distinguish GBM from other diagnoses.4,5,7 Other studies did include EGFR amplification in cohorts of histologically diffuse gliomas (grade II/III),3,9–11,39,40 and several larger studies suggest that at least by prevalence, our findings are generalizable.4–6,41 Our work lends further support to the reclassification of IDH-wildtype astrocytomas as GBM or GBM-like, despite lacking requisite histologic features of microvascular proliferation or necrosis,42 based on the presence of EGFR amplification. This study serves as a practical proof of concept for the clinical use of the new cIMPACT-NOW guidelines, demonstrating that this particular biomarker holds the potential to separate a significant fraction of patients into those with a more benign and those with a more aggressive course. A somewhat higher biopsy rate among EGFR-amplified gliomas along with a prevalence of high-grade neuroimaging features as demonstrated in our study suggest that these tumors may represent undersampled GBM, where the histologic hallmarks of the disease were not well represented in the relatively small amount of diagnostic tissue. However, undersampling alone cannot explain all cases, and it is not possible to distinguish these from gliomas with otherwise aggressive growth. Therefore the overall designation “glioblastoma-like” captures the unique phenotype of the EGFR-amplified, IDH-wildtype, grade II/III gliomas in older patients—or as aptly captured in “diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV,” per cIMPACT-NOW recommendations.8
Indeed, it is important to recognize that the subgroups delineated by our diagnostic algorithm are heterogeneous and composed of tumor types with additional molecular alterations of likely diagnostic and prognostic significance. This is particularly true of the IDH-wildtype, EGFR non-amplified subgroup. While our data show that these tumors demonstrate less aggressive behavior compared with those with EGFR amplification, this may reflect the inclusion of tumors with molecular features of true lower-grade tumors, alongside those with more characteristic high-grade alterations.5,43 Therefore, a role for expanded genomic testing is not entirely obviated by our diagnostic algorithm and we emphasize that an EGFR-focused testing algorithm cannot make these distinctions. In keeping with cIMPACT-NOW recommendations, EGFR testing in IDH-wildtype gliomas (along with chr 7 gain/chr 10 loss or TERT promoter mutation) represents a necessary, but not necessarily sufficient, molecular characterization for all gliomas.
To our knowledge, prior testing approaches for subclassification have not taken into account practical feasibility or financial considerations; arguably these are significant impediments to uniform clinical adoption. While we took these aspects into account, our cost analysis cannot account for the variability of billable costs across practice sites. For example, the cost of comprehensive NGS testing varies widely between the US and Europe. These more comprehensive testing approaches (eg, NGS), once widely adopted, will offer important additional insights—and clinical utility may ultimately justify the higher costs when compared with FISH. Despite improvements in NGS technology and wider clinical adoption, most neuropathology laboratories will not have access to NGS-based copy number calling. In contrast, most diagnostic neuropathology laboratories are already applying FISH (eg, to assess 1p/19q status) and we believe that the relatively succinct nature of the workup presented here can be implemented across several practice settings. Of note, the use of dual-color EGFR/CEP7 FISH simultaneously provides copy number information with respect to the centromere of chromosome 7. In other words, FISH-based analyses can account for loci of interest included in the new cIMPACT-NOW guidelines (in conjunction with chr 10/phosphatase and tensin homolog) as well as other emerging targets (9p/cyclin-dependent kinase inhibitor 2A). Our proposed algorithm is readily scalable to enable meaningful genomic characterization of gliomas.44–47
Therapeutically, EGFR signaling and downstream activation of receptor tyrosine kinase/Ras/phosphatidylinositol-3 kinase pathways have received significant attention in multiple cancer types.48–50 Despite the relative success in lung and breast cancer, EGFR tyrosine kinase inhibitors have not shown significant response rates in gliomas to date,51,52 and antibody-based therapies have been similarly disappointing.53,54 While vaccination for the EGFR variant III gene fusion has also not been a successful strategy,55 preliminary data for an EGFR-targeting antibody–drug conjugate in EGFR-amplified tumors have shown promising results (NCT02573324). Therefore, while EGFR amplification as a target for therapy is currently of limited therapeutic utility, it remains of interest in the context of clinical trials. Our findings therefore also outline a possibly underrecognized subgroup of patients who may benefit from these therapies.
In summary, we present a cost-effective, clinically relevant diagnostic workup to subclassify WHO grade II/III, IDH-wildtype gliomas according to EGFR amplification status. WHO grade II/III, IDH-wildtype gliomas with EGFR gene amplification represent a prognostically relevant and distinct (“glioblastoma-like”) subset of patient with significantly shorter overall survival.
Funding
This work was funded in part by NIH Grant No. R01 CA225655 (J.K.L.), and the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest statement.
None declared.
Authorship statement.
Study design: TAB, JKL; data generation and analysis: TAB, JTJ, OR, NR, NJ, JCD, VN, MMLA, MF, TTB, DNL, AJI, DPC; statistics/verified analytical methods: TAB, JTJ, OR, JKL; writing of first draft: TAB, JTJ, JKL; read and approved final version: all authors.
Supplementary Material
References
- 1. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.. World Health Organization Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2016. [Google Scholar]
- 2. Robinson C, Kleinschmidt-DeMasters BK. IDH1-mutation in diffuse gliomas in persons age 55 years and over. J Neuropathol Exp Neurol. 2017;76(2):151–154. [DOI] [PubMed] [Google Scholar]
- 3. Yan H, Parsons DW, Jin G, et al. . IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Reuss DE, Mamatjan Y, Schrimpf D, et al. . IDH mutant diffuse and anaplastic astrocytomas have similar age at presentation and little difference in survival: a grading problem for WHO. Acta Neuropathol. 2015;129(6):867–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aibaidula A, Chan AK, Shi Z, et al. . Adult IDH wild-type lower-grade gliomas should be further stratified. Neuro Oncol. 2017;19(10):1327–1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reuss DE, Kratz A, Sahm F, et al. . Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol. 2015;130(3):407–417. [DOI] [PubMed] [Google Scholar]
- 7. Ng HK. Prognostic stratification of IDH wild type lower grade gliomas. Neuro Oncol. 2018;20(1):i2. [Google Scholar]
- 8. Brat DJ, Aldape K, Colman H, et al. . cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crespo I, Vital AL, Gonzalez-Tablas M, et al. . Molecular and genomic alterations in glioblastoma multiforme. Am J Pathol. 2015;185(7):1820–1833. [DOI] [PubMed] [Google Scholar]
- 10. Fuller GN, Bigner SH. Amplified cellular oncogenes in neoplasms of the human central nervous system. Mutat Res. 1992;276(3):299–306. [DOI] [PubMed] [Google Scholar]
- 11. Killela PJ, Pirozzi CJ, Reitman ZJ, et al. . The genetic landscape of anaplastic astrocytoma. Oncotarget. 2014;5(6):1452–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen JR, Xu HZ, Yao Y, Qin ZY. Prognostic value of epidermal growth factor receptor amplification and EGFRvIII in glioblastoma: meta-analysis. Acta Neurol Scand. 2015;132(5):310–322. [DOI] [PubMed] [Google Scholar]
- 13. Shinojima N, Tada K, Shiraishi S, et al. . Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–6970. [PubMed] [Google Scholar]
- 14. Tynninen O, Aronen HJ, Ruhala M, et al. . MRI enhancement and microvascular density in gliomas. Correlation with tumor cell proliferation. Invest Radiol. 1999;34(6):427–434. [DOI] [PubMed] [Google Scholar]
- 15. DeWitt JC, Jordan JT, Frosch MP, et al. . Cost-effectiveness of IDH testing in diffuse gliomas according to the 2016 WHO classification of tumors of the central nervous system recommendations. Neuro Oncol. 2017;19(12):1640–1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK.. World Health Organization Classification of Tumours of the Central Nervous System. Lyon: International Agency for Research on Cancer; 2007. [Google Scholar]
- 17. Gevaert O, Mitchell LA, Achrol AS, et al. . Glioblastoma multiforme: exploratory radiogenomic analysis by using quantitative image features. Radiology. 2014;273(1):168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gutman DA, Cooper LA, Hwang SN, et al. . MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. Radiology. 2013;267(2):560–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jain R, Poisson LM, Gutman D, et al. . Outcome prediction in patients with glioblastoma by using imaging, clinical, and genomic biomarkers: focus on the nonenhancing component of the tumor. Radiology. 2014;272(2):484–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhou H, Vallières M, Bai HX, et al. . MRI features predict survival and molecular markers in diffuse lower-grade gliomas. Neuro Oncol. 2017;19(6):862–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zinn PO, Mahajan B, Majadan B, et al. . Radiogenomic mapping of edema/cellular invasion MRI-phenotypes in glioblastoma multiforme. PLoS One. 2011;6(10):e25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chi AS, Batchelor TT, Dias-Santagata D, et al. . Prospective, high-throughput molecular profiling of human gliomas. J Neurooncol. 2012;110(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lennerz JK, Kwak EL, Ackerman A, et al. . MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29(36):4803–4810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sauter G, Maeda T, Waldman FM, Davis RL, Feuerstein BG. Patterns of epidermal growth factor receptor amplification in malignant gliomas. Am J Pathol. 1996;148(4):1047–1053. [PMC free article] [PubMed] [Google Scholar]
- 25. Snuderl M, Eichler AF, Ligon KL, et al. . Polysomy for chromosomes 1 and 19 predicts earlier recurrence in anaplastic oligodendrogliomas with concurrent 1p/19q loss. Clin Cancer Res. 2009;15(20):6430–6437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng Z, Liebers M, Zhelyazkova B, et al. . Anchored multiplex PCR for targeted next-generation sequencing. Nat Med. 2014;20(12):1479–1484. [DOI] [PubMed] [Google Scholar]
- 27. DeWitt J, Jordan JT, Batten J, et al. . Frozen foresight: rapid IDH and MGMT testing in diffuse gliomas initiated at the time of frozen section diagnosis. J Neuropathol Exp Neurol. 2017;76(6):526. [Google Scholar]
- 28. Cerami E, Gao J, Dogrusoz U, et al. . The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gao J, Aksoy BA, Dogrusoz U, et al. . Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ostrom QT, Gittleman H, Fulop J, et al. . CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(Suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Liu X, Xu K, et al. . MRI features can predict EGFR expression in lower grade gliomas: a voxel-based radiomic analysis. Eur Radiol. 2018;28(1):356–362. [DOI] [PubMed] [Google Scholar]
- 32. Brat DJ, Verhaak RG, Aldape KD, et al. ; Cancer Genome Atlas Research Network Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med. 2015;372(26):2481–2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen AL, Holmen SL, Colman H. IDH1 and IDH2 mutations in gliomas. Curr Neurol Neurosci Rep. 2013;13(5):345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eckel-Passow JE, Lachance DH, Molinaro AM, et al. . Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med. 2015;372(26):2499–2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mellinghoff IK, Wang MY, Vivanco I, et al. . Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353(19):2012–2024. [DOI] [PubMed] [Google Scholar]
- 36. Ohgaki H, Dessen P, Jourde B, et al. . Genetic pathways to glioblastoma: a population-based study. Cancer Res. 2004;64(19):6892–6899. [DOI] [PubMed] [Google Scholar]
- 37. Wen PY, Chang SM, Lamborn KR, et al. . Phase I/II study of erlotinib and temsirolimus for patients with recurrent malignant gliomas: North American Brain Tumor Consortium trial 04-02. Neuro Oncol. 2014;16(4):567–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hasselblatt M, Jaber M, Reuss D, et al. . Diffuse astrocytoma, IDH-wildtype: a dissolving diagnosis. J Neuropathol Exp Neurol. 2018;77(6):422–425. [DOI] [PubMed] [Google Scholar]
- 39. Cryan JB, Haidar S, Ramkissoon LA, et al. . Clinical multiplexed exome sequencing distinguishes adult oligodendroglial neoplasms from astrocytic and mixed lineage gliomas. Oncotarget. 2014;5(18):8083–8092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hu X, Martinez-Ledesma E, Zheng S, et al. . Multigene signature for predicting prognosis of patients with 1p19q co-deletion diffuse glioma. Neuro Oncol. 2017;19(6):786–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stichel D, Ebrahimi A, Reuss D, et al. . Distribution of EGFR amplification, combined chromosome 7 gain and chromosome 10 loss, and TERT promoter mutation in brain tumors and their potential for the reclassification of IDHwt astrocytoma to glioblastoma. Acta Neuropathol. 2018;136(5):793–803. [DOI] [PubMed] [Google Scholar]
- 42. Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A. Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol. 2015;129(6):829–848. [DOI] [PubMed] [Google Scholar]
- 43. Ferguson SD, Zhou S, Huse JT, et al. . Targetable gene fusions associate with the IDH wild-type astrocytic lineage in adult gliomas. J Neuropathol Exp Neurol. 2018;77(6):437–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Korshunov A, Sycheva R, Gorelyshev S, Golanov A. Clinical utility of fluorescence in situ hybridization (FISH) in nonbrainstem glioblastomas of childhood. Mod Pathol. 2005;18(9):1258–1263. [DOI] [PubMed] [Google Scholar]
- 45. Koshiyama DB, Trevisan P, Graziadio C, et al. . Frequency and clinical significance of chromosome 7 and 10 aneuploidies, amplification of the EGFR gene, deletion of PTEN and TP53 genes, and 1p/19q deficiency in a sample of adult patients diagnosed with glioblastoma from southern Brazil. J Neurooncol. 2017;135(3):465–472. [DOI] [PubMed] [Google Scholar]
- 46. Lopez-Gines C, Cerda-Nicolas M, Gil-Benso R, et al. . Association of chromosome 7, chromosome 10 and EGFR gene amplification in glioblastoma multiforme. Clin Neuropathol. 2005;24(5):209–218. [PubMed] [Google Scholar]
- 47. Wijnenga MMJ, French PJ, Dubbink HJ, et al. . Prognostic relevance of mutations and copy number alterations assessed with targeted next generation sequencing in IDH mutant grade II glioma. J Neurooncol. 2018;139(2):349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chakravarti A, Zhai G, Suzuki Y, et al. . The prognostic significance of phosphatidylinositol 3-kinase pathway activation in human gliomas. J Clin Oncol. 2004;22(10):1926–1933. [DOI] [PubMed] [Google Scholar]
- 49. Parsons DW, Jones S, Zhang X, et al. . An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321(5897):1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Patel R, Leung HY. Targeting the EGFR-family for therapy: biological challenges and clinical perspective. Curr Pharm Des. 2012;18(19):2672–2679. [DOI] [PubMed] [Google Scholar]
- 51. Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009;16(6):748–754. [DOI] [PubMed] [Google Scholar]
- 52. Rich JN, Rasheed BK, Yan H. EGFR mutations and sensitivity to gefitinib. N Engl J Med. 2004;351(12):1260–1261; author reply 1260. [PubMed] [Google Scholar]
- 53. Bode U, Massimino M, Bach F, et al. . Nimotuzumab treatment of malignant gliomas. Expert Opin Biol Ther. 2012;12(12):1649–1659. [DOI] [PubMed] [Google Scholar]
- 54. Neyns B, Sadones J, Joosens E, et al. . Stratified phase II trial of cetuximab in patients with recurrent high-grade glioma. Ann Oncol. 2009;20(9):1596–1603. [DOI] [PubMed] [Google Scholar]
- 55. Weller M, Butowski N, Tran DD, et al. ; ACT IV trial investigators Rindopepimut with temozolomide for patients with newly diagnosed, EGFRvIII-expressing glioblastoma (ACT IV): a randomised, double-blind, international phase 3 trial. Lancet Oncol. 2017;18(10):1373–1385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.