Abstract
Globins are oxygen-binding heme proteins present in bacteria, protists, fungi, plants, and animals. Their functions have diverged widely in evolution, and include binding, transport, scavenging, detoxification, and sensing of gases like oxygen, nitric oxide, and carbon monoxide. Neuroglobin (Ngb) is a recently discovered monomeric globin with high affinity for oxygen and preferential localization to vertebrate brain. No function for Ngb is known, but its affinity for oxygen and its expression in cerebral neurons suggest a role in neuronal responses to hypoxia or ischemia. Here we report that Ngb expression is increased by neuronal hypoxia in vitro and focal cerebral ischemia in vivo, and that neuronal survival after hypoxia is reduced by inhibiting Ngb expression with an antisense oligodeoxynucleotide and enhanced by Ngb overexpression. Both induction of Ngb and its protective effect show specificity for hypoxia over other stressors. We conclude that hypoxia-inducible Ngb expression helps promote neuronal survival from hypoxic-ischemic insults.
The fate of neurons undergoing hypoxic or ischemic injury is regulated by transcriptional and posttranscriptional events that contribute to competing cell-death and cell-survival programs (1, 2). Survival-promoting events include the transcriptional induction or posttranslational activation of neuroprotective proteins like erythropoietin (3), vascular endothelial growth factor (4), and heme oxygenase (5). In many cases, these are hypoxia-inducible proteins that help to counteract the adverse effects of hypoxia or ischemia by increasing anaerobic metabolism, tissue vascularity, or oxygen delivery (6). Another strategy for promoting the survival of metabolically active tissues like muscle or nerve may involve the tissue-specific expression of intracellular oxygen-binding proteins that can enhance oxygen extraction and intracellular diffusion, or neutralize reactive oxygen species. Examples include myoglobin, in the case of muscle (7), and invertebrate nerve myoglobins (8).
Neuroglobin (Ngb) is a newly discovered vertebrate globin that is expressed most abundantly in neurons (9). Ngb was identified by searching murine and human expressed sequence tag databases for partial globin-like sequences, then cloned, and sequenced to reveal a 151-aa protein with a predicted molecular mass of ≈17 kDa, which exists as a monomer. Human and murine Ngbs show 94% sequence identity at the amino acid level but limited homology to other known globins. For example, there is <21% sequence identity with vertebrate myoglobins and <25% identity with vertebrate hemoglobins. The protein that most closely resembles Ngb (30% amino acid identity) is the intracellular nerve myoglobin of the polychaete annelid worm Aphrodite aculeata (8).
Because Ngb is an oxygen-binding heme protein that is expressed preferentially in cerebral neurons, we investigated its possible involvement in neuronal responses to hypoxia or ischemia. The results indicate that Ngb is induced by neuronal hypoxia and cerebral ischemia and protects neurons from hypoxia in vitro, suggesting that Ngb may have a role in sensing or responding to neuronal hypoxia.
Materials and Methods
Cortical Neuron Culture.
Neuronal cultures were prepared from the cerebral hemispheres of 16-day Charles River CD1 mouse embryos, seeded at 3 × 105 cells per well on 24-well culture dishes precoated with poly-d-lysine, and grown in Eagle's MEM (GIBCO/BRL) with 5% horse serum and 5% FBS (4). Cultures were treated with 10 μM cytosine arabinoside on day 6 and used on day 11, when >95% of cells expressed the neuronal marker, microtubule-associated protein 2. To induce hypoxia, cultures were placed in a modular incubator chamber (Billups-Rothenberg, Del Mar, CA) containing humidified 95% air/5% CO2 (control), or humidified 95% N2/5% CO2 (hypoxic), for 0–24 h at 37°C, and then returned to normoxic conditions for the remainder, if any, of 24 h (10). Both control and hypoxic cultures contained 30 mM glucose. Cell viability was assayed by incubating cultures with 5 mg/ml of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) at 37°C for 2 h and measuring A at 570 nm in solubilized cells by using a Cytofluor Series 4000 multiwell plate-reader (PerSeptive Biosystems, Framingham, MA). In some experiments, results were confirmed by trypan blue exclusion (TBE).
Western Blotting.
Cell lysates were prepared as described (4) and 100-μg protein samples were electrophoresed on 12% SDS/PAGE gels and transferred to poly(vinylidene difluoride) membranes. Membranes were incubated overnight at 4°C with a rabbit polyclonal Ab against Ngb (1:2,000), which was produced by immunizing with a synthetic peptide corresponding to amino acids 35–50 (NH2-CLSSPEFLDHIRKVML-COOH) of mouse Ngb, and affinity-purified by using a SulfoLink kit (Pierce). A horseradish peroxidase-conjugated anti-rabbit secondary Ab (Santa Cruz Biotechnology) and a chemiluminescence substrate system (NEN) were used to visualize the immunolabeled bands (4).
Cytochemistry.
Cultures were fixed with 4% paraformaldehyde and incubated overnight at 4°C with one or more of the following primary Abs: rabbit polyclonal anti-Ngb (1:200), mouse monoclonal anti-neuronal nuclear antigen (NeuN) (1:200, Chemicon), and rabbit polyclonal anti-17–20-kDa caspase-3 cleavage product (1:100, New England Biolabs). The secondary Abs (all 1:200) were fluorescein isothiocyanate-conjugated goat anti-rabbit or anti-mouse IgG (Vector Laboratories) and rhodamine-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch). Controls for nonspecific binding included omitting primary (Fig. 1c) or secondary Abs. In addition, in some cultures, nuclei were labeled with 4′,6-diamidino-2-phenylindole (DAPI) or DNA strand breaks were labeled with the Klenow fragment of DNA polymerase I (Roche) followed by rhodamine avidin D (Vector Laboratories), as described (11). Fluorescence signals were detected with a Nikon E800 epifluorescence microscope by using excitation/emission wavelengths of 535/565 nm for rhodamine (red), 470/505 nm for fluorescein isothiocyanate (green), and 360/400 nm for DAPI (blue). Results were recorded with a Magnifire digital color camera (Optronics). To evaluate the in vivo expression of Ngb, immunohistochemistry was done on cerebral cortical sections from rats subjected to 90 min of focal cerebral ischemia followed by 4–24 h of reperfusion (12), using the same anti-Ngb primary Ab described above (1:200) and a horseradish peroxidase-conjugated goat anti-rabbit secondary Ab (1:1000, Santa Cruz Biotechnology).
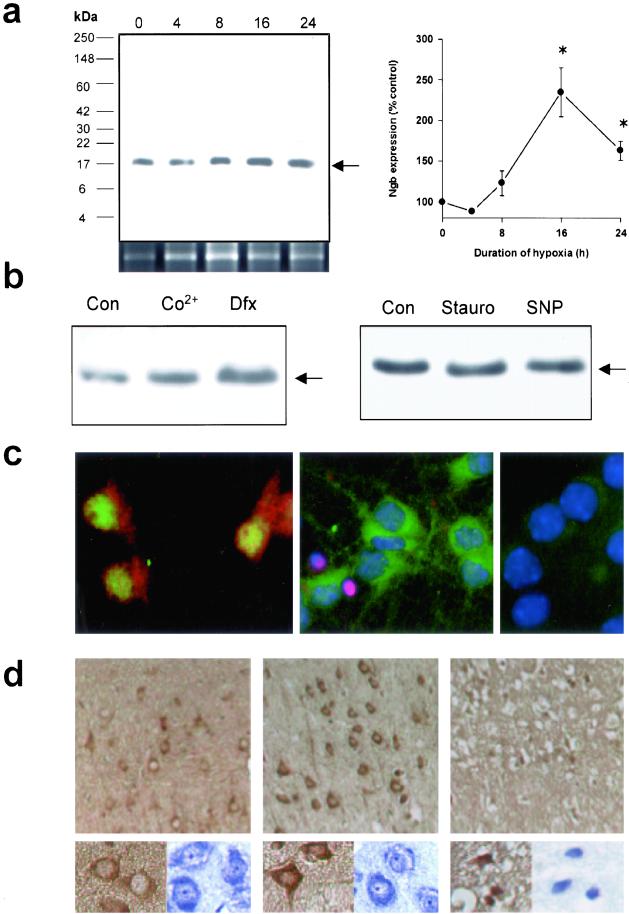
Figure 1.
Neuronal hypoxia and ischemia induce Ngb protein expression. (a) Representative Western blot showing increased Ngb expression in cultured cortical neurons maintained without oxygen for the indicated number of hours (Left). Panel beneath the Western blot shows Ngb mRNA expression over the same time course. Expression of the 17-kDa band (arrow) was quantified by computer densitometry (mean ± SEM, n = 3; *, P < 0.05 relative to 0 h by t test) (Right). (b) Representative Western blots (n = 3) showing increased Ngb expression in cultures treated for 24 h with 300 μM Co2+ or 100 μM Dfx (Left), but no change with 0.1 μM staurosporine (Stauro) or 500 μM SNP (Right). (c) Fluorescence labeling of cultured cortical neurons showing Ngb immunoreactivity (red) in the cytoplasm of cells that express the neuronal nuclear antigen NeuN (green) (Left). Segregation of Ngb expression (green) and DNA damage (detected by labeling with the Klenow fragment of DNA polymerase I, red) into distinct populations, corresponding to viable cells with large nuclei (DAPI staining, blue) and nonviable cells with shrunken nuclei (Center). Preabsorption of the Ab with authentic Ngb peptide antigen abolished immunolabeling (Right). (d) Representative sections from contralateral, nonischemic rat cerebral cortex (Left) and penumbra (Center) or core (Right) of ischemic cerebral cortex at 24 h. Immunostaining for Ngb shows increased Ngb expression in the penumbra; this increased staining is localized to the cytoplasm of normal-appearing, unshrunken cells with neuronal morphology (Center, Insets). Brown, anti-Ngb; blue, cresyl violet. [Original magnification, ×400 (c and Insets to d) and ×200 (d)].
Oligodeoxynucleotide (ODN) Treatment.
A phosphorothioate antisense ODN labeled with fluorescein at the 5′ end and directed against the initial coding region of the target Ngb mRNA (5′-TCCGGGCGCTCCAT-3′, from nucleotides 90–77) was designed based on the mouse Ngb sequence obtained from GenBank (accession no. NM022414). This and a sense sequence (5′-ATGGAGCGCCCGGA-3′, from nucleotides 77–90) were synthesized commercially (Operon Technologies, Alameda, CA) and purified by HPLC. Cultures were transfected with ODNs (1–10 μM) by using FuGENE 6 (Roche), beginning 3 h before the onset of hypoxia. Cultures were analyzed by Western blotting and by MTT cell viability assay, and fluorescence microscopy was used to confirm transfection and examine its relationship to cell death, using caspase-3 activation as a marker.
HN33 Cell Culture and Transfection.
HN33 cells (passage number ≤20) were plated at 1 × 105 cells per well on uncoated, six-well plastic dishes and maintained as described (13, 14). Full-length mouse Ngb cDNA (9) was cloned into a pcDNA 3.1 plasmid with cytomegalovirus promoter (CLONTECH). The recombinant plasmid (pcDNA-Ngb) or vector alone (pcDNA) was transfected into HN33 cells for 48 h by using FuGENE 6 (Roche), followed by screening with G418 (Life Technologies, Grand Island, NY). Overexpression of Ngb was confirmed by Western blot as described above.
Results and Discussion
Hypoxia Induces Ngb Expression.
To test whether Ngb expression is induced in hypoxic cerebral cortical neurons, we generated and affinity-purified a rabbit polyclonal Ab against a synthetic peptide corresponding to amino acids 35–50 of mouse Ngb. This Ab labeled a band on Western blots prepared from mouse cortical neuron cultures at the predicted relative molecular mass of 17,000 (Fig. 1a). When cultures were deprived of oxygen for up to 24 h, expression of this protein increased, as did the abundance of Ngb mRNA, consistent with transcriptional induction. Expression was also increased by 300 μM CoCl2 and by 100 μM deferoxamine (Dfx) (Fig. 1b), which enhance the expression of hypoxia-inducible genes, including the major hypoxia-signaling transcription factor, hypoxia-inducible factor-1α (HIF-1α) (15). In contrast to the effects of hypoxia, CoCl2, and Dfx, other stressors [including staurosporine and the NO donor, sodium nitroprusside (SNP)] did not increase Ngb expression (Fig. 1b), suggesting the specific involvement of hypoxia-signaling pathways in Ngb induction.
Immunocytochemistry with the same anti-Ngb Ab used for Western blotting showed that Ngb was localized to the cytoplasm of cells that expressed the neuronal nuclear antigen NeuN and were therefore neurons (Fig. 1c). In hypoxic cultures stained for Ngb and for DNA damage with the Klenow fragment of DNA polymerase I (11), Ngb was expressed most prominently in undamaged (Klenow-negative) cells. Ngb immunostaining was abolished when the Ab was preabsorbed with authentic Ngb peptide antigen.
To determine whether Ngb expression was also increased by cerebral ischemia in vivo, we immunostained sections from cerebral cortex of mice subjected to focal cerebral ischemia by occlusion of the middle cerebral artery for 90 min followed by reperfusion for 4–24 h as described (12). These sections showed increased Ngb immunoreactivity in the cytoplasm of neurons from the ischemic compared to the nonischemic hemisphere (Fig. 1d). This increase was greatest in the ischemic penumbra and less pronounced in what would evolve into the ischemic core. These findings demonstrate that Ngb is expressed in neurons and that its expression is increased by hypoxia and ischemia, especially in neuronal populations that are destined to survive.
Reducing Neuroglobin Expression Worsens Hypoxic Injury.
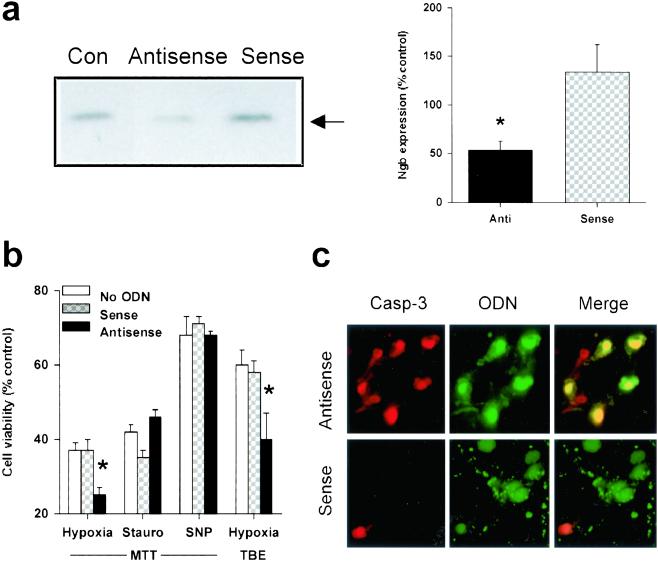
To begin to investigate the possibility that Ngb protects neurons from hypoxia, cultured neurons were transfected with a phosphorothioate antisense ODN directed against the initial coding region of the target Ngb mRNA (5′-TCCGGGCGCTCCAT-3′, from nucleotides 90–77) or with a control sense sequence (5′-ATGGAGCGCCCGGA-3′, from nucleotides 77–90), both labeled with fluorescein at the 5′ end. Transfection efficiency, measured in cultures transfected with the fluorescent Ngb antisense ODN and counterstained with DAPI, was 96 ± 1% (n = 10). Western blots showed that Ngb protein expression was reduced in antisense-transfected compared to sense-transfected or untransfected control cultures (Fig. 2a). The antisense-mediated reduction in Ngb expression was associated with a decrease in the viability of cultured neurons exposed to hypoxia, whether measured by MTT absorbance, which reflects mitochondrial function and is an early and sensitive indicator of cell injury in this model, or TBE, which relates to membrane integrity and declines with more advanced damage (Fig. 2b). In contrast to its effect in hypoxia, Ngb antisense had no effect on the toxicity of staurosporine or SNP. Fluorescence microscopy showed that many antisense-transfected cells, but few sense-transfected cells, co-expressed the 17–20-kDa caspase-3 cleavage product that is generated in neurons undergoing ischemic cell death (16) (Fig. 2c).
Figure 2.
Decreased Ngb expression exacerbates hypoxic neuronal death. (a) Representative Western blot showing decreased Ngb expression compared to untransfected control cells (Con) in cultured cortical neurons treated with an antisense ODN directed against Ngb, but not with a sense ODN (Left). Ngb expression was quantified (mean ± SEM, n = 3) by computer densitometry (*, P < 0.05 relative to Con by t test) (Right). (b) Cell viability, measured by MTT absorbance or TBE, in cultures maintained for 12 h without oxygen or in the presence of 0.1 μM staurosporine (Stauro) or 200–400 μM SNP, under standard conditions (no ODN) or after treatment with 5 μM sense or antisense ODN, added 3 h before the onset of, and present throughout the toxic exposure (n = 3–6). *, P < 0.05 relative to no treatment (t test). (c) Fluorescence labeling of cultured cortical neurons treated with Ngb antisense (Upper) or sense (Lower) ODNs, showing immunoreactivity for the 17–20-kDa caspase-3 cleavage product (Left, red), ODN fluorescence (Center, green), and the merged images (Right, yellow). (Original magnification, ×400). Antisense-transfected cultures show an increase in the proportion of neurons that exhibit caspase-3 cleavage compared to sense-transfected cultures, consistent with the antisense-mediated decrease in cell viability shown in b.
Increasing Neuroglobin Expression Lessens Hypoxic Injury.
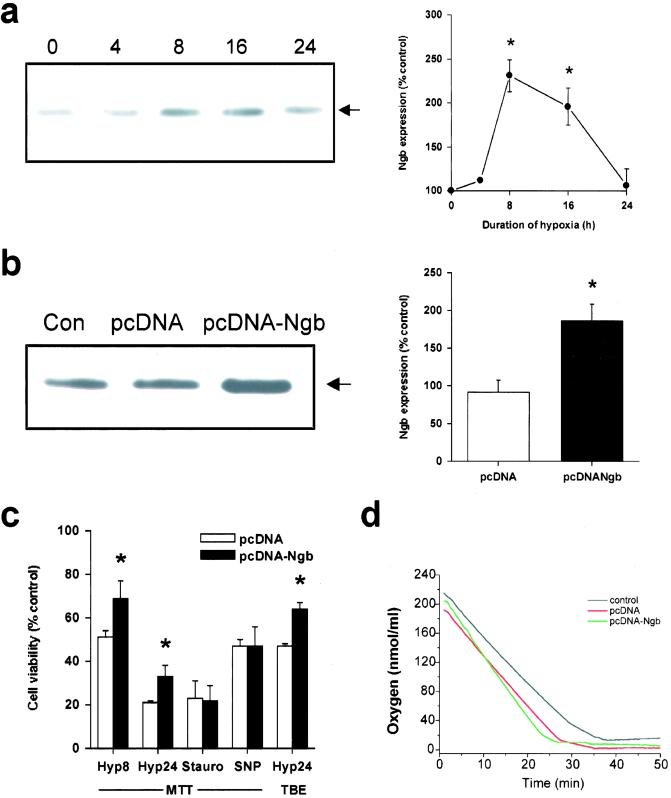
To test further the protective effect of Ngb in hypoxia, full-length mouse Ngb cDNA (9) was cloned into a pcDNA 3.1 plasmid with cytomegalovirus promoter (pcDNA-Ngb) and transfected into and stably expressed in HN33, an immortalized hippocampal neuronal cell line (17). These cells were chosen because they provided high transfection efficiency and because their response to hypoxia is well-characterized (4, 13). Hypoxia increased the expression of Ngb in HN33 cells, with a time course similar to that observed in cultured cortical neurons (Fig. 3a). Transfection with pcDNA-Ngb led to overexpression of Ngb (Fig. 3b) and increased the viability of hypoxic HN33 cells, determined with either MTT or TBE (Fig. 3c). However, pcDNA-Ngb afforded no protection against staurosporine or SNP.
Figure 3.
Overexpression of Ngb reduces hypoxic cell death. (a) Representative Western blot showing increased Ngb expression in cultured HN33 cells maintained without oxygen for the indicated number of hours (Left). Expression of the 17-kDa band (arrow) was quantified by computer densitometry (mean ± SEM, n = 3; *, P < 0.05 relative to 0 h by t test) (Right). (b) pcDNA vector or Ngb-expressing recombinant plasmid (pcDNA-Ngb) was stably transfected into HN33 cells, and the overexpression of Ngb protein in pcDNA-Ngb-transfected cultures was confirmed by Western blotting (Left). Ngb expression was quantified by computer densitometry (mean ± SEM, n = 5; *, P < 0.05 relative to Con by t test) (Right). (c) Cell viability, measured by MTT absorbance or TBE, in pcDNA- or pcDNA-Ngb-transfected cultures maintained for 8 h (Hyp8) or 24 h (Hyp24) without oxygen, or for 24 h in the presence of 0.1 μM staurosporine (Stauro) or 300 μM SNP. *, P < 0.05 relative to untransfected control cultures (t test). (d) Oxygen consumption in untransfected (control) and pcDNA- or pcDNA-Ngb-transfected HN33 cells (5 × 106 cells in 1 ml of DMEM) measured by using a Clark oxygen electrode (Hansatech Instruments, Pentney King's Lynn, U.K.). Each tracing is the average of three independent experiments. The slopes give oxygen consumption (nmol O/ml/min) and were not significantly different across conditions (control, 7.46 ± 0.90; pcDNA, 9.24 ± 1.39; pcDNA-Ngb, 11.87 ± 2.31; P = 0.18 by ANOVA, n = 9).
Possible Mechanisms for Induction of Ngb.
These results are consistent with a role for Ngb as a hypoxia-inducible neuroprotective factor in hypoxic-ischemic injury. How hypoxia stimulates Ngb expression is uncertain, although hypoxia can induce hemoglobin synthesis in invertebrates (18) and may act through HIF-1 to regulate β-globin gene expression during vertebrate development (19). The effects of CoCl2 and Dfx (Fig. 1b) are consistent with involvement of HIF-1 in hypoxic induction of Ngb expression, as is the observation that the 5′-untranslated region of Ngb (GenBank accession number NM 022414) contains several copies of the consensus HIF-1-binding sequence 5′-RCGTG-3′ (20), located 2073, 1977, 1445, 1041, 985, 627, 522, and 64 nucleotides upstream of the transcription initiation site.
Possible Mechanisms for Protection by Ngb.
The manner in which Ngb exerts its neuroprotective effect is also uncertain. One possibility is that, like myoglobin in muscle, it may bind oxygen and facilitate its delivery to mitochondria (7). To evaluate this possibility, we used a Clark oxygen electrode (21) to compare oxygen consumption in control and Ngb-overexpressing HN33 cells (Fig. 3d). Oxygen consumption did not vary significantly across conditions, indicating that Ngb does not increase the rate of oxygen consumption and arguing for a different mode of neuroprotective action. In some respects, this is not surprising because oxygen supply does not normally limit oxygen consumption and because the affinity of Ngb for oxygen may be too high for it to release oxygen under physiological conditions (22), although this is disputed (23, 24). Alternatively, and also by analogy to myoglobin, Ngb might scavenge NO (25), which has been implicated in hypoxic-ischemic neuronal injury (26). However, the failure of Ngb antisense to exacerbate and of Ngb overexpression to protect against SNP toxicity in our model argues against this mechanism. Additional possibilities are that Ngb might be involved in sensing hypoxia and triggering protective cellular responses thereto, or in detoxifying mediators of hypoxic-ischemic injury other than NO, for both of which actions there is precedent among nonvertebrate globins (18).
Conclusion
The recent discovery of Ngb (9) and the results presented here provide evidence for the existence of a novel endogenous neuroprotective mechanism. Understanding how Ngb and other hypoxia-inducible proteins confer neuronal protection may help in the development of improved treatment for ischemic disorders such as stroke.
Acknowledgments
We thank T. Burmester and T. Hankeln for the Ngb plasmid used in this study and D. Nicholls, S. Chalmers, B. Cochran, and G. del Rio for helpful discussions and advice. This work was supported by the National Institute of Neurological Disorders and Stroke, National Institutes of Health, and by the Buck Institute for Age Research.
Abbreviations
- DAPI
4′,6-diamidino-2-phenylindole
- HIF-1
hypoxia-inducible factor-1
- MTT
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- NeuN
neuronal nuclear antigen
- Ngb
neuroglobin
- ODN
oligodeoxynucleotide
- SNP
sodium nitroprusside
- TBE
trypan blue exclusion
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Sharp F R, Lu A, Tang Y, Millhorn D E. J Cereb Blood Flow Metab. 2000;20:1011–1032. doi: 10.1097/00004647-200007000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Graham S H, Chen J. J Cereb Blood Flow Metab. 2001;21:99–109. doi: 10.1097/00004647-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Sakanaka M, Wen T C, Matsuda S, Masuda S, Morishita E, Nagao M, Sasaki R. Proc Natl Acad Sci USA. 1998;95:4635–4640. doi: 10.1073/pnas.95.8.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin K L, Mao X O, Greenberg D A. Proc Natl Acad Sci USA. 2000;97:10242–10247. doi: 10.1073/pnas.97.18.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dore S, Sampei K, Goto S, Alkayed N J, Guastella D, Blackshaw S, Gallagher M, Traystman R J, Hurn P D, Koehler R C, Snyder S H. Mol Med. 1999;5:656–663. [PMC free article] [PubMed] [Google Scholar]
- 6.Lopez-Barneo J, Pardal R, Ortega-Saenz P. Annu Rev Physiol. 2001;63:259–287. doi: 10.1146/annurev.physiol.63.1.259. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki T, Imai K. Cell Mol Life Sci. 1998;54:979–1004. doi: 10.1007/s000180050227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dewilde S, Blaxter M, Van Hauwaert M L, Vanfleteren J, Esmans E L, Marden M, Griffon N, Moens L. J Biol Chem. 1996;271:19865–19870. doi: 10.1074/jbc.271.33.19865. [DOI] [PubMed] [Google Scholar]
- 9.Burmester T, Weich B, Reinhardt S, Hankeln T. Nature (London) 2000;407:520–523. doi: 10.1038/35035093. [DOI] [PubMed] [Google Scholar]
- 10.Koretz B, Ahern K v B, Lustig H S, Greenberg D A. Brain Res. 1994;643:334–337. doi: 10.1016/0006-8993(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 11.Jin K, Chen J, Nagayama T, Chen M, Sinclair J, Graham S H, Simon R P. J Neurochem. 1999;72:1204–1214. doi: 10.1046/j.1471-4159.1999.0721204.x. [DOI] [PubMed] [Google Scholar]
- 12.Longa E Z, Weinstein P R, Carlson S, Cummins R. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- 13.Shi L C, Wang H Y, Friedman E. J Neurochem. 1998;70:1035–1044. doi: 10.1046/j.1471-4159.1998.70031035.x. [DOI] [PubMed] [Google Scholar]
- 14.Jin K L, Mao X O, Greenberg D A. J Mol Neurosci. 2000;14:197–203. doi: 10.1385/JMN:14:3:197. [DOI] [PubMed] [Google Scholar]
- 15.Semenza G L, Wang G L. Mol Cell Biol. 1992;12:5447–5454. doi: 10.1128/mcb.12.12.5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Namura S, Zhu J, Fink K, Endres M, Srinivasan A, Tomaselli K J, Yuan J, Moskowitz M A. J Neurosci. 1998;18:3659–3668. doi: 10.1523/JNEUROSCI.18-10-03659.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee H J, Hammond D N, Large T H, Roback J D, Sim J A, Brown D A, Otten U H, Wainer B H. J Neurosci. 1990;10:1779–1787. doi: 10.1523/JNEUROSCI.10-06-01779.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weber R E, Vinogradov S N. Physiol Rev. 2001;81:569–628. doi: 10.1152/physrev.2001.81.2.569. [DOI] [PubMed] [Google Scholar]
- 19.Bichet S, Wenger R H, Camenisch G, Rolfs A, Ehleben W, Porwol T, Acker H, Fandrey J, Bauer C, Gassmann M. FASEB J. 1999;13:285–295. doi: 10.1096/fasebj.13.2.285. [DOI] [PubMed] [Google Scholar]
- 20.Semenza G L, Jiang B H, Leung S W, Passantino R, Concordet J P, Maire P, Giallongo A. J Biol Chem. 1996;271:32529–32537. doi: 10.1074/jbc.271.51.32529. [DOI] [PubMed] [Google Scholar]
- 21.Minning D M, Gow A J, Bonaventura J, Braun R, Dewhirst M, Goldberg D E, Stamler J S. Nature (London) 1999;401:497–502. doi: 10.1038/46822. [DOI] [PubMed] [Google Scholar]
- 22.Trent J T, III, Watts R A, Hargrove M S. J Biol Chem. 2001;276:30106–30110. doi: 10.1074/jbc.C100300200. [DOI] [PubMed] [Google Scholar]
- 23.Couture M, Burmester T, Hankeln T, Rousseau D L. J Biol Chem. 2001;276:36377–36382. doi: 10.1074/jbc.M103907200. [DOI] [PubMed] [Google Scholar]
- 24.Dewilde S, Kiger L, Burmester T, Hankeln T, Baudin-Creuza V, Aerts T, Marden M C, Caubergs R, Moens L. J Biol Chem. 2001;276:38949–38955. doi: 10.1074/jbc.M106438200. [DOI] [PubMed] [Google Scholar]
- 25.Flögel U, Merx M W, Gödecke A, Decking U K, Schrader J. Proc Natl Acad Sci USA. 2001;98:735–740. doi: 10.1073/pnas.011460298. . (First Published January 2, 2001; 10.1073/pnas.011460298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dawson V L, Kizushi V M, Huang P L, Snyder S H, Dawson T M. J Neurosci. 1996;16:2479–2487. doi: 10.1523/JNEUROSCI.16-08-02479.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]