Abstract
Background.
Medullary thyroid cancer portends poor survival once liver metastasis occurs. We hypothesize that Notch3 overexpression in medullary thyroid cancer liver metastasis will decrease proliferation and growth of the tumor.
Methods.
TT cells were modified genetically to overexpress Notch3 in the presence of doxycycline, creating the TT-Notch3 cell line. Mice were injected intrasplenically with either TT-Notch3 or control vector TT-TRE cells. Each cell line had 3 treatment groups: control with 12 weeks of standard chow, early DOX with doxycycline chow at day 0 and for 70 days thereafter, and late DOX with doxycycline chow at 8 weeks. Each animal underwent micro-computed tomography to evaluate for tumor formation and tumor quantification was performed. Animals were killed at 12 weeks, and the harvested liver was stained with Ki-67, hematoxylin and eosin, and Notch3.
Results.
Induction of Notch3 did not prevent formation of medullary thyroid cancer liver metastases as all mice in the early DOX group developed tumors. However, induction of Notch after medullary thyroid cancer liver tumor formation decreased tumor size, as seen on micro-computed tomography scans (late DOX group). This translated to a 37-fold decrease in tumor volume (P = .001). Notch3 overexpression also resulted in decreased Ki-67 index (P = .038). Moreover, Notch3 induction led to increased areas of neutrophil infiltration and necrosis on hematoxylin and eosin staining of the tumors
Conclusion.
Notch3 overexpression demonstrates an antiproliferative effect on established metastatic medullary thyroid cancer liver tumors and is a potential therapeutic target in treatment.
The mortality associated with thyroid cancer is most commonly from non–well-differentiated subtypes such as medullary thyroid cancer (MTC). MTC is a neuroendocrine neoplasm, and although it only comprises 10% of all thyroid cancers, MTC is responsible for a disproportionate 14% of all thyroid cancer–related deaths.1,2 Effective treatment of MTC focuses mainly on early detection with operative resection involving total thyroidectomy and central neck lymph node dissection, which can allow for near-total cure when the disease is localized and no distant metastases are present. Approximately one half of all patients with MTC, however, will have a tumor extending beyond the thyroid or extending to distant organs.1 The disease-specific survival of MTC patients is clearly correlated with stage of presentation, and the survival in patients with distant metastasis at 5 years is less than 50%.1,3
Total thyroidectomy has been shown to improve survival in all stages of MTC without distant metastases; however, treatments for patients with distant metastasis remain limited. Tyrosine kinase inhibitor (TKI) therapy, while promising in clinical trials, has yet to show demonstrable survival benefit and durability. The most common site of metastasis is the liver, but, unlike other solid neoplasms, the liver metastasis found in MTC is usually small and distributed widely in the liver, thereby precluding curative resection.4 Despite improvements in genetic and diagnostic tools for MTC, neither systemic chemotherapy nor radiation has shown durable clinical responses in patients with metastatic disease.5–7 Previously, we examined the molecular pathway involved in MTC progression and determined Notch3 to be a tumor suppressor in MTC in vitro and in an in vivo subcutaneous model.8 We therefore hypothesize that Notch3 overexpression in MTC liver nodules will result in decreased tumor proliferation and growth in an animal model of metastatic MTC in the liver.
Methods
Establishing the metastatic mouse model
All animal experiments were performed after approval by the University of Wisconsin (Madison, WI) Animal Care and Use Committee. The TT cell line derived from human MTC was provided by Dr Barry D Nelkin (Johns Hopkins University, Baltimore, MD). TT cells were modified genetically to overexpress Notch3 in the presence of doxycycline by subcloning the Notch3 intracellular domain into a pRevTRE vector system (Takara Bio USA, Inc [formerly Clontech] Mountain View, CA) and then transfected with the regulatory plasmid pRevTet-ON and selected as described previously.8 Transfection with empty vector via the same method created the control vector cell line, TT-TRE. The TT cell line was selected as the MTC cell line for our experimental design based on familiarity of use in our laboratory and because of its ability to be transfected with the vector system.
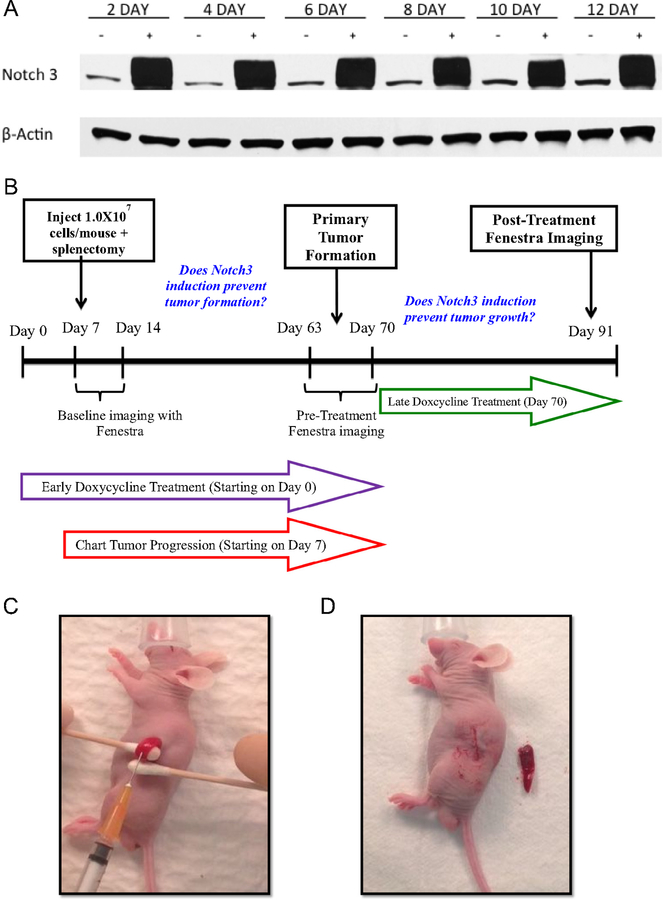
To ascertain the durability of overexpression of our cell line constructs in the presence of doxycycline, TT-TRE and TT-Notch3 cells were plated on 10 cm3 round petri dishes and treated every 48 hours with 10 mL of either plain media or with 1 mg/mL of doxycycline added to media on the day of cell culture. Cells from each group were trypsinized, and cellular pellets were lysed in sample buffer (50 mmol/L tris, 0.15 mol/L NaCl, 0.1% SDS, 1% Nonidet P-40, 0.5% Na/deoxycholate, and 0.6 mmol/L PMSF). Western blotting was then performed as described previously9 to confirm the durable inducibility of the Notch3 protein over time (Fig 1 A).
Fig 1.
A, Western blot analysis of TT-Notch 3 demonstrates a durable induction of the Notch3 protein when treated with doxycycline. B, Study design. C, Technique of injection of MTC cells to the spleen to obtain the liver metastasis mouse model. D, Completion of splenectomy.
With confirmation of the inducible structure, 35 athymic male nude mice were obtained from Charles River Laboratories, Wilmington, MA. The mice were rested for a minimum of 7 days to acclimate to their new vivarium environment to decrease the stress of transport and arrival. All mice were maintained under specific pathogen-free conditions. Six mice were assigned to each cell line group (control, early DOX, late DOX) with only 5 assigned to the TT-TRE control group because 1 mouse died during transportation to our laboratory facility. Mouse operations for implantation of each cell line were divided between 2 days to prevent operator fatigue. On the day of operation, each mouse was anesthetized using inhaled isofluorane, according to institutional standards. Each mouse was placed on its right side with adequate exposure of the left flank. A 3–4-mm incision was made through the skin and subcutaneous tissue and the peritoneum was entered. The spleen was then isolated with the aid of cotton applicators and stabilized for injection. The spleen was injected with 0.1 mL of either TT-Notch3 or TT-TRE solution containing 107 cells in 200 μL of Hanks Buffered Salt Solution (Mediatech, Inc, Manassas, VA) (Fig 1 C) The tumor cells were allowed 3 minutes to enter the circulation of each mouse and the splenic vessels were then tied off and the spleen removed. (Fig 1 D). The fascia was approximated and the skin closed with Vetbond veterinary skin glue (3M, St Paul, MN). Each mouse was then appropriately recovered from anesthesia. Mice were examined every 24 hours for the first week and then twice weekly.
Among the TT-TRE and TT-Notch3 mice, each group was subdivided into 3 treatment groups. One served as a control and was given 12 weeks of regular chow. Another group, the early DOX, was started immediately on a special diet containing 625 mg of doxycycline/ 1 kg feed (Harlan Tekland, Madison, WI) and maintained on this exposure to DOX for 70 days to evaluate whether Notch3 induction could prevent tumor formation. The third group, the late DOX, was given regular chow initially until week 8 (63 days), then switched from regular chow to the doxycycline-containing chow to determine whether Notch3 induction could prevent tumor growth in established liver metastases. The mice were then followed for another 4 weeks. At 12 weeks (day 91), the mice underwent their final imaging and were killed (Fig 1 B). Any mice suffering from heavy tumor burden before 12 weeks were imaged and then killed at the time of discovered illness.
Mouse imaging
Two representative animals from the TT-Notch3 and TT-TRE treatment groups underwent baseline imaging within 7 days of injection to establish normal liver morphology. All animals from each group then underwent micro computed tomography (microCT) after injection of Fenestra VC (MediLumine, Montreal, Quebec, Canada) weight-based contrast at 8 and 12 weeks to evaluate for presence of liver tumors.
Evaluation and quantification of tumor
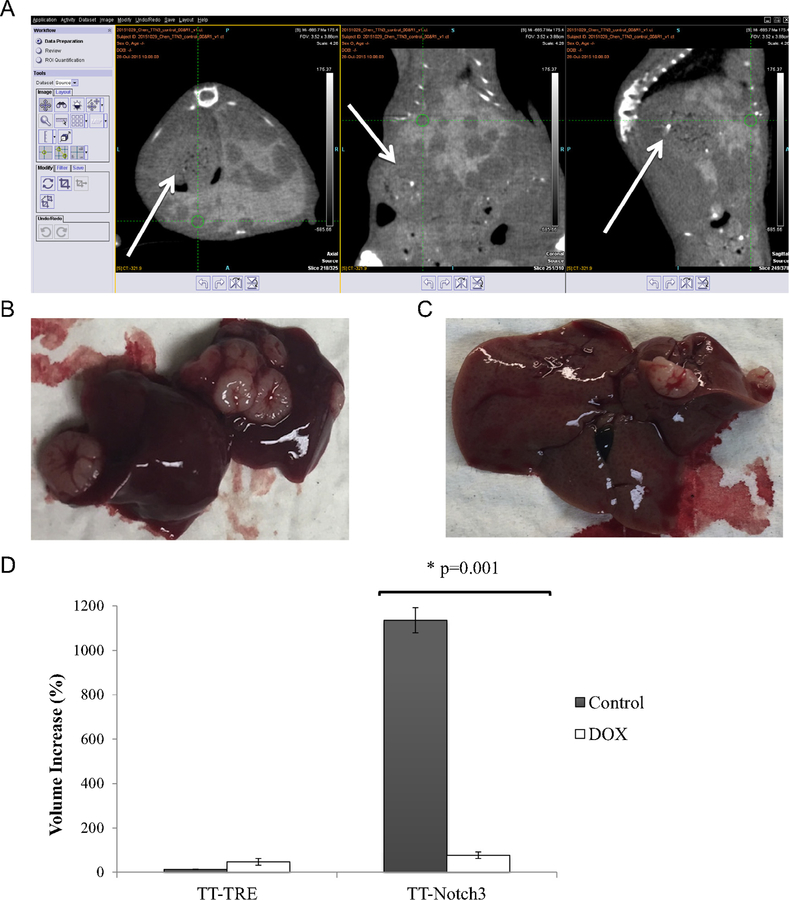
Quantification of tumor volumes was performed using Inveon Research Workplace Software (IRW; Siemens Healthcare, Malvern, PA). The staff of the Small Animal Imaging Facility provided orientation and training in software usage. Computed tomography (CT) scans were loaded onto the software and were analyzed individually, using axial, coronal, and sagittal views to calculate tumor volumes and were measured at each 8 and 12 weeks (Fig 2 A). Animals were killed at 12 weeks, and the entire liver was harvested. The abdominal cavity was also examined at the time of necropsy for metastatic foci outside of the liver parenchyma. Also at 12 weeks, gross correlation was performed between tumors found in the liver specimen and on microCT (Figs 2 B and 2 C).
Fig 2.
Correlation between imaging and gross specimen. A, Representative look at IRW research workplace software. Largest tumor on various cross-sections (arrow). B, Corresponding gross specimens at 12 weeks anteriorly and (C) posteriorly. D, Compared tumor volumes between TT-Notch3 and TT-TRE groups from imaging at 8 and 12 weeks; depicted as percentage volume increased, we see the antiproliferative effect of Notch3 induction between the control and late DOX groups. Early induction of Notch3 did not seem to prevent tumor formation *P < .05 versus control.
Tissue staining and quantification
The harvested liver tissue was fixed in 10% formalin for 2 weeks and then stained for Ki-67 and Notch3 expression (Santa Cruz Laboratories, Santa Cruz, CA), as well as with hemotoxylin and eosin (H&E). Three representative images were taken of each slide at 10 × magnification on a Nikon NIS-Elements D3.10 microscope (Nikon Instruments Inc, Melville, NY). Quantification of Ki-67 staining was performed using ImageJ (National Institutes of Health, Bethesda, MD), where each image was marked manually for percentage of staining. To ensure accuracy, the measurement of each individual slide was performed in triplicate independently by 2 experienced authors; when a discrepancy arose, a third analysis was performed (I.L., S.O., and R.J.S.). Adequacy of H&E and Notch3 staining was reviewed by 2 of the authors for confirmation. (I.L. and R.J.S.).
Statistical analysis
All statistical calculations were performed using SPSS (IBM SPSS Statistics for Windows, v. 22.0; IBM Corp, Armonk, NY.
Results
Early induction of notch3 does not prevent tumor formation
Baseline scans were reviewed and no mice had tumors present within 7 days of injection. All animals in the study developed metastatic MTC liver tumors by 8 weeks. Importantly, the early DOX group developed tumors despite having Notch3 induced. Thus, activation of Notch3 did not prevent the formation of MTC metastatic tumors.
Anti-proliferative effect of notch3 overexpression
We then examined the effects of the induction of Notch3 after formation of MTC liver tumors (late DOX group). In comparing the microCTs, we found no difference in the tumor size after doxycycline induction within the TT-TRE group, as expected. However, we were able to detect the antiproliferative effect of Notch3 induction (P = .001) in the TT-Notch3 mice (Fig 2 D). The TT-Notch3 tumor-bearing mice treated with doxycycline chow had a 37-fold decrease in tumor volume. We found a 97% (34/35) correlation between the gross specimens with radiologic findings when the animals were killed to end the experiment. The 1 tumor not visualized well on microCT was located at the dome of the liver and was only 2 mm in diameter on gross inspection. Therefore, induction of Notch3 after MTC tumor formation was associated with a reduction in tumor growth.
Immunostaining analysis
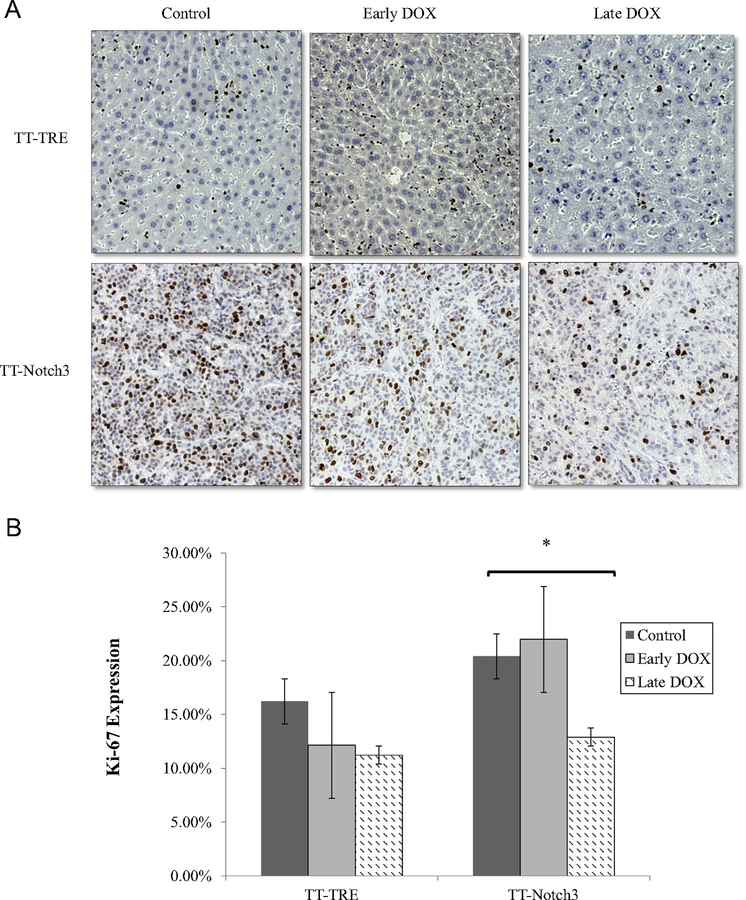
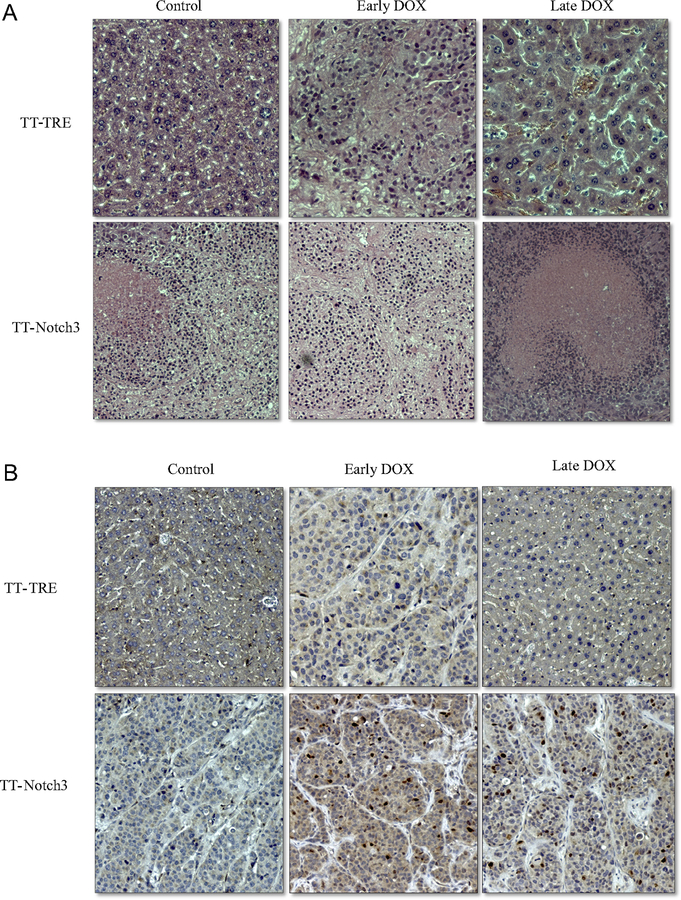
We examined the effect of Notch3 on Ki-67 as a marker of proliferation (Fig 3). As expected, there was no difference in the TT-TRE groups. TT-Notch3 Control versus early DOX group also were statistically similar, confirming the CT scans showing that early DOX treatment had no effect on tumor growth. However, there was a reduction in Ki-67 in the late DOX group (P = .038). These results were consistent with the observations that Notch3 does decrease tumor growth. TT-Notch3 tumor–bearing mice additionally showed increased areas of neutrophilic infiltration on H&E staining with large areas of necrosis, which were not observed in control TT-TRE mice (Fig 4 A). Finally, Notch3 staining proved to be highly specific only in the TT-Notch3 mice that developed tumors, therefore confirming overexpression (Fig 4 B).
Fig 3.
A, Representative immunohistochemistry staining for Ki-67 proliferation index. B, Ki-67 quantification compared within TT-Notch3 and TT-TRE treatment groups demonstrated in the TT-Notch3 mice a decrease in Ki-67 between the control and late DOX treatment groups. *P < .05.
Fig 4.
A, Representative immunohistochemistry staining of tumors for H&E, showing increased neutrophil infiltration and areas of necrosis across the TT-Notch3 group compared with the TT-TRE mice. B, Representative immunohistochemistry staining of tumors for Notch3, revealing highly positive staining only in the TT-Notch3 mice where tumors were formed.
Discussion
Our work demonstrates the potential of Notch3 overexpression for the treatment of metastatic MTC. MTC is a neuroendocrine tumor derived from thyroid c cells, making it distinct from other types of thyroid cancer. Complete surgical resection, when possible, is associated with improved survival.7 The overall expected survival for patients found to have extrathyroidal extension or extracervical metastasis (stage IV) disease is only 55% at 5 years,1 which is far inferior to papillary or follicular subtypes. When liver metastasis occurs, the tumors tend to be miliary and diffuse, thus precluding curative resection as a potential treatment option. Additionally, radioactive iodine has no proven efficacy in MTC given the c-cell origin of this cancer.7 Complete and age-appropriate operative resection for those known to have a genetic predisposition is the only effective treatment for MTC.10 Given the poor prognosis of patients with liver metastases, the inability to obtain a cure by liver resection, and the worse prognosis with radiation,3 the remaining treatments for MTC with liver metastasis have been focused on systemic therapy.
While surgeons may associate MTC with familial autosomal dominant syndromes, such as multiple endocrine neoplasia (MEN) 2A0020and MEN2B, as well as familial MTC, most cases of MTC are sporadic. While familial cases are due universally to germ line mutations in the RET gene, up to 65% of sporadic cases contain somatic RET derangements. RET encodes a tyrosine kinase receptor and, as such, current treatments for metastatic MTC focus on TKI. A phase II clinical trial looking at sorafenib, a multikinase inhibitor targeting RET, and vascular endothelial growth factor receptor was able to accrue 16 patients with sporadic advanced MTC in 2 centers. This trial demonstrated some clinical benefit with sorafenib; however, there was 1 reported patient death and common adverse events, such as diarrhea and skin reactions.11 Vandetanib has also been studied in clinical trials to demonstrate progression-free survival over placebo by less than 1 year but, again, with several reported side effects.12 A more recent phase III trial conducted using carbozantinib, a TKI that also specifically inhibits hepatocyte growth factor receptor, showed improvement of progression-free survival versus placebo. This drug, however, also led to substantial lifestyle-limiting side effects of diarrhea, hand-foot syndrome, nausea, and fatigue.13 Because of considerable toxicities of chemotherapy, systemic radiolabeled anticarinoembryonic antigen antibodies and modified somatostatin analogs have also been studied. A phase II clinical trial looking at this form of therapy demonstrated long-term survival, but only at a single instutition.14 The wide array of clinical trials available highlights the current limitations in treating metastatic MTC. While early diagnosis and prophylactic thyroidectomy have proven highly effective at achieving cure, once disease has become advanced or has metastasized, treatment options remain limited.1
The Notch family of receptors are transmembrane receptors that play fundamental roles in stem cell maintenance and lineage determination.15 While Notch signaling has been known for many years to be vital in determining cell fate, the role of Notch3 as a therapeutic target in cancers is a much more recent discovery. The initial investigation into Notch signaling in cancer focused on malignancies of T cells and the epidermis.16 Components of the Notch pathway parallel markers of thyroid differentiation during development, with decreased expression in poorly differentiated tumors. Therefore, the tumor suppressor capabilities were first seen with overexpression of Notch1.17 The potential of Notch3 in tumor suppression was elucidated by Song et al,18 who identified that a microRNA sequence activates apoptosis, inhibits tumor cell migration, and focuses formation in HeLa cells. Further work revealed the capabilities of Notch3 as a tumor suppressor by controlling cellular senescence.19 Neuroendocrine cells, including thyroid c cells, are heavily regulated by the components of Notch signaling with overexpression of the pathway leading to suppression of c cells and therefore MTC proliferation.20 We only recently discovered the tumor-suppressive role of Notch3 in MTC and showed that Notch3 protein is not expressed in human MTC tumors, but targeted overexpression demonstrated induction of apoptosis and an antiproliferative effect in a mouse model of subcutaneous tumor inplantation.8 Our current study with liver metastases of MTC further expands this finding on metastatic disease.
We have demonstrated a feasible model for metastatic MTC to the liver. Additionally, by using microCT imaging to detect the presence and measure the size of tumors, we were able to watch the progression of these tumors over a longer time scale. Our study, however, is not without limitations. We do not know the potential toxicities of Notch3 overexpression. These promising preclinical results may help to get drugs approved that specifically target Notch3 into early clinical phase I trials. One laboratory is currently investigating AB3, a drug that specifically upregulates Notch3 expression and its antiproliferative potential in neuroendocrine tumors. Certainly, preventing metastasis from occurring rather than treating metastases once they have occurred is preferred; however, we were unable to demonstrate that upregulation of Notch3 prevented tumor formation.
In conclusion, Notch3 upregulation shows a 37-fold decrease in tumor volume compared with control groups in a metastatic liver MTC mouse model. This promising work establishes future investigation into drugs that specifically overexpress Notch3 in addition to targeted delivery of these compounds to tumor sites. Our results revealed that Notch3 receptor is an exciting new biologic target in the armamentarium for metastatic MTC.
The authors acknowledge Justin Jeffery from the Small Animal Imaging Facility of the University of Wisconsin, who performed the mouse imaging. We thank Drew Ronnenburg, staff surgical pathologist, for his assistance in preparing and staining tumor specimens.
Acknowledgments
Supported by NIH grant T32 CA090217–15 (Irene Lou, MD), American Cancer Society Research Scholars Grant, and American Cancer Society MEN2 Professorship.
Footnotes
Presented at the American Association of Endocrine Surgeons, April 2017.
References
- 1.Kebebew E, Ituarte PH, Siperstein AE, Duh QY, Clark OH. Medullary thyroid carcinoma: Clinical characteristics, treatment, prognostic factors, and a comparison of staging systems. Cancer 2000;88:1139–48. [DOI] [PubMed] [Google Scholar]
- 2.Sippel RS, Kunnimalaiyaan M, Chen H. Current management of medullary thyroid cancer. Oncologist 2008;13:539–47. [DOI] [PubMed] [Google Scholar]
- 3.Roman S, Lin R, Sosa JA. Prognosis of medullary thyroid carcinoma: demographic, clinical, and pathologic predictors of survival in 1252 cases. Cancer 2006;107:2134–42. [DOI] [PubMed] [Google Scholar]
- 4.Chen H, Roberts JR, Ball DW, et al. Effective long-term palliation of symptomatic, incurable metastatic medullary thyroid cancer by operative resection. Ann Surg 1998;227:887–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martins RG, Rajendran JG, Capell P, Byrd DR, Mankoff DA. Medullary thyroid cancer: Options for systemic therapy of metastatic disease? J Clin Oncol 2006;24:1653–5. [DOI] [PubMed] [Google Scholar]
- 6.American Thyroid Association Guidelines Task Force, Kloos RT, Eng C, et al. Medullary thyroid cancer: management guidelines of the American thyroid association. Thyroid 2009;19:565–612. [DOI] [PubMed] [Google Scholar]
- 7.Schneider DF, Chen H. New developments in the diagnosis and treatment of thyroid cancer. CA Cancer J Clin 2013;63:374–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jaskula-Sztul R, Eide J, Tesfazghi S, et al. Tumor-suppressor role of Notch3 in medullary thyroid carcinoma revealed by genetic and pharmacological induction. Mol Cancer Ther 2015;14:499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sippel RS, Carpenter JE, Kunnimalaiyaan M, Lagerholm S, Chen H. Raf-1 activation suppresses neuroendocrine marker and hormone levels in human gastrointestinal carcinoid cells. Am J Physiol Gastrointest Liver Physiol 2003;285:G245–54. [DOI] [PubMed] [Google Scholar]
- 10.Shepet K, Alhefdhi A, Lai N, Mazeh H, Sippel R, Chen H. Hereditary medullary thyroid cancer: age-appropriate thyroidectomy improves disease-free survival. Ann Surg Oncol 2013;20:1451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam ET, Ringel MD, Kloos RT, et al. Phase II clinical trial of sorafenib in metastatic medullary thyroid cancer. J Clin Oncol 2010;28:2323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wells SA, Robinson BG, Gagel RF, et al. Vandetanib in patients with locally advanced or metastatic medullary thyroid cancer: a randomized, double-blind phase III trial. J Clin Oncol 2012;30:134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elisei R, Schlumberger MJ, Müller SP, et al. Cabozantinib in progressive medullary thyroid cancer. J Clin Oncol 2013;31:3639–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iten F, Müller B, Schindler C, et al. Response to [90Yttrium-DOTA]-TOC treatment is associated with long-term survival benefit in metastasized medullary thyroid cancer: a phase II clinical trial. Clin Cancer Res 2007;13:6696–702. [DOI] [PubMed] [Google Scholar]
- 15.Wilson A, Radtke F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett 2006;580:2860–8. [DOI] [PubMed] [Google Scholar]
- 16.Nickoloff BJ, Osborne BA, Miele L. Notch signaling as a therapeutic target in cancer: a new approach to the development of cell fate modifying agents. Oncogene 2003;22:6598–608. [DOI] [PubMed] [Google Scholar]
- 17.Ferretti E, Tosi E, Po A, et al. Notch signaling is involved in expression of thyrocyte differentiation markers and is down-regulated in thyroid tumors. J Clin Endocrinol Metab 2008;93:4080–7. [DOI] [PubMed] [Google Scholar]
- 18.Song G, Zhang Y, Wang L. MicroRNA-206 targets notch3, activates apoptosis, and inhibits tumor cell migration and focus formation. J Biol Chem 2009;284:31921–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui H, Kong Y, Xu M, Zhang H. Notch3 functions as a tumor suppressor by controlling cellular senescence. Cancer Res 2013;73:3451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cook M, Yu XM, Chen H. Notch in the development of thyroid C-cells and the treatment of medullary thyroid cancer. Am J Transl Res 2010;2:119–25. [PMC free article] [PubMed] [Google Scholar]