Abstract
Background:
Regular binge drinking is associated with numerous adverse consequences yet the U.S. Food and Drug Administration (FDA) has approved only 4 medications for the treatment of alcohol use disorders (AUDs), and none have been specifically targeted for treating binge drinking. Here we assessed the effectiveness of the dopamine/norepinephrine reuptake inhibitor, bupropion (BUP), alone and in combination with naltrexone (NAL), to reduce binge-like and chronic ethanol intake in mice. While BUP is an FDA-approved drug that is currently used to treat depression and nicotine dependence there has been only limited investigation to assess the ability of BUP to reduce ethanol intake.
Methods:
Male C57BL/6J mice were tested with 20% (v/v) ethanol using “drinking in the dark” (DID) procedures to model binge-like ethanol intake and following intermittent access to ethanol (IAE). In Experiment 1, mice were given intraperitoneal (i.p.) injection of 0, 20, or 40 mg/kg BUP 30-min before DID testing, in Experiment 2 mice were given i.p. injection of vehicle, BUP (20 mg/kg), NAL (3 mg/kg), or BUP + NAL (20 and 3 mg/kg, respectively) 30 min before DID testing, and in Experiment 3 mice were given i.p. injection of 0, 20, 40 or 60 mg/kg BUP 30-min before ethanol access after mice had 16-weeks of IAE.
Results:
BUP dose-dependently blunted ethanol intake with DID procedures and after 16-weeks of IAE. Administration of subthreshold doses of BUP + NAL also reduced binge-like ethanol intake. Finally, BUP failed to reduce consumption of a 3% (w/v) sucrose solution.
Conclusions:
BUP, alone and in combination with NAL, may represent a novel approach to treating binge ethanol intake. We are currently assessing the efficacy of BUP to curb binge drinking in a phase II clinical trial experiment.
Keywords: Alcohol, Bupropion, Naltrexone, Binge, Treatment
INTRODUCTION
Binge drinking is a major public health problem in the United States. The Center for Disease Control (CDC) places alcohol as the number three cause of preventable deaths following nicotine use and being overweight (Mokdad et al., 2004). Binge drinking is operationally defined as the consumption of five or more standard drinks for a man or four or more drinks for a woman in about a two-hour period (NIAAA, 2004). A recent report found that 1 in 6 US adults participates in binge drinking approximately 4-times per month, with an average consumption of 7 drinks per binge (Kanny et al., 2018). Further, over 90% of individuals that drink excessively indicate that they engaged in binge drinking within the last month (Esser et al., 2014). Binge drinking leads to multiple problems, e.g. accidental injury (Gmel et al., 2006), aggressive and violent behavior (Shepherd et al., 2006), and high blood pressure (Fan et al., 2008). Additionally, there is increased risk for developing alcohol dependence in individuals that binge drink frequently (Miller et al., 2007, Hingson et al., 2005, Hingson et al., 2006). Overall, binge drinking contributes to more than half of all deaths attributed to alcohol and to three quarters of the economic cost of excessive alcohol use—binge drinking is a serious public health problem and one that may exceed traditionally defined alcohol dependence in its overall cost to society. Thus, identifying medications to specifically treat binge drinking is of critical significance.
Receptors for two neuropeptides cleaved from the polypeptide precursor proopiomelanocortin (POMC), alpha melanocyte stimulating hormone (α-MSH) and beta-endorphin (β-endorphin), are novel and established, respectively, targets for treating alcohol use disorders (AUDs), including binge drinking (Olney et al., 2014a, Krystal et al., 2001). These neuropeptides are primarily produced in the arcuate nucleus of the hypothalamus (Hadley and Haskell-Luevano, 1999) and a large body of research implicates these neuropeptide systems in the modulation of the neurobiological responses to ethanol (Gianoulakis, 2001, Rasmussen et al., 2002, Olney et al., 2014a). The neuropeptide, α-MSH, along with β-, and γ- melanocyte stimulating hormone, comprise the melanocortin (MC) system (Hadley and Haskell-Luevano, 1999). The MC system exerts its effect by acting though five receptor (MCR) subtypes, MC1R-MC5R. The MC3R and MC4R are the most abundantly expressed MCRs in the brain (Hadley and Haskell-Luevano, 1999). We have shown that central and peripheral administration of Melanotan II (MT-II), a non-specific MC3/MC4R agonist, significantly decreases alcohol (ethanol) consumption without influencing sucrose intake in mice (Navarro et al., 2003, Navarro et al., 2005). Both the MC4R (Navarro et al., 2011) and the MC3R (Olney et al., 2014b) modulate the effect of MTII on ethanol intake. Further, administration of agouti-related protein (AgRP), an endogenous MCR antagonist, significantly increases ethanol intake while genetic deletion of AgRP significantly reduces ethanol drinking in mice (Navarro et al., 2005, Navarro et al., 2009). Most recently, we found that administration of MT-II blunted, while AgRP augmented, binge-like ethanol consumption in mice when delivered directly into the lateral hypothalamus (LH) (Sprow et al., 2016). Ethanol also has direct effects on the MC system, as consumption of an ethanol-containing diet by rats significantly decreases α-MSH protein levels in brain regions implicated in the neurobiological responses to ethanol (Navarro et al., 2008), and intraperitoneal injection of ethanol in mice significantly increases central protein levels of AgRP (Cubero et al., 2010).
β-endorphin, and the associated opioid receptors, have been well-established in the literature as key modulators of neurobiological responses to ethanol (e.g., (Froehlich and Li, 1993, Gianoulakis, 2001). Given the important role of the endogenous opioid system in modulating ethanol intake, it is not surprising that the non-selective opioid receptor antagonist, naltrexone (NAL), is the active agent in 2 of the 4 current medications for treating AUDs that have been approved by the FDA (Garbutt, 2009). Interestingly, the opioid system and the MC system share overlapping anatomical distribution in the central nervous system and there is evidence of functional interactions between these neuropeptide systems. For example, blockade of the MC4R prevents the development of morphine tolerance and dependence (Contreras and Takemori, 1984, Starowicz et al., 2005, Starowicz et al., 2002, Starowicz et al., 2003), and this manipulation also enhances the antinociceptive effects of opiates (Ercil et al., 2005). Furthermore, chronic activation of the opioid system has been shown to decrease brain MC4R mRNA (Alvaro et al., 1996). Supporting a potential interaction between these systems in the modulation of ethanol intake, we have shown that combined administration of MT-II and NAL synergistically blunts ethanol intake in a mouse model of binge-like ethanol drinking (Navarro et al., 2015). Similarly, a combination of NAL and a putative stimulator of POMC/MC signaling, bupropion (BUP), has been successfully tested in pre-clinical and clinical studies, and subsequently approved, for treating eating disorders and obesity (Greenway et al., 2009b). These translational studies indicate that BUP + NAL therapy produces synergistic weight loss which exceeds either BUP or NAL treatment alone (Greenway et al., 2009a, Greenway et al., 2010). Finally, these studies led to the FDA approval in 2014 of Contrave®, a combination of NAL + BUP, (32mg Naltrexone + 360mg Bupropion) for the treatment of obesity (Gohil, 2014).
The similarities between our study combining MT-II and NAL with proof-of-concept studies that led to the approval of Contrave® suggest the exciting possibility that a combination of BUP + NAL may also show efficacy in treating AUDs. However, with the exception of a recent study in rats selectively bred for high alcohol intake (Nicholson et al., 2018) there has been no pre-clinical or clinical evidence that BUP reduces ethanol drinking. Thus, in the present study we assessed the ability of BUP, alone and in combination with NAL, to reduce ethanol intake in a mouse model of acute binge-like ethanol intake and in mice following 16-weeks of intermittent access to ethanol (IAE). BUP, approved by the FDA since 1985, is an antidepressant medication (Ascher et al., 1995) which has also been used off-label to promote smoking cessation (Foley et al., 2006) and in weight loss therapy (Anderson et al., 2002). The ability of BUP to reduce food intake is thought to stem from the ability of this drug to promote central POMC activity (Greenway et al., 2009b). To the best of our knowledge, the present results provide the first pre-clinical evidence that BUP, as well as BUP + NAL, significantly blunts intake in models of binge-like consumption and after IAE.
MATERIAL AND METHODS
Animals
Male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME) were obtained between 6 to 8 weeks of age and weighed an average of 26.4 + 0.23 g (Experiment 1), 26.2 + 0.17 g (Experiment 2), or 31.4 + 0.33 g (Experiment 3) on the first day of pharmacological manipulations. It should be noted that the limited budget provided by the UNC School of Medicine/TraCS Translational Team Science Award (TTSA018P1), the primary funding source for this project, allowed us to propose and conduct studies only with male mice. Mice were individually housed in plastic cages and had ad libitum access to standard rodent chow (Prolab RMH 3000; Purina LabDiet, Inc., St Louis, MO) and water throughout the experiments except where noted. The colony room was maintained at approximately 22°C with a 12 hours light/12 hours dark cycle. All procedures used were in compliance with the National Institute of Health guidelines and all the protocol were approved by the University of North Carolina Institutional Animal Care and Use Committee.
Drugs
95% ethanol (Deacon Laboratories Inc, Prussia, PA, USA) mixed in tap water and sucrose mixed in tap water were used to prepare ethanol (20%, v/v) and sucrose (3%, w/v) solutions, respectively. BUP (bupropion hydrochloride; Sigma-Aldrich, St. Louis, MO) and NAL (naltrexone hydrochloride; Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% sterile saline.
Drinking in the Dark (DID)
To generate binge-like ethanol drinking we used the DID procedure (Thiele et al., 2014, Rhodes et al., 2005). This is a 4-day protocol in which on days 1 to 3, 3-hours into the dark cycle, water bottle are removed from mouse cages and replace by a bottle containing a 20% ethanol solution for 2 hours. Following ethanol access water bottles were returned to the mouse cages. On day 4, the test day, pharmacological drug administration was given 30 minutes before ethanol access, and at the end of this 2-hour ethanol access period tail blood samples were collected for analysis of blood ethanol concentration (BEC).
Intermittent Access to Ethanol (IAE)
For chronic ethanol consumption we used the intermittent access to ethanol (IAE) model using a variation of a previously described model and has been reported to a promote handling-induced convulsions, a dependence-like phenotype (Hwa et al., 2011). The IAE is a 2-bottle choice procedure in which on every-other-day one of two water bottles was removed and replaced by a 20% ethanol solution (20% v/v) for 24 hours. The position of the ethanol bottle was alternated between days to prevent place preferences. Using this procedure mice had 24-hours of access to ethanol bottles on Monday, Wednesday, and Friday of each week over 16-weeks.
Experiment 1: Effect of Bupropion on binge-like ethanol intake and sucrose consumption
On day 4 of the DID protocol animals were weighed and divided into 3 groups based on average ethanol drinking collected on days 1–3 of the DID procedure such that there were no group differences in baseline ethanol intake [F(2, 45) = 0.007, p > 0.05]. Thirty minutes before the ethanol was presented, animals received an intraperitoneal (i.p.) injection of either 20 mg/kg (N=16) or 40 mg/kg (N=16) of BUP or a similar volume (5 ml/kg) of 0.9% saline (N=16), the vehicle group. These doses were chosen based on a previous study that assessed the ability of BUP to improve active avoidance conditioning in mice (Gomez et al., 2016). Mice were returned to their home cages and given 20% ethanol access for 2-hours. Ethanol consumption measures were collected hourly by reading measures from calibrated sipper tubes. After ethanol was removed and consumption measures collected, tail blood samples were taken for BEC assessment. After 3-days of rest these same mice were given 4-days of access to a 3% sucrose solution using the same DID procedures noted above and were then re-distributed to the 3 drug-dose groups on day 4 based on average sucrose consumption on days 1–3. The same doses of BUP used for the binge-like ethanol consumption study were used for the sucrose intake study (N=16/group).
Experiment 2: Effect of a Bupropion + Naltrexone cocktail binge-like ethanol intake
To evaluate the effect of the combination of BUP and NAL on binge-like ethanol drinking we used the DID protocol as described above. Animals were distributed into 4 groups based on average ethanol consumption on days 1–3 of the DID procedure such that there were no group differences in baseline ethanol intake [F(3, 46) = 0127, p > 0.05]: Vehicle (0.9% saline; N=12); BUP alone (20 mg/kg; N=13); NAL alone (3 mg/kg; N=12) and the combined NAL (3 mg/kg) + BUP (20 kg/kg) group (N=12). The 3 mg/kg dose of NAL was chosen based on our previous research (Navarro et al., 2015). Thirty minutes before ethanol access on day 4 of the DID procedure mice received an i.p. injection consistent with their group in a 5 ml/kg volume. Immediately after drug treatment water was removed from each cage and replaced with a bottle containing 20% ethanol for 2 hours. Consumption measures were collected hourly. After ethanol access tail blood sample were taken for BEC analysis. After a 3-day rest period, mice were given a 4-day period with DID procedures but with 3% sucrose. Animals were distributed into 4 groups based on average sucrose consumption on days 1–3: Vehicle (0.9% saline; N=12); BUP alone (20 mg/kg; N=13); NAL alone (3 mg/kg; N=12) and the combined NAL (3 mg/kg) + BUP (20 kg/kg) group (N=12). Thirty minutes before sucrose access on day 4 of the DID procedure mice received an i.p. injection consistent with their group.
Experiment 3: Effect of Bupropion on ethanol intake following 16-weeks of intermittent access to ethanol
Following 16-weeks of IAE as described above, animals were distributed into 4 groups based on average ethanol consumption such that there were no group differences in baseline ethanol intake [F(3, 46) = 0.116, p > 0.05] and 24 hours after the last IAE period mice were injected with either 0.9% vehicle (N=12), 20 mg/kg BUP (N=12), 40 mg/kg BUP (N=13), or 60 mg/kg BUP (N=13). Then, 30-minutes later and 3-hours into the dark cycle water bottles were removed and mice were given access to a bottle containing a 20% ethanol solution. We used a single bottle approach on the test day over a 2-hour period so that we could more easily make comparisons to the DID studies. Consumption measures were collected hourly. After the 2-hour test ethanol consumption measures were collected and tail blood samples were collected to assess BEC levels.
Statistical Analysis
To obtain a measure that corrected for individual differences in body weight, grams (g) per kilogram (kg) of ethanol by each animal were calculated. Ethanol preference ratios were calculated as volume of ethanol solution consumed divided by total fluid (ethanol + water) intake. For sucrose consumption, milliliter (ml) of solution per kilogram was calculated. For all experiments, differences in consumption and BECs were analyzed using analysis of variance (ANOVA). With significant interaction effects, or main effects in the absence of significant interactions, post hoc comparisons were performed using the Tukey HSD test to parse out group differences. Analyses were performed using SPSS software (IBM Corp. in Armonk, NY). In all cases, p < 0.05 (2-tailed) was used to indicate statistical significance.
RESULTS
Experiment 1: Effect of Bupropion on binge-like ethanol intake and sucrose consumption
The results from Experiment 1 are presented in Fig. 1. As is evident in Fig. 1A and B, BUP dose-dependently blunted binge-like ethanol consumption during the first hour of testing, and this effect dissipated during the second hour. A repeated-measure 3 × 2 (BUP dose x hours) ANOVA performed on ethanol consumption data revealed a main effect of BUP dose [F(2, 45) = 10.62, p < 0.001] and significant main effect of hours [F(1, 45) = 15.58, p < 0.001], and a significant interaction effect between BUP dose and hours [F(2, 45) = 5.99, p = 0.005]. Post hoc analyses on the first hour of testing revealed that relative to the vehicle injection, both the 20 mg/kg (p = 0.045) and the 40 mg/kg (p < 0.001) doses of BUP significant reduced binge-like ethanol intake. Further, the 40 mg/kg BUP group drank significantly less ethanol than the 20 mg/kg BUP group (p = 0.012). However, at the second hour of testing Tukey HSD analysis failed to find significant differences between the vehicle condition and the groups treated with either the 20 mg/kg or 40 mg/kg dose of BUP (p > 0.05 in both cases). Fig. 1C shows cumulative ethanol consumption over the 2-hour test. A Tukey HSD analysis revealed the relative to vehicle treatment the 40 mg/kg dose of BUP significantly blunted binge-like ethanol consumption over the 2-hour test (p < 0.001). Fig. 1D shows the BECs that were associated with each group immediately after the 2-h DID test. A one-way ANOVA performed on these data was significant [F(2, 45) = 17.58, p < 0.001], and post hoc tests showed that relative to the vehicle treated group, both the 20 mg/kg and 40 mg/kg BUP-treated groups showed significantly lower BECs (p < 0.001 in both cases). There were no group differences in consumption of 3% sucrose during the first (Fig. 1E) and second (Fig. 1F) hours of consumption, nor were there differences in total 3% sucrose consumption (Fig. 1G). Consistently, a repeated-measure 3 × 2 (BUP dose x hours) ANOVA performed on sucrose consumption data failed to indicate significant main effects of BUP dose [F(2, 45) = 1.58, p > 0.05], hours [F(1, 45) = 0.79, p < 0.001], or an interaction between BUP dose and hours [F(2, 45) = 0.09, p > 0.05].
Fig. 1:
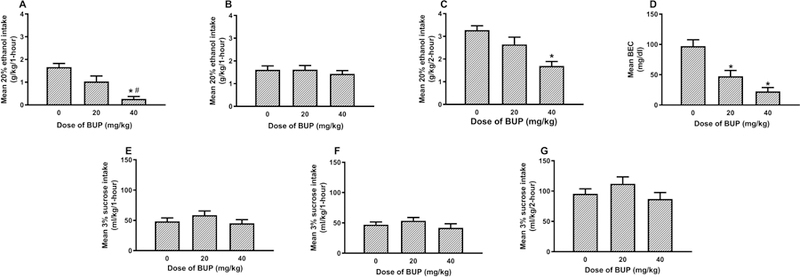
Binge-like ethanol consumption of a 20% (v/v) ethanol solution with DID procedures beginning 30-minutes after administration of vehicle (0 mg/kg) or BUP (20 or 40 mg/kg; i.p.). Panels A and B show ethanol consumption during the first and second hours of testing, respectively, and panel C shows cumulative ethanol consumption over the 2-hour test. Panel D shows BECs that were assessed immediately after the 2-hour test. Panels E and F show consumption of a 3% (w/v) sucrose solution during the first and second hours of test, respectively, and panel G shows cumulative sucrose consumption over the 2-hour test. Data are presented as means + SEM, and * = p < 0.05 relative to the vehicle group. # = p <0.05 relative to the 20 mg/kg group.
Experiment 2: Effect of a Bupropion + Naltrexone cocktail binge-like ethanol intake
Results from Experiment 2 are presented in Fig. 2. Fig. 2A and B show binge-like ethanol consumption during the first and second hours of testing, respectively, and Fig. 2C shows cumulative ethanol consumption over the 2-hour test. A repeated-measures, 4 × 2 (drug group x hours) ANOVA performed on ethanol consumption data revealed main effects of drug group [F(3, 46) = 3.95, p = 0.02] and hours [F(1, 46) = 4.28, p = 0.04] and a significant interaction between these variable [F(3, 46) = 4.32, p = 0.009]. Post hoc tests showed that only the combined 20 mg/kg BUP + 3 mg/kg NAL group (p = 0.002) drank significant less ethanol during the first hour of testing relative to the vehicle group. Notably, these results contract with previous data showing that doses of NAL at 3 mg/kg or less blunted binge-like ethanol intake in similar DID paradigms (Navarro et al., 2015, Kamdar et al., 2007). During the second hour of testing post hoc tests revealed no group differences. Tukey HSD testing performed on cumulative ethanol consumption showed that relative to the vehicle group only the group treated with the combined 20 mg/kg BUP + 3 mg/kg NAL drank significantly less ethanol (p = 0.048).Though there was a trend for reduced BECs in the drug treated groups relative to the vehicle group (Fig. 2D), a one-way ANOVA run on these data failed to achieve statistical significance [F(3, 46) = 2.32, p > 0.05].
Fig. 2:
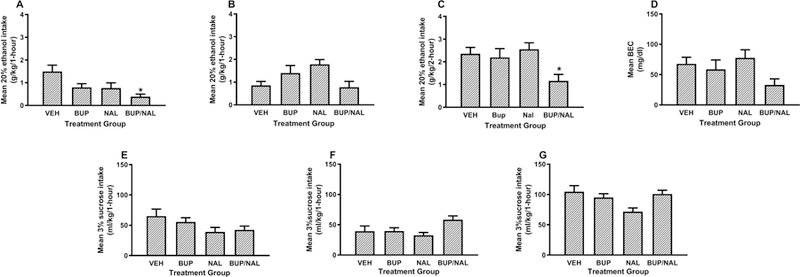
Binge-like ethanol consumption of a 20% (v/v) ethanol solution with DID procedures beginning 30-minutes after administration of vehicle (VEH), BUP alone (20 mg/kg; i.p.), NAL alone (3 mg/kg, i.p.) or combined BUP/NAL (20 mg/kg and 3 mg/kg, respectively; i.p.). Panels A and B show ethanol consumption during the first and second hours of testing, respectively, and panel C shows cumulative ethanol consumption over the 2-hour test. Panel D shows BECs that were assessed immediately after the 2-hour test. Panels E and F show consumption of a 3% (w/v) sucrose solution during the first and second hours of test, respectively, and panel G shows cumulative sucrose consumption over the 2-hour test. Data are presented as means + SEM, and * = p < 0.05 relative to the vehicle group.
Consumption of 3% sucrose during the first and second hours of testing are presented in Fig. 2E and F, and cumulative consumption over the 2-hour test are presented in Figure 2G. A repeated-measures, 4 × 2 (drug group x hours) ANOVA performed on these data failed to find a significant main effect of drug group [F(3, 46) = 1.22, p > 0.05], however the main effect of hours [F(1, 46) 6.05, p = 0.018], and the interaction between variable [F(3, 46) = 7.58, p < 0.001] both achieved statistical significance. However, post hoc Tukey HSD tests failed to find any group differences during the first and second hours of testing, and over total 2-hour consumption.
Experiment 3: Effect of Bupropion on ethanol intake following 16-weeks of intermittent access to ethanol
Data from Experiment 3 are presented in Fig. 3. Fig. 3A shows average daily ethanol consumption over the 16 weeks of IAE, and Fig. 2B shows average weekly ethanol preference ratios over the 16 weeks of IAE. Figs. 3C and D show ethanol consumption during the first and second hours of testing following BUP administration, respectively, and Fig. 3E shows cumulative consumption over the 2-hour test. A repeated-measures, 4 × 2 (BUP dose x hours) ANOVA performed on these data revealed a main effect of BUP dose [F(3, 46) = 9.11, p < 0.001] and a significant main effect of hours [F(1, 46) = 51.2, p < 0.001], but the interaction between these variables failed to achieve statistical significance [F(3, 46) = 0.3, p > 0.05]. Post hoc Tukey HSD tests showed that while the group treated with the 20 mg/kg dose of BUP failed to differ significantly from the vehicle treated group during the first hour of testing (p > 0.05), both the groups treated with 40 mg/kg of BUP (p = 0.017) and 60 mg/kg dose of BUP (p = 0.003) drank significantly less ethanol than the vehicle-treated group. Further, the 60 mg/kg BUP group (p = 0.32) drank significantly less ethanol than the 20 mg/kg BUP group. During the second hour of testing, the 60 mg/kg BUP group drank significantly less ethanol than both the vehicle (p = 0.028) and the 20 mg/kg BUP (p = 0.036) groups. Tukey HSD tests performed on cumulative consumption data showed that relative to the vehicle group both the 40 mg/kg BUP (p = 0.021) and the 60 mg/kg BUP (p < 0.001) groups drank less ethanol. Further, relative to the 20 mg/kg BUP group the 60 mg/kg BUP group groups drank less ethanol (p < 0.001). Finally, BEC data from this study are presented in Fig. 3F. A one-way ANOVA performed on these data achieved statistical significance [F(3, 46) = 5.49, p = 0.003], and post hoc tests indicated that while the group treated with 20 mg/kg BUP failed to differ from the vehicle group (p > 0.05), both the groups treated with 40 mg/kg of BUP (p = 0.032) and 60 mg/kg of BUP (p = 0.003) showed significantly lower BECs relative to the vehicle groups.
Fig. 3:
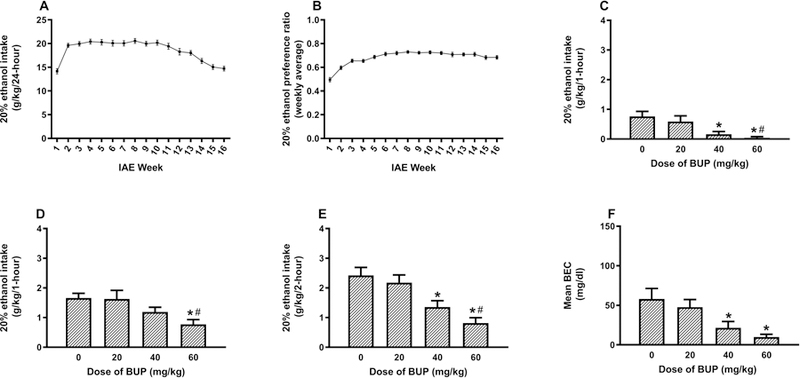
Consumption of a 20% (v/v) ethanol solution beginning 30-minutes after administration of vehicle (0 mg/kg) or BUP (20, 40, or 60 mg/kg; i.p.) in mice with 16-days of prior experience with IAE. Panel A shows average daily ethanol consumption, and Panel B shows ethanol preference ratios, averaged over each of the 16-weeks of testing. Panels C and D show ethanol consumption during the first and second hours of testing, respectively, and panel E shows cumulative ethanol consumption over the 2-hour test. Panel F shows BECs that were assessed immediately after the 2-hour test. Data are presented as means + SEM, and * = p < 0.05 relative to the vehicle group. # = p < 0.05 relative to the 20 mg/kg group.
DISCUSSION
The results obtained in the present studies show that in male C57BL/6J mice, administration of BUP significantly reduced binge-like ethanol drinking in a dose-dependent manner. At least at lower doses (40 mg/kg and below) this effect was specific to ethanol consumption as BUP failed to significantly reduce sucrose intake; however, as we did not assess the effects of the 60 mg/kg dose of BUP on sucrose intake thus we cannot rule out non-specific effects of this high dose. Furthermore, the combination of subthreshold doses of BUP (20 mg/kg) and NAL (3 mg/kg), when given in combination, reduced binge-like ethanol drinking in mice while failing to significantly reduce ethanol intake when given alone. Importantly, this combination of BUP and NAL failed to influence sucrose drinking. Additionally, when we tested the effect of BUP on ethanol intake after 16-weeks of IAE, we found that like the DID study, BUP significantly reduced ethanol drinking. When taken together, these data provide the first pre-clinical evidence the BUP and BUP + NAL reduce ethanol intake in animal models of binge and chronic ethanol consumption at doses that do not alter the acute consumption of the caloric and salient reinforcer, sucrose.
Interestingly, the effects of 40 mg/kg BUP and 20 mg/kg BUP + 3 mg/kg NAL on ethanol intake were restricted to the first hour of ethanol intake. This relatively short-term effect likely stems for the rapid metabolism of BUP, where the half-life of this drug is 1.8 + 0.2 hours in mice (Welch et al., 1987). However, this is of little concern from a therapeutic point-of-view as extended release forms of BUP have been developed for use in humans (Fava et al., 2005). It is also of interest to consider that observation that the 40 mg/kg dose of BUP, and the combination of 20 mg/kg dose of BUP + 3 mg/kg dose of NAL effectively reduced binge-like ethanol drinking in mice without altering sucrose drinking, a stimulus with caloric content. This is noteworthy as both pre-clinical and clinical studies suggest that BUP is effective in reducing food intake and body weight, particularly when combined with NAL (Greenway et al., 2009b, Guerdjikova et al., 2017). However, higher doses of BUP may be necessary to reduce food intake. For example, a recent report showed that while a 20 mg/kg dose of BUP reduced food intake in rats, a 50 mg/kg dose was used to reduce food intake in mice (Clapper et al., 2013). This raises the interesting possibility that BUP and/or BUP + NAL therapy may be effective against binge drinking at doses that do not alter food intake, allowing physicians the benefit of targeting binge alcohol drinking independent of effects on eating behaviors.
It has previously been shown that BUP + NAL therapy produces synergistic weight loss which exceeds either BUP or NAL treatment alone (Greenway et al., 2009a, Greenway et al., 2010), work that supported the FDA approval of Contrave ®. Because of these observations here we provided an initial assessment of combined BUP + NAL on binge-like ethanol drinking in mice and we showed that this drug combination effectively reduces binge-like ethanol intake using subthreshold doses of each drug. Consistently, it was recently reported that doses of BUP and NAL (10 mg/kg each) failed to reduce voluntary ethanol intake in rats selectively bred for high ethanol intake when administered alone but when administered together reduce ethanol intake, suggesting potential synergistic interactions (Nicholson et al., 2018). However, our limited initial assessment prevents us from making statements about synergistic interactions and more thorough studies will be necessary to address synergy. We have previously shown that MT-II, which is a non-selective MCR agonist, synergistically augments the ability of NAL to blunt excessive ethanol intake in a mouse model of binge drinking (Navarro et al., 2015). These data suggest that pharmaceutical compounds that stimulate POMC-derived MC signaling may be good candidates to reduce binge drinking and to augment the effectiveness of a currently FDA-approved drug for treating AUDs.
The neurobiological mechanisms underlying the actions of BUP on ethanol intake are currently unknown, but one possibility is that BUP stimulates MC signaling, a system which we have shown to modulate ethanol intake (Olney et al., 2014a). Activity of the POMC system is influenced by both dopamine (DA) and norepinephrine (NE) (Billes and Cowley, 2007, Billes and Cowley, 2008). BUP is a DA and NE reuptake inhibitor that augments DA/NE signaling (Roitman et al., 2010, Foley et al., 2006, Nomikos et al., 1992). Interestingly, it has been shown that BUP, and to a greater degree BUP + NAL, stimulates murine POMC neurons in vitro (Greenway et al., 2009b), which in turn would lead to stimulation of MC activity and promote the reduction of ethanol intake. The increased effectiveness of BUP to reduce feeding when combined with NAL has been hypothesized to involved an interaction between the opioid and POMC systems at the level of the arcuate nucleus of the hypothalamus (Greenway et al., 2009b). Since POMC-derived β-endorphin reduces the activity of POMC by binding to auto-inhibitory opioid receptors located on POMC cells in the arcuate nucleus (Kelly et al., 1990, Loose and Kelly, 1990), when BUP is giving in combination with NAL, an opioid antagonist, NAL blocks the opioid receptors on the POMC cells thus preventing the auto-inhibitory feedback produced by β-endorphin, and as a result, the ability of BUP to stimulate POMC activity, and by extension the MC signaling, goes unopposed. A similar theoretical mechanism may also explain our current findings and our previous observations of synergistic interactions between a MCR agonist and NAL in blunting binge-like ethanol intake (Navarro et al., 2015), and it will be important to more thoroughly examine the interactions of BUP and NAL in future studies.
Two of the 4 FDA-approved medications for treating AUDs are NAL-based therapies, however there are data showing that NAL produces only modest reductions of ethanol drinking relative to placebo controls (Del Re et al., 2013). Thus, an approach that increases the effectiveness of NAL in managing excessive ethanol drinking would have high clinical value. In this project, we provide novel evidence that BUP is an effective treatment for reducing ethanol intake using two pre-clinical models of ethanol consumption and that a combination of subthreshold doses of BUP and NAL also blunts binge-like ethanol intake. Collectively, our pre-clinical observations raise the possibility that BUP alone and/or in combination with NAL may be effective treatments against AUDs, particularly problematic binge drinking. To this end, we are currently in the midst of conducting phase II clinical trial studies with BUP and combined BUP + NAL to assess the potential utility of these approaches in treating binge drinking in humans.
ACKNOWLEDGEMENTS
This research was funded by NIH grant AA013573, AA015148, and AA022048 and a UNC School of Medicine/TraCS Translational Team Science Award (TTSA018P1).
Footnotes
The authors have no conflicts of interest related to the research presented in this manuscript, financial or otherwise, to declare.
REFERENCES
- Alvaro JD, Tatro JB, Quillan JM, Fogliano M, Eisenhard M, Lerner MR, Nestler EJ, Duman RS (1996) Morphine down-regulates melanocortin-4 receptor expression in brain regions that mediate opiate addiction. Mol Pharmacol 50:583–591. [PubMed] [Google Scholar]
- Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM (2002) Bupropion SR enhances weight loss: a 48-week double-blind, placebo- controlled trial. Obes Res 10:633–641. [DOI] [PubMed] [Google Scholar]
- Ascher JA, Cole JO, Colin JN, Feighner JP, Ferris RM, Fibiger HC, Golden RN, Martin P, Potter WZ, Richelson E, et al. (1995) Bupropion: a review of its mechanism of antidepressant activity. J Clin Psychiatry 56:395–401. [PubMed] [Google Scholar]
- Billes SK, Cowley MA (2007) Inhibition of dopamine and norepinephrine reuptake produces additive effects on energy balance in lean and obese mice. Neuropsychopharmacology 32:822–834. [DOI] [PubMed] [Google Scholar]
- Billes SK, Cowley MA (2008) Catecholamine reuptake inhibition causes weight loss by increasing locomotor activity and thermogenesis. Neuropsychopharmacology 33:1287–1297. [DOI] [PubMed] [Google Scholar]
- Clapper JR, Athanacio J, Wittmer C, Griffin PS, D’Souza L, Parkes DG, Roth JD (2013) Effects of amylin and bupropion/naltrexone on food intake and body weight are interactive in rodent models. Eur J Pharmacol 698:292–298. [DOI] [PubMed] [Google Scholar]
- Contreras PC, Takemori AE (1984) Antagonism of morphine-induced analgesia, tolerance and dependence by alpha-melanocyte-stimulating hormone. JPET 229:21–26. [PubMed] [Google Scholar]
- Cubero I, Navarro M, Carvajal F, Lerma-Cabrera JM, Thiele TE (2010) Ethanol-induced increase of agouti-related protein (AgRP) immunoreactivity in the arcuate nucleus of the hypothalamus of C57BL/6J, but not 129/SvJ, inbred mice. Alcohol Clin Exp Res 34:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Re AC, Maisel N, Blodgett J, Finney J (2013) The declining efficacy of naltrexone pharmacotherapy for alcohol use disorders over time: a multivariate meta-analysis. Alcohol Clin Exp Res 37:1064–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ercil NE, Galici R, Kesterson RA (2005) HS014, a selective melanocortin-4 (MC4) receptor antagonist, modulates the behavioral effects of morphine in mice. Psychopharmacology (Berl) 180:279–285. [DOI] [PubMed] [Google Scholar]
- Esser MB, Hedden SL, Kanny D, Brewer RD, Gfroerer JC, Naimi TS (2014) Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev Chronic Dis 11:E206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan AZ, Russell M, Naimi T, Li Y, Liao Y, Jiles R, Mokdad AH (2008) Patterns of alcohol consumption and the metabolic syndrome. J Clin Endocrinol Metab 93:3833–3838. [DOI] [PubMed] [Google Scholar]
- Fava M, Rush AJ, Thase ME, Clayton A, Stahl SM, Pradko JF, Johnston JA (2005) 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry 7:106–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KF, DeSanty KP, Kast RE (2006) Bupropion: pharmacology and therapeutic applications. Expert Rev Neurother 6:1249–1265. [DOI] [PubMed] [Google Scholar]
- Froehlich JC, Li TK (1993) Recent developments in alcoholism:opioid peptides. Recent Dev Alcohol 11:187–205. [PubMed] [Google Scholar]
- Garbutt JC (2009) The state of pharmacotherapy for the treatment of alcohol dependence. J Subst Abuse Treat 36:S15–23; quiz S24–15. [PubMed] [Google Scholar]
- Gianoulakis C (2001) Influence of the endogenous opioid sytem on high alcohol consumption and genetic predisposition to alcoholism. J Psychiatry Neurosci 26:304–318. [PMC free article] [PubMed] [Google Scholar]
- Gmel G, Bissery A, Gammeter R, Givel JC, Calmes JM, Yersin B, Daeppen JB (2006) Alcohol-attributable injuries in admissions to a swiss emergency room--an analysis of the link between volume of drinking, drinking patterns, and preattendance drinking. Alcohol Clin Exp Res 30:501–509. [DOI] [PubMed] [Google Scholar]
- Gohil K (2014) Pharmaceutical approval update. P T 39:746–772. [PMC free article] [PubMed] [Google Scholar]
- Gomez MC, Redolat R, Carrasco MC (2016) Differential effects of bupropion on acquisition and performance of an active avoidance task in male mice. Behav Processes 124:32–37. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Dunayevich E, Tollefson G, Erickson J, Guttadauria M, Fujioka K, Cowley MA (2009a) Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J Clin Endocrinol Metab 94:4898–4906. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, Kim DD, Dunayevich E (2010) Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 376:595–605. [DOI] [PubMed] [Google Scholar]
- Greenway FL, Whitehouse MJ, Guttadauria M, Anderson JW, Atkinson RL, Fujioka K, Gadde KM, Gupta AK, O’Neil P, Schumacher D, Smith D, Dunayevich E, Tollefson GD, Weber E, Cowley MA (2009b) Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring) 17:30–39. [DOI] [PubMed] [Google Scholar]
- Guerdjikova AI, Walsh B, Shan K, Halseth AE, Dunayevich E, McElroy SL (2017) Concurrent Improvement in Both Binge Eating and Depressive Symptoms with Naltrexone/Bupropion Therapy in Overweight or Obese Subjects with Major Depressive Disorder in an Open-Label, Uncontrolled Study. Adv Ther 34:2307–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley ME, Haskell-Luevano C (1999) The proopiomelanocortin system. Ann NY Acad Sci 885:1–21. [DOI] [PubMed] [Google Scholar]
- Hingson R, Heeren T, Winter M, Wechsler H (2005) Magnitude of alcohol-related mortality and morbidity among U.S. college students ages 18–24: changes from 1998 to 2001. Annu Rev Public Health 26:259–279. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR (2006) Age of alcohol-dependence onset: associations with severity of dependence and seeking treatment. Pediatrics 118:e755–763. [DOI] [PubMed] [Google Scholar]
- Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA (2011) Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res 35:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS (2007) Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology (Berl) 192:207–217. [DOI] [PubMed] [Google Scholar]
- Kanny D, Naimi TS, Liu Y, Lu H, Brewer RD (2018) Annual Total Binge Drinks Consumed by U.S. Adults, 2015. Am J Prev Med 54:486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Loose MD, Ronnekleiv OK (1990) Opioids hyperpolarize beta-endorphin neurons via mu-receptor activation of a potassium conductance. Neuroendocrinology 52:268–275. [DOI] [PubMed] [Google Scholar]
- Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA, Veterans Affairs Naltrexone Cooperative Study G (2001) Naltrexone in the treatment of alcohol dependence. N Engl J Med 345:1734–1739. [DOI] [PubMed] [Google Scholar]
- Loose MD, Kelly MJ (1990) Opioids act at mu-receptors to hyperpolarize arcuate neurons via an inwardly rectifying potassium conductance. Brain Res 513:15–23. [DOI] [PubMed] [Google Scholar]
- Miller JW, Naimi TS, Brewer RD, Jones SE (2007) Binge drinking and associated health risk behaviors among high school students. Pediatrics 119:76–85. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL (2004) Actual causes of death in the United States, 2000. JAMA 291:1238–1245. [DOI] [PubMed] [Google Scholar]
- Navarro M, Carvajal F, Lerma-Cabrera JM, Cubero I, Picker MJ, Thiele TE (2015) Evidence that Melanocortin Receptor Agonist Melanotan-II Synergistically Augments the Ability of Naltrexone to Blunt Binge-Like Ethanol Intake in Male C57BL/6J Mice. Alcohol Clin Exp Res 39:1425–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Chen AS, Chen HY, Knapp DJ, Breese GR, Marsh DJ, Thiele TE (2005) Effects of melanocortin receptor activation and blockade on ethanol intake: A possible role for the melanocortin-4 receptor. Alcohol Clin Exp Res 29:949–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Breese GR, Thiele TE (2008) Decreased immunoreactivity of the melanocortin neuropeptide alpha-melanocyte-stimulating hormone (alpha-MSH) after chronic ethanol exposure in Sprague-Dawley rats. Alcohol Clin Exp Res 32:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Knapp DJ, Thiele TE (2003) MTII-induced reduction of voluntary ethanol drinking is blocked by pretreatment with AgRP-(83–132). Neuropeptides 37:338–344. [DOI] [PubMed] [Google Scholar]
- Navarro M, Cubero I, Ko L, Thiele TE (2009) Deletion of agouti-related protein blunts ethanol self-administration and binge-like drinking in mice. Genes Brain Behav 8:450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro M, Lerma-Cabrera JM, Carvajal F, Lowery EG, Cubero I, Thiele TE (2011) Assessment of voluntary ethanol consumption and the effects of a melanocortin (MC) receptor agonist on ethanol intake in mutant C57BL/6J mice lacking the MC-4 receptor. Alcohol Clin Exp Res 35:1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIAAA (2004) National Institute on Alcohol Abuse and Alcoholism Council approves definition of binge drinking, in Series National Institute on Alcohol Abuse and Alcoholism Council approves definition of binge drinking, Vol. 3, NIAAA Newsletter. [Google Scholar]
- Nicholson ER, Dilley JE, Froehlich JC (2018) Co-Administration of Low-Dose Naltrexone and Bupropion Reduces Alcohol Drinking in Alcohol-Preferring (P) Rats. Alcohol Clin Exp Res 42:571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomikos GG, Damsma G, Wenkstern D, Fibiger HC (1992) Effects of chronic bupropion on interstitial concentrations of dopamine in rat nucleus accumbens and striatum. Neuropsychopharmacology 7:7–14. [PubMed] [Google Scholar]
- Olney JJ, Navarro M, Thiele TE (2014a) Targeting central melanocortin receptors: a promising novel approach for treating alcohol abuse disorders. Front Neurosci 8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JJ, Sprow GM, Navarro M, Thiele TE (2014b) The protective effects of the melanocortin receptor (MCR) agonist, melanotan-II (MTII), against binge-like ethanol drinking are facilitated by deletion of the MC3 receptor in mice. Neuropeptides 48:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen DD, Boldt BM, Wilkinson CW, Mitton DR (2002) Chronic daily ethanol and withdrawal: 3. Forebrain pro-opiomelanocortin gene expression and implications for dependence, relapse, and deprivation effect. Alcoholism: Clinical & Experimental Research 26:535–546. [PubMed] [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav 84:53–63. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Wescott S, Cone JJ, McLane MP, Wolfe HR (2010) MSI-1436 reduces acute food intake without affecting dopamine transporter activity. Pharmacol Biochem Behav 97:138–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JP, Sutherland I, Newcombe RG (2006) Relations between alcohol, violence and victimization in adolescence. J Adolesc 29:539–553. [DOI] [PubMed] [Google Scholar]
- Sprow GM, Rinker JA, Lowery-Gointa EG, Sparrow AM, Navarro M, Thiele TE (2016) Lateral hypothalamic melanocortin receptor signaling modulates binge-like ethanol drinking in C57BL/6J mice. Addict Biol 21:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starowicz K, Obara I, Przewlocki R, Przewlocka B (2005) Inhibition of morphine tolerance by spinal melanocortin receptor blockade. Pain 117:401–411. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Przewlocki R, Gispen WH, Przewlocka B (2002) Modulation of melanocortin-induced changes in spinal nociception by mu-opioid receptor agonist and antagonist in neuropathic rats. Neuroreport 13:2447–2452. [DOI] [PubMed] [Google Scholar]
- Starowicz K, Sieja A, Bilecki W, Obara I, Przewlocka B (2003) The effect of morphine on MC4 and CRF receptor mRNAs in the rat amygdala and attenuation of tolerance after their blockade. Brain Res 990:113–119. [DOI] [PubMed] [Google Scholar]
- Thiele TE, Crabbe JC, Boehm SL 2nd (2014) “Drinking in the Dark” (DID): a simple mouse model of binge-like alcohol intake. Curr Protoc Neurosci 68:9 49 41–49 49 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RM, Lai AA, Schroeder DH (1987) Pharmacological significance of the species differences in bupropion metabolism. Xenobiotica 17:287–298. [DOI] [PubMed] [Google Scholar]


