Abstract
The trans-activating response DNA-binding protein 43 (TDP-43) is a transcriptional repressor and splicing factor. TDP-43 is normally mostly in the nucleus, although it shuttles to the cytoplasm. Mutations in TDP-43 are one cause of familial amyotrophic lateral sclerosis (ALS). In neurons of these patients, TDP-43 forms cytoplasmic aggregates. In addition, wild-type TDP-43 is also frequently found in neuronal cytoplasmic aggregates in patients with neurodegenerative diseases not caused by TDP-43 mutations. TDP-43 expressed in yeast causes toxicity and forms cytoplasmic aggregates. This disease model has been validated because genetic modifiers of TDP-43 toxicity in yeast have led to the discovery that their conserved genes in humans are ALS genetic risk factors. While how TDP-43 is associated with toxicity is unknown, several studies find that TDP-43 alters mitochondrial function. We now report that TDP-43 is much more toxic when yeast are respiring than when grown on a carbon source where respiration is inhibited. However, respiration is not the unique target of TDP-43 toxicity because we found that TDP-43 retains some toxicity even in the absence of respiration. We found that H2O2 increases the toxicity of TDP-43, suggesting that the reactive oxygen species associated with respiration could likewise enhance the toxicity of TDP-43. In this case, the TDP-43 toxicity targets in the presence or absence of respiration could be identical, with the reactive oxygen species produced by respiration activating TDP-43 to become more toxic or making TDP-43 targets more vulnerable.
Keywords: TDP-43, amyotrophic lateral sclerosis, yeast, respiration, mitochondria
Communication
The trans-activating response DNA-binding protein 43 (TDP-43) is a nucleic acid binding protein that functions as a transcriptional repressor, splicing factor and in translational regulation. TDP-43 is normally found mostly in the nucleus, although it shuttles between the nucleus and the cytoplasm. Mutations in TARDBP, the gene encoding TDP-43, are one cause of familial amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD). In neurons of these patients, TDP-43 is no longer found in the nucleus, but instead forms cytoplasmic aggregates. In addition, wild-type TDP-43 is also frequently found in cytoplasmic aggregates in neurons of patients with other neurodegenerative diseases or with ALS/FTD not caused by TDP-43 mutations1;2;3;4.
How TDP-43 aggregates are associated with toxicity is the subject of intense research. Evidence links TDP-43 toxicity with both inhibition of the ubiquitin proteasome system5, and inhibition of lysosome and endosomal activity6; 7. Also, several studies find that TDP-43 alters mitochondrial function 8; 9; 10; 11; 12; 13; 14; 15 Overexpression of TDP-43 in a number of model organisms causes neurodegeneration similar to that seen in ALS patients. In motor neurons of TDP-43 transgenic mice, mitochondria were found in cytoplasmic inclusions or in abnormal juxta-nuclear aggregates and were missing in motor axon termini9; 10; 11. In mammalian neuron-like cell culture, TDP-43 localized to mitochondria and caused mitophagy. Likewise, in a mouse model, TDP-43 co-localization with motor neuron mitochondria was enhanced by TDP-43 ALS mutations and this co-localization was associated with inhibited mitochondrial function13; 14. Also, overexpressed TDP-43 in human cell culture binds to, and inhibits maturation of, mitochondrial RNA causing a phenotype similar to that seen in cells deficient in mitochondrial RNase P15. Finally, mutations in the mitochondrial intermembrane protein, CHCHD10 (yeast homolog MIX17) have recently been associated with sporadic and familial ALS and FDT 16; 17; 18; 19; 20; 21; 22 and functional CHCHD10 appears to help keep TDP-43 in the nucleus away from mitochondria23.
There is also evidence that TDP-43 expression in yeast causes mitochondrion-dependent apoptosis and that TDP-43 toxicity requires respiratory capacity8. TDP-43 expressed in yeast causes toxicity and forms cytoplasmic aggregates24. Furthermore, conserved genetic modifiers of this toxicity have been validated in higher organisms and have led to the discovery of human ALS genetic risk factors25. This confirms the usefulness of the yeast model.
To examine the effect of respiration on TDP-43 toxicity we used the fact that yeast turns off respiration in dextrose media where it instead uses glycolysis to grow26. We found that TDP-43-GFP expressed with a TET promoter aggregated in yeast cells whether grown on dextrose, where respiration is inhibited; galactose, where respiration is not inhibited and both fermentation and respiration is present26; or glycerol or ethanol where only respiration and not fermentation is present. However, the TET controlled TDP-43 was only toxic on galactose, glycerol or ethanol where respiration is present. Also, the previously described TDP-43 aggregation and induction of cell elongation5 was much more pronounced on media with respiration (galactose and glycerol) vs. media without respiration (dextrose) (Fig. 1ab). Despite this, there was no significant difference in the level of soluble TDP-43 found in boiled lysates of dextrose vs. galactose or glycerol grown cells (Fig. 1c). To test if the difference in sensitivity to TDP-43 was caused by the slower cell division rate in galactose vs. dextrose, we compared the toxicity in cells grown on dextrose at room temperature with cells grown on galactose at 30°C. Although cells grew at approximately the same rate under these two conditions, TDP-43 toxicity was pronounced on galactose but not dextrose plates (Supplementary Fig. 1).
Fig. 1.
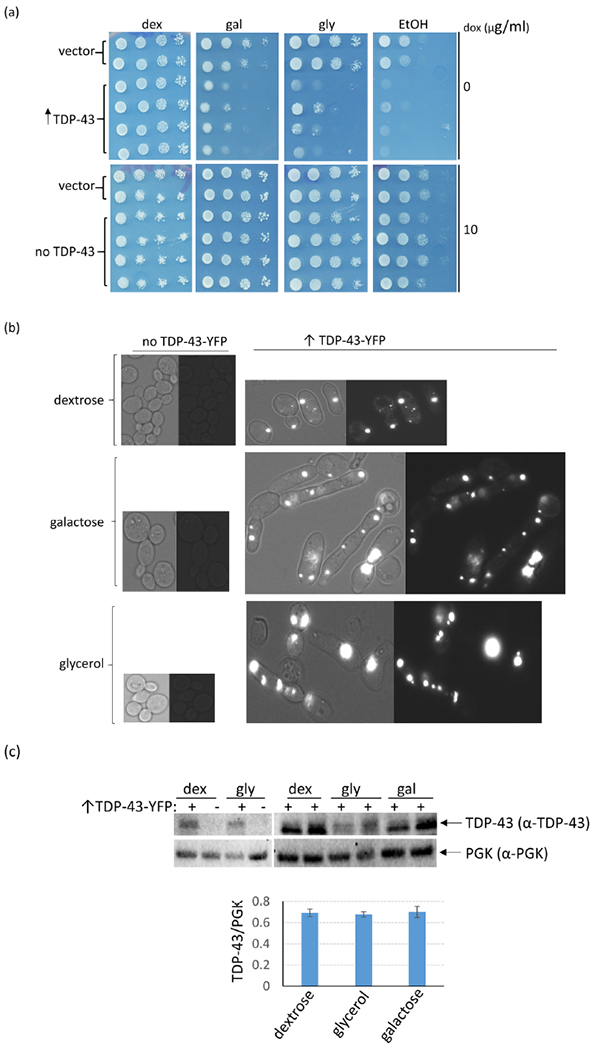
TDP-43 overexpression causes more toxicity and cell elongation in cells grown in media that allows respiration vs. cells grown in the absence of respiration. (a) Yeast cells (L2910 [pin−] [RHO+] 74D-694) transformed with the doxycycline-repressible CEN TET-TDP-43-YFP TRP1 (p2223), or vector control (pl576) plasmid were obtained on plasmid selective dextrose plates with 10 μg/ml of doxycycline (dox) to inhibit expression of TDP-43-YFP. Normalized suspensions of cells were serially diluted in water (1/10) and were spotted on plasmid selective 2% dextrose (dex), 2% galactose (gal), 2% glycerol (gly), and 2% ethanol (EtOH) plates without doxycycline (0) and with 10 μg/ml of doxycycline to respectively express or not express TDP-43-YFP. The plates were photographed after 4 (dextrose) or 6 (galactose, glycerol or ethanol) d of incubation at 30°C. (b) TDP-43 aggregates in galactose, glycerol and dextrose but causes more aggregation and cell elongation in galactose or glycerol vs. dextrose media. Cells from TDP-43-YFP inducing plates (↑TDP-43-YFP) were imaged with a fluorescence microscope at the same exposure and are shown at the same magnification. Left images show bright field with fluorescent, right images show just fluorescence. Control cells with no TDP-43-YFP expression (no TDP-43-YFP) are also shown. The increased aggregation and elongation on galactose and glycerol caused by TDP-43-YFP expression was very obvious. (c) Expression of TET controlled TDP-43-YFP is not enhanced on glycerol or galactose medium. The level of TDP-43-YFP was compared in L2910 transformants with doxycycline-repressible - TDP-43-YFP (p2223) grown in 2% dextrose (dex) vs. 2% glycerol (gly) or 2% raffinose + 2% galactose (gal) plasmid selective media. Cells grown overnight in medium containing 8 μg/ml of doxycycline were diluted to OD600 = 0.5 in plasmid selective dex, gly or gal without doxycycline, grown for 24 h, harvested, and lysed for immunoblotting35. Normalized cell lysates boiled for 5 mins with 2% SDS in 80 mM DTT sample buffer were resolved on 10% SDS-PAGE followed by immunoblotting with rabbit polyclonal α-TDP-43 antibody (1:3000, Proteintech Group). Also, PGK, yeast 3-Phosphoglycerate Kinase, was detected with anti PGK antibodies (1:10,000, Novex) as an internal control (3c upper). Immunoblot signals for TDP-43-YFP and PGK were quantified and converted into % ratios of TDP43-YFP to PGK (3c lower). Standard error shown was calculated from 3 independent immunoblots. Gateway destination vector pl576 was made by dropping reading frame B gateway cassette (http://www.lifetechnologies.com) into pCM18436. TDP-43-YFP from entry vector pDONR TDP-43-YFP (Addgene plasmid # 27470) was then cloned into pl576 using an LR reaction, creating doxycycline-repressible CEN TET-TDP-43-YFP TRP1 (p2223).
One explanation could be that TDP-43 only inhibits respiration, which only affects growth when cells are respiring. Alternatively, respiration could be required for TDP-43 to be toxic, e.g. by modifying the TDP-43 protein. Indeed, oxidative stress has been shown to increase disulfide cross-linking, acetylation, and aggregation of TDP-43 in mammalian cells27. Furthermore, oxidative stress has been proposed to promote acetylation of TDP-43, causing reduced binding of TDP-43 to RNA and increased levels of cytoplasmic, phosphorylated, aggregated TDP-4328.
However, we eliminated both of the above hypotheses because we were able to detect TDP-43 toxicity in the absence of respiration on dextrose by expressing TDP-43-YFP with the strong CUP1 promoter 29; 30. To do this, we obtained an array of integrants of a plasmid expressing CUP1-TDP43-YFP in [PIN+] 74D-694. As expected, due to differences in the number of tandem plasmid repeats present31, different integrants showed different levels of toxicity (high, medium and low). The more toxic integrants clearly showed toxicity on dextrose (Supplementary Fig. 2). Each integrant was cured of [PIN+] by growth on guanidine HCl32. Then, since yeast lacking functional mitochondria are viable we made all the [PIN+] and [pin−] [RHO+] integrants containing functional mitochondria into [rho0] cells lacking functional mitochondrial. We then compared the level of toxicity caused by overexpressed TDP-43 in these isogenic [RHO+] and [rho0] strains grown on dextrose (dex) where respiration is inhibited. We found that TDP-43 was toxic and retained the same level of expression in both [RHO+] and [rho0] cells (Fig. 2abc). There was also no obvious difference in the appearance of TDP-43 aggregates (Fig. 2d). As we reported previously5 TDP-43 is more toxic in the presence of the [PIN+] prion. This was clear when cells were grown on dextrose or galactose (Fig. 2a), but the effect of [PIN+] on TDP-43 toxicity in cells grown on glycerol was minor and not reproducible (not shown). Since the [rho0] cells can never respire and the [RHO+] cells do not respire on dextrose, this clearly establishes that there is a TDP-43 toxicity target in yeast distinct from respiration and that respiration is not required for this TDP-43 toxicity.
Fig. 2.
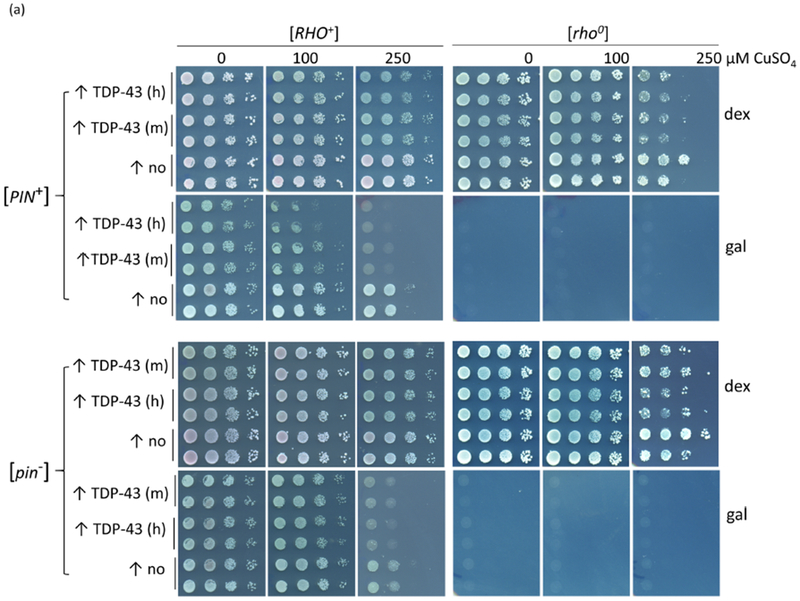
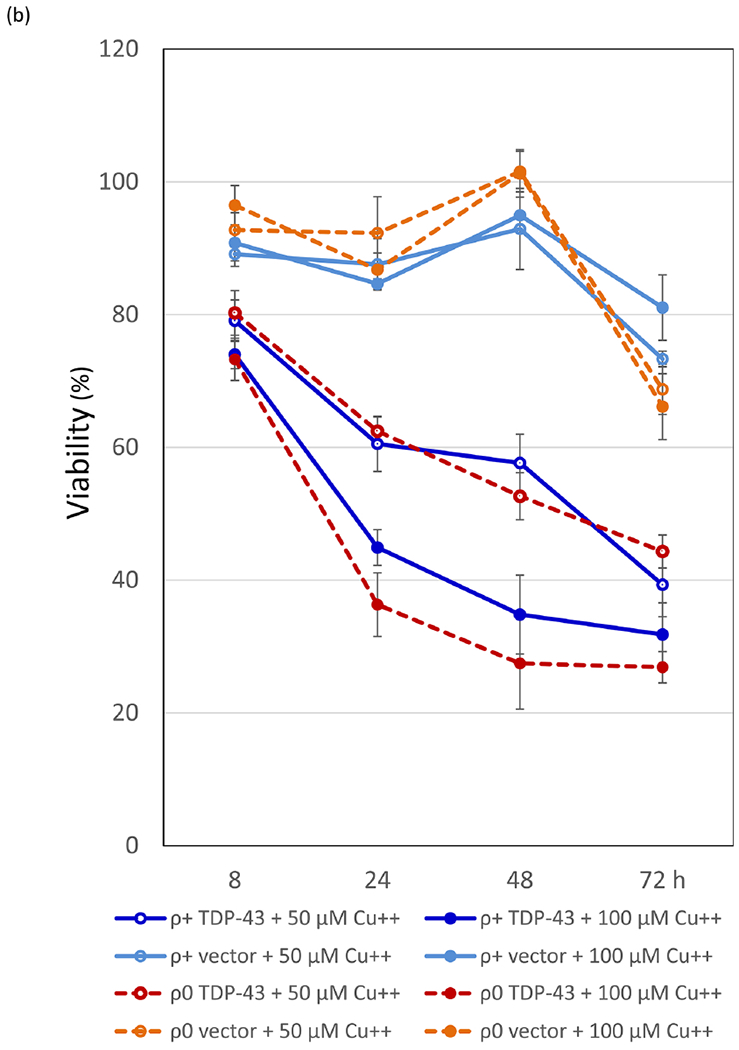
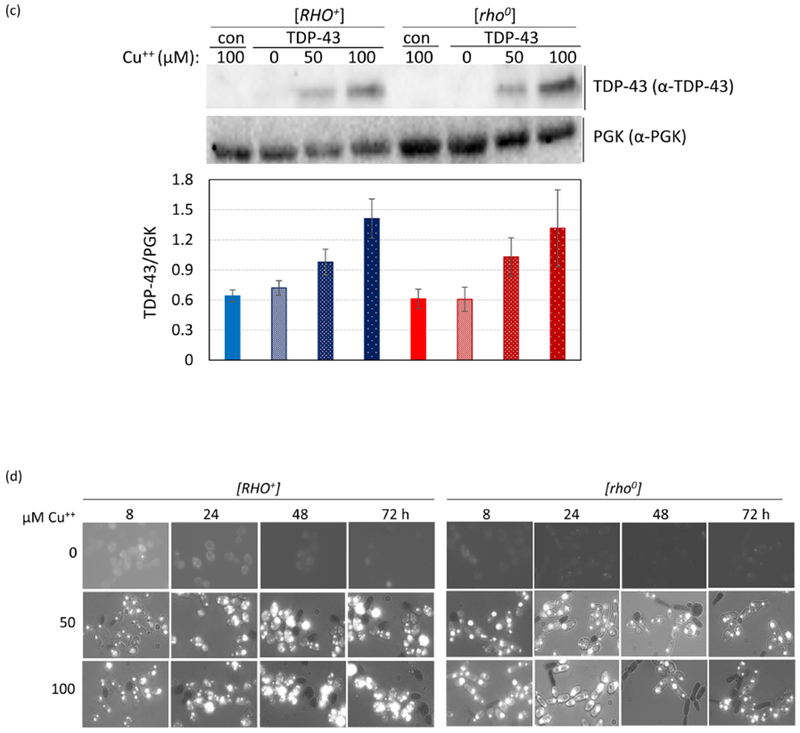
TDP-43-YFP is still toxic in non-respiring cells and in cells lacking functional mitochondria. (a) Spot test measure of TDP-43-YFP toxicity. CUP1-TDP-43-YFP in p2261 (pAG305 CUP1::TDP43-YFP) was integrated at the LEU2 locus in [RHO+] [PIN+] and [RHO+] [pin−] 74D-694 cells. Isogenic [rho0] versions of the CUP1-TDP-43-YFP integrants were acquired by growing [RHO+] integrants on YPD with 0.4 mg/ml ethidium bromide. This method has been shown to cause the loss of all mitochondrial DNA37. The lack of functional mitochondria was verified as cells failed to grow on glycerol medium. To assay for TDP-43-YFP toxicity, two independent subclones of [RHO+] and [rho0] CUP1-TDP-43-YFP medium (m) and high (h) level toxicity integrants (Supplementary Fig. 2) (↑TDP-43) and control empty integrants (↑no) grown on dextrose medium without CuSO4 were normalized, serially diluted (1/10) and spotted on dextrose (dex) and galactose (gal) plates including 0, 100, and 250 μM CuSO4. Plates with [rho0] cells were allowed to grow longer than plates with [RHO+] cells and galactose plates were grown longer than dextrose plates prior to scanning. Reduced growth indicated toxicity. As expected [rho0] cells failed to grow on gal. Integrants were constructed by transforming cells with purified linear integrating vector pCUP1-TDP-43-YFP (p2261) that had been cut in the LEU2 gene with BstXI and control integration vector without TDP-43-YFP, pAG305 GAL1-ccdB (Addgene plasmid #14137) that was cut similarly, and selecting for transformants on integrant selective dextrose plates. Integration was verified by observing TDP-43-YFP aggregation in transformants in dextrose medium with 50 μM CuSO4. To generate p2261, the Hpal-Pmel fragment containing the TET promoter from pAG305 TET-TDP-43-YFP (p2237) was switched with the CUP1 promoter from p1988 (pCUP1-SOD1-GFP) cut with the same restriction site. Plasmid pAG305 TET-TDP-43-YFP (p2237) was made by replacing the GAL promoter in pAG305 GAL-ccdB (Addgene plasmid # 14137) with PCR amplified HpaI-XbaI TET promotor from pCM184. Integrants contain different numbers of tandem plasmids which caused different levels of toxicity. (b) Viability measure of TDP-43-YFP toxicity. Cytotoxicity of the CUP1 driven TDP-43-YFP in both [RHO+] and [rho0] cells was determined by colony forming units8. Overnight cultures of a TDP-43-YFP integrant associated with a high level of toxicity (supplementary Fig. 2) or vector controls grown without CuSO4 were inoculated into liquid synthetic dextrose media with 0, 50 or 100 μM CuSO4 to a final OD600 of 0.5. Samples were taken thereafter at 8, 24, 48, and 72 h and colony forming units determined on synthetic dextrose plates lacking CuSO4. The number of colonies grown after 3 d of incubation at 30°C were counted and converted into viability (%) calculated as ratio of cells grown in TDP-43-YFP inducing media (with 50 or 100 μM CuSO4) over cells grown in non-inducing media (0 μM CuSO4). Error bars present the standard error calculated from 3 independent subclones of integrants. (c) No significant difference in levels of TDP43-YFP expression in [RHO+] vs. [rho0] CUP1-TDP-43-YFP integrated strains. Isogenic [RHO+] vs. [rho0] high toxicity integrants taken from dextrose plates were inoculated into 50 ml dextrose containing 0, 50, or 100 μM CuSO4 to an OD600 of 0.5. Cells were harvested and lysed at 24 h. Normalized cell lysates were boiled, resolved and immunoblotted as shown in Fig. 1c. The intensity of each protein band (upper) was scanned and the ratio between TDP-43-YFP and PGK was calculated (lower). Bars represent standard error calculated from 3 independent immunoblots. (d) TDP-43-YFP aggregates similarly in [RHO+] and [rho0] CUP1-TDP-43-YFP integrated strains. Samples from 2b were visualized with fluorescent microscope at the same exposure. No obvious visual differences were seen.
To test the idea that increased levels of reactive oxygen species associated with respiration could be the cause of the observed increase in TDP-43 toxicity in the presence of respiration, we looked at the effects of H2O2, a source of reactive oxygen species, and N-Acetyl L-cysteine (NAC) an antioxidant, on TDP-43 toxicity. Using an integrant with low TDP-43 toxicity, we saw no toxicity of cells grown for 24 h with either 2mM H2O2 without TDP-43 expression (Fig. 3a left) or with expression of TDP-43 with 250 μM CuSO4 in dextrose medium without H2O2 (Fig. 3a right rows marked 0). However, the combination of 24 h of 2mM H2O2 and induction of TDP-43 with 250 μM CuSO4 did cause toxicity (Fig. 3a right row marked 2). This was true in both [RHO+] or [rho0] cells. We also observed a dramatic increase in the size of TDP-43 aggregates in cells treated with H2O2 (Fig. 3b), and this was not due to an increase in the cellular level of TDP-43 (Fig. 3c). Possibly, this was caused by free radical oxygen stress, which has been shown to cause an increase in protein aggregation33; 34. Also, we saw reduced toxicity of cells in the presence of the antioxidant NAC and this was quite dramatic in the presence of TDP-43 (Fig. 4ab). However, the level of TDP-43 in cells was dramatically reduced in the presence of NAC (Figs. 3c and 4c). If NAC directly reduced expression of TDP-43 that would explain the reduced toxicity. Likewise if NAC inhibited aggregation of TDP-43, the unaggregated TDP-43 would be expected to be rapidly degraded again explaining reduced toxicity.
Fig. 3.
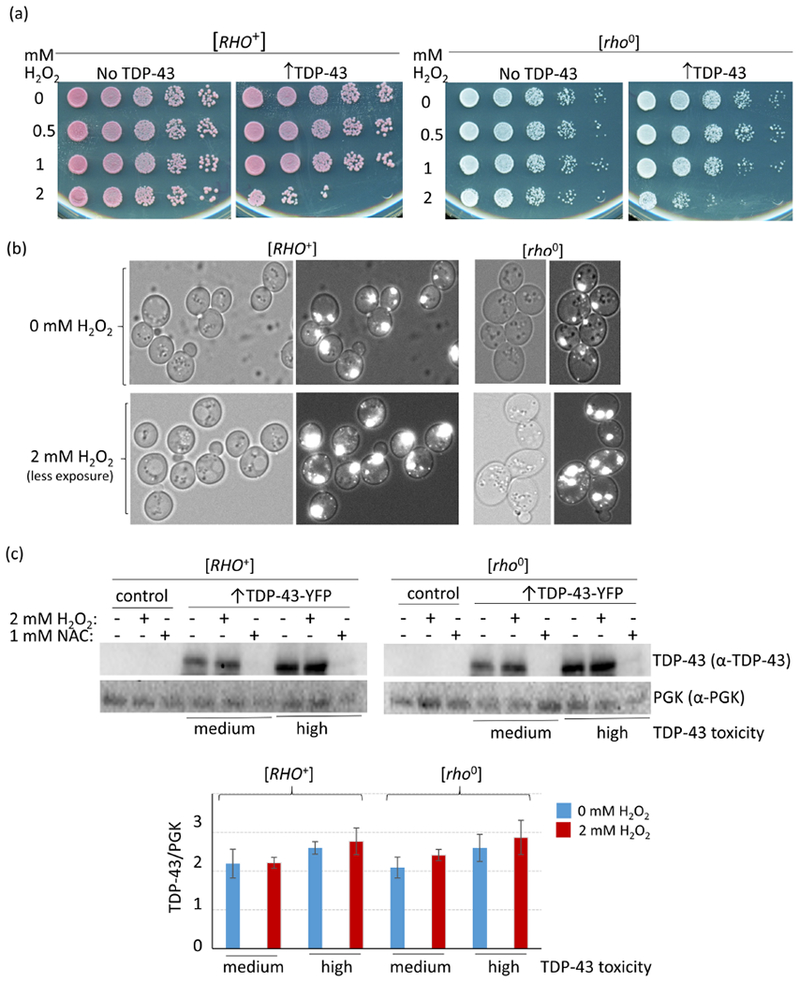
H2O2 enhances TDP-43 toxicity and aggregation. (a) H2O2 enhances TDP-43 toxicity. [RHO+] and [rho0] versions of strain 74D-694 with integrated CUP1-TDP-43-YFP (↑TDP-43) associated with a low level of toxicity (Supplementary Fig. 2), or vector control (No TDP-43), were grown to OD600 = 0.5 in synthetic dextrose non-inducing medium and then for an additional 24 h after the simultaneous addition of CuSO4 to 250 μM to induce TDP-43-YFP expression and H2O2 to the concentrations listed. Serial dilutions of cells were then spotted on plates lacking CuSO4 and H2O2 and photographed after growth. (b) H2O2 enhances TDP-43 aggregation. Cells from the liquid cultures used in (a) were examined under a fluorescent microscope. Left shows bright field, right is fluorescence plus bright field. Since the intensity of TDP-43-YFP aggregates was much brighter in H2O2 treated cells than in cells without H2O2 treatment, images of H2O2 treated cells were taken with less exposure than cells without H2O2 treatment to allow visualization of dots. (c) The level of TDP-43 in cells is unchanged by the addition of H2O2, but is dramatically reduced by the addition of NAC. Freshly grown subclones of medium and high level toxicity CUP-TDP-43-YFP integrants or control (empty vector) were inoculated at OD600 = 0.5 into 50 mL plasmid selective dextrose media with 250 μM CuSO4 and either 2 mM H2O2 or 1 mM NAC. Cells were harvested and lysed after 24 h growth. Normalized cell lysates were boiled, resolved and immunoblotted as shown in Fig. 1c. The protein level was compared between cells treated or not treated with H2O2 or NAC. The ratio between scanned TDP-43-YFP and PGK signals for control (blue) and H2O2 (red) cells is shown. Bars represent standard error calculated from 3 independent immunoblots.
Fig. 4.
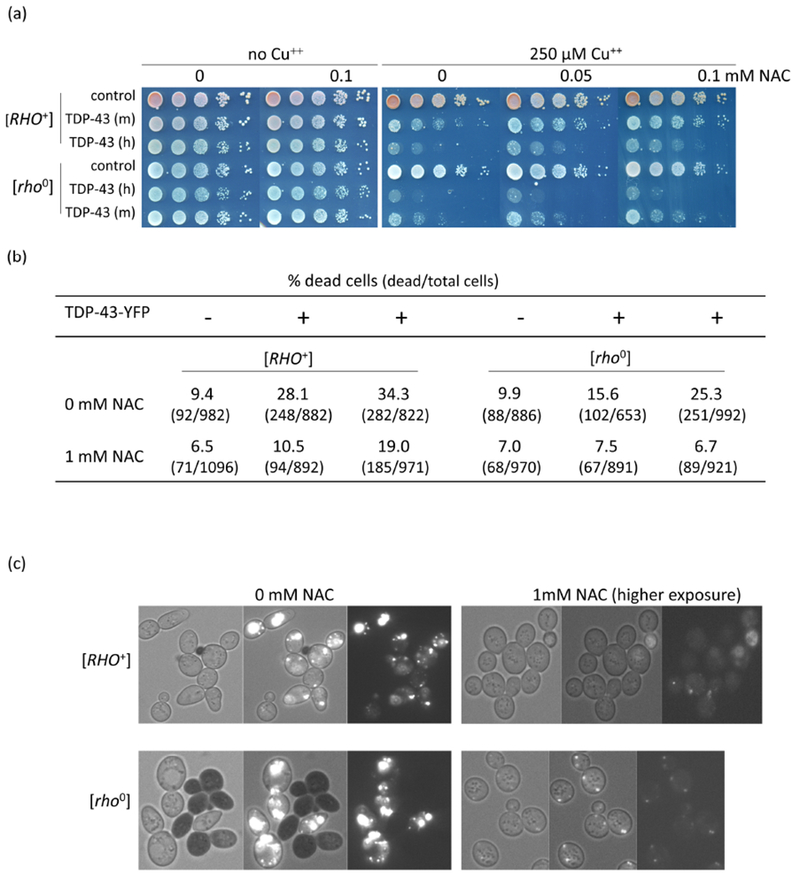
Antioxidant reduces TDP-43 level and toxicity. (a) Antioxidant reduces TDP-43 growth inhibition. Isogenic [RHO+] and [rho−] versions of CUP1-TDP-43-YFP (TDP-43) integrants with medium (m) and high (h) levels of toxicity and vector (control) grown on plasmid selective medium were normalized, serially diluted (1/10) and spotted on plates with the indicated levels of CuSO4 and antioxidant, NAC (N-acetyl-L-cysteine, Sigma). Plates were photographed after 4 days of incubation at 30°C. (b) NAC reduces lethality due to TDP-43. An integrant of CUP1-TDP-43-YFP (TDP-43) with high toxicity and vector (control) grown on synthetic dextrose medium without CuSO4 were inoculated to OD600 = 0.5 in dextrose medium with (+) or without (−) 250 μM CuSO4 and 0 mM or 1 mM NAC. After 24 h growth cells were stained with trypan blue to identify dead cells5 and counted. (c) NAC reduces TDP-43-YFP level. Cells from (b) were photographed with bright field (left), fluorescence and bright field (middle) and just fluorescence (right). TDP-43-YFP fluorescence was barely observed in NAC treated cells even at the very high exposure shown. Black cells indicate trypan blue stained dead cells.
The prevalence of TDP-43 aggregation in patients with a variety of neurodegenerative diseases makes it critical to understand how this is associated with toxicity. Our data shows that toxicity is enhanced in the presence of respiration, but that TDP-43 remains toxic even in the absence of respiration. This is consistent with the hypothesis that TDP-43 targets the same cellular components in the presence or absence of respiration, and that the reactive oxygen species produced by respiration either activates TDP-43 to become more toxic or makes the same TDP-43 targets more vulnerable.
Supplementary Material
Supplementary Fig. 1. Slow growth does not enhance TDP-43 toxicity. Three independent transformants of [PIN+] 74D-694 (L1749) with either TET-TDP-43-YFP (TDP-43) or empty vector (v) were taken from plasmid selective media without TDP-43-YFP expression, normalized to OD600 = 3 and serially diluted (1/10 ) and spotted on dextrose (dex) or galactose (gal) plasmid selective inducing media (lacking doxycycline). Dextrose and galactose plates were respectively incubated at room temperature (RT) or 30°C for the number of days indicated prior to being photographed.
Supplementary Fig. 2. CUP1 controlled TDP-43-YFP integrants show differences in toxicity. Cells of different [PIN+] integrants were normalized and spotted on plasmid selective dextrose plates containing 0, 50, and 250 μM CuSO4 as shown. Integrants were scored as having low, medium and high toxicity.
Highlights.
How TDP-43 effects amyotrophic lateral sclerosis toxicity is unknown.
In yeast, TDP-43 toxicity and aggregation is enhanced in cells that are respiring.
TDP-43 still aggregates and is toxic in cells that are not respiring.
Hydrogen peroxide enhances TDP-43 toxicity.
Respiration may make TDP-43 more toxic and/or make its targets more vulnerable.
Acknowledgements
We thank Aaron Gitler, Stanford U. for plasmids and Ruben Dagda, U. of Nevada, Reno, and Martin Duennwald, Western U. Ca, for helpful ideas. This work was supported National Institutes of Health Grant R01GM056350 (SWL).
Abbreviations
- TDP-43
trans-activating response DNA-binding protein 43
- ALS
amyotrophic lateral sclerosis
- dex
dextrose
- gal
galactose
- dox
doxycycline
- CuSO4
copper sulfate
- H2O2
hydrogen peroxide
- NAC
N-Acetyl L-cysteine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dormann D & Haass C (2011). TDP-43 and FUS: a nuclear affair. Trends Neurosci 34, 339–48. [DOI] [PubMed] [Google Scholar]
- 2.Baloh RH (2011). TDP-43: the relationship between protein aggregation and neurodegeneration in amyotrophic lateral sclerosis and frontotemporal lobar degeneration. FEBS J 278, 3539–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee AC, Gavett BE, Stern RA, Nowinski CJ, Cantu RC, Kowall NW, Perl DP, Hedley-Whyte ET, Price B, Sullivan C, Morin P, Lee HS, Kubilus CA, Daneshvar DH, Wulff M & Budson AE (2010). TDP-43 proteinopathy and motor neuron disease in chronic traumatic encephalopathy. J Neuropathol Exp Neurol 69, 918–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robinson JL, Geser F, Stieber A, Umoh M, Kwong LK, Van Deerlin VM, Lee VM & Trojanowski JQ (2013). TDP-43 skeins show properties of amyloid in a subset of ALS cases. Acta Neuropathol 125, 121–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SK, Hong JY, Arslan F, Kanneganti V, Patel B, Tietsort A, Tank EMH, Li X, Barmada SJ & Liebman SW (2017). Overexpression of the essential Sis1 chaperone reduces TDP-43 effects on toxicity and proteolysis. PLoS Genet 13, e1006805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leibiger C, Deisel J, Aufschnaiter A, Ambros S, Tereshchenko M, Verheijen BM, Buttner S & Braun RJ (2018). Endolysosomal pathway activity protects cells from neurotoxic TDP-43. Microb Cell 5, 212–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leibiger C, Deisel J, Aufschnaiter A, Ambros S, Tereshchenko M, Verheijen BM, Buttner S & Braun RJ (2018). TDP-43 controls lysosomal pathways thereby determining its own clearance and cytotoxicity. Hum Mol Genet 27, 1593–1607. [DOI] [PubMed] [Google Scholar]
- 8.Braun RJ, Sommer C, Carmona-Gutierrez D, Khoury CM, Ring J, Buttner S & Madeo F (2011). Neurotoxic 43-kDa TAR DNA-binding protein (TDP-43) triggers mitochondrion-dependent programmed cell death in yeast. J Biol Chem 286, 19958–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shan X, Chiang PM, Price DL & Wong PC (2010). Altered distributions of Gemini of coiled bodies and mitochondria in motor neurons of TDP-43 transgenic mice. Proc Natl Acad Sci U S A 107, 16325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magrane J, Cortez C, Gan WB & Manfredi G (2014). Abnormal mitochondrial transport and morphology are common pathological denominators in SOD1 and TDP43 ALS mouse models. Hum Mol Genet 23, 1413–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu YF, Gendron TF, Zhang YJ, Lin WL, D’Alton S, Sheng H, Casey MC, Tong J, Knight J, Yu X, Rademakers R, Boylan K, Hutton M, McGowan E, Dickson DW, Lewis J & Petrucelli L (2010). Wild-type human TDP-43 expression causes TDP-43 phosphorylation, mitochondrial aggregation, motor deficits, and early mortality in transgenic mice. J Neurosci 30, 10851–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong K, Li Y, Duan W, Guo Y, Jiang H, Li W & Li C (2012). Full-length TDP-43 and its C-terminal fragments activate mitophagy in NSC34 cell line. Neurosci Lett 530, 144–9. [DOI] [PubMed] [Google Scholar]
- 13.Wang W, Li L, Lin WL, Dickson DW, Petrucelli L, Zhang T & Wang X (2013). The ALS disease-associated mutant TDP-43 impairs mitochondrial dynamics and function in motor neurons. Hum Mol Genet 22, 4706–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang W, Wang L, Lu J, Siedlak SL, Fujioka H, Liang J, Jiang S, Ma X, Jiang Z, da Rocha EL, Sheng M, Choi H, Lerou PH, Li H & Wang X (2016). The inhibition of TDP-43 mitochondrial localization blocks its neuronal toxicity. Nat Med 22, 869–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Izumikawa K, Nobe Y, Yoshikawa H, Ishikawa H, Miura Y, Nakayama H, Nonaka T, Hasegawa M, Egawa N, Inoue H, Nishikawa K, Yamano K, Simpson RJ, Taoka M Yamauchi Y, Isobe T & Takahashi N (2017). TDP-43 stabilises the processing intermediates of mitochondrial transcripts. Sci Rep 7, 7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dols-Icardo O, Nebot I, Gorostidi A, Ortega-Cubero S, Hernandez I, Rojas-Garcia R, Garcia-Redondo A, Povedano M, Llado A, Alvarez V, Sanchez-Juan P, Pardo J, Jerico I, Vazquez-Costa J, Sevilla T, Cardona F, Indakoechea B, Moreno F, Fernandez-Torron R, Munoz-Llahuna L, Moreno-Grau S, Rosende-Roca M, Vela A, Munoz-Blanco JL, Combarros O, Coto E, Alcolea D, Fortea J, Lleo A, Sanchez-Valle R, Esteban-Perez J, Ruiz A, Pastor P, Lopez De Munain A, Perez-Tur J & Clarimon J (2015). Analysis of the CHCHD10 gene in patients with frontotemporal dementia and amyotrophic lateral sclerosis from Spain. Brain 138, e400. [DOI] [PubMed] [Google Scholar]
- 17.Johnson JO, Glynn SM, Gibbs JR, Nalls MA, Sabatelli M, Restagno G, Drory VE, Chio A, Rogaeva E & Traynor BJ (2014). Mutations in the CHCHD10 gene are a common cause of familial amyotrophic lateral sclerosis. Brain 137, e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muller K, Andersen PM, Hubers A, Marroquin N, Volk AE, Danzer KM, Meitinger T, Ludolph AC, Strom TM & Weishaupt JH (2014). Two novel mutations in conserved codons indicate that CHCHD10 is a gene associated with motor neuron disease. Brain 137, e309. [DOI] [PubMed] [Google Scholar]
- 19.Zhang M, Xi Z, Zinman L, Bruni AC, Maletta RG, Curcio SA, Rainero I, Rubino E, Pinessi L, Nacmias B, Sorbi S, Galimberti D, Lang AE, Fox S, Surace EI, Ghani M, Guo J, Sato C, Moreno D, Liang Y, Keith J, Traynor BJ, St George-Hyslop P & Rogaeva E (2015). Mutation analysis of CHCHD10 in different neurodegenerative diseases. Brain 138, e380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chio A, Mora G, Sabatelli M, Caponnetto C, Traynor BJ, Johnson JO, Nalls MA, Calvo A, Moglia C, Borghero G, Monsurro MR, La Bella V, Volanti P, Simone I, Salvi F, Logullo FO, Nilo R, Battistini S, Mandrioli J, Tanel R, Murru MR, Mandich P, Zollino M, Conforti FL, Brunetti M, Barberis M, Restagno G, Penco S & Lunetta C (2015). CHCH10 mutations in an Italian cohort of familial and sporadic amyotrophic lateral sclerosis patients. Neurobiol Aging 36, 1767 e3–1767 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronchi D, Riboldi G, Del Bo R, Ticozzi N, Scarlato M, Galimberti D, Corti S, Silani V, Bresolin N & Comi GP (2015). CHCHD10 mutations in Italian patients with sporadic amyotrophic lateral sclerosis. Brain 138, e372. [DOI] [PubMed] [Google Scholar]
- 22.Jiao B, Xiao T, Hou L, Gu X, Zhou Y, Zhou L, Tang B, Xu J & Shen L (2016). High prevalence of CHCHD10 mutation in patients with frontotemporal dementia from China. Brain 139, e21. [DOI] [PubMed] [Google Scholar]
- 23.Woo JA, Liu T, Trotter C, Fang CC, De Narvaez E, LePochat P, Maslar D, Bukhari A, Zhao X, Deonarine A, Westerheide SD & Kang DE (2017). Loss of function CHCHD10 mutations in cytoplasmic TDP-43 accumulation and synaptic integrity. Nat Commun 8, 15558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson BS, McCaffery JM, Lindquist S & Gitler AD (2008). A yeast TDP-43 proteinopathy model: Exploring the molecular determinants of TDP-43 aggregation and cellular toxicity. Proc Natl Acad Sci U S A 105, 6439–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elden AC, Kim HJ, Hart MP, Chen-Plotkin AS, Johnson BS, Fang X, Armakola M, Geser F, Greene R, Lu MM, Padmanabhan A, Clay-Falcone D, McCluskey L, Elman L, Juhr D, Gruber PJ, Rub U, Auburger G, Trojanowski JQ, Lee VM, Van Deerlin VM, Bonini NM & Gitler AD (2010). Ataxin-2 intermediate-length polyglutamine expansions are associated with increased risk for ALS. Nature 466, 1069–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.De Deken RH (1966). The Crabtree effect: a regulatory system in yeast. J Gen Microbiol 44, 149–56. [DOI] [PubMed] [Google Scholar]
- 27.Cohen TJ, Hwang AW, Unger T, Trojanowski JQ & Lee VM (2012). Redox signalling directly regulates TDP-43 via cysteine oxidation and disulphide cross-linking. EMBO J 31, 1241–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen TJ, Hwang AW, Restrepo CR, Yuan CX, Trojanowski JQ & Lee VM (2015). An acetylation switch controls TDP-43 function and aggregation propensity. Nat Commun 6, 5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ecker DJ, Khan MI, Marsh J, Butt TR & Crooke ST (1987). Chemical synthesis and expression of a cassette adapted ubiquitin gene. J Biol Chem 262, 3524–7. [PubMed] [Google Scholar]
- 30.Thiele DJ (1988). ACE1 regulates expression of the Saccharomyces cerevisiae metallothionein gene. Mol Cell Biol 8, 2745–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plessis A & Dujon B (1993). Multiple tandem integrations of transforming DNA sequences in yeast chromosomes suggest a mechanism for integrative transformation by homologous recombination. Gene 134, 41–50. [DOI] [PubMed] [Google Scholar]
- 32.Liebman SW, Bagriantsev SN & Derkatch IL (2006). Biochemical and genetic methods for characterization of [PIN+] prions in yeast. Methods 39, 23–34. [DOI] [PubMed] [Google Scholar]
- 33.David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL & Kenyon C (2010). Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol 8, e1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weids AJ, Ibstedt S, Tamas MJ & Grant CM (2016). Distinct stress conditions result in aggregation of proteins with similar properties. Sci Rep 6, 24554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bagriantsev SN, Gracheva EO, Richmond JE & Liebman SW (2008). Variant-specific [PSI+] infection is transmitted by Sup35 polymers within [PSI+] aggregates with heterogeneous protein composition. Mol Biol Cell 19, 2433–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gari E, Piedrafita L, Aldea M & Herrero E (1997). A set of vectors with a tetracycline-regulatable promoter system for modulated gene expression in Saccharomyces cerevisiae. Yeast 13, 837–48. [DOI] [PubMed] [Google Scholar]
- 37.Goldring ES, Grossman LI, Krupnick D, Cryer DR & Marmur J (1970). The petite mutation in yeast. Loss of mitochondrial deoxyribonucleic acid during induction of petites with ethidium bromide. J Mol Biol 52, 323–35. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Slow growth does not enhance TDP-43 toxicity. Three independent transformants of [PIN+] 74D-694 (L1749) with either TET-TDP-43-YFP (TDP-43) or empty vector (v) were taken from plasmid selective media without TDP-43-YFP expression, normalized to OD600 = 3 and serially diluted (1/10 ) and spotted on dextrose (dex) or galactose (gal) plasmid selective inducing media (lacking doxycycline). Dextrose and galactose plates were respectively incubated at room temperature (RT) or 30°C for the number of days indicated prior to being photographed.
Supplementary Fig. 2. CUP1 controlled TDP-43-YFP integrants show differences in toxicity. Cells of different [PIN+] integrants were normalized and spotted on plasmid selective dextrose plates containing 0, 50, and 250 μM CuSO4 as shown. Integrants were scored as having low, medium and high toxicity.


