Abstract
Background:
Major postoperative complications are associated with increased cost and mortality. The complexity of electronic health records (EHR) overwhelms physicians’ ability to use the information for optimal and timely preoperative risk-assessment. We hypothesized that data-driven, predictive risk algorithms implemented in an intelligent decision support platform simplify and augment physicians’ risk-assessment.
Methods:
This prospective, non-randomized pilot study of 20 physicians at a quaternary academic medical center compared the usability and accuracy of preoperative risk-assessment between physicians and MySurgeryRisk, a validated, machine-learning algorithm, using a simulated workflow for the real-time, intelligent decision-support platform. We used area under the receiver operating characteristic curve (AUC) to compare the accuracy of physicians’ risk-assessment for six postoperative complications before and after interaction with the algorithm for 150 clinical cases.
Results:
The AUC of the MySurgeryRisk algorithm ranged between 0.73 and 0.85 and was significantly better than physicians’ initial risk-assessments (AUC between 0.47 and 0.69) for all postoperative complications except cardiovascular. After interaction with the algorithm, the physicians significantly improved their risk-assessment for acute kidney injury and for an intensive care unit admission greater than 48 hours, resulting in a net improvement of reclassification of 12% and 16%, respectively. Physicians rated the algorithm easy to use and useful.
Conclusions:
Implementation of a validated, MySurgeryRisk computational algorithm for real-time predictive analytics with data derived from the EHR to augment physicians’ decision making is feasible and accepted by physicians. Early involvement of physicians as key stakeholders in both design and implementation of this technology will be crucial for its future success.
INTRODUCTION
Postoperative complications increase odds of 30-day mortality, lead to greater readmission rates, and require greater utilization of health care resources.1–5 Prediction of postoperative complications for individual patients is increasingly complex due to the need for rapid decision-making coupled with the constant influx of dynamic physiologic data in electronic health records (EHR). Risk-communication tools and scores are continually being developed to convert the large amount of available EHR data into a usable format, but it is unclear if these tools are able to change users’ perceptions of risk.6
Two commonly used and validated risk scores for surgical patients, the National Surgical Quality Improvement score and the Physiological and Operative Severity Score for the enUmeration of Mortality and morbidity, provide risk stratifications for selected postoperative complications.7, 8 Although these scoring systems have been proven reliable, they have not been automated or integrated uniformly into the EHR, because they require elaborate data collection and calculations.9 Other risk scores frequently integrated into EHR, such as the Modified Early Warning Score or Rothman Index, are designed to alert health care providers to all at-risk patients; however, these risk scores often have high false positive rates and do not differentiate between risks of specific postoperative complications.1, 10 Interestingly, studies comparing how physicians’ clinical judgment compares to these risk models for predicting surgical complications are lacking.
Recently we validated the machine learning algorithm MySurgeryRisk which predicts preoperative risk for major postoperative complications using EHR data. The algorithm is integrated into the clinical workflow through the intelligent, perioperative platform for real-time analytics of routine clinical data and prospective data collection for the model retraining.11–13
In this prospective pilot, study we compared the usability and accuracy of preoperative risk-assessment between physicians and the MySurgeryRisk algorithm using a simulated workflow for the real-time, intelligent, decision support platform. We tested the hypothesis that physicians will gain knowledge from interaction with the algorithm and improve the accuracy of their risk-assessments.
MATERIALS AND METHODS
The Institutional Review Board and Privacy Office of the University of Florida (UF) approved this study (#2013-U-1338, #5–2009). Written informed consent was obtained from all participants.
Study Design
This prospective, non-randomized, interventional pilot study of 20 surgical intensivists (attending physicians or trainees in anesthesiology and surgical fellowships) at a single academic quaternary care institution was designed to assess the usability and accuracy of the MySurgeryRisk algorithm for preoperative risk assessment using a simulated workflow for the real-time, intelligent, decision support platform.
The MySurgeryRisk algorithm is a validated, machine-learning algorithm that predicts preoperative risk for major complications using existing clinical data in the EHR data with a high sensitivity and a high specificity.11–13 Development and validation of the algorithm is described in detail in Bihorac et al.11 We designed an intelligent platform such that the MySurgeryRisk algorithm can be implemented in real time to provide an augmented preoperative risk-assessment for inpatient surgical cases at University of Florida. Prior to operation, the platform autonomously integrates and transforms existing EHR data to run the MySurgeryRisk algorithm in real time and calculates risk-probabilities for major complications. The output of the algorithm is presented to the surgeons scheduled to perform the operations using an interactive interface that resides on the web portal within the platform and allows user feedback (Figure 1A-D).11 This pilot study was performed prior to the launch of this real time platform to evaluate its usability and performance. We simulated the real-time workflow of this platform for 150 patient cases to allow us to study the participants interaction with the results of the algorithm in a same way as they would with fully functional real time platform.11 We selected new cases from a large, retrospective, longitudinal database of adult patients age 18 years or older admitted to University of Florida Health (UF Health) for greater than 24 h after any type of inpatient surgical procedure from the years 2000 to 2010.11, 12, 14 The selected cases were not used for the development of the algorithm reported previously. 11 For each case, we had a complete electronic health record from which we used available preoperative data as an input for both algorithm and the physicians’ risk-assessment, while the clinical data related to the hospitalization after operation were used to determine whether complications occurred (detailed description of the algorithm input data and assessment of complications is provided in Bihorac et al.11). Physicians and algorithm were blinded to the observed outcomes of the cases.
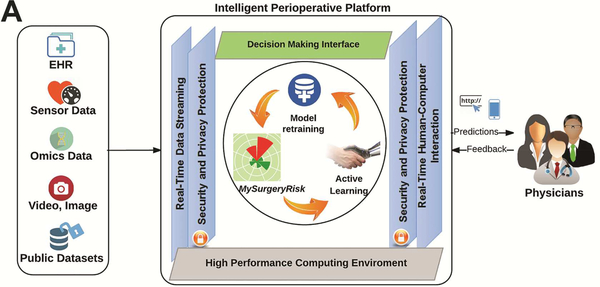
Figure 1A.
Design of Intelligent Perioperative Platform that hosts MySurgeryRisk Algorithm, reprinted with permission from Annals of Surgery, Bihorac et al.11
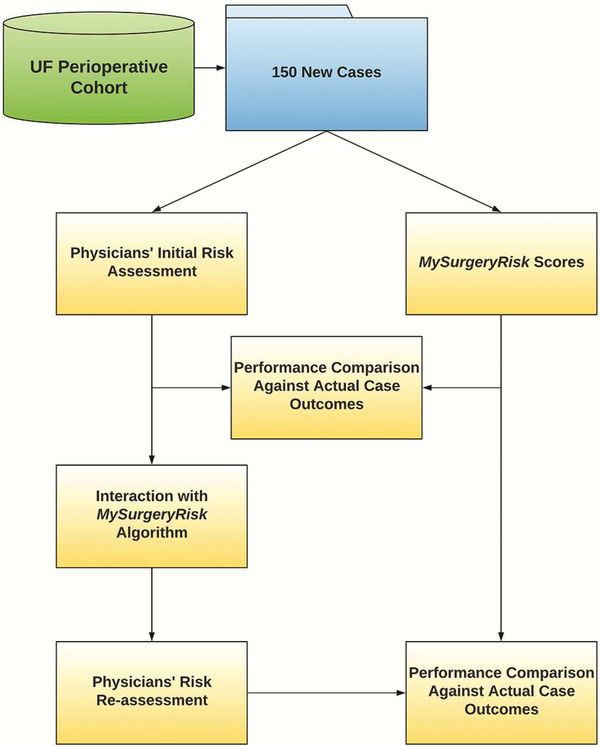
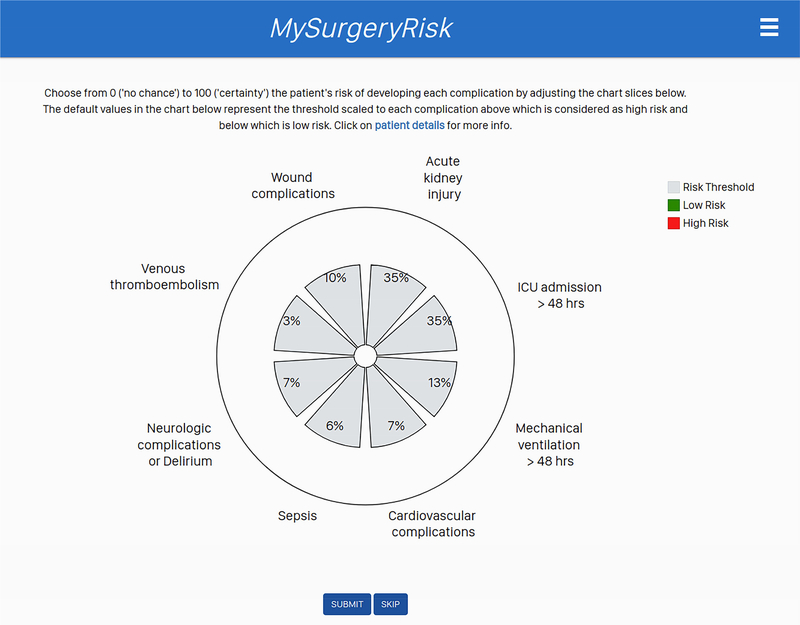
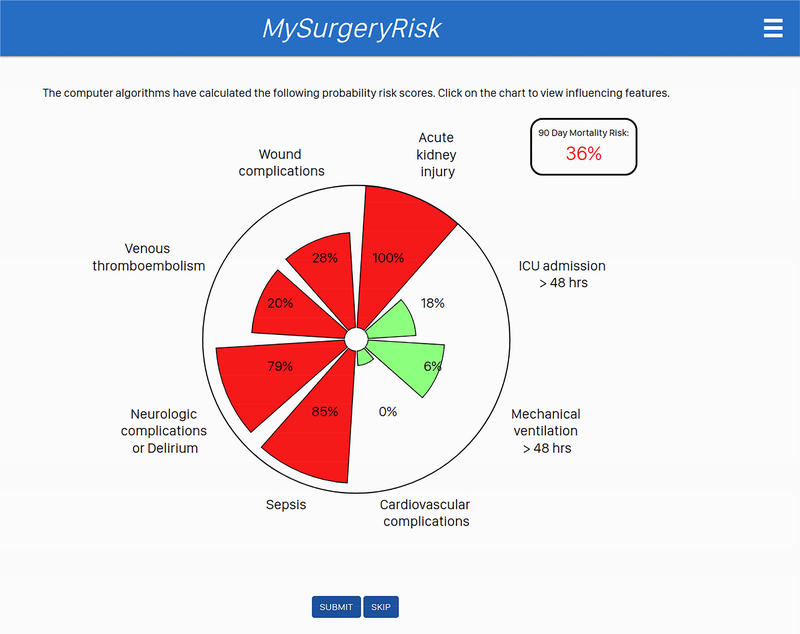
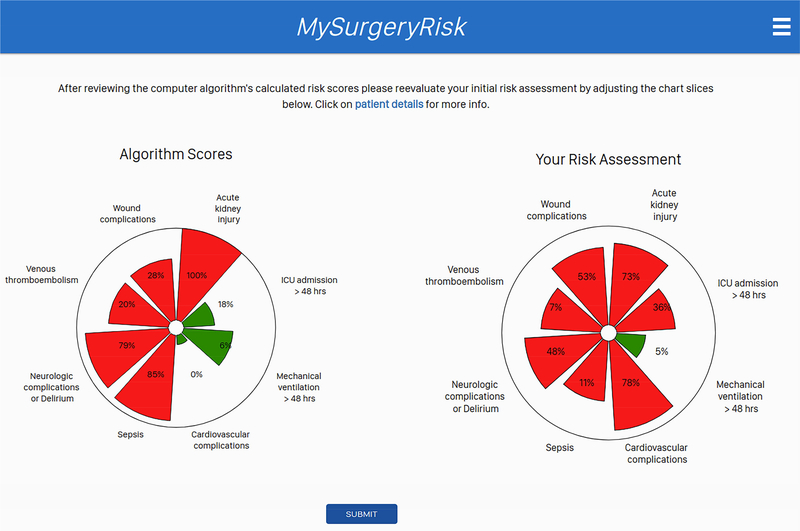
Each physician evaluated 8 to 10 ten individual cases and provided a risk-assessment for each complication both before and after seeing the scores of the MySurgeryRisk algorithm (Figure 2). All evaluations were performed on a personal laptop during a single, “think aloud” individual session with a research coordinator who assisted with the use of the interactive interface of the platform. The physicians had access to all available preoperative data that was also used as an input for MySurgeryRisk algorithm. For each case, we summarized the preoperative clinical data as a brief clinical vignette similar to a progress note available in the patient’s chart. On review of a case, the physicians were asked to assess the absolute risk for each of the six complications ranging from 0 to 100% using a sliding pie chart for data input. For each complication, we used a previously determined threshold11 to determine whether the assigned absolute risk classified the patient into low or high-risk group as reflected in the change of color from green to red on the pie chart (Figure 1B). After the initial risk assessment, physicians were presented with absolute risk scores and risk groups calculated by MySurgeryRisk algorithm (Figure 1C). Each score was accompanied with the display of the top three features that were the most important contributors to the calculated risk for the individual patient. Finally, the physicians were asked to repeat their risk-assessment for the same patient in order to assess whether the interaction with the algorithm would change their perception of the risk. They used a similar interactive pie chart to re-enter the absolute risk cores for each complication (Figure 1D). At the end of the session. we surveyed the physicians regarding the usability of the algorithm and web interface.
Figure 2.
Flowchart of the study design.
Figure 1B.
The interactive interface for physicians to input their initial assessment of absolute risk for each complication.
Figure 1C.
The interactive interface displaying absolute risk scores and risk groups calculated by MySurgeryRisk algorithm to physicians. Each score was accompanied with the display of the top three features that were the most important contributors to calculated risk for the individual patient.
Figure 1D.
The interactive interface for physicians to input their repeated assessment of the absolute risk after reviewing MySurgeryRisk scores for the same case.
At study enrollment, we evaluated each physician’s decision-making style and numeracy skills with a validated Cognitive Reflection Test (CRT) and a numeracy assessment test15–17. The CRT consists of three questions and was validated against other measures of cognitive reflection.17 A lesser score on the CRT indicated a more impulsive decision-making preference and a strong reliance on intuition, while a greater score indicated a reflective thinker with a more cautious decision-making preference and less reliance on intuition.17 The numeracy assessment test measured the physician’s ability to understand and use numbers, while a greater score indicated an increased ability to use numeric data.
Statistical Analysis
In order to increase the number of cases for complications with a low prevalence in the original cohort, we selected patient cases with observed 30-day mortality and matched them with patient cases without an observed 30-day mortality in 5:1 ratio. This approach allowed us to increase the number of cases with observed 30-day mortality in testing the cohort to 16% compared to 3% in original cohort. As expected, this strategy also resulted in an increase of the prevalence for other complications (Table 2). Each of the 150 cases was treated as an independent observation. The study had 80% power to detect at least a 10% difference between the algorithm and the physician risk-assessments while assuming a standard deviation of 10%.11,12,14
Table 2.
Summary of reference cohort and testing cohort.
| Overall reference cohort (N=10,000) | Testing cohort (n=150) | |
|---|---|---|
| Demographic features | ||
| Age, median (25th-75th) | 56 (43, 68) | 62 (51, 70) |
| Female sex, n (%) | 4898 (49) | 73 (49) |
| Race, n (%) | ||
| White | 8032 (80) | 125 (83) |
| African-American | 1235 (12) | 19 (13) |
| Hispanic | 338 (3) | 4 (3) |
| Other | 210 (2) | 1 (1) |
| Primary insurance group, n (%) | ||
| Medicare | 3845 (38) | 71 (47) |
| Medicaid | 1294 (13) | 17 (11) |
| Private | 4088 (41) | 57 (38) |
| Uninsured | 773 (8) | 5 (3) |
| Socio-economic features | ||
| Neighborhood characteristics | ||
| Rural area, n (%) | 3156 (32) | 50 (33) |
| Total population, median (25th-75th) | 17176 (10002, 27782) | 16192 (9812, 25971) |
| Median income, median (25th-75th) | 33221 (28400, 40385) | 33293 (28589, 41410) |
| Total proportion of African-Americans (%), mean (SD) | 15 (15) | 16 (16) |
| Total proportion of Hispanic (%), mean (SD) | 6 (6) | 5 (6) |
| Population proportion below poverty (%), mean (SD) | 15 (8) | 14 (8) |
| Distance from residency to hospital (km), median (25th75th) | 54.1 (26.7, 117.7) | 47.8 (21.9, 99.4) |
| Comorbidity features | ||
| Charlson's comorbidity index (CCI), median (25th-75th) | 1 (0, 2) | |
| Cancer, n (%) | 2025 (20) | 27 (18) |
| Diabetes, n (%) | 1620 (16) | 25 (17) |
| Chronic pulmonary disease, n (%) | 1573 (16) | 30 (20) |
| Peripheral vascular disease, n (%) | 1131 (11) | 26 (17) |
| Cerebrovascular disease, n (%) | 823 (8) | 28 (19) |
| Congestive heart failure, n (%) | 781 (8) | 20 (13) |
| Myocardial infarction, n (%) | 650 (7) | 17 (11) |
| Liver disease, n (%) | 503 (5) | 10 (7) |
| Operative features | ||
| Admission | ||
| Weekend admission, n (%) | 142 (14) | 26 (17) |
| Admission source, n (%) | ||
| Emergency room | 2637 (27) | 45 (30) |
| Outpatient setting | 5900 (60) | 76 (51) |
| Transfer | 1334 (14) | 29 (19) |
| Emergent surgery status, n (%) | 4545 (45) | 80 (53) |
| Surgery type, n (%) | ||
| Cardiothoracic surgery | 1284 (13) | 22 (15) |
| Non-cardiac general surgery | 3905 (39) | 54 (36) |
| Neurologic surgery | 1713 (17) | 30 (20) |
| Specialty surgery | 2959 (30) | 41 (27) |
| Other surgery | 139 (1) | 3 (2) |
| Preoperative and admission day laboratory results | ||
| Estimated reference glomerular filtration rate (mL/min/1.73 m2), median (25th-75th) | 91.9 (71.6, 107.8) | 85.8 (56.2, 102.6) |
| Automated urinalysis, urine protein (mg/dL), n (%) | ||
| Missing | 8330 (83) | 124 (83) |
| <30 | 1109 (11) | 15 (10) |
| ≥30 | 561 (6) | 11( 7) |
| Number of complete blood count tests, n (%) | ||
| 0 | 2954 (30) | 34 (23) |
| 1 | 5526 (55) | 88 (59) |
| ≥2 | 1520 (15) | 28 (18) |
| Hemoglobin, g/dL median (25th-75th) | 11.7 (10.2, 13.3) | 11.4 (9.7, 13.0) |
| Hematocrit, median (25th-75th) | 34.4 (30.1, 38.6) | 33.4 (29.1, 37.6) |
| Admission day medications | ||
| Admission day medication groups (top 10 categories), n (%) | ||
| Betablockers | 2333 (23) | 42 (28) |
| Diuretics | 1124 (11) | 19 (13) |
| Statins | 1122 (11) | 23 (15) |
| Aspirin | 671 (7) | 12 (8) |
| Angiotensin-converting-enzyme inhibitors | 979 (10) | 13 (9) |
| Vasopressors and inotropes | 551 (6) | 15 (10) |
| Bicarbonate | 406 (4) | 15 (10) |
| Antiemetics | 5717 (57) | 84 (56) |
| Aminoglycosides | 687 (7) | 5 (3) |
| Corticosteroids | 1218 (12) | 23 (15) |
| Outcomes | ||
| Postoperative complications, n (%) | ||
| Acute kidney injury during hospitalization | 3869 (39) | 82 (55) |
| Intensive care unit admission > 48 hours | 3161 (32) | 74 (49) |
| Mechanical ventilation > 48 hours | 1313 (13) | 55 (37) |
| Wound complications | 1085 (11) | 17 (11) |
| Neurological complications or delirium | 773 (8) | 26 (17) |
| Cardiovascular complications | 724 (7) | 43 (29) |
| Sepsis | 536 (5) | 39 (26) |
| Venous thromboembolism | 287 (3) | 11 (7) |
| Mortality, n (%) | ||
| 30-day mortality | 335 (3) | 24 (16) |
| 3-month mortality | 668 (7) | 41 (27) |
| 6-month mortality | 919 (9) | 46 (31) |
| 12-month mortality | 1261 (13) | 48 (32) |
| 24-month mortality | 1687 (17) | 55 (37) |
We calculated the area under the receiver operating characteristic curve (AUC) to test the accuracy of both the MySurgeryRisk algorithm and the physicians’ risk-assessments for predicting the occurrence of each of the six complications separately. For each case, we compared both the initial and repeated (after reviewing the MySurgeryRisk scores) physician risk-assessments to the MySurgeryRisk scores against the true occurrence of each complication. The change in accuracy between the initial and the repeated physician’s risk-assessments were compared using the DeLong test.18 The net improvement in reclassification was calculated to measure the improvement in the physicians’ risk-reassessments after interaction with the algorithm.19 We calculated the misclassification rate as a proportion of cases misclassified in a wrong risk group based on observed outcome for each complication. A case was considered as misclassified if the physician’s assessment of absolute risk classified the patient into a low risk group for positive cases, where the complication was observed or into a high risk group for negative cases where the complication was not observed, We determined thresholds of absolute risk separating low and high risk groups based on prevalence of each complication in the original cohort, with values similar to the previously reported thresholds11 (0.32 for intensive care unit admission greater than 48 h, 0.26 for acute kidney injury, 0.13 for mechanical ventilation greater than 48 h, 0.07 for cardiovascular complications, 0.05 for severe sepsis, and 0.034 for 30day mortality). The proportion of misclassified cases between physicians stratified by specialty or training status were compared using Fisher’s exact test. The correlation between years of practice and the average misclassification rate for physicians was calculated using the Spearman correlation. A t-test was used to test the hypothesis that the mean absolute difference in risk-assessment score, after interaction with the MySurgeryRisk algorithm was different than 0. All statistical analyses were performed using SAS software (v.9.4, Cary, N.C.) and R software (v 3.4.0, https://www.r-project.org/).
RESULTS
Comparison between the physicians’ initial risk-assessment and the MySurgeryRisk algorithm
Twenty physicians provided risk-assessment scores for six postoperative complications for total of 150 patient cases. Fourteen of the physicians were attending physicians, the remainder were residents or fellows, with an average of 13 years of experience. Ninety percent had high numeracy skills on the numeracy assessment. The majority, 70%, scored in the intermediate range for the decision-making style reflecting a balance between intuitive and reflective decision-making. Only 15% of physicians scored in the impulsive decision-making range with strong reliance on intuition, while the other 15% were in the reflective thinker range with more cautious decision-making preference and less reliance on intuition (Table 1).
Table 1.
Physician Characteristics.
| Physician characteristics | N=20 |
|---|---|
| Female sex, n (%) | 5 (25) |
| Age (years), n (%) | |
| <=30 | 2 (10) |
| 31–40 | 10 (50) |
| 41–50 | 4 (20) |
| >50 | 4 (20) |
| Attending physicians, n (%) | 14 (70) |
| Specialty, n (%) | |
| Anesthesiology/Emergency Medicine | 16 (80) |
| Surgery | 4 (20) |
| Years since graduation, mean (SD) | 13 (10) |
| High numeracy score (≥9)*, n (%) | 18 (90) |
| Cognitive Reflection Test score†, n (%) | |
| Low score (0): Non-reflective thinker with unquestioning reliance on intuition | 3 (15) |
| Intermediate score (1–2): Balance between reflective thinking and intuition | 14 (70) |
| High score (3): Reflective thinker whose initial intuition is tempered by analysis | 3 (15) |
The numeracy assessment is a measure of ability to understand and use numeric data
Cognitive Reflection Test is a validated measure of decision-making preference; differentiating impulsive decision-making preferences from reflective decision-making preferences
As expected, the prevalence of postoperative complications among 150 cases was greater than in the reference population as a result of selection process and ranged between 16% for 30day mortality and 49% for ICU admission > 48 hours (Table 2). The MySurgeryRisk algorithm (AUCs ranged from 0.64 to 0.85) was more accurate in predicting the risk for complications compared to the initial physicians’ risk-assessments (AUCs ranged between 0.47 and 0.69) with greater AUCs for predicted absolute risks for all complications (p<0.002 each) except cardiovascular (Table 3). Compared to the MySurgeryRisk algorithm, the physicians were more likely to underestimate the risk of ICU stay and AKI for positive cases, for which complications occurred (their assessment of absolute risk was less than the MySurgeryRisk score). In contrast, the physicians overestimated the risk of mortality, cardiovascular complications, and severe sepsis for negative cases, for which complications did not occur (their assessment of absolute risk was greater than of MySurgeryRisk).
Table 3.
Comparison between Physicians’ Initial Risk Assessement and My Surgery Risk Alogorithm Prediction.
| Postoperative complications | Prevalence of positive cases with complications among 150 cases, n (%) | Physicians’ first risk assessment AUC (95% CI) | MySurgeryRisk Algorithm risk score AUC (95% CI) | p-value for difference in AUC |
|---|---|---|---|---|
| Intensive care unit admission longer than 48 hours | 74 (49) | 0.69 (0.61, 0.77) | 0.84 (0.78, 0.90) | <0.001 |
| Acute kidney injury | 57 (38) | 0.65 (0.56, 0.74) | 0.79 (0.72, 0.87) | 0.002 |
| Mechanical ventilation greater than 48 hours | 55 (37) | 0.66 (0.57, 0.75) | 0.85 (0.79, 0.91) | <0.001 |
| Cardiovascular complications | 43 (29) | 0.54 (0.44, 0.65) | 0.64 (0.55, 0.73) | 0.09 |
| Severe sepsis | 39 (26) | 0.54 (0.44, 0.64) | 0.78 (0.69, 0.87) | <0.001 |
| 30-day mortality | 24 (16) | 0.47 (0.36, 0.57) | 0.73 (0.64, 0.83) | <0.001 |
MySurgeryRisk algorithm and physicians evaluated absolute risk for complications ranging from 0 (no risk) to 100 (complete certainty of risk).
Abbreviations: AUC, area under the receiver operator characteristic curve; CI, confidence interval
Among physicians, the rate of misclassifying patients in a wrong risk category based on the observed outcome (low risk group for patients with observed outcome and high-risk group for patients without outcome) ranged from 28% for severe sepsis to 64% for the 30day mortality (Table 4). We observed no significant difference in misclassification rate between attending physicians and trainees. The years of practice correlated with the misclassification rate for predicting risk for acute kidney injury with more experienced physicians having a lesser rate (r=−0.63, P=0.01). The proportion of cases where the physicians’ assessment of absolute risk was more accurate than the algorithm (for cases where the complication was observed, the physicians estimated a greater absolute risk and for cases where the complication was not observed, the physicians estimated lesser absolute risk) ranged widely from 20% when predicting mortality to 46% when predicting risk for AKI (29% for severe sepsis, 33% for mechanical ventilation greater than 48 hours, 35% for cardiovascular complications, and 39% for intensive care unit admission greater than 48 hours). We observed no difference based on physician specialty or training status,
Table 4.
Misclassification rate of physicians’ initial assessment of absolute risk for postoperative complications
| Intensive care unit admission greater than 48 hours | Acute kidney injury | Mechanical ventilation greater than 48 hours | Cardiovascular complications | Severe sepsis | Thirty-day mortality | |
|---|---|---|---|---|---|---|
| Misclassification rate | ||||||
| Physicians’ initial risk assessment | ||||||
| Overall | 37 (4) | 41 (0.04) | 43 (4) | 57 (4) | 63 (4) | 65 (4) |
| By physician’s specialty | ||||||
| Surgery | 38 (8) | 59 (8)* | 44 (8) | 69 (7) | 67 (8) | 77 (7) |
| Anesthesiology/Emergency Medicine | 37 (5) | 35 (5) | 42 (5) | 52 (5) | 61 (5) | 60 (5) |
| By training status | ||||||
| Attending physicians | 36 (5) | 38 (5) | 40 (5) | 52 (5) | 61 (5) | 61 (5) |
| Trainees | 41 (8) | 51 (8) | 49 (8) | 68 (7) | 68 (7) | 73 (7) |
| Correlation between years of practice and misclassification rate, r (p-value) | −0.13 (0.63) | −0.63 (0.01) | −0.27 (0.30) | −0.14 (0.60) | 0.03 (0.92) | −0.43 (0.09) |
Data represents proportion of misclassified cases as percent and its standard error in parenthesis. Correlation between years of practice and average misclassification rate for physicians was calculated using Spearman correlation.
A case was considered as misclassified if MySurgeryRisk algorithm risk score or physician’s assessment of absolute risk classified patient into low risk group for positive cases, where the complication was observed or into high risk group for negative cases, where the complication was not observed, Thresholds separating low and high-risk groups were 0.32 for Intensive care unit admission greater than 48 hours , 0.26 for acute kidney injury, 0.13 for mechanical ventilation greater than 48 hours, 0.07 for cardiovascular complications, 0.05 for severe sepsis, and 0.034 for 30-day mortality.
P< 0.05 using Fisher’s exact test. No significant difference (p>0.05) was observed in proportion of misclassified cases between attending physicians and trainees.
Change in the physicians’ risk-assessment after interaction with the MySurgeryRisk algorithm
To assess whether physicians changed their perception of absolute risk after reviewing the MySurgeryRisk scores, we compared their initial and repeated risk-assessments. In greater than 75% of clinical cases, physicians responded to interaction with the algorithm by changing their risk-assessment score, and the majority of the new scores were closer to the MySurgeryRisk score. The average change in the physicians’ absolute risk-perception ranged between 8% and 10% (Table 5). Compared to their initial risk-assessment, the accuracy of the physician’s repeated risk-assessments improved after interaction with the algorithm with an increase in AUC between 2% and 5% for all complications except 30day mortality. The improvement in AUC for predicting cardiovascular complications before and after their interaction was the only complication that was statistically significant, increasing by 5% (Table 6). The calculated net improvement in reclassification (net percentages of correctly reclassified cases after interaction with the algorithm) showed a statistically significant improvement for AKI and ICU admission greater than 48 hours with 12% and 16% cases correctly reclassified, respectively.
Table 5.
Change in physicians’ perception of absolute risk after interaction with MySurgeryRisk algorithm
| Postoperative complications | Physician changed absolute risk assessment after interaction with MySurgeryRisk algorithm, n (%) | Physician changed absolute risk assessment closer to MySurgeryRisk algorithm, n (%) | Difference in physicians’ assessment of absolute risk after interaction with MySurgeryRisk algorithm, mean (SD) |
|---|---|---|---|
| Intensive care unit admission greater than 48 hours | 122 (81) | 91 (75) | 10 (13)* |
| Acute kidney injury | 123 (82) | 88 (72) | 8 (11) * |
| Mechanical ventilation greater than 48 hours | 120 (80) | 85 (71) | 10 (13) * |
| Cardiovascular complications | 124 (83) | 94 (76) | 8 (10) * |
| Severe sepsis | 120 (80) | 91 (76) | 8 (11) * |
| 30-day mortality | 114 (76) | 83 (73) | 7 (10) * |
MySurgeryRisk algorithm and physicians evaluated absolute risk for complications ranging from 0 (no risk) to 100 (complete certainty of risk).
Data represent the number and percentages of cases satisfying the criteria.
P<0.001 when testing the hypothesis that the mean absolute difference in risk assessment score after interaction with MySurgeryRisk
Algorithm is different than 0 using a t-test.
Table 6.
Comparison between Physicians’ Initial and repeated absolute risk assessment after interaction with MySurgeryRisk Algorithm.
| Postoperative complications | Physicians’ initial risk assessment AUC (95% CI) | Physicians’ risk re-assessment AUC (95% CI) | p-value for difference in AUC | Net reclassification improvement, % (95% CI) |
|---|---|---|---|---|
| Intensive care unit admission greater than 48 hours | 0.69 (0.61, 0.77) | 0.71 (0.62, 0.79) | 0.452 | 16.0 (3.0, 29.6) † |
| Acute kidney injury | 0.65 (0.56, 0.74) | 0.69 (0.60, 0.77) | 0.064 | 12.4 (1.0, 23.8) † |
| Mechanical ventilation greater than 48 hours | 0.66 (0.57, 0.75) | 0.70 (0.61, 0.80) | 0.074 | 0.8 (−10.9, 9.3) |
| Cardiovascular complications | 0.54 (0.44, 0.65) | 0.59 (0.49, 0.69) | 0.039* | 5.1 (−2.9, 13.1) |
| Severe sepsis | 0.54 (0.44, 0.64) | 0.59 (0.50, 0.69) | 0.063 | 7.8 (−5.9, 21.6) |
| 30-day mortality | 0.47 (0.36, 0.57) | 0.49 (0.39, 0.60) | 0.276 | −1.0 (−11.6, 9.6) |
MySurgeryRisk algorithm and physicians evaluated absolute risk for complications ranging from 0 (no risk) to 100 (complete certainty of risk).
Abbreviations: AUC, area under the receiver operator characteristic curve; CI, confidence interval.
p-value < 0.05. The change in AUC for repeated physicians' risk-assessment after interaction with MySurgeryRisk Algorithm was tested using the DeLong test.
p-value < 0.05 for testing null hypothesis of net improvement in reclassification being equal to 0 using chi-square distribution with 1 degree of freedom
Although the study size was too small for formal comparison, decision-making attitudes as classified by the Cognitive Reflection Test appear to play a role in physician interaction with the algorithm. Reflective decision makers changed their scores more frequently than intuitive decision makers. This change was most noticeable in cases in which complications did not occur. Half of the physicians completed a written post-test survey, with both Likert scale and free response questions administered to assess the usability of the MySurgeryRisk algorithm in a simulated workflow for the real-time, intelligent decision-support platform. Half of the respondents found the algorithm helpful with decision-making process, while 25% were neutral (Table 7), and the majority listed tablet and website-based applications during clinics and ICU rounds as the best way to access the algorithm. Two physicians reported they would use the MySurgeryRisk algorithm for counseling patients preoperatively.
Table 7.
Participant Feedback Survey.
| Physicians’ feedback | ||||
|---|---|---|---|---|
| Usability Questions | Agree | Neutral | Disagree | |
| Easy to use | 8 | 1 | 1 | |
| Helps with decision making | 5 | 2 | 3 | |
| Would use a version of the algorithm | 5 | 3 | 2 | |
| Algorithm was helpful and innovative | 5 | 2 | 3 | |
| Personal computer | Tablet/phone | Website | EHR | |
| Best device for this application | 1 | 6 | 6 | 1 |
| Clinic/ICU | Preoperatively | Postoperatively | Home/Office | |
| Best location to use the algorithm* | 7 | 2 | 1 | 1 |
Based on a summary of free response text
DISCUSSION
In this pilot usability study, the previously validated MySurgeryRisk algorithm implemented in simulated workflow for real time intelligent platform predicted postoperative complications with equal or greater accuracy than our sample of physicians using readily available clinical data from the EHR. Interestingly, physicians were more likely than the algorithm to both underestimate the risk of postoperative complications for cases where complications actually occurred and overestimate risk for cases where complications did not occur. Although lacking in statistical significance for all complications, the interaction with the MySurgeryRisk algorithm resulted in a change in the physicians’ risk-perceptions and improvement in the AUC and net scores for reclassification for the tested postoperative complications. Establishing users’ attention, facilitating information processing, and updating risk-perceptions remains a challenge for all types of risk-assessment tools.6 It appears the algorithm was able to address these challenges, because in a majority of the cases, physicians changed their risk-assessments in response to MySurgeryRisk. We attribute this success due to the trust instilled by the transparent nature of the MySurgeryRisk interface, which highlights important clinical variables contributing to the calculated risks of postoperative complications. The algorithm is deployed currently in a real time, intelligent platform integrated in the clinical workflow for autonomous surgical risk-prediction as a part of a single-center, prospective clinical trial at the University of Florida.13
Physicians’ abilities to predict postoperative outcomes and the comparison of physicians’ risk scores to that of automated predictive risk scores and systems have not been studied extensively and the existing studies have produced mixed findings.12, 13, 15, 16, 20, 21 Among several studies that compared differential diagnosis generators, symptom checkers, and automated electrocardiograms with physicians, the algorithms showed improved accuracy in “less acute” and more “common” scenarios, but in general, physicians had better diagnostic accuracy.20, 21 Studies specific to colorectal and hepatobiliary surgery showed that the surgeon’s gut feeling was more accurate than the POSSUM score to predict postoperative mortality.22, 23 Ivanov et al showed that physicians tended to overestimate risk of postoperative mortality and prolonged ICU stay in patients undergoing coronary artery bypass surgery when compared to a statistical model.24 Detsky et al. showed that the accuracy for ICU physicians’ prediction of in-hospital mortality, return to home at 6 months, and 6-month cognitive function varied considerably and was only slightly better than random.25 The computational algorithm as a greater-capacity and lesser-cost information processing service is a logical next step to augment physicians’ decision-making for rapid identification of patients at risk in the perioperative period.26 Our algorithm outperformed the physicians’ initial risk-assessment of postoperative complications (except for cardiovascular complications) in the majority of cases. When examining the rates of misclassification of events and non-events, physicians tended to overestimate the risks of sentinel events such as 30-day mortality and cardiovascular events likely due to the associated emotional, personal, and professional consequences of failing to recognize these risks.
Physician’s decision-making style can influence their perception of risk and the use of information from decision tools such as algorithms. In spite of our small sample size, we observed that physicians who scored at the extreme of the cognitive reflection test reacted differently in response to interaction with the algorithm. Reasons for this are not known, but studies of decision-making preferences by Frederick et al suggest that this observation could be due to time spent on the risk assessment and the willingness to reassess the decision-making process as assessed by the cognitive reflection test that measures people’s ability to resist their first instinct.17 A high score indicates a reflective thinker whose initial intuition is tempered by analysis and who takes more time to reflect on the risk-probabilities and the information provided by the algorithm. Although our participants had high numeracy scores, it has been demonstrated that even those individuals are likely to make numeric mistakes on relatively simple questions.15, 16
Our study has several limitations. Data used for the MySurgeryRisk algorithm, although more than sufficient in size to have well-fitting and precise models, was collected from a single center. Results may not be generalizable where patient characteristics differ dramatically. Second, the number of physician participants (n=20) was small and homogeneous, making it difficult to provide a robust statistically significant estimate of physician decision-making preferences based on the cognitive reflection test and numeric assessment. We did not compare the accuracy of physician risk-assessments between subgroups, such as the sex or experience level of the physician due to the small sample size and the inability to make inference based on those comparisons. Third, it is plausible that physician risk-assessment was positively influenced by the MySurgeryRisk patient case presentation in summary format, because it is often difficult to find relevant predictive information in the large amount of data in the EHR. Whether an even more optimal way to present numeric clinical data would improve risk-assessment needs to be to clarified in further studies. The MySurgeryRisk algorithm performance is independent of the enrichment of the testing cohort with positive cases, however, physician assessment may be improved due to the increased number of rare complications and specifically for sentinel events. Although physicians also may or may not have estimated the risks more accurately if they had exposure to an increased amount of patient data, we specifically designed our study to reflect everyday practice and routine preoperative risk-assessment environment.
A majority of respondents to the post-test survey found the system easy to use, helpful for decision-making, and appropriate for the clinical environment. Reasons for physician nonresponse to the written post-test survey are unclear and may be related to physician opinion of the algorithm or simply reflect lack of time needed to complete the questionnaire. We have integrated the post-test survey into physician use of the algorithm in our current, prospective follow-study in order to stream line it further and obtain a greater response rate. We continue to further refine the algorithm, allowing participants to input their own assessments into the computational algorithm to facilitate two-way knowledge-transfer and allow models to “learn” from participants. We anticipate expanding our range of complications to allow for greater personalization specific to individual patients and to include the algorithm risk-assessment scores into the EHR. Our larger, prospective clinical evaluation of the algorithm in multiple real-time environments to assess algorithm and participant performance, ease of use in clinical decision-making, and the potential for further decreases in postoperative complications is ongoing.13
Prediction of major postoperative complications is complex and multifactorial; in this pilot study, we have demonstrated that implementation of a validated, computational algorithm for real time predictive analytics with EHR data to augment physicians decision-making is feasible and accepted by physicians. While our study suggests that the low-cost, high-capacity, information-processing power of computational algorithms within an EHR may augment the accuracy of physicians’ risk-assessment, larger studies will be needed to confirm this assumption. The implementation of an autonomous platform for real time analytics and communication with physicians in a perioperative clinical workflow would greatly simplify and augment the perioperative risk-assessment and stratification of patients. With the advance of data science and digitalization of medical records, this type of advanced analytics27, 28 is coming of age for perioperative medicine, and early involvement of physicians as key stakeholders in both design and implementation of this technology will be crucial for its future success.
ACKNOWLEDGMENTS
Meghan Brennan and Sahil Puri contributed equally to the manuscript.
We would like to acknowledge our research colleagues Dmytro Korenkevych, PhD and Paul Thottakkara who have assisted us in this project with development of algorithm risk scores, and Daniel W Freeman for graphical art.
We want to thank Gigi Lipori and University of Florida Integrated Data Repository team for assistance with data retrieval.
Source of Funding: A.B., T.O.B., P.M., X.L., and D.Z.W. were supported by RO1GM110240 from the NIGMS. A.B. and T.O.B. were supported by Sepsis and Critical Illness Research Center Award P50 GM-111152 from the NIGMS. T.O.B. has received a grant (97071) from Clinical and Translational Science Institute, University of Florida. This work was supported in part by the NIH/NCATS Clinical and Translational Sciences Award to the University of Florida UL1 TR000064. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. University of Florida and A.B., T.O.B. , X.L., P.M., and Z.W. have a patent pending on real-time use of clinical data for surgical risk prediction using machine learning models in MySurgeryRisk algorithm.
Footnotes
Conflicts of Interest: NONE
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Tepas JJ, Rimar JM, Hsiao AL, Nussbaum MS. Automated analysis of electronic medical record data reflects the pathophysiology of operative complications. Surgery. 2013;154:918–24. [DOI] [PubMed] [Google Scholar]
- 2.Hobson C, Ozrazgat-Baslanti T, Kuxhausen A, Thottakkara P, Efron PA, Moore FA, et al. Cost and Mortality Associated With Postoperative Acute Kidney Injury. Ann Surg. 2015;261:1207–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lagu T, Rothberg MB, Shieh MS, Pekow PS, Steingrub JS, Lindenauer PK. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–61. [DOI] [PubMed] [Google Scholar]
- 4.Silber JH, Rosenbaum PR, Trudeau ME, Chen W, Zhang X, Kelz RR, et al. Changes in prognosis after the first postoperative complication. Med Care. 2005;43:122–31. [DOI] [PubMed] [Google Scholar]
- 5.Hobson C, Singhania G, Bihorac A. Acute Kidney Injury in the Surgical Patient. Crit Care Clin. 2015;31:705–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harle C, Padman R, Downs J. The impact of web-based diabetes risk calculators on information processing and risk perceptions. AMIA Annu Symp Proc. 2008:283–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Copeland GP, Jones D, Walters M. POSSUM: a scoring system for surgical audit. Br J Surg. 1991;78:355–60. [DOI] [PubMed] [Google Scholar]
- 8.Gawande AA, Kwaan MR, Regenbogen SE, Lipsitz SA, Zinner MJ. An Apgar score for surgery. J Am Coll Surg. 2007;204:201–8. [DOI] [PubMed] [Google Scholar]
- 9.ACS NSQIP Surgical Risk Calculator. 2007–2018.
- 10.Hollis RH, Graham LA, Lazenby JP, Brown DM, Taylor BB, Heslin MJ, et al. A Role for the Early Warning Score in Early Identification of Critical Postoperative Complications. Ann Surg. 2016;263:918–23. [DOI] [PubMed] [Google Scholar]
- 11.Bihorac A, Ozrazgat-Baslanti T, Ebadi A, Motaei A, Madkour M, Pardalos PM, et al. MySurgeryRisk: Development and Validation of a Machine-learning Risk Algorithm for Major Complications and Death After Surgery. Ann Surg. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thottakkara P, Ozrazgat-Baslanti T, Hupf BB, Rashidi P, Pardalos P, Momcilovic P, et al. Application of Machine Learning Techniques to High-Dimensional Clinical Data to Forecast Postoperative Complications. PLoS One. 2016;11:e0155705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Z, Bhat R, Yuan X, Freeman D, Baslanti T, Bihorac A, et al. Intelligent Perioperative System: Towards Real-time Big Data Analytics in Surgery Risk Assessment. arXiv. 2017. [DOI] [PMC free article] [PubMed]
- 14.United States Census Bureau: American FactFinder 2010.
- 15.Lipkus IM, Samsa G, Rimer BK. General performance on a numeracy scale among highly educated samples. Med Decis Making. 2001;21:37–44. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz LM, Woloshin S, Black WC, Welch HG. The role of numeracy in understanding the benefit of screening mammography. Ann Intern Med. 1997;127:966–72. [DOI] [PubMed] [Google Scholar]
- 17.Frederick S Cognitive reflection and decision making. J Econ Perspect. 2005;19:25–42. [Google Scholar]
- 18.Delong ER, Delong DM, Clarkepearson DI. Comparing the Areas under 2 or More Correlated Receiver Operating Characteristic Curves - a Nonparametric Approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 19.Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;30:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon K, Okin PM, Kligfield P. Diagnostic performance of a computer-based ECG rhythm algorithm. J Electrocardiol. 2005;38:235–8. [DOI] [PubMed] [Google Scholar]
- 21.Semigran HL, Levine DM, Nundy S, Mehrotra A. Comparison of Physician and Computer Diagnostic Accuracy. Jama Intern Med. 2016;176:1860–1. [DOI] [PubMed] [Google Scholar]
- 22.Markus PM, Martell J, Leister I, Horstmann O, Brinker J, Becker H. Predicting postoperative morbidity by clinical assessment. Brit J Surg. 2005;92:101–6. [DOI] [PubMed] [Google Scholar]
- 23.Hartley MN, Sagar PM. The surgeon’s ‘gut feeling’ as a predictor of post-operative outcome. Ann R Coll Surg Engl. 1994;76:277–8. [PubMed] [Google Scholar]
- 24.Ivanov J, Borger MA, David TE, Cohen G, Walton N, Naylor CD. Predictive accuracy study: comparing a statistical model to clinicians’ estimates of outcomes after coronary bypass surgery. Ann Thorac Surg. 2000;70:162–8. [DOI] [PubMed] [Google Scholar]
- 25.Detsky ME, Harhay MO, Bayard DF, Delman AM, Buehler AE, Kent SA, et al. Discriminative Accuracy of Physician and Nurse Predictions for Survival and Functional Outcomes 6 Months After an ICU Admission. JAMA. 2017;317:2187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao L, Mark DB. Clinical prediction models: Are we building better mousetraps? J Am Coll Cardiol. 2003;42:851–3. [DOI] [PubMed] [Google Scholar]
- 27.Stead WW. Clinical implications and challenges of artificial intelligence and deep learning. JAMA. 2018;320:1107–8. [DOI] [PubMed] [Google Scholar]
- 28.Shickel B, Tighe PJ, Bihorac A, Rashidi P. Deep EHR: A Survey of Recent Advances in Deep Learning Techniques for Electronic Health Record (EHR) Analysis. IEEE Journal of Biomedical and Health Informatics. 2018;22:1589–604. [DOI] [PMC free article] [PubMed] [Google Scholar]