Abstract
Numerous genetic alterations have been identified during prostate cancer progression. The influence of environmental factors, particularly the diet, on acceleration of tumor progression is largely unknown. Expression levels and/or activity of Src kinase are highly elevated in numerous cancers including advanced stages of prostate cancer. In this study, we demonstrate that high fat diets promoted pathological transformation mediated by the synergy of Src and androgen receptor in vivo. Additionally, a diet high in saturated fat significantly enhanced proliferation of Src-mediated xenograft tumors in comparison with a diet high in unsaturated fat. The saturated fatty acid palmitate, a major constituent in a high fat diet, significantly up-regulated the biosynthesis of palmitoyl-CoA in cancer cells in vitro and in xenograft tumors in vivo. The exogenous palmitate enhanced Src-dependent mitochondrial beta-oxidation. Additionally, it elevated the amount of C16-ceramide and total saturated ceramides, increased the level of Src kinase localized in the cell membrane, and Src mediated downstream signaling, such as the activation of MAPK and FAK. Our results uncover how the metabolism of dietary palmitate cooperates with elevated Src kinase in acceleration of prostate tumor progression.
Keywords: Palmitate, fatty acid metabolism, Src kinase, prostate cancer
Introduction
Prostate cancer is the most common cancer in men and one of the leading causes of cancer-related deaths in developed countries (1). Diet is considered a critical environmental risk factor in prostate cancer progression (2). Numerous epidemiological studies indicate that obesity, higher body mass index, or adult weight gain significantly increases the risk of aggressive prostate cancer, mortality, and recurrence of prostate cancer (3–8). A high fat diet (HFD) or certain dietary saturated fatty acids (FAs) including palmitic acid (PA) and myristic acid (MA) are associated with advanced stages of prostate cancer and increase the risk of prostate cancer-specific mortality (3,5,7,9). Incorporation of excess free FAs into cancer cell membranes promotes cancer invasion by reduction of cell-cell contact and increase of surface adhesion (10). However, the interaction of dietary factors with oncogenic events to regulate tumor progression is not well-understood.
Numerous genetic alterations including aberrant expression and/or loss of tumor suppressor genes have been identified in the initiation and progression of prostate tumors (11). Among those oncogenic events, over-expression and/or activation of Src kinase is common in advanced stages of prostate cancer (12,13). Src kinase is a pleiotropic activator to mediate numerous signal transduction pathways initiated by G-protein coupled receptors, beta 1-integrin, growth factor receptors, and others (14,15). The activation of Src kinase promotes cell survival, proliferation, migration, and invasion in cancer development, and facilitates epithelial-to-mesenchymal transition during cancer progression (16). Our previous study indicated that over-expression of Src kinase synergizes with androgen receptor (AR) in promoting prostate tumorigenesis (17,18). Additionally, Src mediated AR activation promotes development of androgen independent prostate cancer (13). Quantitative mass spectrometry analysis reveals that 50% of metastatic castration resistant prostate cancer patients have enrichment of Src tyrosine kinase, and >80% of patients are candidates for Src-inhibitor treatment (19).
Our previous studies have shown that a high fat diet accelerates Src-mediated tumorigenesis. Particularly, dietary MA significantly increases biosynthesis of myristoyl-CoA, expression levels of myristoylated Src, and enhances its kinase activity (20). PA is a major saturated fatty acid component in a Western diet (21). In this study, we demonstrate that a diet high in saturated fat promotes growth of Src-mediated tumors and pathological transformation. Dietary PA could fuel Src-dependent beta-oxidation, and increase Src mediated signaling. These results demonstrate a collaboration of Src-mediated signaling with dietary palmitate in promoting tumor progression.
MATERIALS AND METHODS
Plasmid construction and lentiviral production
Lentiviral vector for over-expression of Src(WT), Src(Y529F), Src(WT)+AR, and AR were constructed as previously reported using the FUCRW parental vector (17,18). The TRE/Src(Y529F) doxycycline inducible lentiviral vector was previously created using pTK380 as a parental vector (22). Co-expression of Src(WT) and AR in a lentiviral vector was created by removing the fragment between BamH1 and Pme1 from pCSCMV-AR vector and replacing it with Src(WT). Therefore, the created lentiviral vector expressed c-Src under the ubiquitin promoter, and AR under the CMV promoter without RFP or GFP markers. shRNA-Src construct was used to knockdown the human Src gene as previously reported (18). Lentiviral production and infection were performed as described previously (23). Lentivirus usage followed all safety guidelines and regulations according to the University of Georgia.
Cell lines and cell culture
PC-3, 22Rv1, DU145 cells, and PNT2 (normal prostate epithelial cells) were purchased from ATCC and cultured in ATCC-recommended media supplemented with 10% fetal bovine serum and 1% antibiotic solution (100 units/mL penicillin, 100 μg/mL streptomycin) in a humidified 5% CO2-containing atmosphere at 37 xC. PC-3 cells were transduced with shRNA-control or shRNA-Src by lentiviral infection. The stable cell lines with knock down of Src kinase were created for the xenograft tumor study.
SYF1 (Src−/−Yes−/−Fyn−/−) mouse fibroblast cells expressing c-Src were created by transduction of SYF1 cells with Src(WT) through lentiviral infection (bicistronic vector containing RFP as a marker) (17). RFP-expressing cells were isolated by the FACSAria cell sorter (BD Biosciences) to exclude the uninfected cells. HEK 293T cells carrying rtTA were transduced with TRE/Src(Y529F) by lentiviral infection. The stable cell line expressing Src(Y529F) kinase could be induced by addition of doxycycline (1 μg/mL) in DMEM medium with 10% FBS (HyClone, Logan, UT). All cell lines in this study were tested by the Universal Mycoplasma Detection Kit (ATCC catalog no. 30–1012K) and determined to be mycoplasma free. The cell lines were used within no more than 20 passages, and SYF1 cells and 293T-rtTA cells were used within no more than 35 passages.
Xenograft tumors
For PC-3 xenograft tumors, a clonal PC-3 cell expressing shRNA-Control or shRNA-Src was selected and expanded in F-12K medium with 10% FBS. 3×105 cells were mixed with 50 μL of collagen type I (pH 7.4) and inoculated on the both lateral flanks of SCID mice by subcutaneous injection. Host mice carrying PC-3 xenografts were placed on a 10% low fat diet, 40% unsaturated fat diet, or 40% saturated fat diet for 8 weeks designed by Research Diets (New Brunswick, NJ) (Supplemental Table 1–2). Tumor sizes were measured (length × length × width /2) with Vernier calipers once a week. Body weight and food intake were also measured weekly. After 8 weeks, the animals were sacrificed and the tumors were harvested and weighed. Collected tumors and tissues were stored in 10% buffered formalin for the histology analysis and in a deep freezer for the biochemistry assays.
For setting up the 22Rv1 xenograft tumors, 22Rv1 cells were transduced with Src(WT) by lentiviral infection and the transduced cells [22Rv1+Src(WT)] or 22Rv1 cells were cultured in RPMI 1640 medium with 10% FBS. Cells (2×105 cells per xenograft) were mixed with 50 μL of collagen type I (pH 7.4) and inoculated on the both lateral flanks of SCID mice by subcutaneous injection. Host mice carrying 22Rv1 or 22Rv1+Src(WT) were placed on with 10% LF or 40% HSF diet for 7 or 8 weeks. 22Rv1+Src(WT) xenograft tumors from the 40% HSF diet group were sacrificed at 7 weeks due to tumor burden. Tumors were measured as described above.
Prostate regeneration assay
The prostate regeneration assay has been well-described previously (24). In brief, prostate tissues were isolated from 8–12 week-old C57BL/6J male mice and were minced into small pieces. The tissues were digested by collagenase in DMEM medium for at least 1 hour at 37°C, and further digested by 0.5% trypsin for 5–10 minutes. The prostate tissue becomes single cells after the above processes. The isolated primary prostate cells were transduced with Src(WT)+AR by lentiviral infection. The transduced cells (3×105 cells/graft) were combined with urogenital sinus mesenchyme (UGSM) (3×105 cells/graft) and mixed with 25 μL of collagen type I (adjusted to pH 7.0). After overnight incubation, the cell mixture formed as a graft, and was implanted under the kidney capsule in SCID mice by survival surgery.
To isolate UGSM cells, 16.5 days mice embryo were obtained from pregnant mothers. Urogenital sinus epithelium cells were separated and removed from UGSM cells by micro-dissection. UGSM cells were grown in DMEM medium containing 5% FBS, 5% Nu serum, 1% antibiotic solution (100 units/mL penicillin, 100 μg/mL streptomycin), and 1mg/ml insulin at 37 °C in a 5% CO2 atmosphere. UGSM cells were not used after 5 passages. Of note, primary prostate cells isolated from the adult tissue will develop into tubules in the regenerated prostate tissues due to differentiation of adult stem cells. However, UGSM cells will only develop into stromal cells in the regenerated prostate tissue.
After 3 days of implantation, host SCID mice carrying Src(WT)+AR transduced grafts were placed on 10% (Cat#: D12450J), 45% (Cat#: D12451), or 60% (Cat#: D12492) fat diet designed by Research Diets (Supplemental table 3–4). Body weight and food intake were measured once a week. All animals were sacrificed at 12 weeks after grafts were implanted. The grafts, liver and fat tissues were collected and stored for histology analysis.
C57BL/6J and CB.17SCID/SCID (SCID) mice were purchased from Taconic (Hudson, NY). All animals were maintained and used according to the surgical and experimental procedures of the protocol A2013 03–008. The protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Georgia
Acyl-CoA analysis by LC-MS/MS
Quantification of acyl-CoAs including decanoyl- (C10:0), lauroyl- (C12:0), myristoyl-CoA (C14:0), and palmitoyl-CoA (C16:0) has been described in a previous study (25). Acyl-CoAs were extracted from cells or tumor tissue by the addition of 2 mL methanol (with 15 μL of 25 μg/mL pentadecanoyl-CoA as an internal standard) at −80 °C for 15 min. Standards of the four acyl CoAs prepared from ammonium salts were used (Avanti Polar Lipids, Alabaster, AL). The collected samples and standards were subjected to LC–MS/MS analysis using an HPLC system (with 1100 binary pump) (Santa Clara, CA) coupled with a Waters Micromass Quattro Micro triple quadrupole mass spectrometer. The details of the simultaneous detection of acyl-CoAs and internal standard were described in previous publications (20,25).
Protein preparation and antibodies
PC-3, 293T, or 22Rv1 derived cells were serum-starved for 24 hours, then cultured with 2% fatty acid free BSA and individual fatty acids overnight. Protein lysate was extracted using RIPA buffer containing 50 mM Tris-HCl pH 7.4, 150 mM NaCl, 5 mM EDTA, 10% glycerol, 1% Triton X-100, 0.1% SDS, 0.5% sodium deoxycholate, protease inhibitor cocktail and phosphatase inhibitor cocktail 1 and 2. Src kinase and downstream signaling proteins were analyzed by Western blot. Antibodies against total Src, phospho-Src(Y416), FAK, pFAK(Y925), GAPDH, and Caveolin-1 were purchased from Cell Signaling Technology (Beverly, MA). Antibodies against Androgen receptor (AR) and Erk2 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). All other reagents were purchased from Sigma Chemical Co. (St. Louis, MO) and Calbiochem (San Diego, CA).
Immunohistochemistry (IHC)
Xenografts and prostate regenerated tissue samples were dissected and fixed in 10% buffered formalin for histology and IHC analysis. Hematoxylin and eosin (H&E) staining and IHC analysis were performed as described previously (17,23). Briefly, formalin-fixed/paraffin-embedded grafts and tissues were sectioned at 4 μm thickness and mounted on positively charged microscope slides. Sections were stained with H&E, and IHC analysis was performed as follows: primary antibodies and dilutions for Ki-67 (1:400), Src (1:250), AR (1:200), CK8 (1:1000) and CK5 (1:1000) were used. Illuminated and fluorescent images were taken with dissecting and fluorescence microscopes.
Protein fractionation
Fractions of detergent resistant and non-detergent resistant membranes (DRM) were prepared using a slightly modified procedure described previously (26). Briefly, Src(WT)-expressing SYF1 cells were serum-starved for 24 hours, then cultured with 2% fatty acid free BSA with or without 400 μM palmitic acid overnight. Protein lysate was extracted by TNF lysis buffer containing 50 mM Tris, 150 mM NaCl and 2 mM EDTA pH 7.4, protease inhibitor cocktail and phosphatase inhibitor cocktails, and homogenized using a syringe with a 25-gauge needle (20 strokes). The lysate was centrifuged at 14,000 rpm for 20 min and the supernatant was defined as the cytosolic fraction (Cyt). Pellets were re-suspended in TNE lysis buffer containing 60 mM octyl β-D-glucopyranoside and incubated on ice for 60 min, followed by centrifugation at 14,000 rpm for 20 min. The supernatant was defined as the total membrane fraction (TM).
To isolate the detergent soluble and insoluble protein lysate after the Cyt fraction was removed as described in the above, pellets were re-suspended in TNE lysis buffer containing 1% Triton X-100 for 60 min in ice. The soluble fraction (S) also called the non-DRM was obtained after being centrifuged at 14,000 rpm for 20 min at 4 °C. The insoluble fraction (I) was rinsed twice with TNE lysis buffer and re-suspended in TNE lysis buffer containing 60 mM octyl β-D-glucopyranoside and incubated 30 min in ice. After centrifugation at 14,000 rpm for 20 min at 4 °C, the supernatant was collected as the insoluble fraction.
Measurements of mitochondrial respiration
PC-3 cells expressing shRNA-Control or shRNA-Src were seeded on a XF24 cell culture microplate at a density of 4×104 cells/well with F-12K medium and cultured in a humidified 5% CO2-containing atmosphere at 37 °C overnight. 22Rv1 cells expressing control vector or Src(WT) were seeded on a XF24 cell culture microplate at a density of 6×104 cells/well.
The cells were washed with KHB media (111 mM NaCl, 4.7 mM KCl, 1.25 mM CaCl2, 2 mM MgSO4, 1.2 mM NaH2PO4 supplemented with 2.5 mM glucose, 0.5 mM carnitine, and 5 mM HEPES, adjusted to pH 7.4) and cultured with 400 μL/well of KHB medium in a non-CO2 incubator for 45 min at 37 °C. Oligomycin (3 μM), FCCP (5 μM), and rotenone/antimycin A (2 μM) were loaded into the microplate. The cells were incubated with palmitate-BSA or BSA for 15 min at 37 °C and then the microplate was inserted into the XF24 analyzer. Three or four baseline measurements were recorded before sequential injection of oligomycin (ATP synthase complex inhibitor), FCCP (carbonyl cyanide-p-trifluoromethoxy-phenylhydrazone, an uncoupling agent that allows maximum electron transport), and rotenone/antimycin A (complex I and III inhibitors blocking mitochondrial respiration) and the mitochondrial respiration rate was measured.
Lipidomics analysis
The cells were cultured in DMEM medium with 2% BSA with control (DMSO), 400 μM palmitic acid, or 400 μM decanoic acid for 24 hours and cell pellets were collected. Additionally, PC-3 cell xenografts derived from 10%, 45%, and 60% fat diets were homogenized in homogenization buffer (pH 7.4) containing 0.25 M sucrose, 25 mM KCl, 50 mM Tris, and 0.5 mM EDTA. N-acyl-sphingosine/dihydrosphingosine (ceramide/dihydroceramide) molecular species were identified and quantified by HPLC-MS/MS (high performance liquid chromatography tandem mass spectroscopy) in the Lipidomics Shared Resource Core at the Medical University of South Carolina (MUSC) as previously described (27). All ceramide values were normalized with C16:0 ceramide in the control or lipid phosphate. Samples were measured in triplicate.
Statistical Analysis
All results were expressed as the standard error of the mean (SEM). Differences between groups were examined for statistical significance using the Student’s t test. All t tests were performed at the two-sided 0.05 level for significance. *: p<0.05; **: p<0.01.
RESULTS
High fat diet (HFD) accelerates Src-mediated pathological transformation and tumor progression.
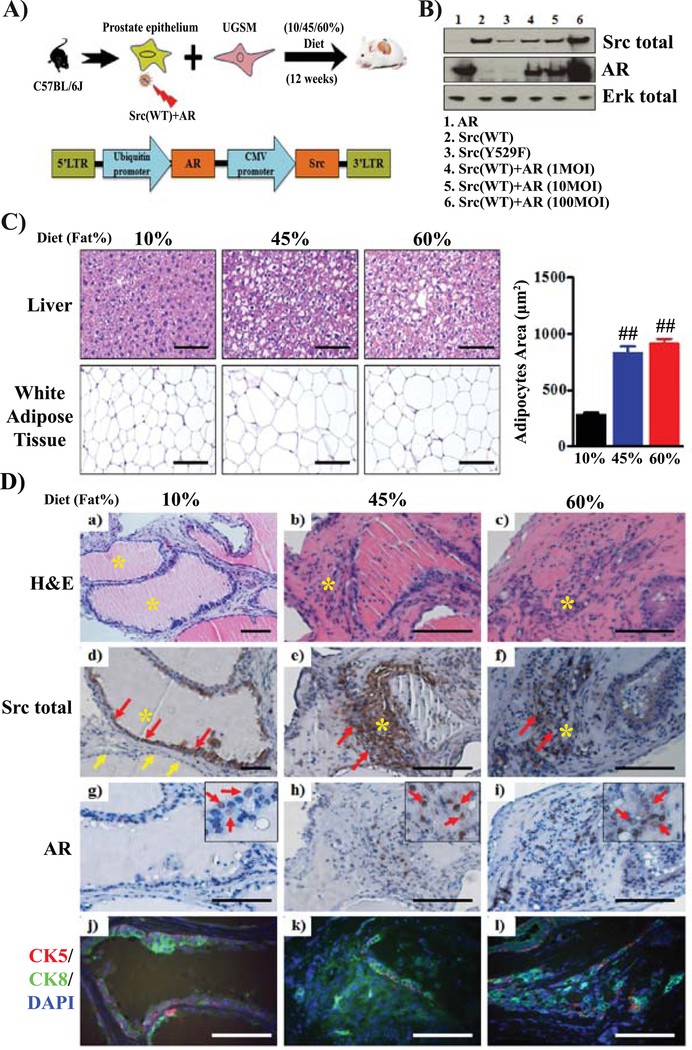
We have previously shown that crosstalk of c-Src and AR increases levels of activated Src kinase and induces invasive prostate cancer (17). We cloned c-Src and AR in a bicistronic lentiviral vector (Fig. 1A–B). In this study, we examine if a high fat diet accelerates Src mediated prostate tumor progression using the prostate tissue regeneration model (Fig. 1A). The size of adipocytes in white adipose tissue and the amount of fat accumulated in hepatocytes were higher in host SCID mice fed the 45% or 60% fat diets compared to mice on the 10% fat diet (Supplemental Table 1–2) (Fig. 1C). While the regenerated tissues transduced with Src and AR in the 10% fat diet group contained normal prostate tubules (Fig. 1D–a), Src(WT)+AR transformed tissues contained tumorigenic cells that invaded into the neighboring stromal microenvironment in the 45 and 60% fat diet groups (Fig.1D–b and c). The elevated expression levels of Src kinase (Fig.1D–d to f) and AR (Fig.1D–g to I, and Fig. S1) were confirmed in the regenerated tubules (red arrows). Additionally, the regenerated tissues transduced with Src (WT) and AR in the 45 or 60% fat diet groups showed an expansion of CK8+ cells (Fig. 1D–k and l) in comparison with those expressing CK8+ in the luminal compartment and CK5+ cells in the basal membrane in the 10% fat diet group (Fig.1D–i). The data suggest that a HFD promotes the synergy of Src(WT)+AR mediated prostate tumorigenesis.
FIGURE 1. High saturated fat diet promotes the synergy of Src(WT)+AR induced prostate tumorigenesis.
(A) Schematic of the in vivo prostate regeneration assay. Prostate tissues were isolated from C57BL/6J mice and were dissociated into single cells. The isolated primary prostate epithelial cells were transduced with Src(WT) and AR by lentiviral infection. The bicistronic lentiviral vector for co-overexpression of Src(WT) and AR was created as shown in the scheme. The transduced prostate epithelial cells were mixed with urogenital mesenchymal cells (UGSM). The cell mixture was implanted under the renal capsule of SCID mice. The SCID mice carrying the grafts were fed a 10%, 45%, or 60% fat diet for 12 weeks (n=4 per group). The dietary components including the amount and type of fatty acids are listed in Supplemental Table 1 and 2. (B) Co-overexpression of Src(WT) and AR in lentiviral vector. 293T cells were transduced with AR, Src(WT), Src(Y529F), or AR+Src(WT) (MOI =1, 10, or 100) by lentiviral infection. Expression levels of AR, Src, and Erk were examined by immunoblotting. (C) Representative images of H&E staining of liver and white adipose tissue derived from the host SCID mice on 10%, 45%, or 60% fat diets after 12 weeks in (A). High fat diets (45% and 60% fat diets) increase the size of white adipose cells and lipid deposits in the liver cells. (D) The regenerated tissues from (A) were subjected to H&E and IHC staining. Representative H&E (a-c) and IHC staining of total Src (d-f), AR (g-i), and co-staining of CK5 (basal cell marker)/ CK8 (luminal cell marker)/DAPI (j-l) in tumors are displayed (scale bar, 100 μm). Yellow asterisks indicate the regenerated tubules or tissue transduced with Src(WT)+AR, based on elevated levels of Src and/or AR in IHC. Red arrows indicate the regenerated tissues being transduced with Src(WT)+AR and yellow arrows indicate the neighboring control tubule not being transduced.
A high saturated fat diet accelerates Src kinase-mediated prostate tumor progression.
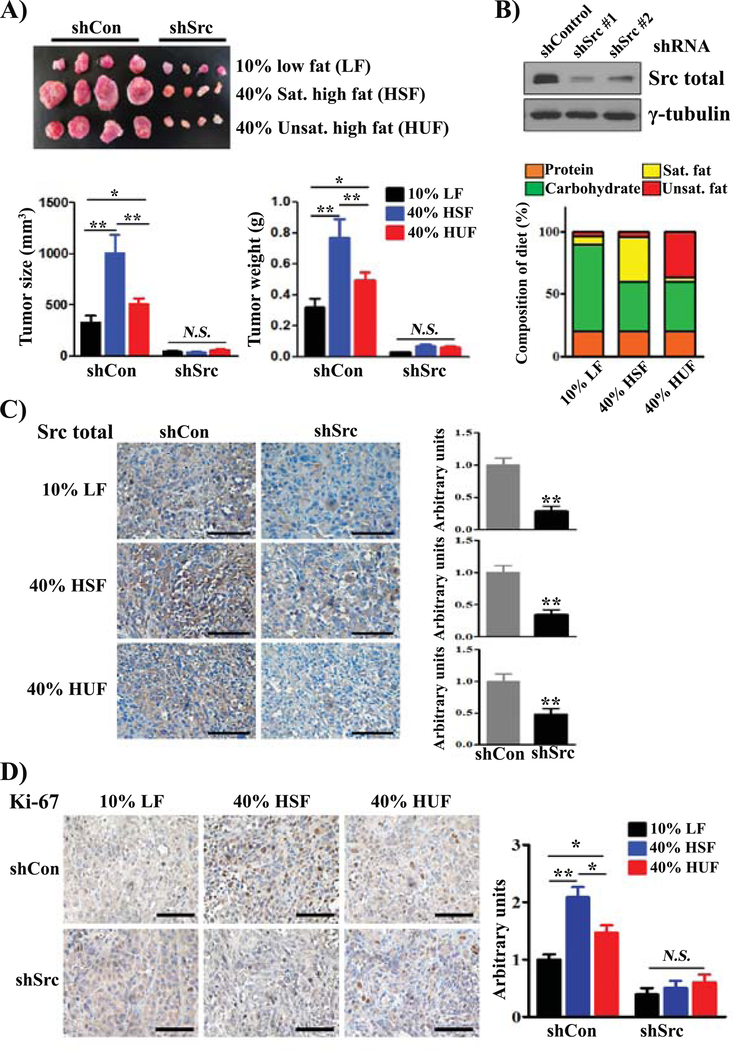
We further determined if saturated fat plays a more important role than unsaturated fat in accelerating Src-mediated prostate tumor growth. We have previously shown that PC-3 exhibited higher expression levels of activated Src in comparison with other prostate cancer cell lines (20). Host SCID mice with PC-3 xenografts expressing shRNA-control or shRNA-Src were placed on 10% low fat (LF), 40% high saturated fat (HSF), or 40% high unsaturated fat (HUF) diets (Fig. 2A–B). All three diets contain the same total amount of calories, but 36% of the calories come from saturated fat in the 40% HSF diet and 36% from unsaturated fat in the 40% HUF diet (Supplemental Table S3–S4, Fig. 2B). The size and weight of xenograft tumors expressing shRNA-Src were significantly lower compared to the control regardless of the diet (Fig. 2A). Additionally, the size and weight of PC-3 xenograft tumors expressing shRNA-control were significantly elevated in both 40% HSF and HUF diets in comparison with 10% LF diet (Fig. 2A). This suggests that acceleration of tumor growth by dietary fat was mediated by Src kinase. Furthermore, the data also indicate that saturated fat plays a more significant role in acceleration of PC-3 xenograft tumors compared to unsaturated fat (Fig. 2A).
FIGURE 2. Src kinase mediates high saturated fat diet accelerated prostate tumor progression.
(A) PC-3 cells were transduced with shRNA-Control or shRNA-Src by lentiviral infection. The transduced cells were implanted into the flank sides of SCID mice subcutaneously (n = 9 per group). The mice were placed on a 10% low fat diet (LF), 40% saturated fat (HSF) diet, or 40% unsaturated fat (HUF) diet for 8 weeks. The dietary components including the amount and type of fatty acids are listed in Supplemental Table 3 and 4. All the diets contain 20% of total calories from proteins. The 10% LF diet contained 70% of the total calories from carbohydrate and 6% from saturated fat and 4% from unsaturated fat. Both the 40% HSF and HUF contain 40% of total calories from carbohydrate. While the 40% HSF has 36% of calories derived from saturated fat and 4% from unsaturated fat, the 40% HUF has 4% of calories from saturated fat and 36% from unsaturated fat. Representative images, sizes and weights of xenograft tumors are shown. (B) PC-3 cells were transduced with shRNA-control or shRNA-Src#1 or #2 by lentiviral infection. The expression levels of Src kinase were analyzed by immunoblotting. (C-D) Expression levels of Src kinase (C) and Ki-67 (D) in PC-3 xenograft tumors in panel A were examined by IHC staining (scale bars, 200 μm). *: p < 0.05; **: p < 0.01; N.S.: not significant.
Downregulation of Src kinase levels in xenograft tumors expressing shRNA-Src in all three diets were further confirmed (Fig. 2C). Levels of the cell proliferation marker Ki-67 were elevated in 40% HSF shRNA-control tumors relative to 10% LF or 40% HUF shRNA-control tumors (Fig. 2D). These markers were suppressed in shRNA-Src tumors from all three different diets. Collectively, the data support that a HSF diet accelerates tumor progression by elevation of Src mediated cancer cell proliferation.
A high fat diet synergizes with ectopically expressed Src kinase in tumor progression.
To investigate if a high fat diet promotes tumor progression with ectopically expressed Src in androgen receptor positive cancer cells, 22Rv1 cells were transduced with control vector or Src(WT). Host SCID mice carrying 22Rv1 xenografts or 22Rv1+Src(WT) were placed on a 10% low-fat (LF) or 40% high saturated fat diet (HSF) (Fig. S2A–B). The size and weight of 22Rv1 xenograft tumors showed no significant difference between the LFD and the HSF diet (Fig. S2A). Similarly, expression levels of Ki67 in tumors were not significantly different between diets. However, the size and weight of 22Rv1+Src(WT) xenograft tumors from the HSF diet group were significantly elevated in comparison with those from the LFD (Fig. S2B). The ectopic expression of Src was confirmed by IHC. Expression levels of Ki67 (positive staining in the nucleus) was elevated in 22Rv1+Src(WT) xenograft tumors from the HSF diet group in comparison with those from the LF diet (Fig.S2C). The data suggest that high saturated fat synergizes with ectopic Src kinase to promote tumor progression.
Exogenous PA elevates the levels of palmitoyl-CoA.
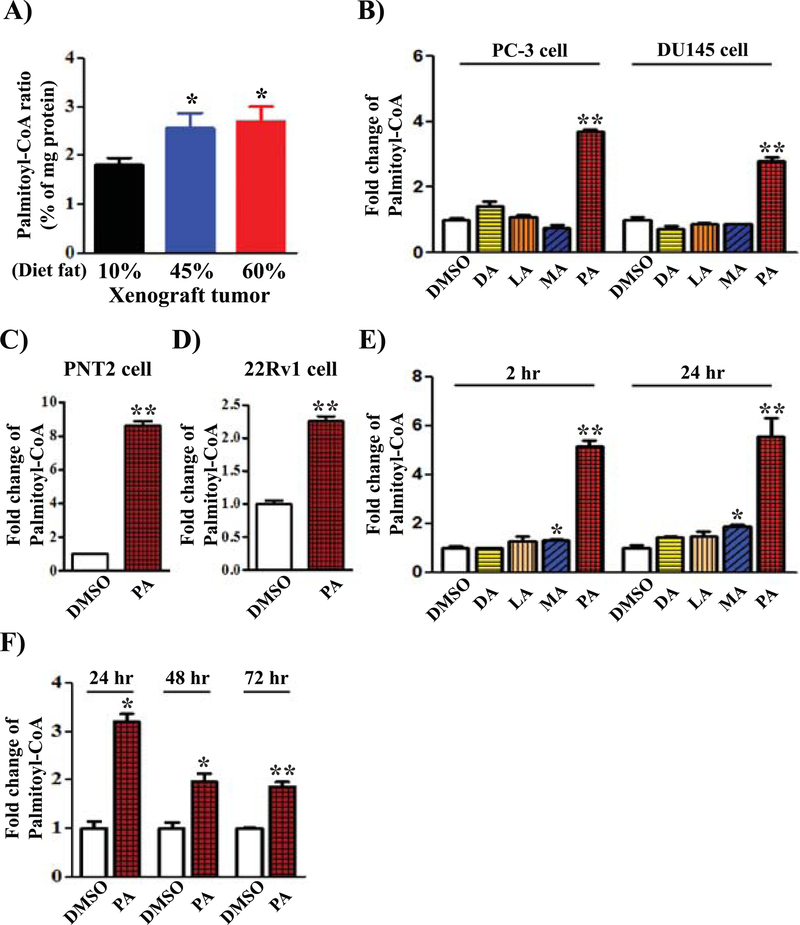
PA is one of the major sources of saturated fat in the tested high fat diet (Supplemental Table S2 and S4). Exogenous PA must be converted into palmitoyl-CoA to further participate in downstream metabolism (28). We examined the levels of palmitoyl-CoA in PC-3 xenograft tumors derived from different fat diets. Indeed, palmitoyl-CoA levels were significantly increased in xenograft tumors from 45% or 60% fat diets in comparison with those from the 10% fat diet (Fig. 3A). To examine whether other saturated fatty acids contribute to the biosynthesis of palmitoyl-CoA, we determined the levels of palmitoyl-CoA in prostate cancer cells grown with various fatty acids including decanoic acid (DA, C10:0), lauric acid (LA, C12:0), myristic acid (MA, C14:0), or palmitic acid (PA, C16:0). The amount of cellular palmitoyl-CoA (C16:0) was markedly increased only after addition of PA (Fig. 3B). Similarly, increased levels of palmitoyl-CoA after the addition of PA was also observed in PNT (normal prostate epithelium) and 22Rv1 cells (Fig. 3C–D). While exogenous PA significantly elevated the production of palmitoyl-CoA and was detected at 2 hours or 24 hours after addition of PA to the medium, other fatty acids such as MA only led to a minor increase of palmitoyl-CoA (Fig. 3E). The levels of palmitoyl-CoA were maintained after 24 hours but decreased with extended incubation time (48 hours and 72 hours) (Fig. 3F). Exogenous PA also did not change levels of other short-chain CoAs such as decanoyl-CoA or lauroyl-CoA (Supplemental Fig. S3). The results suggest that only a very small portion of shorter chain FAs could contribute to the palmitoyl-CoA pool. Palmitoyl-CoA levels were mainly derived from exogenous PA.
FIGURE 3. Biosynthesis of acyl-CoAs in prostate cancer cells and xenograft tumors.
(A) The levels of palmitoyl-CoA in PC-3 xenograft tumors from mice on 10%, 45%, or 60% fat diet were analyzed by LC/MS-MS. The amount of palmitoyl-CoA was standardized to the amount of total protein in the tumors. Seven tumors per group were analyzed. (B) PC-3 or DU145 cells were grown in DMEM medium with 10% FBS, and treated with DMSO (control), decanoic acid (DA, C10:0), lauric acid (LA, C12:0), myristic acid (MA, C14:0), or palmitic acid (PA, C16:0) for 24 hours. (C-D) PNT2 or 22Rv1 cells were grown in RPMI1640 medium with 10% FBS and treated with DMSO or palmitic acid (PA, C16:0) for 24 hours. (E) 293T cells expressing doxycycline inducible Src(Y529F) were treated with decanoic acid (DA, C10:0), lauric acid (LA, C12:0), myristic acid (MA, C14:0), or palmitic acid (PA, C16:0) for 2 and 24 hours. Cellular palmitoyl-CoA was extracted as described in the Materials and Methods. Levels of cellular palmitoyl-CoA were analyzed by LC/MS-MS. The amount of cellular palmitoyl-CoA from different fatty acid treatments was divided with that in the DMSO control. Therefore, the ratio of palmitoyl-CoA in the DMSO treatment group was set as 1. (F) 293T cells expressing doxycycline inducible Src(Y529F) were treated with DMSO (Control) or palmitic acid (PA, C16:0) for 24, 48, 72 hours. The levels of palmitoyl-CoA were analyzed and compared with the levels in the DMSO control. The ratio of palmitoyl-CoA in the DMSO treatment group was set as 1 (three repeats in each group).
Collaboration of exogenous PA with Src kinase in regulation of beta-oxidation in the mitochondria.
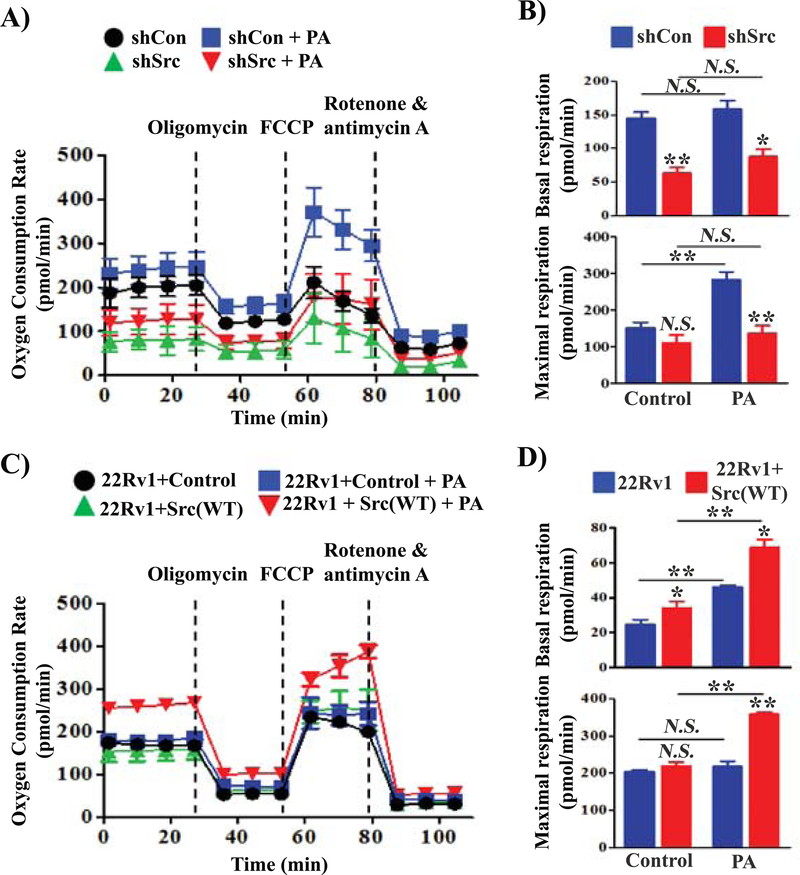
Palmitoyl-CoA is a major substrate for ATP production through beta-oxidation in the mitochondria. Given that PC-3 cells have higher endogenous Src kinase activity than other prostate cancer cell lines, we examined whether Src kinase was involved in PA-stimulated mitochondrial respiration including the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) in PC-3 cells. Knockdown of Src significantly inhibited the OCR (Fig. 4A and Fig. 4B top panel) and ECAR (Fig. S4A and S4B top panel) independent of PA during basal respiration. Exogenous PA led to a 2-fold increase of the OCR (Fig. 4A and Fig. 4B bottom panel) and ECAR rate in PC-3 cells (Fig. S4A and Fig. S4B bottom panel), but not in PC-3 cells expressing shRNA-Src in the maximal respiration phase, or in 293T cells (normal cells) (Supplemental Fig. S5). During maximal respiration, knockdown of Src also significantly inhibited the OCR and ECAR even under the stimulation of PA.
FIGURE 4. Src kinase synergizes with exogenous palmitic acid to regulate mitochondrial respiration in prostate cancer cells.
(A-B) PC-3 cells were transduced with shRNA-Control or shRNA-Src by lentiviral infection. 4 × 104 of the transduced PC-3 cells were plated in each well of the XF24 cell culture plate. (C-D) 22Rv1 cells were transduced with control vector (Control) or Src(WT) by lentiviral infection. 6 × 104 of the transduced 22Rv1 cells were plated in each well of the XF24 cell culture plate. The cells were grown with 10% FBS overnight. The medium was replaced with substrate-limited medium to prime the cells for utilization of exogenous PA and tested with XF Palmitate-BSA FAO Substrate. Oxygen consumption rate (OCR) was measured using a Seahorse Extracellular Flux (XF) Analyzer after sequential injection of oligomycin (OM, 3 μM), FCCP (5 μM), and rotenone & antimycin (R/A, 2 μM). Basal respiration and maximal respiration were calculated based on the data from Panel A or C for PC-3 cells (B) and 22Rv1 cell (D), respectively. Data are represented as mean ± SD (n=5). *: p<0.05; **: p<0.01; N.S., not significant.
Additionally, we examined if exogenous PA collaborates with ectopic levels of Src kinase in mitochondrial respiration. 22Rv1 cells were transduced with Src(WT) or control vector by lentiviral infection. Over-expression of Src kinase increased the OCR (Fig. 4C and Fig. 4D top panel) and ECAR (Fig. S4C and Fig.S4D top panel) in basal respiration. Exogenous PA significantly increased the OCR (Fig. 4C and Fig. 4D bottom panel) and ECAR rate in 22Rv1+Src(WT) (Fig. S4C and Fig. S4D bottom panel), but not in 22Rv1+control in the maximal respiration phase. Collectively, the data suggest that exogenous PA synergizes with Src kinase mediated β-oxidation, thereby regulating mitochondrial activity and ATP production to fuel the growth of cancer cells.
Exogenous PA promotes Src kinase levels in the cell membrane.
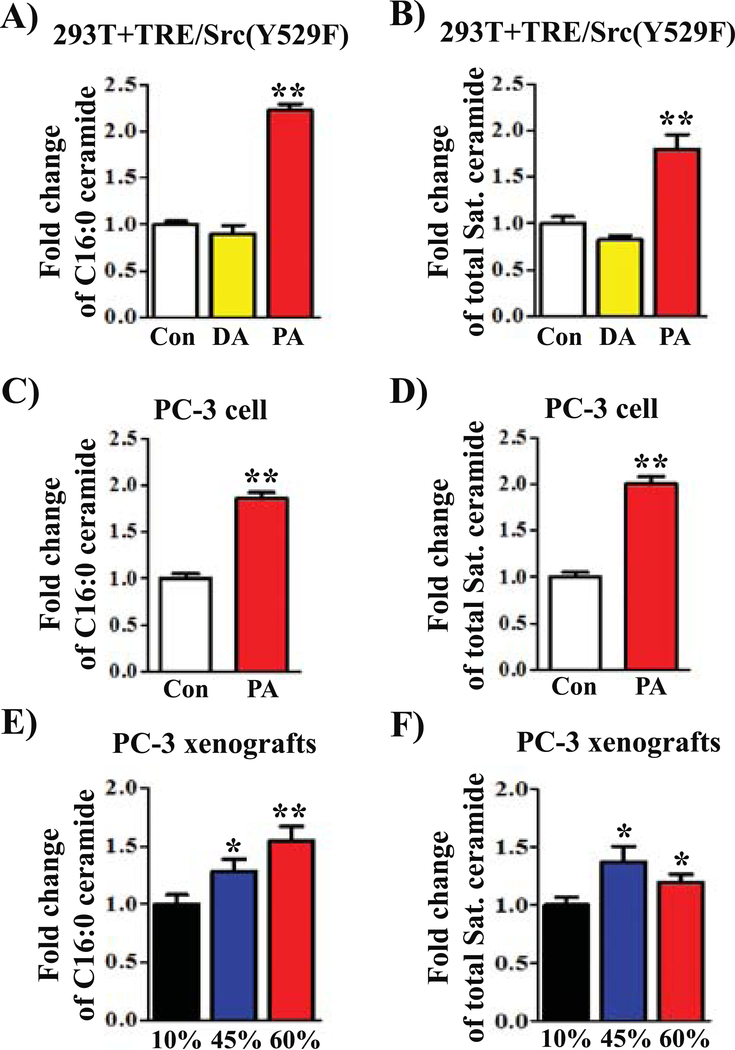
Palmitoyl-CoA is a substrate for ceramide synthesis (29). We next analyzed if exogenous PA affects cellular ceramide compositions. As expected, lipidomics analysis of ceramide species with the N-acyl-chain bearing the C18-sphingoid backbone showed that exogenous PA led to a significant increase in C16:0 ceramide (including C16:0 dihydroceramide) (Fig. 5 A and C) and the total amount of saturated ceramides (Fig. 5B and D, and Supplemental Fig. S6A–B). No major alterations in the ceramide composition were detected by addition of decanoic acid (DA) (Fig. 5A–B, and Supplemental Fig. S6A). Additionally, PC-3 xenograft tumors from both 45% and 60% fat diets significantly increased C16:0 ceramide and the total amount of saturated ceramides as well (p<0.05) (Fig. 5E–F, and Supplemental Fig. S6C).
FIGURE 5. Exogenous palmitic acid or a high fat diet change ceramide compositions.
(A-D) 293T cells expressing Src(Y529F) (A-B) and PC-3 prostate cancer cells (C-D) were grown in DMEM medium with DMSO control (Con), 400 μM palmitic acid (PA, C16:0), or 600 μM decanoic acid (DA, C10:0) for 24 hours. The cells were collected for lipidomics analysis. (E-F) PC-3 xenograft tumors from mice placed on 10%, 45%, or 60% fat diets were collected for ceramide analysis. Ceramide profiles (also see the Supplemental Figure 4) were measured by HPLC-MS/MS described in Materials and Methods. Fold changes of C16:0 ceramide (A, C, and E) and total saturated ceramide profiles (B, D, and F) were calculated. The amount of the control treatment was set as 1. *: p<0.05; **: p<0.01.
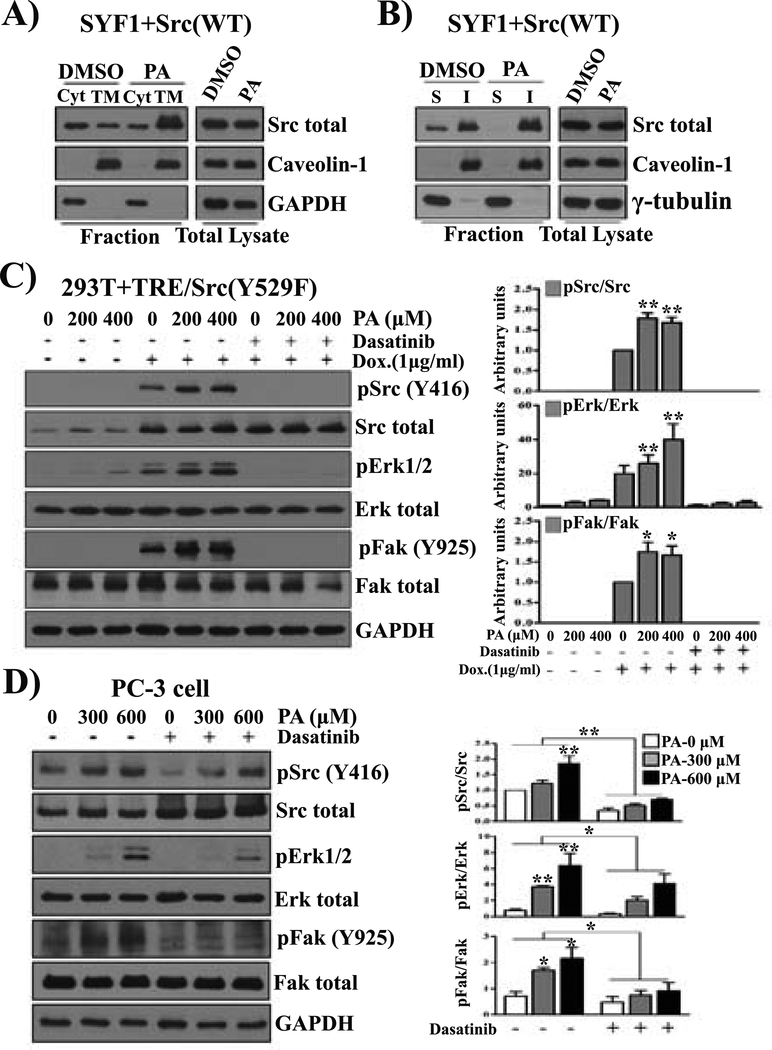
Ceramide compositions determine the formation of detergent resistant lipid raft microdomains in the cell membrane and subsequently the association of Src kinase in the membrane (30). We determined that Src levels in the cell membrane were affected by exogenous PA. Indeed, exogenous PA significantly increased Src levels in the total membrane fraction (Fig. 6A) and the detergent-resistant cell membrane (Fig. 6B).
FIGURE 6. Exogenous palmitic acid promotes Src mediated oncogenic signaling and localization at the cell membrane.
(A-B) Exogenous palmitic acid (PA) increases Src levels at the cell membrane fraction. SYF1 cells expressing Src(WT) were cultured in DMEM with 2% fatty acid free BSA. Cells were treated with or without 400 μM PA for 24 hours. Expression levels of Src kinase in the cytosol (Cyt) and the cell membrane (TM) fraction. Caveolin-1 and GAPDH were used as markers for TM and Cyt, respectively (A). Additionally, expression levels of Src kinase in the detergent soluble (S) and insoluble (I) membrane fractions were determined by immunoblotting. Tubulin and caveolin-1 were used as markers for detergent soluble (S) and insoluble (I) fractions, respectively (B). (C) 293T cells expressing doxycycline-inducible Src(Y529F) (293T+TRE/Src(Y529F) were grown in DMEM with 2% fatty acid free BSA. After treatment with dasatinib (10 nM) for 1 hour, the cells were further treated with DMSO, 200, or 400 μM of PA with/without doxycycline (1 μg/mL) for 24 hours. (D) PC-3 cells were grown in F-12K medium with 2% fatty acid free BSA and treated with DMSO, 300, or 600 μM of PA or PA and dasatinib (10 nm) for 24 hours. The levels of total Src, pSrc(Y416), total Fak, pFak(Y925), total Erk, and pErk1/2 were analyzed by Western blot. The data represent three independent experiments.
Exogenous PA enhances Src kinase downstream oncogenic signaling.
We examined if PA contributes to Src-mediated oncogenic signaling. MAPK and activation of FAK are downstream of Src mediated signaling (15,16). Based on the physiological concentration of PA in the blood 8 hours after a meal (1 g fat/kg body weight) of healthy men, 200 μM and 400 μM PA were examined (31). The levels of pERK and pFAK increased with the induction of pSrc by the addition of doxycycline, and the activity was significantly elevated by an increase in PA (from 0 to 200 or 400 μM) (Fig. 6C). Additionally, the induced pERK and pFAK expression levels were inhibited by dasatinib, suggesting that PA induced MAPK and FAK signaling is dependent on Src kinase activity. A high concentration of PA (400 μM) also induced Src independent pERK expression (Fig. 6C). However, the same or higher concentrations of decanoic acid (DA) or lauric acid (LA) (Supplemental Fig. S7) did not enhance the phosphorylation of Src or its mediated pERK or pFAK. Similarly, PA elevated the expression levels of pSrc, pERK, and pFAK in a dose-dependent manner (300 and 600 μM), and elevated pERK and pFAK was inhibited by dasatinib in PC-3 prostate cancer cells (Fig. 6D). It also increased levels of pSrc, pERK, and pFAK in 22Rv1+Src(WT), but not in 22Rv1+vector cells (Fig. S8). Collectively, these results indicate that exogenous PA collaborates with Src-mediated oncogenic signaling.
DISCUSSION
Epidemiological studies regarding the association of diet with the progression of aggressive prostate cancer is very controversial due to a lack of molecular understanding. Our study demonstrates that a diet high in saturated fat significantly promotes activated Src-mediated tumor progression in the Src(WT)+AR induced tumors and Src-mediated PC-3 xenograft tumors. Both tumor models carry elevated Src kinase activity as in our previous studies (17,20). This study supports a collaboration between dietary factors and dysregulated oncogenic alterations in cancer progression. Given the fact that Src activation is common in advanced stages of prostate cancer, and over-expression or activation of Src kinase promotes proliferation, invasion, and migration of cancer cells (12), the interaction of Src activity and saturated fat at least partially reflects the epidemiological observation showing the association of dietary factors with an increased risk of tumor occurrence or cancer mortality (32). The interaction of oncogenic events with a HFD has also been reported in other oncogenic events, such as loss of PTEN mediated prostate cancer. High fat diets promote the differentiation of basal cells to luminal cells to initiate PTEN mediated hyperplasia (33), and exacerbate prostate neoplasia by increasing the inflammatory response (34), or accelerating metastasis in loss of PTEN-mediated tumors (35). Our studies and others emphasize the importance of dietary choice for cancer patients and indicate that a diet low in saturated fat is potentially beneficial in reducing the risk of progression of aggressive cancer.
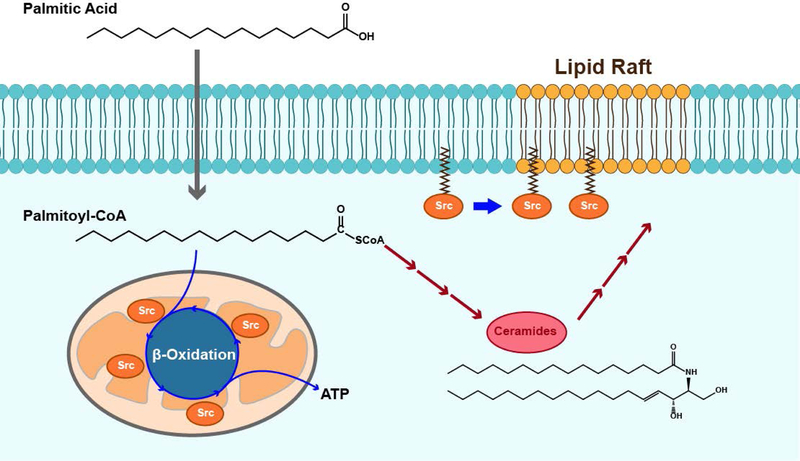
Prostate cancer has aberrant lipid and fatty acid metabolism (36). This study further supports our previous evidence that saturated fat exacerbates Src-mediated prostate tumor progression. Myristate and palmitate are two of the main saturated fatty acids in a high saturated fat diet, which potentially are fuel for metabolism in cancer cells. While exogenous myristate enhances Src-mediated oncogenic signaling through elevation of myristoylated Src kinase levels (20), our data demonstrate that dietary palmitate increases levels of cellular palmitoyl-CoA, and the elevation of palmitoyl-CoA fuels mitochondrial beta-oxidation to increase ATP production (Fig. 7), which will potentially provide energy for the proliferation of cancer cells. Src kinase mediates PA induced beta-oxidation, and reciprocally, increased mitochondrial beta-oxidation enhances Src activation and metastasis (37). Therefore, the collaborative interaction of Src kinase with beta-oxidation activity in mitochondria provide a biological mechanism to promote tumor progression.
FIGURE 7. Mechanism of Src mediated prostate tumor progression stimulated by dietary palmitic acid.
Exogenous PA is converted into palmitoyl-CoA. Src kinase mediates mitochondrial ATP production under the stimulation of exogenous palmitoyl-CoA in the catabolic process. In addition, exogenous palmitoyl-CoA participates in ceramide synthesis, particularly increasing C16:0-ceramide and total saturated ceramides, which may alter the membrane structure. The alteration might lead to increased levels of Src kinase in the cell membrane or detergent resistant cell membrane and elevate Src-mediated oncogenic signaling including the phosphorylation of MAPK and FAK. The combination of these effects could accelerate Src-mediated tumor progression.
Dietary PA leading to an elevation of cellular palmitoyl-CoA levels could change ceramide compositions in the cellular anabolic process (29), and potentially alter cell membrane structure (Fig. 7). Ceramides are one of the important elements in assembling lipid rafts in the cellular membrane (30). Dietary saturated FAs regulate the composition of ceramides, glycerophospholipids, sphingolipids, and other lipids in cancer cells and tumors (38–40), and thereby the structure of lipid rafts in the cytoplasmic membrane (41). We observed that an elevated amount of C16:0 ceramide is associated with a high fat diet or exogenous PA. The production of C16:0 ceramide induced by a high fat diet is dependent on ceramide synthase 6 (CerS6) (42,43). Activation of Src kinase can further increase the expression of CerS6, and change the structure of lipid raft microdomains and subsequently promote cell transformation (44). The ceramide enriched membrane domain promotes reorganization of receptor molecules, potassium channels or recruits intracellular signal mediators for effective cell signal initiation or transduction (45). Our results are also consistent with the evidence that a high fat diet causes c-Src partitioning and activation in detergent resistant membranes and inhibits insulin signaling (46).
Palmitoyl-CoA as an immediate metabolite of dietary PA could also directly be involved in the palmitoylation of an array of proteins, and promote these proteins’ mediated tumor progression (47,48). For example, dietary PA could elevate levels of palmitoylated proteins, such as Sonic Hedgehog protein (Shh). Blockade of Shh palmitoylation sufficiently inhibits its signaling by targeting Hedgehog acyltransferase (49). Another example, such as the palmitoylation of EGFR could regulate its mediated AKT signaling, and enhance the survival of tumor cells in lung cancer (50). Therefore, targeting palmitoylation provides a therapeutic approach for treatment of numerous diseases.
A diet high in unsaturated fat, albeit to a lesser degree than a high saturated fat diet, promotes the growth of xenograft tumors in comparison with a low fat diet. Numerous reports have shown that unsaturated fatty acids, such as docosahexaenoic acid (DHA) and n-3 polyunsaturated fatty acids (n-3 PUFAs) induces apoptosis by up-regulation of syndecan-1 expression, and decreases phosphorylation of the 3-phosphoinositide-dependent kinase 1/AKT/Bad pathway (51). However, other studies also report that dietary n-3 PUFAs fail to reduce tumor progression in PB-ErbB-2xPTEN(+/−) mice (52). The inconsistency might be oncogenic event dependent. Nevertheless, dietary choice has been recognized as an important factor to influence prostate cancer progression (2). Our studies provide biological mechanisms demonstrating that the interaction of dietary saturated FAs including myristate and palmitate with an important oncogenic event such as the increased levels of active Src, could accelerate tumor progression in prostate cancer.
Supplementary Material
Acknowledgments
This work was supported by NIH (R01CA172495) and DOD (W81XWH-15-1-0507) to HC.
Footnotes
The authors disclose no potential conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, and Jemal A (2017) Cancer Statistics, 2017. CA: a cancer journal for clinicians 67, 7–30 [DOI] [PubMed] [Google Scholar]
- 2.Labbe DP, Zadra G, Ebot EM, Mucci LA, Kantoff PW, Loda M, and Brown M (2015) Role of diet in prostate cancer: the epigenetic link. Oncogene 34, 4683–4691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Calle EE, Rodriguez C, Walker-Thurmond K, and Thun MJ (2003) Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 348, 1625–1638 [DOI] [PubMed] [Google Scholar]
- 4.Gong Z, Neuhouser ML, Goodman PJ, Albanes D, Chi C, Hsing AW, Lippman SM, Platz EA, Pollak MN, Thompson IM, and Kristal AR (2006) Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev 15, 1977–1983 [DOI] [PubMed] [Google Scholar]
- 5.Cao Y, and Ma J (2011) Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 4, 486–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, and Leitzmann MF (2007) Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 109, 675–684 [DOI] [PubMed] [Google Scholar]
- 7.Hu MB, Xu H, Bai PD, Jiang HW, and Ding Q (2014) Obesity has multifaceted impact on biochemical recurrence of prostate cancer: a dose-response meta-analysis of 36,927 patients. Med Oncol 31, 829. [DOI] [PubMed] [Google Scholar]
- 8.Joshu CE, Mondul AM, Menke A, Meinhold C, Han M, Humphreys EB, Freedland SJ, Walsh PC, and Platz EA (2011) Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 4, 544–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein MM, Kasperzyk JL, Mucci LA, Giovannucci E, Price A, Wolk A, Hakansson N, Fall K, Andersson SO, and Andren O (2012) Dietary fatty acid intake and prostate cancer survival in Orebro County, Sweden. Am J Epidemiol 176, 240–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le TT, Huff TB, and Cheng JX (2009) Coherent anti-Stokes Raman scattering imaging of lipids in cancer metastasis. BMC Cancer 9, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, and Gerald WL (2010) Integrative genomic profiling of human prostate cancer. Cancer cell 18, 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Z, Dai B, Jiang T, Xu K, Xie Y, Kim O, Nesheiwat I, Kong X, Melamed J, Handratta VD, Njar VC, Brodie AM, Yu LR, Veenstra TD, Chen H, and Qiu Y (2006) Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer cell 10, 309–319 [DOI] [PubMed] [Google Scholar]
- 13.Chen Y, Sawyers CL, and Scher HI (2008) Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol 8, 440–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas SM, and Brugge JS (1997) Cellular functions regulated by Src family kinases. Annual review of cell and developmental biology 13, 513–609 [DOI] [PubMed] [Google Scholar]
- 15.Martin GS (2001) The hunting of the Src. Nature reviews. Molecular cell biology 2, 467–475 [DOI] [PubMed] [Google Scholar]
- 16.Aleshin A, and Finn RS (2010) SRC: a century of science brought to the clinic. Neoplasia 12, 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cai H, Babic I, Wei X, Huang J, and Witte ON (2011) Invasive Prostate Carcinoma Driven by c-Src and Androgen Receptor Synergy. Cancer Res 71, 862–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S, Alsaidan OA, Goodwin O, Li Q, Sulejmani E, Han Z, Bai A, Albers T, Beharry Z, Zheng YG, Norris JS, Szulc ZM, Bielawska A, Lebedyeva I, Pegan SD, and Cai H (2017) Blocking Myristoylation of Src Inhibits Its Kinase Activity and Suppresses Prostate Cancer Progression. Cancer Res 77, 6950–6962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Drake JM, Graham NA, Lee JK, Stoyanova T, Faltermeier CM, Sud S, Titz B, Huang J, Pienta KJ, Graeber TG, and Witte ON (2013) Metastatic castration-resistant prostate cancer reveals intrapatient similarity and interpatient heterogeneity of therapeutic kinase targets. Proc Natl Acad Sci U S A 110, E4762–4769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, Yang X, Li Q, Wu M, Costyn L, Beharry Z, Bartlett MG, and Cai H (2017) Myristoylation of Src kinase mediates Src-induced and high-fat diet-accelerated prostate tumor progression in mice. The Journal of biological chemistry 292, 18422–18433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicolosi RJ (1997) Dietary fat saturation effects on low-density-lipoprotein concentrations and metabolism in various animal models. The American journal of clinical nutrition 65, 1617S–1627S [DOI] [PubMed] [Google Scholar]
- 22.Haack K, Cockrell AS, Ma H, Israeli D, Ho SN, McCown TJ, and Kafri T (2004) Transactivator and structurally optimized inducible lentiviral vectors. Mol Ther 10, 585–596 [DOI] [PubMed] [Google Scholar]
- 23.Xin L, Ide H, Kim Y, Dubey P, and Witte ON (2003) In vivo regeneration of murine prostate from dissociated cell populations of postnatal epithelia and urogenital sinus mesenchyme. Proc Natl Acad Sci U S A 100 Suppl 1, 11896–11903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukacs RU, Goldstein AS, Lawson DA, Cheng D, and Witte ON (2010) Isolation, cultivation and characterization of adult murine prostate stem cells. Nature protocols 5, 702–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang X, Ma Y, Li N, Cai H, and Bartlett MG (2017) Development of a Method for the Determination of Acyl-CoA Compounds by Liquid Chromatography Mass Spectrometry to Probe the Metabolism of Fatty Acids. Analytical chemistry 89, 813–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adam RM, Yang W, Di Vizio D, Mukhopadhyay NK, and Steen H (2008) Rapid preparation of nuclei-depleted detergent-resistant membrane fractions suitable for proteomics analysis. BMC Cell Biol 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bielawski J, Szulc ZM, Hannun YA, and Bielawska A (2006) Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods 39, 82–91 [DOI] [PubMed] [Google Scholar]
- 28.Grevengoed TJ, Klett EL, and Coleman RA (2014) Acyl-CoA metabolism and partitioning. Annual review of nutrition 34, 1–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogretmen B, and Hannun YA (2004) Biologically active sphingolipids in cancer pathogenesis and treatment. Nature reviews. Cancer 4, 604–616 [DOI] [PubMed] [Google Scholar]
- 30.Gulbins E, and Kolesnick R (2003) Raft ceramide in molecular medicine. Oncogene 22, 7070–7077 [DOI] [PubMed] [Google Scholar]
- 31.Tholstrup T, Sandstrom B, Bysted A, and Holmer G (2001) Effect of 6 dietary fatty acids on the postprandial lipid profile, plasma fatty acids, lipoprotein lipase, and cholesterol ester transfer activities in healthy young men. The American journal of clinical nutrition 73, 198–208 [DOI] [PubMed] [Google Scholar]
- 32.Richman EL, Kenfield SA, Chavarro JE, Stampfer MJ, Giovannucci EL, Willett WC, and Chan JM (2013) Fat intake after diagnosis and risk of lethal prostate cancer and all-cause mortality. JAMA internal medicine 173, 1318–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwon OJ, Zhang B, Zhang L, and Xin L (2016) High fat diet promotes prostatic basal-to-luminal differentiation and accelerates initiation of prostate epithelial hyperplasia originated from basal cells. Stem cell research 16, 682–691 [DOI] [PubMed] [Google Scholar]
- 34.Liu J, Ramakrishnan SK, Khuder SS, Kaw MK, Muturi HT, Lester SG, Lee SJ, Fedorova LV, Kim AJ, Mohamed IE, Gatto-Weis C, Eisenmann KM, Conran PB, and Najjar SM (2015) High-calorie diet exacerbates prostate neoplasia in mice with haploinsufficiency of Pten tumor suppressor gene. Molecular metabolism 4, 186–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen M, Zhang J, Sampieri K, Clohessy JG, Mendez L, Gonzalez-Billalabeitia E, Liu XS, Lee YR, Fung J, Katon JM, Menon AV, Webster KA, Ng C, Palumbieri MD, Diolombi MS, Breitkopf SB, Teruya-Feldstein J, Signoretti S, Bronson RT, Asara JM, Castillo-Martin M, Cordon-Cardo C, and Pandolfi PP (2018) An aberrant SREBP-dependent lipogenic program promotes metastatic prostate cancer. Nature genetics 50, 206–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, Daniels G, Lee P, and Monaco ME (2014) Lipid metabolism in prostate cancer. Am J Clin Exp Urol 2, 111–120 [PMC free article] [PubMed] [Google Scholar]
- 37.Park JH, Vithayathil S, Kumar S, Sung PL, Dobrolecki LE, Putluri V, Bhat VB, Bhowmik SK, Gupta V, Arora K, Wu D, Tsouko E, Zhang Y, Maity S, Donti TR, Graham BH, Frigo DE, Coarfa C, Yotnda P, Putluri N, Sreekumar A, Lewis MT, Creighton CJ, Wong LC, and Kaipparettu BA (2016) Fatty Acid Oxidation-Driven Src Links Mitochondrial Energy Reprogramming and Oncogenic Properties in Triple-Negative Breast Cancer. Cell reports 14, 2154–2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah C, Yang G, Lee I, Bielawski J, Hannun YA, and Samad F (2008) Protection from high fat diet-induced increase in ceramide in mice lacking plasminogen activator inhibitor 1. The Journal of biological chemistry 283, 13538–13548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, and Cowart LA (2012) Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest 122, 3919–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Louie SM, Roberts LS, Mulvihill MM, Luo K, and Nomura DK (2013) Cancer cells incorporate and remodel exogenous palmitate into structural and oncogenic signaling lipids. Biochimica et biophysica acta 1831, 1566–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hattersley KJ, Hein LK, and Fuller M (2013) Lipid composition of membrane rafts, isolated with and without detergent, from the spleen of a mouse model of Gaucher disease. Biochemical and biophysical research communications 442, 62–67 [DOI] [PubMed] [Google Scholar]
- 42.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Bluher M, Kronke M, and Bruning JC (2014) Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20, 678–686 [DOI] [PubMed] [Google Scholar]
- 43.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, and Summers SA (2014) CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab 20, 687–695 [DOI] [PubMed] [Google Scholar]
- 44.Kajiwara K, Yamada T, Bamba T, Fukusaki E, Imamoto F, Okada M, and Oneyama C (2014) c-Src-induced activation of ceramide metabolism impairs membrane microdomains and promotes malignant progression by facilitating the translocation of c-Src to focal adhesions. Biochem J 458, 81–93 [DOI] [PubMed] [Google Scholar]
- 45.Bollinger CR, Teichgraber V, and Gulbins E (2005) Ceramide-enriched membrane domains. Biochimica et biophysica acta 1746, 284–294 [DOI] [PubMed] [Google Scholar]
- 46.Holzer RG, Park EJ, Li N, Tran H, Chen M, Choi C, Solinas G, and Karin M (2011) Saturated fatty acids induce c-Src clustering within membrane subdomains, leading to JNK activation. Cell 147, 173–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Resh MD (2017) Palmitoylation of proteins in cancer. Biochem Soc Trans 45, 409–416 [DOI] [PubMed] [Google Scholar]
- 48.Ko PJ, and Dixon SJ (2018) Protein palmitoylation and cancer. EMBO Rep 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Petrova E, Rios-Esteves J, Ouerfelli O, Glickman JF, and Resh MD (2013) Inhibitors of Hedgehog acyltransferase block Sonic Hedgehog signaling. Nat Chem Biol 9, 247–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ali A, Levantini E, Teo JT, Goggi J, Clohessy JG, Wu CS, Chen L, Yang H, Krishnan I, Kocher O, Zhang J, Soo RA, Bhakoo K, Chin TM, and Tenen DG (2018) Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non-small cell lung cancer. EMBO Mol Med 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu Y, Sun H, Owens RT, Gu Z, Wu J, Chen YQ, O’Flaherty JT, and Edwards IJ (2010) Syndecan-1-dependent suppression of PDK1/Akt/bad signaling by docosahexaenoic acid induces apoptosis in prostate cancer. Neoplasia 12, 826–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vissapragada S, Ghosh A, Ringer L, Salinas P, Brophy A, Peaceman D, Kallakury B, Banerjee PP, Fricke ST, Helfrich W, Lee YC, Pestell R, Scherer P, Tanowitz HB, Avantaggiati ML, Hilakivi-Clarke L, Lisanti MP, Rodriguez OC, and Albanese C (2010) Dietary n-3 polyunsaturated fatty acids fail to reduce prostate tumorigenesis in the PB-ErbB-2 x Pten(+/−) preclinical mouse model. Cell cycle 9, 1824–1829 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.