Abstract
Background-
Tricuspid regurgitation (TR) is a significant contributor to morbidity and mortality in hypoplastic left heart syndrome (HLHS). The goal of this study was to characterize the dynamic annular motion of the tricuspid valve (TV) in HLHS with a Fontan circulation and assess relation to TV function.
Methods-
Tricuspid annuli (TA) of 48 patients with HLHS with a Fontan circulation were modeled at end-diastole, mid-systole, end-systole and mid-diastole using transthoracic 3D echocardiograms and custom code in 3D Slicer. The angle of the anterior papillary muscle (APM) relative to the annular plane in each systolic phase was also measured.
Results-
Imaging was performed 5.0 [2 – 11] years after Fontan operation. The TA varies in shape significantly throughout the cardiac cycle, changing in sphericity (p<0.001), but not in annular height or bending angle. In univariate modeling, patients with significant TR had larger changes in septo-lateral diameter, lateral quadrant area, and posterior quadrant area (all p<0.05), as well as lower (more laterally directed) APM angles (p<0.001) than patients with mild or less TR. In multivariate modeling, a 1 mm/BSA0.5 increase in the maximum change in septo-lateral diameter was associated with a 1.7-fold increase in having moderate or greater TR, while a 10 degree decrease in APM angle at mid-systole was associated with an almost 2.5-fold increase in moderate or greater TR (all p ≤ 0.01).
Conclusions-
The TA in HLHS with a Fontan circulation changes in shape significantly throughout the cardiac cycle but remains relatively planar. Increased change in septo-lateral diameter and decreased APM angle are strongly associated with the presence of TR. These findings may inform annuloplasty methods and subvalvular interventions in these complex patients.
Keywords: Congenital Heart Disease, Echocardiography, Valvular Heart Disease
INTRODUCTION
In hypoplastic left heart syndrome (HLHS), the right ventricle (RV) acts as the systemic ventricle and the tricuspid valve (TV) functions as the systemic atrioventricular valve. In this setting, hemodynamically significant tricuspid regurgitation (TR) has been identified as a significant contributor to morbidity and mortality.[1–3] TR is associated with RV dilation and dysfunction, as well as structural changes in the TV annulus, leaflets, and subvalvular structure.[4, 5]
In adults, an understanding of static and dynamic three-dimensional (3D) structure of the mitral valve has progressed significantly with the use of 3D echocardiography (3DE).[6–8] The dynamic structure of the mitral valve annulus has been shown to differ with varying pathology,[9, 10] and this has been translated into morphology-directed therapies such as non-planar annuloplasty rings and informed location of annuloplasty placement.[11, 12]
Several studies have now characterized the static TV annular and leaflet structure in HLHS. Aberrations in TV structure have been associated with TV regurgitation and even patient survival.[4, 5, 13] However, the dynamic motion of the tricuspid annulus (TA) in older HLHS children with a Fontan circulation has not been robustly characterized. A more detailed understanding of the TA motion may inform the design of TV annuloplasty in this vulnerable population.
Therefore, our primary goal was to define the dynamic annular shape and motion of the tricuspid valve in HLHS with a Fontan circulation using 3DE. We hypothesized that the TA dynamics of patients with hemodynamically significant (moderate or greater) TR would differ from those with mild or less TR. We also investigated the relationship of the anterior papillary muscle(APM) angle to annular shape and the presence of significant TR.
MATERIAL AND METHODS
Subjects
In January 2016, acquisition of transthoracic 3DE images of the TV became part of the standard clinical echo lab protocol for HLHS at the Children’s Hospital of Philadelphia. An institutional database was utilized to retrospectively identify patients with HLHS with a Fontan circulation in whom transthoracic 3DE of the tricuspid valve had been previously performed. Exclusion criteria included previous TV repair, presence of significant stitch artifact, lack of inclusion of the entire TA and subvalvular apparatus in the acquisition, and inability to delineate the TA. No patients had significant arrhythmia including atrial fibrillation. This study was performed according to a protocol approved by the institutional review board at the Children’s Hospital of Philadelphia.
Transthoracic Image Acquisition
3DE images of the tricuspid valve had been acquired as per lab protocol using sector narrowed Full Volume or 3D Zoom mode with a wide field of view. EKG gated acquisitions were obtained when patient cooperation allowed. Transthoracic X7 or X5 probes were used with the Philips IE33 and EPIQ 7 ultrasound systems (Philips Medical, Andover, MA).
Two-Dimensional Echocardiography
Qualitative assessment of ventricular function was drawn from clinical interpretations. Post hoc RV diastolic and systolic area and fractional area change (FAC) were assessed by a single observer (AN). RV area was measured from the apical 4-chamber view by tracing the RV endocardium in systole and diastole, and FAC percentage was calculated as ((end-diastolic(ED) area – end-systolic(ES) area)/ED area) x 100. TR was assessed by two dimensional echocardiography (2DE) by qualitative grading by a single observer (MJ). The severity of TR was graded subjectively as follows: trivial (no regurgitation to narrow single jet), mild (multiple narrow jets), moderate (wide jet reaching the midportion of the right atrium), and severe (wide jet reaching the back wall of the right atrium). Vena contracta was measured in parasternal long axis and apical views by a single observer (MJ).
Annular Curve Modeling
Annular modeling and quantification were performed using custom code run in 3D Slicer(www.slicer.org) as detailed in the online supplement (Figures S1-S13).[14] 3DE images were exported to Philips Digital Imaging and Communications in Medicine (DICOM) format, converted to Cartesian DICOM in QLAB (Philips Medical, Andover, MA) and imported into 3D Slicer. We have previously validated scaling and registration of 3DE data in 3D Slicer.[15] In total, 4 equally spaced cardiac phases were modeled. EKG was not available in Cartesian DICOM; timing within the cardiac cycle was determined by valve position. Analysis began with the identification of anatomic landmarks and selection of the ED frame (first frame with TV closed) and the ES frame (last frame before the TV starts to open). The mid-systolic(MS) frame was defined as the frame midway between the ED and ES frames. Similarly, the mid-diastolic (MD) frame was defined as the frame halfway between the ES and ED frames. The annular curve was traced then smoothed using Fourier smoothing (Figure 1).
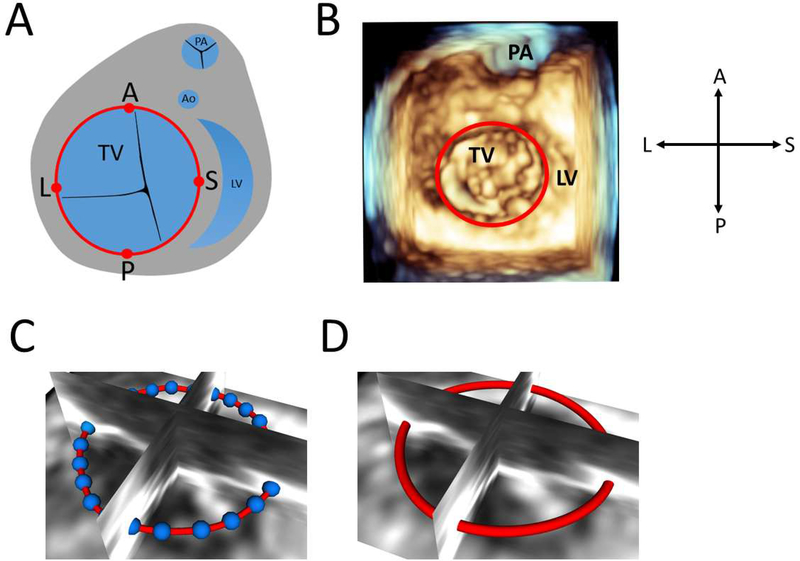
Figure 1. Creation of Tricuspid Valve Annulus Contour.
A. Schematic diagram of tricuspid valve annulus(red) with assignment of anterior, posterior, septal, and lateral annular points; B. Volume rendered view of tricuspid valve with annulus(red) viewed from ventricle; C. 36 points(blue) placed to define valve annulus viewed from atrium; D. Resampled points to generate a smoothed annulus contour(red) viewed from atrium.
Annular Quantification
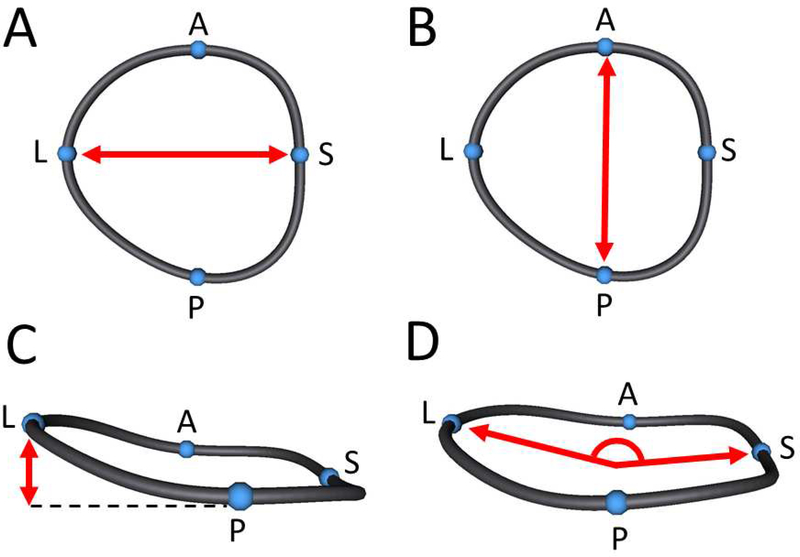
Points restricted to the smoothed curve were then assigned anatomically based on the ultrasound image (Figure 1). The septo-lateral(S-L) and anterior-posterior(A-P) diameters were calculated for each TA in each phase (Figures 2A, and 2B). Annular height was calculated as the distance from the highest point on the annulus to the lowest point on the annulus relative to a least squares annular plane (Figure 2C). Sphericity was defined as the ratio of A-P diameter to S-L diameter. Bending angle in the anterior and posterior direction was calculated by fitting a least-squares plane to the anterior portion of the TA as well as the posterior portion of the TA and calculating the angle between them. Similarly, the bending angle between the septal and lateral portions of the TA was calculated (Figure 2D). Annular 3D area was calculated by fitting a least area manifold surface to the valve similar to the form a “soap bubble” would take on the annulus.
Figure 2. Tricuspid Annular Measurements.
A. Septo-lateral diameter measurement; B. Anterior-posterior diameter measurement; C. Annular height measurement; D. Septo-lateral bending angle measurement.
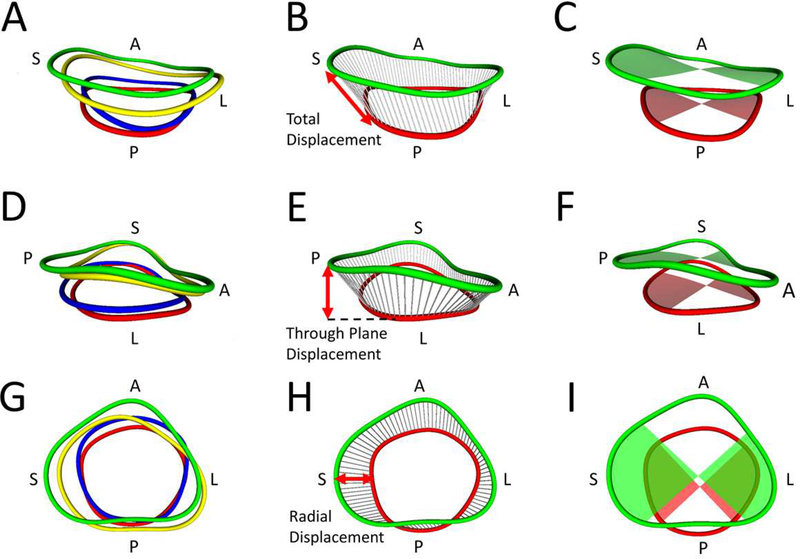
To further quantify changes in annular shape, the full annular area was divided into quadrants centered on the anterior, lateral, septal, and posterior markers (Figure 3C, 3F, 3I). To quantify annular motion, chords were created between points on the annulus at different cardiac phases. The mean translation over the chords in a given quadrant was calculated (Figure 3B), then broken down into through annular plane and radial components (Figure 3E, 3H).
Figure 3. Metrics of Annular Movement and Area by Quadrant.
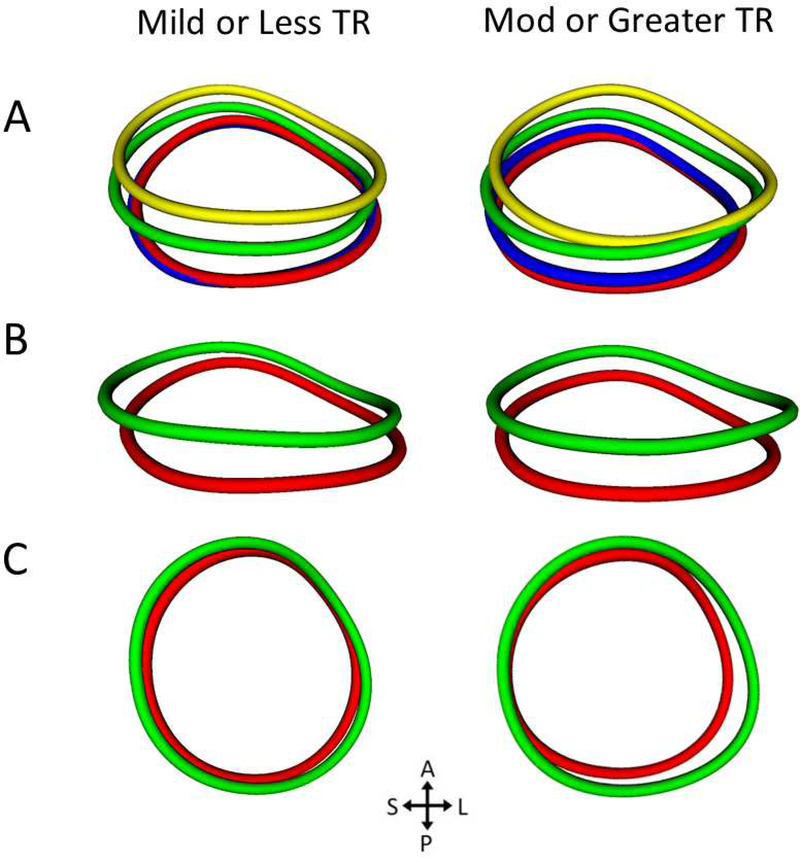
A. All four annulus contours from a posterior view (green = end-diastole(ED), blue = mid-systole(MS), red = end-systole(ES), yellow = mid-diastole(MD); B. Total displacement (chords) measurement from ED to ES; C. Septal and lateral quadrant areas at ED and ES; D. All four annulus contours from a lateral view; E. Through plane displacement (chords) from ED to ES; F. Anterior and posterior quadrant areas at ED and ES; G. All four annulus contours from an atrial view; H. Radial displacement (chords) measurement from ED to ES; I. Septal and lateral quadrant areas at ED and end ES.
To compare normalized annular curves across our cohort, generalized Procrustes analysis was applied to the curves in order to align the different annular shapes for the 4 different phases of the cardiac cycle (Figure 6).[16] APM angle was measured relative to the annular plane, using multiplane reconstruction in 3D Slicer to ensure visualization of the APM (Figure 4). The posterior and septal papillary muscles were not consistently seen and therefore these angles were not measured.
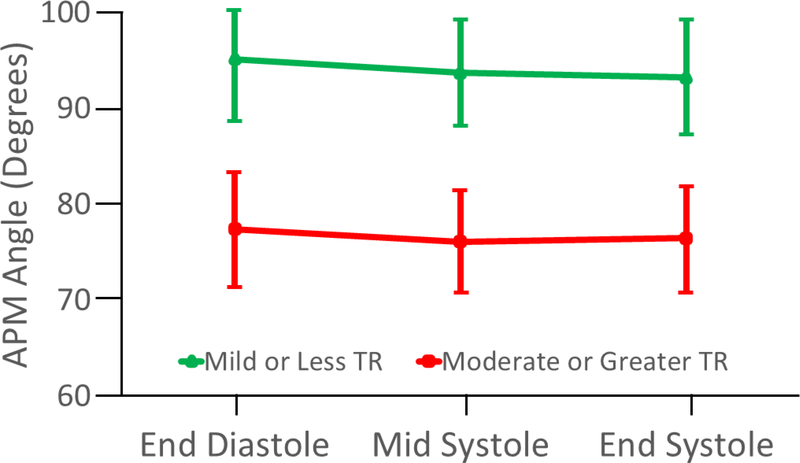
Figure 6. Comparison of Anterior Papillary Muscle Angles by Tricuspid Regurgitation Group.
Data shown as Mean (± 95% confidence interval). Differences between TR group p < 0.001 at each cardiac phase. APM = anterior papillary muscle; TR = tricuspid regurgitation.
Figure 4. Comparison of the Average Shape of Tricuspid Annulus by TR Group Using Procrustes Analysis.
A. Average TA shape in 4 cardiac phases viewed from an inferior atrial view (green = end-diastole(ED), blue = mid-systole(MS), red = end-systole(ES), yellow = mid-diastole(MD)); B. Comparison of average annular shape in ED and ES from an inferior atrial view; C. Same comparison from an atrial view. Note the larger septo-lateral change and expansion of the lateral and posterior quadrant areas in the moderate or greater TR group in ED. TA = tricuspid annulus TR = tricuspid regurgitation; Mod = moderate.
Statistics
Cardiac output and valve area are linearly related to BSA. Valve area is related to the square of valve radius (and other linear metrics).[17, 18] As such annular area measures were normalized to BSA, and linear measures were normalized to BSA0.5. Continuous variables are presented as median [IQR]. Friedman’s Test was utilized to compare annular metrics throughout the cardiac cycle. Comparisons of continuous variables between groups were made using Mann-Whitney U-test. Linear regression was used to determine the strength of the relationship between continuous variables. In order to quantify the maximum change in metrics over the whole cardiac cycle we defined maximum change as the maximum value (ED or MD) in cardiac cycle minus the minimum value in cardiac cycle (MS or ES). Systolic change was defined as the difference between ED and ES. Tricuspid regurgitation grades were dichotomized into mild or less TR, or moderate or greater TR for all analysis. The data was explored with mixed effects logistic regression and general estimating equations (for multi-phase data), but ultimately a multivariate logistic regression model was created. Variables were identified from univariate associates of moderate or greater TR (p<0.1). A bivariate screen was performed to evaluate the association between variables of interest and moderate or greater TR. Due to a constrained study population, a limited number of variables could be included, and a threshold of p<0.1 was chosen. Inter-observer and intra-observer reproducibility of annular and APM angle was assessed in 8 subjects, at 4 cardiac phases. The same observer re-measured all the parameters at least 1 month from the initial evaluation and a second observer performed all measurements without knowledge of the results of the first observer. Intra-observer and inter-observer reproducibility were quantified on this set of 32 curves and angles using the intra-class correlation coefficient (ICC). All analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
The TA of 48 patients with HLHS with a Fontan circulation were modeled at ED, MS, ES, and MD. Patients ranged in age from 2 years old to 28 years old (Table 1). 3DE were obtained a median of 5.0 [2 – 11] years after Fontan operation. Thirty-five patients had qualitatively normal RV systolic function, 9 had mildly diminished RV systolic function, and 4 had moderately diminished RV systolic function. Although qualitative grading of right ventricular function was rated as normal in the majority of patients, the median fractional area change was 32.2%. Twenty patients had qualitative grades of moderate or greater TR and 28 had mild or less TR. The average time required for annular curve creation, marker placement, and scripted calculation was 5 minutes per phase.
Table 1.
Demographics
| Variable | Median [IQR] |
|---|---|
| Age (y) | 7.5 [5.0–13] |
| Female Gender (n, %) | 14 [29%] |
| Height (cm) | 121 [105–151] |
| Weight (kg) | 22.6 [16.5–40.4] |
| BSA (m2) | 0.87 [0.69–1.30] |
| Heart Rate (bpm) | 85 [70–96] |
| SBP (mm Hg) | 103 [95–114] |
| DBP (mm Hg) | 61 [54–66] |
| MBP (mm Hg) | 75 [71–80] |
| Mitral and Aortic Anatomy | |
| Mitral Stenosis/Aortic Stenosis (n, %) | 9 (19%) |
| Mitral Stenosis/Aortic Atresia (n, %) | 15 (31%) |
| Mitral Atresia/Aortic Atresia (n, %) | 23 (48%) |
| Mitral Atresia/Aortic Stenosis (n, %) | 1 (2%) |
| Years after Fontan Surgery (y) | 5 [2–11] |
| Qualitative RV Systolic Function | |
| Normal (n, %) | 35 (74%) |
| Mildly Diminished (n, %) | 9 (18%) |
| Moderately Diminished (n, %) | 4 (8%) |
| Quantitative RV Function | |
| RV End Diastolic Area (cm2/m2) | 21.1 [17.9–25.9] |
| RV End Systolic Area (cm2/m2) | 14.9 [10.8–17.5] |
| Fractional Area Change (%) | 32.2 [25.9–38.5] |
| Tricuspid Regurgitation | |
| Mild or Less (n, %) | 28 (58%) |
| Moderate or Greater (n, %) | 20 (42%) |
| LV Area (cm2/m2) | 1.29 [0.46–2.96] |
| Frame Rate (Hz) | 33 [27–33] |
Values listed are Median [IQR], BSA = body surface area, SBP = systolic blood pressure, DBP = diastolic blood pressure, MBP = mean blood pressure, MS = mitral stenosis, AS = aortic stenosis, MA = mitral atresia, AA = aortic atresia, RV = right ventricular, LV = left ventricle.
Dynamic Changes in Annular Shape
TA area, A-P diameter, S-L diameter, and circumference all changed significantly throughout the cardiac cycle (Table 2; Figure 3), decreasing from diastole to systole. The sphericity was lower in diastole than systole, indicating expansion of the S-L diameter relative to the A-P diameter in diastole.
Table 2.
Tricuspid Annular Dimensions Throughout the Cardiac Cycle
| ------------------SYSTOLE------------------ | ---DIASTOLE--- | |||||
|---|---|---|---|---|---|---|
| Variable | End-Diastole | Mid-Systole | End-Systole | Mid-Diastole | Max Change % | p Value |
| A-P Diameter (cm/m) | 3.38 [3.04–3.96] | 3.27 [3.06–3.95] | 3.28 [3.05–3.99] | 3.36 [3.08–3.86] | 6.96 [1.70–9.71] | 0.01 |
| S-L Diameter (cm/m) | 3.60 [3.21–4.12] | 3.51 [3.04–4.07] | 3.41 [3.11–4.03] | 3.66 [3.28–4.24] | 9.92 [4.96–14.9] | <0.001 |
| Sphericity (A-P/S-L) | 0.94 [0.84–1.08] | 1.00 [0.90–1.10] | 1.00 [0.85–1.11] | 0.93 [0.81–1.03] | 2.25 [−3.18–8.22] | <0.001 |
| Circumference (cm/m) | 11.3 [10.4–13.1] | 10.9 [9.89–12.7] | 10.8 [10.0–12.7] | 11.4 [10.4–13.0] | 5.44 [4.01–11.1] | <0.001 |
| Annular Height (cm/m) | 0.43 [0.33–0.54] | 0.38 [0.30–0.51] | 0.42 [0.33–0.53] | 0.44 [0.34–0.56] | 21.8 [7.98–50.8] | 0.26 |
| AH to A-P Diameter Ratio | 0.13 [0.10–0.15] | 0.11 [0.09–0.15] | 0.13 [0.09–0.15] | 0.12 [0.11–0.16] | 22.6 [6.38–53.5] | 0.18 |
| AH to S-L Diameter Ratio | 0.12 [0.09–0.14] | 0.11 [0.08–0.17] | 0.11 [0.09–0.17] | 0.11 [0.10–0.15] | 18.0 [1.23–47.5] | 0.54 |
| A-P Bending Angle (degrees) | 166 [159–171] | 165 [159–172] | 166 [157–172] | 165 [156–170] | 2.47 [−0.51–5.77] | 0.83 |
| S-L Bending Angle (degrees) | 165 [160–170] | 164 [159–170] | 164 [155–172] | 164 [158–170] | 3.14 [0.48–5.87] | 0.78 |
| Area (cm2/m2) | 9.57 [8.35–13.2] | 8.82 [7.59–12.4] | 8.84 [7.43–12.4] | 9.60 [8.12–12.7] | 12.3 [8.15–22.9] | <0.001 |
| Anterior Quadrant Area (cm2/m2) | 2.61 [2.18–3.34] | 2.31 [1.90–2.92] | 2.29 [1.95–3.12] | 2.69 [2.24–3.39] | 17.1 [11.1–29.9] | <0.001 |
| Posterior Quadrant Area (cm2/m2) | 2.16 [1.76–2.64] | 2.15 [1.76–2.75] | 2.03 [1.71–2.60] | 2.06 [1.80–2.66] | 9.26 [1.17–20.8] | 0.01 |
| Septal Quadrant Area (cm2/m2) | 2.56 [2.14–3.29] | 2.32 [1.92–3.02] | 2.19 [1.82–2.80] | 2.59 [2.07–3.15] | 22.4 [13.0–30.9] | <0.001 |
| Lateral Quadrant Area (cm2/m2) | 2.32 [1.95–3.24] | 2.14 [1.87–3.05] | 2.25 [1.92–2.99] | 2.58 [1.93–3.31] | 12.4 [6.49–25.4] | <0.001 |
Values listed are Median [IQR], AH = annular height, AP = anterior-posterior, SL = septo-lateral.
End-Diastole: First frame tricuspid valve closed, End-Systole: Last frame tricuspid valve is closed before opening
Mid-Systole: Frame between End-Diastole and End-Systole, Mid-Diastole: Frame between End-Systole and End-Diastole
The magnitude of the variations in annular height were, on average, small, and the mean tricuspid valve annulus shape was relatively planar (Table 2). Annular height, annular height to A-P diameter ratio, annular height to S-L diameter ratio, A-P bending angle, and S-L bending angle did not change significantly throughout the cardiac cycle (Table 2).
Associations of Annular Shape and Dynamics with Tricuspid Regurgitation
Subjects were stratified into two groups; mild or less TR, or moderate or greater TR. Vena contracta size was larger in the moderate or greater TR group (Table 3). Demographic and 2D echocardiographic assessment of function did not differ between the mild or less TR group and the moderate or greater TR group, with the exception of the number of years after Fontan surgery which was greater in the moderate or greater TR group (Table 3).
Table 3.
Demographic and 2D Echocardiographic Characteristics by Tricuspid Regurgitation Group
| Variable | Mild or Less TR (n=28) | Moderate or Greater TR (n=20) | p Value |
|---|---|---|---|
| Age (y) | 7 [4–12] | 11 [6–15] | 0.05 |
| Years after Fontan Surgery (y) | 4 [1–9.5] | 7.5 [3.5–13] | 0.04 |
| Female Gender (n, %) | 10 [35.7%] | 4 [20.0%] | 0.24 |
| Height (cm) | 116 [99–144] | 143 [109–156] | 0.08 |
| Weight (kg) | 20.8 [15.7–35.8] | 35.3 [17.9–47.6] | 0.10 |
| BSA (m2) | 0.80 [0.65–1.20] | 1.18 [0.73–1.45] | 0.08 |
| Heart Rate (bpm) | 89.5 [73–99] | 80.5 [70–92] | 0.23 |
| SBP (mm Hg) | 103 [93.5–111] | 103 [97–121] | 0.39 |
| DBP (mm Hg) | 56.5 [54–64] | 62.5 [60–67] | 0.10 |
| MBP (mm Hg) | 72.8 [68.7–78.7] | 75.5 [72.7–85.7] | 0.15 |
| Mitral and Aortic Anatomy | 0.64 | ||
| Mitral Stenosis/Aortic Stenosis (n, %) | 5 (18) | 4 (20) | |
| Mitral Stenosis/Aortic Atresia (n, %) | 9 (32) | 6 (30) | |
| Mitral Atresia/Aortic Atresia (n, %) | 14 (50) | 9 (45) | |
| Mitral Atresia/Aortic Stenosis (n, %) | 0 (0) | 1 (5) | |
| Long Axis Vena Contracta (mm/m) | 0.08 [0–0.15] | 0.47 [0.36–0.64] | <0.001 |
| Four Chamber Vena Contracta (mm/m) | 0.11 [0.03–0.22] | 0.51 [0.40–0.73] | <0.001 |
| Qualitative RV Function | |||
| Normal (n, %) | 23 (57) | 12 (30) | |
| Mildly Diminished (n, %) | 4 (14) | 5 (25) | |
| Moderately Diminished (n, %) | 1 (4) | 3 (15) | |
| Quantitative RV Function | |||
| RV End Diastolic Area (cm2/m2) | 20.3 [16.7–24.9] | 23.1 [18.0–30.2] | 0.13 |
| RV End Systolic Area (cm2/m2) | 14.6 [10.6–16.7] | 16.1 [10.8–20.6] | 0.19 |
| Fractional Area Change (%) | 33.5 [24.0–39.2] | 31.7 [28.3–35.6] | 0.58 |
| LV Area (cm2/m2) | 1.14 [0.43–3.38] | 1.68 [0.60–2.78] | 0.43 |
| Frame Rate (Hz) | 33.0 [26.5–33.0] | 33.0 [29.0–33.0] | 0.65 |
Values listed are Median [IQR]
BSA = body surface area, SBP = systolic blood pressure, DBP = diastolic blood pressure, MBP = mean blood pressure
MS = mitral stenosis, AS = aortic stenosis, MA = mitral atresia, AA = aortic atresia, RV = right ventricular, LV = left ventricle
Associations of annular metrics to TR group are shown in Table 4 and Table S2. ED annular area and both maximum and ED-ES annular area change were greater in the moderate or greater TR group. Maximum circumference change and ED-ES change were greater in the moderate or greater TR group. Maximum S-L ED diameter, ED-ES change, and maximum change were all greater in the moderate or greater TR group. There was no difference in the A-P diameter between the two TR groups at any phase, but ED-ES change was larger in the moderate or greater TR group. There were no differences in annular height, annular height to A-P ratio, A-P bending angle, annular height to S-L ratio, or S-L bending angle between the TR groups at any phase (Table S1).
Table 4.
Annular Dimensions by Tricuspid Regurgitation Group
| Variable | Mild or Less TR (n=28) | Moderate or Greater TR (n=20) | p Value |
|---|---|---|---|
| Total Annulus Area(cm2/m2) | |||
| ED | 8.83 [8.24–10.93] | 10.96 [8.79–15.35] | 0.047 |
| MS | 8.37 [7.59–10.95] | 9.91 [7.83–14.66] | 0.23 |
| ES | 8.40 [7.63–10.61] | 9.24 [7.32–14.43] | 0.36 |
| ED-ES | 0.42 [−0.11–1.07] | 1.23 [0.77–1.83] | 0.001 |
| Maximum Change | 0.92 [0.49–1.30] | 1.71 [1.28–2.41] | 0.02 |
| Maximum % Change | 9.3 [5.4–16.1] | 16.4 [11.3–28.4] | 0.007 |
| Circumference (cm/m) | |||
| ED | 10.85 [10.39–12.15] | 11.86 [10.65–14-56] | 0.09 |
| MS | 10.58 [9.94–12.00] | 11.30 [9.81–13.95] | 0.30 |
| ES | 10.57 [10.13–11.86] | 10.95 [9.82–13.76] | 0.39 |
| ED-ES | 2.10 [0.24–4.89] | 7.16 [3.69–9.57] | 0.009 |
| Maximum Change | 4.94 [2.46–7.61] | 9.87 [7.43–14.33] | 0.008 |
| Maximum % Change | 4.1 [2.2–7.5] | 10.5 [6.0–13-7] | <0.001 |
| Antero-posterior Diameter (cm/m) | |||
| ED | 3.28 [3.09–3.51] | 3.65 [2.89–4.58] | 0.13 |
| MS | 3.21 [3.06–3.65] | 3.56 [2.91–4.38 | 0.31 |
| ES | 3.21[3.08–3.63] | 3.44 [2.83–4.24] | 0.62 |
| ED-ES | 0.05 [−0.14–0.17] | 0.23 [0.06–0.33] | 0.01 |
| Maximum Change | 0.19 [0.30–0.28] | 0.29 [0.09–0.48] | 0.06 |
| Maximum % Change | 6.5 [1.0–8.62] | 7.2 [3.5–13.5] | 0.16 |
| Septo-lateral Diameter (cm/m) | |||
| ED | 3.43 [3.16–4.00] | 3.86 [3.60–4.63] | 0.03 |
| MS | 3.33 [2.99–3.84] | 3.64 [3.26–4.23] | 0.18 |
| ES | 3.29 [3.12–3.79] | 3.55 [3.19–4.31] | 0.16 |
| ED-ES | 0.07 [−0.01–0.30] | 0.21 [0.15–3.90] | 0.015 |
| Maximum Change | 0.22 [0.07–0.40] | 0.43 [0.34–0.64] | 0.001 |
| Maximum % Change | 5.7[1.9–12.9] | 10.7 [9.1–20.4] | 0.006 |
Values listed as Median [IQR], TR = tricuspid regurgitation
ED=End-diastole, MS=Mid-Systole, ES=End-Systole, MD=Mid-Diastole
Maximum change = maximum value in cardiac cycle (MD or ED) minus minimum value in cardiac cycle (MS or ES) Maximum Percent Change is maximum Change / minimum Value expressed as a percent
Metrics of annular quadrant area and movement compared by TR group are shown in Table 5 and Table S3. Metrics of lateral and posterior quadrant area change and radial motion were greater in the moderate or greater TR group than in the mild or less TR group.
Table 5.
Annular Quadrant Areas and Movement by Tricuspid Regurgitation Group
| Variable | Mild or Less TR (n=28) | Moderate or Greater TR (n=20) | p Value |
|---|---|---|---|
| Anterior Quadrant | |||
| ED Area (cm2/m2) | 2.46 [2.01–3.05] | 2.88 [2.22–4.19] | 0.20 |
| MS Area (cm2/m2) | 2.24 [1.97–2.70] | 2.72 [1.84–3.70] | 0.36 |
| ES Area (cm2/m2) | 2.27 [1.95–2.85] | 2.66 [1.90–3.64] | 0.40 |
| ED-ES Area Change (cm2/m2) | 0.17 [0.06–0.42] | 0.36 [0.10–0.49] | 0.07 |
| Maximum Area Change (cm2/m2) | 0.37 [0.20–0.56] | 0.58 [0.30–0.86] | 0.05 |
| Maximum Area Percent Change (%) | 16.6 [9.8–21.8] | 32.5 [13.3–35.6] | 0.09 |
| Mean ED-ES Radial Displacement (cm/m) | 0.08 [0.00–0.15] | 0.11[0.01–0.18] | 0.08 |
| Posterior Quadrant | |||
| ED Area (cm2/m2) | 2.04 [1.77–2.29] | 2.54 [1.71–3.31] | 0.11 |
| MS Area (cm2/m2) | 2.00[1.77–2.46] | 2.31 [1.67–3.40] | 0.41 |
| ES Area (cm2/m2) | 2.00 [1.77–2.54] | 2.04 [1.58–3.10] | 0.75 |
| ED-ES Area Change (cm2/m2) | −0.02[−0.21–0.21] | 0.20 [0.01–0.39] | 0.008 |
| Maximum Area Change (cm2/m2) | 0.12[−0.03–0.27] | 0.30 [0.10–0.60] | 0.02 |
| Maximum Area Percent Change (%) | 7.4 [−1.7–15.4] | 14.1 [4.5–25.0] | 0.03 |
| Mean ED-ES Radial Displacement (cm/m) | 0.01 [−0.11–0.09] | 0.13 (0.00–0.17] | 0.02 |
| Septal Quadrant | |||
| ED Area (cm2/m2) | 2.43 [2.08–3.00] | 2.78 [2.23–4.00] | 0.15 |
| MS Area (cm2/m2) | 2.24[2.88–1.92] | 2.46[1.91–3.66] | 0.36 |
| ES Area (cm2/m2) | 2.13[1.78–2.68] | 2.30[1.88–3.51] | 0.33 |
| ED-ES Area Change (cm2/m2) | 0.37 [0.16–0.49] | 0.41 [0.21–0.73] | 0.37 |
| Maximum Area Change (cm2/m2) | 0.41 [0.23–0.57] | 0.43 [0.34–0.76] | 0.24 |
| Maximum Area Percent Change (%) | 20.3 [11.7–27.5] | 21.0 [12.2–35.3] | 0.61 |
| Mean ED-ES Radial Displacement (cm/m) | 0.19 [0.10–0.25] | 0.12 [0.04–0.62] | 0.23 |
| Lateral Quadrant | |||
| ED Area (cm2/m2) | 2.16 [1.89–2.62] | 2.79 [2.09–4.19] | 0.03 |
| MS Area (cm2/m2) | 2.00 [1.87–2.71] | 2.54 [1.81–3.72] | 0.20 |
| ES Area (cm2/m2) | 2.17 [1.93–2.66] | 2.49 [1.79–3.82] | 0.29 |
| ED-ES Area Change(cm2/m2) | 0.00 [−0.02–0.10] | 0.29 [−0.06–0.53] | 0.006 |
| Maximum Area Change (cm2/m2) | 0.07 [0.21–0.33[ | 0.60 [0.26–0.91] | 0.003 |
| Maximum Area Percent Change (%) | 10.1 [3.1–15.1] | 22.4 [11.9–36.8] | 0.003 |
| Mean ED-ES Radial Displacement (cm/m) | −0.09 [−0.15 –0.04] | 0.06 [−0.03–0.17] | 0.002 |
Values listed as Median [IQR], TR = tricuspid regurgitation, ED=End-diastole, MS=Mid-Systole, ES=End-Systole, MD=Mid-Diastole
Maximum change = maximum value in cardiac cycle (MD or ED) minus minimum value in cardiac cycle (MS or ES)
Maximum Percent Change = maximum change divided by minimum value expressed as a percent
The mean normalized annular shapes of each TR group were generated by Procrustes analysis and are shown in Figure 4. These visualizations demonstrate greater lateral annular expansion in diastole and greater radial translation of the lateral annulus throughout the cardiac cycle in the moderate or greater TR group compared to the mild or less TR group.
Association of Anterior Papillary Muscle Angle to Tricuspid Regurgitation
APM angle relative to the annular plane was determined at ED, MS, and ES as diagrammed in Figure 5. Patients with moderate or greater TR had a lower (more laterally directed) median APM angle at each measured phase compared to patients with mild or less TR (P<0.001; Figure 6). There was no significant relationship between 2D RV area and APM angle at any phase (all R< 0.22, p> 0.17). ED and MS APM angles were weakly inversely associated with larger S-L diameter in the same phase (R=−0.30, p=0.04); R=−0.32, p=0.03). ED, MS, and ES APM angles were weakly inversely associated with change in the lateral quadrant area (ED: R =−0.41, p=0.005; MS: R=−0.36, p=0.02; ES: R=−0.46, p=0.001). Older age was weakly inversely associated with APM angle at ES and ED (R= −0.37, p=0.01; R= −0.31, p=0.04). There was no significant relationship between end-diastolic left ventricle (LV) area and APM angles at any phase (all R< 0.18, p > 0.2).
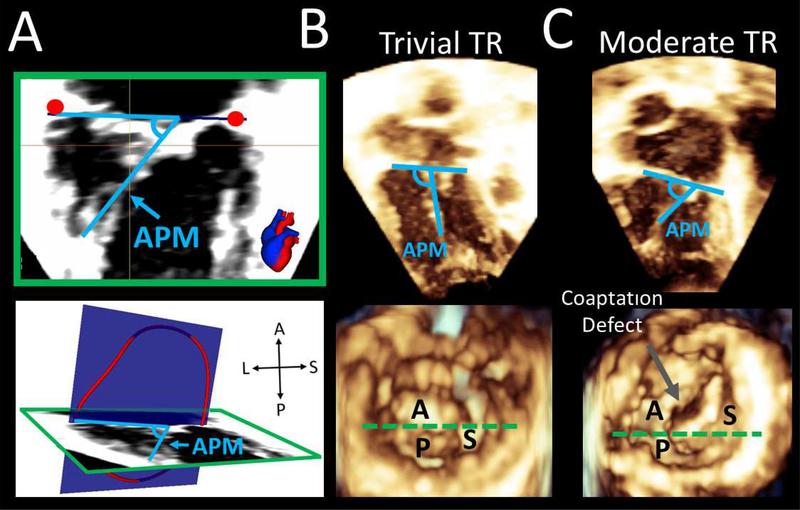
Figure 5. Measurement and Comparison of Anterior Papillary Muscle Angles During Systole.
A. Measurement of anterior papillary muscle (APM) angle relative to annular plane in apical 4 chamber view and ventricular 3D view; B. APM measurement from 4 chamber and ventricular 3D view in a patient with trivial TR. Note obtuse APM angle relative to annular plane; C. 4 chamber and ventricular view in patient with moderate TR. Note acute APM angle relative to annular plane and coaptation defect. Dotted green line in Figures B and C represents plane in Figure A ventricular view. A= anterior leaflet; P = posterior leaflet; S = septal leaflet; APM = anterior papillary muscle; TR = tricuspid regurgitation.
Multivariate Modeling Tricuspid Regurgitation
A multivariate logistic regression model was created by selecting from univariate associates of moderate or greater TR. Maximum change in TA S-L diameter and mid-systolic APM angle were independently associated with moderate or greater TR (Table 6, c-statistic 0.90, Model p < 0.001).
Table 6.
Multivariable Model of Annular and Subvalvular Metrics Associated with Moderate or Greater Regurgitation
| Variable | OR | 95% CI | P Value |
|---|---|---|---|
| Mid Systolic APM Angle (Decrease by 10 degrees) | 2.49 | [1.34–4.65] | 0.004 |
| Maximum Septo-lateral Diameter Change (Increase by 1 mm/BSA0.5) | 1.73 | [1.12–2.68] | 0.01 |
Maximum change = Maximum value in cardiac cycle (MD or ED) minus minimum value in cardiac cycle (MS or ES) c-statistic = 0.90, model p < 0.001. No other univariate associates of TR p < 0.1 when incorporated into multivariate model OR = Odds ratio for moderate or greater tricuspid regurgitation relative to mild or less tricuspid regurgitation APM = anterior papillary muscle
Observer Variability
Intra-user variability was acceptable by ICC, ranging from 0.80–0.99 with an average of 0.92. Inter-user variability was also acceptable, ranging from 0.60–0.98 with an average of 0.83 (Table S4). Measurements with the greatest inter-user variability included antero-posterior bending angle (ICC= 0.61), mean anterior radial displacement (ICC= 0.62), and mean lateral through plane displacement (ICC= 0.60).
DISCUSSION
In HLHS, the 3D annular and leaflet shape of the TV has been shown to differ from that in the normal biventricular heart. During the course of surgically palliated HLHS, the RV is subject to systemic pressures, altered ventricular-ventricular interactions, increased volume load, and increased afterload. One or more of these factors may contribute to aberrations in TV structure and function.[5, 13] Previous studies have shown that the 3D shape of the TA and TV leaflets in HLHS is highly correlated with TV competence and survival.[4, 5, 13] Further, both TV structure and the mechanism of TV dysfunction may differ across age and stage of palliation.[4, 13, 19] Adding to this provocative literature, we present a comprehensive evaluation of the dynamic TA shape and movement in older HLHS patients with a Fontan circulation. Our major findings, in a HLHS Fontan cohort whose single ventricles have been supporting the circulation for an average of a decade, were that 1) the dynamic change in TA S-L diameter and 2) a more laterally directed APM angle together are independently associated with moderate or greater TR. These findings may be relevant to the design of annuloplasty and subvalvular interventions.
Dynamic Annular Motion of the Tricuspid Valve in HLHS with a Fontan Circulation
In normal children with a biventricular circulation, the RV “wraps” around the higher pressure, “bullet shaped” LV.[20] The LV is thought to compress the TA S-L dimension which may facilitate coaptation along the anteroseptal and posteroseptal commissures during ventricular systole.[20, 21] In contrast, in HLHS, the absence of a functional LV leads to a more circular annulus(wider in S-L distance than normal) throughout the cardiac cycle.[13] Consistent with these studies, we found that TA was both larger in the S-L dimension than the A-P dimension and had greater change in the S-L direction throughout the cardiac cycle than in the A-P direction. This suggests that in HLHS patients with a Fontan circulation the facilitation of S-L TV coaptation afforded by normal biventricular dynamics may be lost.[5, 13]
Annular height and bending angle have been shown to be important to mitral valve and tricuspid valve function and are thought to play a role in decreasing leaflet stress.[5, 8, 13, 20] In our cohort, the annulus was essentially flat and did not vary significantly throughout the cardiac cycle. This is in contrast to normal children, but consistent with previous findings in older patients with HLHS, suggesting that by the Fontan stage, the TV leaflets in HLHS may be exposed to increased stress.[13, 20]
Association of Annular and Papillary Muscle Structure to Tricuspid Regurgitation
Tricuspid regurgitation in HLHS has been identified as a significant contributor to morbidity and mortality.[1–3, 22, 23] In our cohort, patients with moderate or greater TR had larger ED S-L diameter, and larger change in S-L diameter and lateral quadrant area throughout the cardiac cycle consistent with loss of the protective annular mechanics described in normal biventricular hearts. A lower, more laterally directed APM angle was also highly associated with moderate or greater TR.
Our study is retrospective and cannot infer causation. However, we speculate that a valve leaflet area that is sufficient to provide septo-anterior and septo-posterior leaflet coaptation when the change in S-L distance is small may become inadequate with significant phasic change in the S-L diameter(expansion in diastole), resulting in a coaptation defect at the anterior-septal and posterior-septal coaptation surface during systole(Figure S15).[5, 13] In the subvalvular region, the dominant APM has chordal attachments to both the anterior and posterior leaflet edges. A more laterally directed APM vector could increase lateral tethering of the anterior and posterior leaflets, further preventing full coaptation of the “lateral” anterior and posterior leaflets with the septal leaflet (Figure S16).[5, 13, 24]
Our finding that the APM angle is associated with TV function in HLHS may vary with patient age and stage of repair. In younger HLHS patients, APM angle has not been associated with TR.[4, 19] However, Colen et al recently demonstrated a trend toward a more laterally directed papillary muscle angle from pre-stage 1 to pre-superior cavopulmonary anastomosis, which we speculate may progress to significance as observed in our older cohort with a Fontan circulation.[19] This highlights that TA dynamics and mechanisms of TR in HLHS may vary and change with age, and as such, defining patientspecific mechanisms of TR may be crucial.
Association of Right Ventricular Size and Function with Annular Dynamics
We did not demonstrate an association of ventricular function with TA dynamics or TR grade. However, the number of patients with significant RV dysfunction in our series was small. Although decreased ventricular function and ventricular dilation are commonly invoked as a mechanism of TR in HLHS, no study to our knowledge has been able to demonstrate an association of TR with 2DE-derived parameters of global RV function.[5, 19] Similarly, RV dilation is a logical mechanism by which APM angle may decrease, but neither our study nor previous studies in this population have demonstrated this relationship.[13, 19] These discrepancies may be related to the complex shape of the RV in the single ventricle and limitations of 2DE imaging. Future longitudinal studies including 3D quantification of the entire ventricular-valvular complex in this population may help to elucidate these complex dynamics. In particular, given that the dynamic changes in 3D valve circumference, valve area, and S-L diameter differed between TR groups, 3D assessment of basal right ventricular geometry and deformation may inform our understanding of the TA dynamics, and ultimately TV function.[25]
Implications for Surgical Repair
Repair of the regurgitant TV in HLHS remains extremely challenging.[26] Defining the mechanisms of TR is difficult, with both 2DE and surgical inspection having significant limitations in this complex population.[27] Our findings suggest that addressing the increased dynamic change of the S-L TA dimension and the lower, more laterally directed APM angle may be important in HLHS patients with a Fontan circulation and significant TR. Substantiating our findings, in-vitro and ex-vivo studies have suggested that aberration of these two factors can independently create TR.[28, 29] As such, a restrictive suture annuloplasty or rigid annuloplasty ring directed at reducing S-L movement of the TA and reducing the lateral and posterior quadrant areas may be therapeutic. Notably, the two most common surgical interventions in this population are posterior leaflet obliteration which involves a posteroseptal to posterolateral annuloplasty, and De Vega annuloplasty from the anteroseptal to the posteroseptal commissure. These interventions restrict different parts of the TA, but may partially “normalize” the TA changes seen in our moderate or greater TR cohort.[24, 30] An improved understanding of the pathological TA perturbations in the HLHS population could further guide optimization of general annuloplasty approaches and perhaps facilitate patient-specific annuloplasty design.[24, 30, 31]
Measures of annular height and bending angles were not associated with moderate or greater TR in our cohort, unlike the adult mitral valve where loss of the hyperbolic paraboloid shape has been highly associated with the development of regurgitation and chordal rupture.[6, 8] In younger patients with HLHS, bending angle has been shown to be associated with greater TR, but by the Fontan stage we speculate that this line of defense may have already been lost. As such, it is unclear if surgical techniques (non-planar rings) to reestablish the normal non-planar shape will be beneficial in the Fontan population.[4, 5]
Finally, while there are differences in the TA of patients with and without moderate or greater TR, our findings suggest that annuloplasty alone may be insufficient to restore normal tricuspid function even with structurally normal TV leaflets.[28, 29] Patients in our moderate or greater TR cohort had significantly more laterally directed APM. Papillary muscle relocation was introduced to treat ischemic mitral regurgitation in adults and has been associated with long-term benefits in LV remodeling and valve geometry.[32, 33] Papillary angle intervention has been explored in the TV in a biventricular ex-vivo porcine model.[29] Notably, in the porcine model APM approximation toward the apex and septum (increased APM angle) reduced TR more than annuloplasty alone, and reduced both the S-L diameter and the area of the TA.[29] This surgical strategy has been reported clinically for TV repair in HLHS, and our findings support further exploration of such novel subvalvular interventions.[24, 34]
Limitations
Our study is retrospective. As such, we can only associate the suggested annular and papillary muscle changes to moderate or greater TR, but cannot determine if those changes caused TR, or if TR preceded and potentiated those changes. However, there is evidence that annular changes, independent of ventricular volume, can cause atrioventricular valve regurgitation, and that annular and subvalvular interventions can independently reduce tricuspid valve regurgitation.[29, 35] Limited visualization of the full RV and tricuspid leaflets prohibited analysis of leaflet shape and assessment of 3D ventricular volumes. Assessment of RV size and function by 2D is limited and utilization of 3D RV volumes may provide more accurate quantification. Our sample size limits power for multi-variate modeling as well as generalization. We had few patients with severe TR or severe RV dysfunction, limiting our power to detect the potential effects of ventricular function on annular dynamics, as well as potentially introducing selection bias. We did not assess TA dynamics or subvalvular structure in a normal biventricular control group. Assessment of TR severity in HLHS is difficult as there are no quantitative grading guidelines in children. 3D color images and hence 3D vena contracta were not consistently available in our retrospective cohort. We examined the annular shape at 4 different points in the cardiac cycle, but not every frame of the cine image. In HLHS the posterior and septal papillary muscles are small and have a highly variable anatomy. As such, only the APM was reliably visualized, consistent with the limitations seen in other studies.[13, 36] Separating the displacement of the TA from the relative movement of the heart is complex and better methods of tracking the TA are needed. EKG was not available during our modeling. RV dyssynchrony can influence both function and annular motion but we did not assess ventricular dyssynchrony as part of this study. We did not validate our 3DE-based measures relative to tomographic imaging, and there may be technique-based differences in measurements.[37] Our analyses were done semi-automatically, which is time-consuming and contributes to variability; in the future, fully automated tools could improve these limitations.[38]
CONCLUSIONS
The TA in HLHS after the Fontan operation varies in shape significantly throughout the cardiac cycle, varying in sphericity while remaining relatively planar. Larger change in S-L diameter throughout the cardiac cycle and acute, laterally directed APM angle together are associated with the presence of TR, potentially providing insight for annuloplasty design and novel subvalvular interventions.
Supplementary Material
Figure S1: Demonstration of Point Placement and Creation of an Unsmoothed Annular Curve
Figure S2: Fourier Smoothed Annular Curve
Figure S3: Annular Point Assignment. A. Assignment of annular points on a tricuspid valve diagram; B. Assignment of annular points on annular curve shown within the echo image in 3D Slicer.
Figure S4: Septo-lateral and Anterior-posterior Diameter Measurements
Figure S5: 3D Visualization of Least Squares Annular Plane (blue)
Figure S6: Annular Height Measurement
Figure S7: Septo-lateral Bending Angle Measurement
Figure S8: Annular Circumference Measurement
Figure S9: Total 3D Annular Area Measurement
Figure S10: 3D Annular Area Measurements Divided by Quadrant
Figure S11: Demonstration of Chord Quantification to Analyze Annular Motion of the Septal Quadrant. A. Total displacement measurement; B. Radial displacement measurement; C. Through plane displacement measurement.
Figure S12: Demonstration of Anterior Papillary Muscle (APM) Angle Measurement Relative to the Annular Plane. Top: Red dots represent annular plane in cross section, with blue line representing annular plane in cross section. Bottom: Perspective view of the same in 3 dimensions.
Figure S13: Demonstration of Procrustes Analysis Comparing Tricuspid Annular Shape in Patients with and without Significant TR Throughout the Cardiac Cycle
Figure S14. Potential Mechanism of Association of Significant TR to Change in Septo-Lateral Annular Diameter. Note the greater septo-lateral diameter change and expansion of the lateral tricuspid annulus in the valve with significant TR compared to the valve with no TR. Tricuspid annulus shown in red. TR = tricuspid regurgitation.
Figure S15. Potential Mechanism of Association of Significant TR to Anterior Papillary Muscle Angle (APM). A. Diagram of APM angle in a valve with no TR; B. Diagram of APM angle in a valve with significant TR. Note the larger lateral tethering angle in the valve with no TR. TR = tricuspid regurgitation, Red Dots = Annulus in cross section, Dotted line = annular least squares plane
Highlights.
We modeled the dynamic annular structure of the tricuspid valve in HLHS with a Fontan circulation.
Annulus dimensions and shape vary throughout the cardiac cycle.
Larger change in septo-lateral diameter and acute anterior papillary muscle angle are strongly associated with TR at the Fontan stage.
Acknowledgments:
We would like to acknowledge the 3D core sonographer group at the Children’s Hospital of Philadelphia (CHOP) whose clinically acquired images were the foundation for this work.
Funding: This work was supported by the Department of Anesthesia and Critical Care at the Children’s Hospital of Philadelphia, the National Institute of Biomedical Imaging and Bioengineering (NIBIB) (P41 EB015902), Cancer Care Ontario with funds provided by the Ontario Ministry of Health and Long-Term Care, and Natural Sciences and Engineering Research Council of Canada, and NIH K01HL125521.
Abbreviations:
- 2D
Two Dimensional
- 2DE
Two-Dimensional Echocardiography
- 3D
Three Dimensional
- 3DE
Three-Dimensional Echocardiography
- A-P
Anterior-Posterior
- APM
Anterior Papillary Muscle
- DICOM
Digital Imaging and Communications in Medicine
- Echo
Echocardiography
- ED
End-diastole
- ES
End-systole
- HLHS
Hypoplastic Left Heart Syndrome
- ICC
Intraclass Correlation Coefficient
- LV
Left Ventricle
- MD
Mid-diastole
- MS
Mid-systole
- RV
Right Ventricle
- S-L
Septo-lateral
- TA
Tricuspid Annulus (Annuli)
- TR
Tricuspid Regurgitation
- TV
Tricuspid Valve
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, et al. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:896–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tabbutt S, Ghanayem N, Ravishankar C, Sleeper LA, Cooper DS, Frank DU, et al. Risk factors for hospital morbidity and mortality after the Norwood procedure: A report from the Pediatric Heart Network Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144:882–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ugaki S, Khoo NS, Ross DB, Rebeyka IM, Adatia I. Tricuspid valve repair improves early right ventricular and tricuspid valve remodeling in patients with hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2013;145:446–50. [DOI] [PubMed] [Google Scholar]
- [4].Kutty S, Colen T, Thompson RB, Tham E, Li L, Vijarnsorn C, et al. Tricuspid regurgitation in hypoplastic left heart syndrome: mechanistic insights from 3-dimensional echocardiography and relationship with outcomes. Circ Cardiovasc Imaging. 2014;7:765–72. [DOI] [PubMed] [Google Scholar]
- [5].Takahashi K, Inage A, Rebeyka IM, Ross DB, Thompson RB, Mackie AS, et al. Real-time 3dimensional echocardiography provides new insight into mechanisms of tricuspid valve regurgitation in patients with hypoplastic left heart syndrome. Circulation. 2009;120:1091–8. [DOI] [PubMed] [Google Scholar]
- [6].Lee AP, Hsiung MC, Salgo IS, Fang F, Xie JM, Zhang YC, et al. Quantitative analysis of mitral valve morphology in mitral valve prolapse with real-time 3-dimensional echocardiography: importance of annular saddle shape in the pathogenesis of mitral regurgitation. Circulation. 2013;127:832–41. [DOI] [PubMed] [Google Scholar]
- [7].Mahmood F, Matyal R. A quantitative approach to the intraoperative echocardiographic assessment of the mitral valve for repair. Anesth Analg. 2015;121:34–58. [DOI] [PubMed] [Google Scholar]
- [8].Salgo IS, Gorman JH 3rd, Gorman RC, Jackson BM, Bowen FW, Plappert T, et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation. 2002;106:711–7. [DOI] [PubMed] [Google Scholar]
- [9].Bartels K, Thiele RH, Phillips-Bute B, Glower DD, Swaminathan M, Kisslo J, et al. Dynamic indices of mitral valve function using perioperative three-dimensional transesophageal echocardiography. J Cardiothorac Vasc Anesth. 2014;28:18–24. [DOI] [PubMed] [Google Scholar]
- [10].Grewal J, Suri R, Mankad S, Tanaka A, Mahoney DW, Schaff HV, et al. Mitral annular dynamics in myxomatous valve disease: new insights with real-time 3-dimensional echocardiography. Circulation. 2010;121:1423–31. [DOI] [PubMed] [Google Scholar]
- [11].Jimenez JH, Liou SW, Padala M, He Z, Sacks M, Gorman RC, et al. A saddle-shaped annulus reduces systolic strain on the central region of the mitral valve anterior leaflet. J Thorac Cardiovasc Surg. 2007;134:1562–8. [DOI] [PubMed] [Google Scholar]
- [12].Mahmood F, Gorman JH 3rd, Subramaniam B, Gorman RC, Panzica PJ, Hagberg RC, et al. Changes in mitral valve annular geometry after repair: saddle-shaped versus flat annuloplasty rings. Ann Thorac Surg. 2010;90:1212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Nii M, Guerra V, Roman KS, Macgowan CK, Smallhorn JF. Three-dimensional tricuspid annular function provides insight into the mechanisms of tricuspid valve regurgitation in classic hypoplastic left heart syndrome. J Am Soc Echocardiogr. 2006;19:391–402. [DOI] [PubMed] [Google Scholar]
- [14].Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin JC, Pujol S, et al. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Scanlan AB, Nguyen AV, Ilina A, Lasso A, Cripe L, Jegatheeswaran A, et al. Comparison of 3D Echocardiogram-Derived 3D Printed Valve Models to Molded Models for Simulated Repair of Pediatric Atrioventricular Valves. Pediatr Cardiol. 2018;39:538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Pouch AM, Vergnat M, McGarvey JR, Ferrari G, Jackson BM, Sehgal CM, et al. Statistical assessment of normal mitral annular geometry using automated three-dimensional echocardiographic analysis. Ann Thorac Surg. 2014;97:71–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol (1985). 2005;99:445–57. [DOI] [PubMed] [Google Scholar]
- [18].Prakash A, Lacro RV, Sleeper LA, Minich LL, Colan SD, McCrindle B, et al. Challenges in echocardiographic assessment of mitral regurgitation in children after repair of atrioventricular septal defect. Pediatr Cardiol. 2012;33:205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Colen T, Kutty S, Thompson RB, Tham E, Mackie AS, Li L, et al. Tricuspid Valve Adaptation during the First Interstage Period in Hypoplastic Left Heart Syndrome. J Am Soc Echocardiogr. 2018;31:624–33. [DOI] [PubMed] [Google Scholar]
- [20].Nii M, Roman KS, Macgowan CK, Smallhorn JF. Insight into normal mitral and tricuspid annular dynamics in pediatrics: a real-time three-dimensional echocardiographic study. J Am Soc Echocardiogr. 2005;18:805–14. [DOI] [PubMed] [Google Scholar]
- [21].Fukuda S, Gillinov AM, Song JM, Daimon M, Kongsaerepong V, Thomas JD, et al. Echocardiographic insights into atrial and ventricular mechanisms of functional tricuspid regurgitation. Am Heart J. 2006;152:1208–14. [DOI] [PubMed] [Google Scholar]
- [22].Barber G, Helton JG, Aglira BA, Chin AJ, Murphy JD, Pigott JD, et al. The significance of tricuspid regurgitation in hypoplastic left-heart syndrome. Am Heart J. 1988;116:1563–7. [DOI] [PubMed] [Google Scholar]
- [23].Elmi M, Hickey EJ, Williams WG, Van Arsdell G, Caldarone CA, McCrindle BW. Longterm tricuspid valve function after Norwood operation. J Thorac Cardiovasc Surg. 2011;142:1341–7 e4. [DOI] [PubMed] [Google Scholar]
- [24].Bautista-Hernandez V, Brown DW, Loyola H, Myers PO, Borisuk M, del Nido PJ, et al. Mechanisms of tricuspid regurgitation in patients with hypoplastic left heart syndrome undergoing tricuspid valvuloplasty. J Thorac Cardiovasc Surg. 2014;148:832–8. [DOI] [PubMed] [Google Scholar]
- [25].Topilsky Y, Khanna A, Le Tourneau T, Park S, Michelena H, Suri R, et al. Clinical context and mechanism of functional tricuspid regurgitation in patients with and without pulmonary hypertension. Circ Cardiovasc Imaging. 2012;5:314–23. [DOI] [PubMed] [Google Scholar]
- [26].Pigula FA, Mettler B. Management of Tricuspid Regurgitation in Patients With Hypoplastic Left Heart Syndrome. Semin Thorac Cardiovasc Surg. 2017;29:64–9. [DOI] [PubMed] [Google Scholar]
- [27].Bharucha T, Honjo O, Seller N, Atlin C, Redington A, Caldarone CA, et al. Mechanisms of tricuspid valve regurgitation in hypoplastic left heart syndrome: a case-matched echocardiographic-surgical comparison study. Eur Heart J Cardiovasc Imaging. 2013;14:135–41. [DOI] [PubMed] [Google Scholar]
- [28].Spinner EM, Shannon P, Buice D, Jimenez JH, Veledar E, Del Nido PJ, et al. In vitro characterization of the mechanisms responsible for functional tricuspid regurgitation. Circulation. 2011;124:920–9. [DOI] [PubMed] [Google Scholar]
- [29].Yamauchi H, Vasilyev NV, Marx GR, Loyola H, Padala M, Yoganathan AP, et al. Right ventricular papillary muscle approximation as a novel technique of valve repair for functional tricuspid regurgitation in an ex vivo porcine model. J Thorac Cardiovasc Surg. 2012;144:235–42. [DOI] [PubMed] [Google Scholar]
- [30].Ohye RG, Gomez CA, Goldberg CS, Graves HL, Devaney EJ, Bove EL. Repair of the tricuspid valve in hypoplastic left heart syndrome. Cardiol Young. 2006;16 Suppl 3:21–6. [DOI] [PubMed] [Google Scholar]
- [31].Dinh DC, Gurney JG, Donohue JE, Bove EL, Hirsch JC, Devaney EJ, et al. Tricuspid valve repair in hypoplastic left heart syndrome. Pediatr Cardiol. 2011;32:599–606. [DOI] [PubMed] [Google Scholar]
- [32].Fattouch K, Castrovinci S, Murana G, Dioguardi P, Guccione F, Nasso G, et al. Papillary muscle relocation and mitral annuloplasty in ischemic mitral valve regurgitation: midterm results. J Thorac Cardiovasc Surg. 2014;148:1947–50. [DOI] [PubMed] [Google Scholar]
- [33].Fattouch K, Lancellotti P, Castrovinci S, Murana G, Sampognaro R, Corrado E, et al. Papillary muscle relocation in conjunction with valve annuloplasty improve repair results in severe ischemic mitral regurgitation. J Thorac Cardiovasc Surg. 2012;143:1352–5. [DOI] [PubMed] [Google Scholar]
- [34].Myers PO, Yamauchi H, Baird CW, Fynn-Thompson F, Emani S, del Nido PJ. Papillary Muscle Approximation to Address Tricuspid Tethering: Efficacy and Safety from Iatrogentic Stenosis in a Case Control Study. J Am Coll Cardiol. 2012;59:E762. [Google Scholar]
- [35].Yamauchi H, Feins EN, Vasilyev NV, Shimada S, Zurakowski D, Del Nido PJ. Creation of nonischemic functional mitral regurgitation by annular dilatation and nonplanar modification in a chronic in vivo swine model. Circulation. 2013;128:S263–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Stamm C, Anderson RH, Ho SY. The morphologically tricuspid valve in hypoplastic left heart syndrome. Eur J Cardiothorac Surg. 1997;12:587–92. [DOI] [PubMed] [Google Scholar]
- [37].Praz F, Khalique OK, Dos Reis Macedo LG, Pulerwitz TC, Jantz J, Wu IY, et al. Comparison between Three-Dimensional Echocardiography and Computed Tomography for Comprehensive Tricuspid Annulus and Valve Assessment in Severe Tricuspid Regurgitation: Implications for Tricuspid Regurgitation Grading and Transcatheter Therapies. J Am Soc Echocardiogr. 2018;31:1190–202. [DOI] [PubMed] [Google Scholar]
- [38].Pouch AM, Aly AH, Lasso A, Nguyen AV, Scanlan AB, McGowan FX, et al. Image Segmentation and Modeling of the Pediatric Tricuspid Valve in Hypoplastic Left Heart Syndrome. Lect Notes Comput Sc. 2017;10263:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Demonstration of Point Placement and Creation of an Unsmoothed Annular Curve
Figure S2: Fourier Smoothed Annular Curve
Figure S3: Annular Point Assignment. A. Assignment of annular points on a tricuspid valve diagram; B. Assignment of annular points on annular curve shown within the echo image in 3D Slicer.
Figure S4: Septo-lateral and Anterior-posterior Diameter Measurements
Figure S5: 3D Visualization of Least Squares Annular Plane (blue)
Figure S6: Annular Height Measurement
Figure S7: Septo-lateral Bending Angle Measurement
Figure S8: Annular Circumference Measurement
Figure S9: Total 3D Annular Area Measurement
Figure S10: 3D Annular Area Measurements Divided by Quadrant
Figure S11: Demonstration of Chord Quantification to Analyze Annular Motion of the Septal Quadrant. A. Total displacement measurement; B. Radial displacement measurement; C. Through plane displacement measurement.
Figure S12: Demonstration of Anterior Papillary Muscle (APM) Angle Measurement Relative to the Annular Plane. Top: Red dots represent annular plane in cross section, with blue line representing annular plane in cross section. Bottom: Perspective view of the same in 3 dimensions.
Figure S13: Demonstration of Procrustes Analysis Comparing Tricuspid Annular Shape in Patients with and without Significant TR Throughout the Cardiac Cycle
Figure S14. Potential Mechanism of Association of Significant TR to Change in Septo-Lateral Annular Diameter. Note the greater septo-lateral diameter change and expansion of the lateral tricuspid annulus in the valve with significant TR compared to the valve with no TR. Tricuspid annulus shown in red. TR = tricuspid regurgitation.
Figure S15. Potential Mechanism of Association of Significant TR to Anterior Papillary Muscle Angle (APM). A. Diagram of APM angle in a valve with no TR; B. Diagram of APM angle in a valve with significant TR. Note the larger lateral tethering angle in the valve with no TR. TR = tricuspid regurgitation, Red Dots = Annulus in cross section, Dotted line = annular least squares plane