Abstract
Purpose:
Racial/ethnic disparities in severe maternal morbidity (SMM) are substantial, but little is known about whether these disparities are changing over time or the role of maternal and obstetric factors.
Methods:
We examined disparities in SMM prevalence and trends using linked birth certificate and delivery discharge records from Californian births during 1997-2014 (n = 8,252,025).
Results:
The prevalence of SMM was highest in non-Hispanic (NH) Black women (1.63%), lowest in NH White women (0.84%), and increased from 1997-2014 by approximately 170% in each racial/ethnic group. The magnitude of SMM disparities remained consistent over time. Compared to NH White women, the adjusted risk of SMM was higher in women who identified as Hispanic (RR 1.14; 95% CI 1.12, 1.16), Asian/Pacific Islander (RR 1.23; 95% CI 1.20, 1.26), NH Black (RR 1.27; 95% CI 1.23, 1.31), and American Indian/Alaska Native (RR 1.17; 95% CI 1.04, 1.31), accounting for comorbidities, anemia, cesarean birth, and other maternal characteristics.
Conclusions:
The prevalence of SMM varied considerably by race/ethnicity but increased at similarly high rates among all racial/ethnic groups. Comorbidities, cesarean birth, and other factors did not fully explain the disparities in SMM, which remained persistent over time.
Keywords: maternal health, maternal mortality, minority health, risk factors, vulnerable populations, cesarean section, obesity, anemia
INTRODUCTION
An estimated 14 in every 1000 women hospitalized for delivery in the U.S. experienced severe maternal morbidity (SMM) in 2014 – a nearly twofold increase from 1994.1 These women suffered hemorrhage, embolism, stroke, and other serious complications that have significant short- and long-term health consequences.2 Women who identify as racial or ethnic minorities have experienced the greatest burden of SMM,3 but little is understood about contributors to this disparity or how it has changed over time. Creanga et al.4 reported that non-Hispanic Black, Hispanic, Asian/Pacific Islander, and American Indian/Alaska Native women had 2.1, 1.3, 1.2, and 1.7 times higher risk of SMM, respectively, compared to non-Hispanic White women in a multi-state study of U.S. deliveries 2008-2010. One prior study has suggested that the trend in SMM may vary by race/ethnicity, reporting that SMM prevalence increased 1998-2012 in a national sample by 74%, 93%, 92%, and 58% in non-Hispanic White, non-Hispanic Black, Hispanic, and Asian/Pacific Islander women, respectively.5
Racial/ethnic health disparities are complex and reflect multiple levels of inequities – from patient characteristics to health-care policies.6 Maternal comorbidities (particularly obesity, hypertension, and diabetes) and cesarean birth are frequently identified as major patient-level contributors to SMM and its rise over time.1,2,7-9 These factors have all increased across recent decades,10-12 and tend to be more common among minority groups.5,7,11,13 In comparison to non-Hispanic White women, obesity and diabetes are more prevalent in women who are non-Hispanic Black, Hispanic, Asian/Pacific Islander, or American Indian/Alaska Native.13,14 Additionally, hypertensive disorders, asthma, anemia, and cesarean birth are all more common in non-Hispanic Black women, who also experience the largest burden of SMM.4,11,13-16 Therefore, it is reasonable to expect that these comorbidities, along with cesarean birth, may contribute to disparities in SMM prevalence and trends. However, the contributions of patient-level factors to disparities in either the prevalence or trends of SMM are uncertain.
Achieving racial/ethnic equity in maternal health will require strategies that target multiple levels of health determinants.6 In this study, we leveraged a high-quality, population-based dataset of delivery discharge records and birth certificates to fill gaps in the understanding of disparities in SMM and patient-level contributors. Our objective was to examine racial/ethnic disparities in the overall prevalence and temporal trend of SMM.
MATERIAL AND METHODS
Study population
This cohort study used data from all recorded live births that occurred in California from January 1, 1997 to December 31, 2014 (N = 9,353,996). Birth certificate records were previously linked to delivery discharge records by the California Office of Statewide Health Planning and Development (1997-2011) and the California Maternal Quality Care Collaborative (CMQCC) (2012-2014). This study included all births ≥20 weeks’ gestation with linked birth certificate and maternal delivery discharge records and complete information on study variables (Supplemental Figure 1). Because births of multiples resulted in duplicate maternal records, we selected for the first record in such cases. The State of California Committee for the Protection of Human Subjects and the Stanford University Research Compliance Office approved the study protocol.
Measures
The outcome of interest was SMM during delivery hospitalization, measured using the Severe Maternity Morbidity Index.1 The CDC and its partners created the index for use in administrative data. It includes 18 indicators of organ-system dysfunction that likely represent specific, well-defined severe events, such as blood transfusion, acute respiratory distress syndrome, and acute renal failure.7 We used International Classification of Disease Clinical Modification 9th Revision (ICD-9-CM) diagnosis and procedure codes to identify these indicators in discharge records (Supplemental Table 1). We also studied a subset of SMM that excluded those cases for which the only indication was a blood transfusion (“transfusion-only cases”). Blood transfusion is the only qualifying indicator for approximately half of SMM cases because postpartum hemorrhage is by far the most common cause of SMM.2,17 However, the number of units of blood transfused is not available in administrative data, and thus transfusion-only cases may include some less severely ill women; this is likely to be more common among women with anemia upon admission.18 Prior studies have also suggested that blood transfusions may be driving the rise in SMM.1,19
Maternal race/ethnicity was collected on the birth certificate and grouped as Hispanic (Hispanic/Spanish/Latina), non-Hispanic White, non-Hispanic Black (Black or African American), Asian/Pacific Islander (Indian, Chinese, Filipino, Japanese, Korean, Vietnamese, Native Hawaiian, Guamanian/Chamorro, Samoan, or other Asian/Pacific Islander), American Indian/Alaska Native, or Other based on available data and prior studies.4 We hereafter refer to non-Hispanic White and non-Hispanic Black as White and Black.
Maternal comorbidity was measured using an index created by Bateman et al.20 We modified the index by removing ICD-9-CM diagnosis codes that are also in the SMM Index (eclampsia [642.6x] and sickle cell disease with crisis [282.41, 282.62, 282.64, 282.69]). The comorbidity index includes previous cesarean birth, advanced maternal age, multiple gestation, placental conditions, drug and alcohol abuse, and several preexisting and gestational conditions. Anemia prior to delivery is often on the direct path to SMM in that it increases the chance of blood transfusion, particularly when combined with cesarean birth. For that reason, we examined the role of anemia separately from other comorbidities. Given the possible misclassification of anemia secondary to postpartum hemorrhage as a preexisting condition, we only included anemia cases (ICD-9-CM 648.20-648.23) with a diagnosis reported as present-on-admission for the delivery hospitalization.21 Cesarean birth was identified either in the discharge record (ICD-9-CM procedure code 74) or on the birth certificate. Prepregnancy body mass index (BMI) was examined as an additional risk factor in a sensitivity analysis conducted in data from 2007-2014. In 2007, California implemented the revised U.S. birth certificate, which collects prepregnancy weight and height. Prepregnancy BMI (kg/m2) was categorized as underweight (<18.5), normal weight (18.5-24.9), overweight (25-29.9), obesity class 1 (30-34.9), obesity class 2 (35-39.9), and obesity class 3 (≥40). We used causal diagrams and prior knowledge to select covariates from available data to include in regression models.4,17,22-24 They included educational attainment, expected method of payment for delivery, trimester prenatal care began, parity, and preterm birth (<37 weeks’ gestation). We recognized that nearly all of the variables in this study temporally occur after race and thus might be considered mediators of an association between racial identity and SMM.25 The findings are therefore limited in interpretation to how SMM for a racial/ethnic group would change if the distribution of the ‘mediators’ (e.g., comorbidity) were set to that of White women (the reference). This interpretation makes the assumption of no unmeasured confounding between each covariate and SMM.25
Statistical analysis
We compared the distributions of maternal and delivery characteristics among racial/ethnic groups in the study population and between included and excluded subjects. To evaluate disparities in the prevalence of SMM, we conducted multivariable logistic regression models with White women as the reference group because they had the lowest risk of SMM. Estimated odds ratios approximated risk ratios because of the rarity of SMM. We adjusted the models for delivery year and sequentially adjusted for sociodemographic/obstetric factors, comorbidity, anemia, and cesarean birth to estimate their contribution to the disparities and because the risk factors often happen in a sequential order.25 In study years during which prepregnancy BMI was collected (2007-2014), we replicated the above models with additional adjustment for prepregnancy BMI.
We next evaluated racial/ethnic differences in the trend of SMM. The annual prevalence of SMM for each racial/ethnic group was calculated and plotted. We grouped every 3 years of data and conducted the same regression models as described above separately in each 3-year time interval. We grouped the years to increase statistical power and smooth the plots. Risk ratios for SMM comparing each racial/ethnic minority group to White women were estimated and plotted over time. We performed all analyses above for SMM including and excluding transfusion-only cases. We also performed a post hoc sensitivity analysis to assess if correlation among sibling births affected the results. We conducted this analysis using generalized estimating equations with an exchangeable correlation matrix in a random sample of 100,000 births during 1997-2011 due to computational limitations and lack of information to link siblings after 2011. During 1997-2011, there were 6,902,025 births to 5,057,098 women and this degree of clustering did not affect the results. All analyses were conducted in R 3.4.4. and SAS 9.4.
RESULTS
The study included 8,252,025 live births during 1997-2014. Fifty percent of women in the study were Hispanic, 30% were White, 12% were Asian or Pacific Islander, 5% were Black, 0.3% were American Indian or Alaska Native, and 3% were multi-race or other. The prevalence of SMM was highest in Black women and lowest in White women (Table 1). Cesarean birth, anemia, and high comorbidity were most common in Black women, and high prepregnancy BMI was most common in American Indian/Alaska Native women. Differences between included and excluded subjects were minimal, except that women excluded from the study had a higher prevalence of SMM (1.19% vs. 1.05%), lower educational attainment, and delivered in earlier years than those who were included (Supplemental Table 2).
Table 1.
Distribution of maternal and delivery variables among racial/ethnic groups, California, 1997-2014 (N = 8,252,025).
| Hispanic | Non- Hispanic White |
Asian/ Pacific Islander |
Non- Hispanic Black |
American Indian/ Alaska Native |
|
|---|---|---|---|---|---|
| n = 4,087,859 |
n = 2,506,883 |
n = 961,585 | n = 444,255 | n = 26,598 | |
| % | % | % | % | % | |
| Severe maternal morbidity | 1.09 | 0.84 | 1.10 | 1.63 | 1.30 |
| Severe maternal morbidity excluding transfusion-only cases | 0.48 | 0.42 | 0.51 | 0.82 | 0.55 |
| Cesarean birth | 29.1 | 29.2 | 29.3 | 33.5 | 28.9 |
| Anemia | 5.2 | 3.7 | 4.6 | 10.2 | 5.4 |
| Prepregnancy BMI group | |||||
| Underweight | 2.7 | 4.1 | 8.9 | 3.9 | 3.0 |
| Normal weight | 41.3 | 56.6 | 66.2 | 40.2 | 36.9 |
| Overweight | 30.0 | 22.4 | 17.5 | 26.5 | 25.5 |
| Obesity class 1 | 16.0 | 9.8 | 5.3 | 15.2 | 17.4 |
| Obesity class 2 | 6.5 | 4.5 | 1.5 | 7.6 | 9.5 |
| Obesity class 3 | 3.6 | 2.7 | 0.6 | 6.5 | 7.7 |
| Comorbidity score | |||||
| 0 | 68.4 | 60.2 | 60.9 | 60.5 | 63.0 |
| 1-2 | 27.6 | 34.0 | 33.4 | 31.7 | 30.2 |
| 3-4 | 2.8 | 4.4 | 4.4 | 5.5 | 5.0 |
| ≥5 | 1.3 | 1.3 | 1.3 | 2.3 | 1.8 |
| Maternal age at delivery | |||||
| <20 y | 12.9 | 5.6 | 2.6 | 14.2 | 14.3 |
| 20-24 y | 27.5 | 16.9 | 9.7 | 28.7 | 29.1 |
| 25-29 y | 27.3 | 26.0 | 25.6 | 24.9 | 26.1 |
| 30-34 y | 20.1 | 29.5 | 36.5 | 19.1 | 19.1 |
| 35-39 y | 9.9 | 17.5 | 20.8 | 10.4 | 9.1 |
| ≥40 y | 2.4 | 4.5 | 4.8 | 2.8 | 2.3 |
| Mother born in the U.S. | 40.8 | 86.3 | 19.3 | 91.5 | 98.0 |
| Maternal education | |||||
| Did not complete high school | 46.3 | 9.2 | 7.2 | 17.9 | 24.8 |
| High school graduate or equivalent | 29.9 | 25.0 | 17.7 | 37.6 | 39.9 |
| Some college – no degree | 16.3 | 24.6 | 20.3 | 14.5 | 24.2 |
| College degree or higher | 7.5 | 41.2 | 54.8 | 29.9 | 11.1 |
| Private insurance expected as delivery payment method | 30.6 | 73.1 | 71.3 | 40.7 | 40.2 |
| Trimester prenatal care began | |||||
| 1st trimester | 81.6 | 88.8 | 87.2 | 80.5 | 72.9 |
| 2nd trimester | 14.9 | 9.1 | 10.4 | 15.5 | 20.3 |
| 3rd trimester or none | 3.6 | 2.0 | 2.4 | 3.9 | 6.7 |
| Preterm birth | 9.1 | 8.2 | 8.6 | 13.2 | 10.4 |
| Twin/multiple birth | 1.1 | 2.1 | 1.5 | 2.1 | 1.4 |
| Obstetric history | |||||
| Primiparous | 34.9 | 43.6 | 46.6 | 39.0 | 35.4 |
| Multiparous with cesarean history | 14.6 | 12.3 | 11.6 | 14.8 | 14.1 |
| Multiparous without cesarean history | 50.5 | 44.2 | 41.8 | 46.3 | 50.6 |
Compared to White women, the unadjusted risk of SMM was 23% higher in Hispanic women, 22% higher in Asian/Pacific Islander women, 92% higher in Black women, and 54% higher in American Indian/Alaska Native women (Table 2). Statistical adjustment for sociodemographic/obstetric factors, comorbidity, anemia, and cesarean birth attenuated, but did not eliminate, the elevated risk of SMM in Hispanic, Black, and American Indian/Alaska Native women. The risk ratios for SMM in Asian/Pacific Islander women did not meaningfully change with adjustment for risk factors. Relative racial/ethnic differences were smaller for the outcome of SMM excluding transfusion-only cases, except for Black women. After full statistical adjustment, the relative Black-White difference for SMM was 1.27 (95% CI: 1.23, 1.31); after excluding transfusion-only cases, it was 1.44 (95% CI: 1.39, 1.50). Additional adjustment for prepregnancy BMI had a minimal impact on estimated risk ratios in sensitivity analysis conducted in study years 2007-2014 (Supplemental Table 3).
Table 2.
Associations between race/ethnicity and severe maternal morbidity, California, 1997-2014.
| Risk Ratio for Racial/Ethnic Group (95% CI) | |||||
|---|---|---|---|---|---|
| Non-Hispanic White |
Hispanic | Asian/ Pacific Islander |
Non-Hispanic Black |
American Indian/ Alaska Native |
|
| Severe Maternal Morbidity | |||||
| Regression model terms* | |||||
| Unadjusted | Reference | 1.23 (1.21, 1.25) |
1.22 (1.19, 1.25) |
1.92 (1.87, 1.97) |
1.54 (1.38, 1.72) |
| Covariates | Reference | 1.12 (1.10, 1.14) |
1.20 (1.16, 1.23) |
1.70 (1.65, 1.74) |
1.41 (1.27, 1.58) |
| Comorbidity + covariates | Reference | 1.19 (1.17, 1.22) |
1.26 (1.23, 1.30) |
1.59 (1.54, 1.63) |
1.33 (1.19, 1.48) |
| Anemia + comorbidity + covariates | Reference | 1.14 (1.12, 1.26) |
1.19 (1.16, 1.22) |
1.29 (1.25, 1.33) |
1.28 (1.15, 1.43) |
| Cesarean birth + anemia + comorbidity + covariates | Reference | 1.14 (1.12, 1.16) |
1.23 (1.20, 1.26) |
1.27 (1.23, 1.31) |
1.29 (1.15, 1.44) |
| Severe Maternal Morbidity – Excluding Cases with only Blood Transfusion | |||||
| Regression model terms | |||||
| Unadjusted | Reference | 1.10 (1.07, 1.13) |
1.16 (1.12, 1.20) |
1.93 (1.86, 2.01) |
1.30 (1.10, 1.53) |
| Covariates | Reference | 1.03 (1.00, 1.06) |
1.07 (1.03, 1.11) |
1.75 (1.68, 1.82) |
1.26 (1.07, 1.49) |
| Comorbidity + covariates | Reference | 1.10 (1.07, 1.13) |
1.13 (1.09, 1.18) |
1.63 (1.57, 1.70) |
1.18 (1.00, 1.40) |
| Anemia + comorbidity + covariates | Reference | 1.08 (1.05, 1.11) |
1.10 (1.06, 1.14) |
1.47 (1.41, 1.53) |
1.16 (0.98, 1.37) |
| Cesarean birth + anemia + comorbidity + covariates | Reference | 1.07 (1.04, 1.10) |
1.14 (1.09, 1.18) |
1.44 (1.39, 1.50) |
1.17 (0.99, 1.38) |
CI, confidence interval
All models adjusted for delivery year. Covariates included education, expected payment method for delivery, country of birth, prenatal care, parity, and preterm birth. Comorbidity was measured by an index of conditions.20
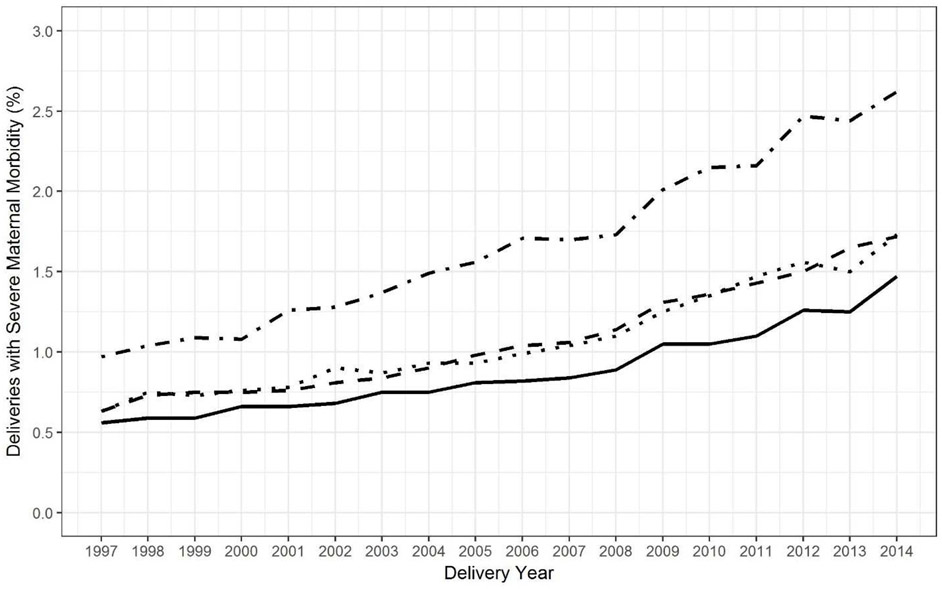
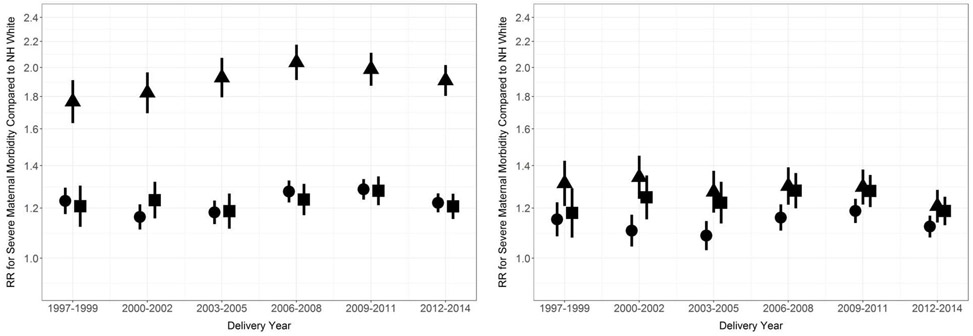
The prevalence of SMM increased in all racial/ethnic groups from 1997-2014 (Figure 1). SMM increased by 179% in Black women, 173% in Hispanic women, 175% in Asian/Pacific Islander women, and 163% in White women. (Trends of SMM in American Indian/Alaska Native women were not analyzed because there were only 10-35 cases per year.) There was minimal variation over time in the relative racial/ethnic differences in SMM, with or without adjustment for risk factors (Figure 2).
Figure 1. Observed trend in severe maternal morbidity by race/ethnicity, California, 1997-2014.
Non-Hispanic Black (· -), Hispanic (- -), Asian/Pacific Islander (· ·), non-Hispanic White (—).
Figure 2. Relative racial/ethnic disparity in severe maternal morbidity compared to non-Hispanic White women, California, 1997-2014.
Unadjusted (left) and adjusted (right). Hispanic (circle), Non-Hispanic Black (triangle), Asian/Pacific Islander (square). Vertical lines represent 95% confidence intervals. Adjustment set included education, expected payment method for delivery, country of birth, prenatal care, parity, preterm birth, comorbidity, anemia, and cesarean birth.
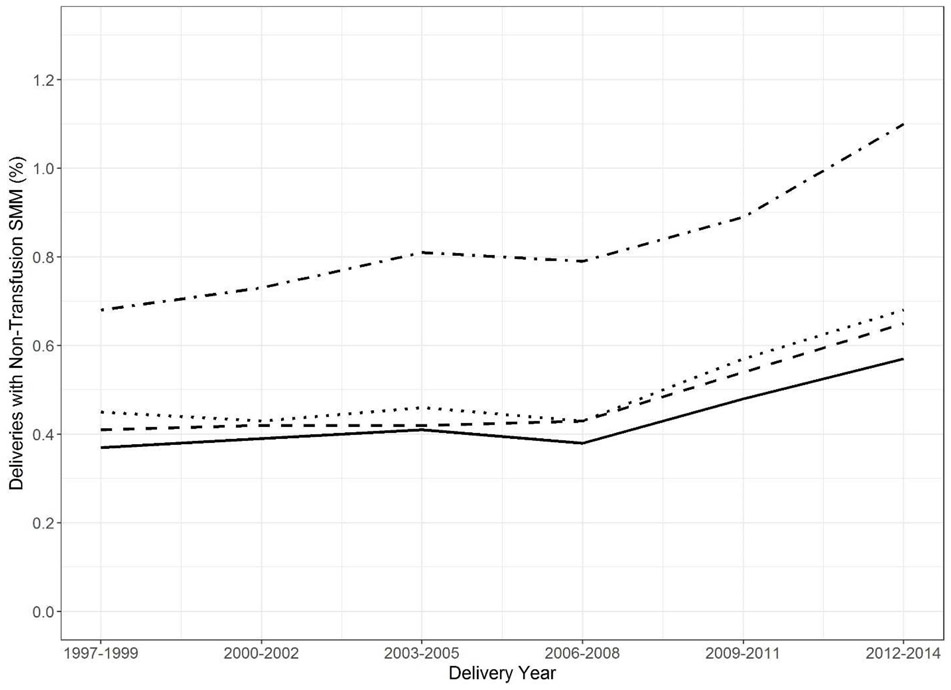
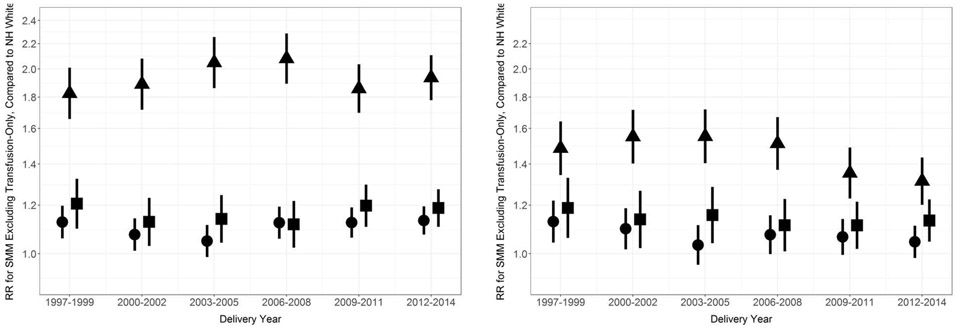
We found that the outcome of SMM excluding transfusion-only cases did not meaningfully increase in prevalence until after 2006-2008, for any racial/ethnic group (Figure 3). From 2008 to 2014, SMM excluding transfusion-only cases increased by 54% in Black women, 44% in Hispanic women, 53% in Asian/Pacific Islander women, and 63% in White women. Similar to results for SMM, minimal variation over time was present in the unadjusted and adjusted relative racial/ethnic differences in SMM excluding transfusion-only cases (Figure 4).
Figure 3. Observed trend in severe maternal morbidity, excluding blood transfusion-only cases, California, 1997-2014.
Non-Hispanic Black (· -), Hispanic (- -), Asian/Pacific Islander (· ·), non-Hispanic White (—).
Figure 4. Relative racial/ethnic disparity in severe maternal morbidity, excluding blood transfusion-only cases, compared to non-Hispanic White women, California, 1997-2014.
Unadjusted (left) and adjusted (right). Hispanic (circle), Non-Hispanic Black (triangle), Asian/Pacific Islander (square). Vertical lines represent 95% confidence intervals. Adjustment set included education, expected payment method for delivery, country of birth, prenatal care, parity, preterm birth, comorbidity, anemia, and cesarean birth.
DISCUSSION
The prevalence of severe maternal morbidity nearly tripled among all racial/ethnic groups in California from 1997 to 2014. Substantial racial/ethnic disparities in SMM were persistent over time, even after accounting for sociodemographic and obstetric characteristics, comorbidities, anemia, and cesarean birth. However, these risk factors did affect the magnitude of the observed disparities, particularly for women who identified as non-Hispanic Black or American Indian/Alaska Native. These findings add to limited evidence on maternal health inequities and underscore their complexity.
Recently, calls to action to address racial and ethnic disparities in maternal morbidity and mortality have emerged.6,26 However, no systematic ongoing data collection for population-based maternal morbidity in the U.S. exists, and inconsistent definitions of maternal morbidity, along with lack of risk factor data, have limited research.7 This study used an index developed by the CDC and its partners to identify SMM cases consistently across studies using diagnosis and procedure codes from hospitalization discharge records.1,2,7 Using this index in the California birth cohort, we observed rates of SMM by race/ethnicity similar to those reported in national inpatient samples.4,27 Previously, Creanga et al.4 found that adjustment for several sociodemographic factors and chronic conditions slightly reduced the disparity in SMM for Black and American Indian/Alaska Native women. We used measures available in a linked birth certificate-hospitalization discharge database to assess the additional impact of anemia, cesarean birth, prepregnancy BMI, and other sociodemographic and obstetric factors. We found these factors attenuated the disparity for all minority groups, except for Asian/Pacific Islander women. Given these findings, further study is needed of maternal health outcomes in Asian/Pacific Islander women – a highly heterogeneous population in California.
Risk factor adjustment in this study had the largest effect on the Black-White disparity in SMM. Adjustment for anemia, in particular, substantially reduced the relative Black-White disparity. Anemia at the time of delivery admission may increase the risk of cesarean birth and of particular importance for SMM, increase the risk of transfusion following cesarean birth.15,28 The prevalence of anemia was 10% in Black women, 5% in Hispanic women, and 4% in White women; in a national study, these rates were 18%, 6%, and 2%, respectively.15 A research challenge is that birth complications, such as hemorrhage, can also result in anemia. To address this issue as best as possible with hospitalization discharge data, we used ICD-9-CM diagnosis codes for anemia complicating pregnancy or childbirth reported as present-on-admission for delivery. Reporting of present-on-admission in California patient discharge data was previously found to have 74% agreement with medical records, which is similar to the validity of maternal diagnoses and procedures in hospitalization discharge databases.21,29 Anemia is a treatable condition, and our findings highlight a potential area of further investigation in studies with detailed information on anemia and timing of diagnosis.
The patient-level risk factors examined in this study did not fully explain the substantial racial/ethnic disparities in SMM. This finding may point to the understudied roles of health care delivery systems, communities, and chronic stress.6 Researchers have recently demonstrated a significant impact of hospital quality on disparities in SMM.30-32 In New York City, Howell et al.31,32 estimated that hospital differences may contribute up to 37% of the Hispanic-White disparity in SMM and up to 48% of the Black-White disparity. Personally mediated racism and stress during pregnancy have also been linked to disparities in preterm birth and low birthweight,33 which often c-ooccur with SMM. Institutionalized and internalized racism likely also contribute to these disparities.34 Together, the current evidence underscores the combined effects of multiple levels of factors on maternal health disparities and directions for future research and public health initiatives.
The national prevalence of SMM increased substantially during this study’s time period (1997-2014).1 We found that this trend was similar across racial/ethnic groups, which was also found in a recent government report,27 but not in a separate study.5 We found that this observation persisted after adjustment for a wide range of individual-level risk factors. These results suggest that changes in the characteristics of women giving birth have not affected disparities in SMM over time, despite increasing trends in maternal comorbidities and SMM.1,35 Previously, we found that prepregnancy health and cesarean birth were associated with SMM, but were not associated with an increasing trend in SMM in California during 2007 to 2014.12 Additionally, the Black-White disparity in maternal mortality in California has remained substantial, despite a decline of 57% in the overall maternal mortality rate from 2006 to 2013.36 The persistence of the disparities highlights the need for initiatives that specifically target maternal health inequities.
Blood transfusion is the only qualifying indicator for approximately half of SMM cases, and may be driving the national increase in SMM.1,27 Furthermore, racial/ethnic disparities in SMM may differ when the transfusion-only cases are excluded.4 In our study, SMM increased across the entire study period whereas SMM excluding transfusion-only cases only increased after 2006. Additionally, the adjusted risk ratio for the Black-White disparity in SMM was 1.27 for all SMM and 1.44 after excluding transfusion-only cases. For the other racial/ethnic subpopulations, the disparity was lower when transfusion-only cases were excluded. In a multi-state sample 2008-2010, the adjusted relative Black-White disparity in SMM was 2.1 for all SMM and 2.4 for SMM after excluding transfusion-only cases.4 We adjusted for additional factors in this study, which may explain our lower estimates.
This study should be interpreted in view of its design. Although large administrative datasets make it possible to study racial/ethnic disparities in rare outcomes like SMM, they are also subject to a number of limitations. Rare pregnancy complications were likely underreported,37 and although we attempted to isolate preexisting anemia, postpartum cases may have been miscoded as preexisting. If such misclassification was non-differential, bias in measures of association would be expected toward the null. If misclassification was differential, bias could be toward or away from the null. Cesarean birth is a well-known contributor to SMM,2,7 but in certain cases could occur after SMM. We used sequential adjustment in our analyses to consider cesarean birth separately, but could not study the timing of events during the same hospitalization; thus, this aspect of our results should be interpreted with caution. The CDC Index used to identify SMM cases in our study was previously found to have reasonable validity in California, but likely overestimates cases compared to medical record abstraction because of blood transfusions for non-severe complications.18 However, results were overall consistent for the outcome of SMM excluding transfusion-only cases. Our dataset also did not include information on several factors likely related to disparities in SMM, such as measures of stress and racism. We grouped races and ethnicities for sufficient group sizes and to compare with related studies but recognize the heterogeneity within these groups, which deserves further study.
CONCLUSIONS
Health disparities are a public heath priority, but disparities in severe maternal morbidity have remained persistent. The current findings suggest that individual-level factors contribute to, but do not fully explain, these disparities. Additionally, changes in the characteristics of pregnant women – including increases in comorbidities – have not affected racial/ethnic differences in severe maternal morbidity over time. These findings are informative for research and initiatives aimed at advancing equity in maternal health. In particular, our study supports public health endeavors to consider factors beyond those captured in administrative datasets, including patient-reported measures of stress, structural racism, and cultural congruency between health-care providers and patients.
Supplementary Material
Acknowledgments
FUNDING SOURCES
The Eunice Kennedy Shriver National Institute of Child Health and Development (F32HD091945), Stanford Maternal and Child Health Research Institute, and the National Institute of Nursing Research (R01NR017020) provided funding for this study.
Abbreviations
- BMI
body mass index
- CMQCC
California Maternal Quality Care Collaborative
- ICD-9-CM
International Classification of Disease Clinical Modification 9th Revision
- SMM
severe maternal morbidity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Centers for Disease Control and Prevention. Severe maternal morbidity in the United States. 2017; https://www.cdc.gov/reproductivehealth/maternalinfanthealth/severematernalmorbidity.html#anchor_SMM. Accessed September 26, 2018.
- 2.Callaghan WM, Creanga AA, Kuklina EV. Severe maternal morbidity among delivery and postpartum hospitalizations in the United States. Obstet Gynecol. 2012;120(5):1029–1036. [DOI] [PubMed] [Google Scholar]
- 3.Holdt Somer SJ, Sinkey RG, Bryant AS. Epidemiology of racial/ethnic disparities in severe maternal morbidity and mortality. Semin Perinatol. 2017;41(5):258–265. [DOI] [PubMed] [Google Scholar]
- 4.Creanga AA, Bateman BT, Kuklina EV, Callaghan WM. Racial and ethnic disparities in severe maternal morbidity: a multistate analysis, 2008-2010. Am J Obstet Gynecol. 2014;210(5):435 e431–438. [DOI] [PubMed] [Google Scholar]
- 5.Metcalfe A, Wick J, Ronksley P. Racial disparities in comorbidity and severe maternal morbidity/mortality in the United States: an analysis of temporal trends. Acta Obstet Gynecol Scand. 2018;97(1):89–96. [DOI] [PubMed] [Google Scholar]
- 6.Howell EA, Brown H, Brumley J, et al. Reduction of peripartum racial and ethnic disparities: a conceptual framework and maternal safety consensus bundle. Obstet Gynecol. 2018;131(5):770–782. [DOI] [PubMed] [Google Scholar]
- 7.Creanga AA, Berg CJ, Ko JY, et al. Maternal mortality and morbidity in the United States: where are we now? J Womens Health (Larchmt). 2014;23(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berg CJ, McKay AP, Qin C, Callaghan WM. Overview of maternal morbidity during hospitalization for labor and delivery in the United States: 1993-1997 and 2001-2005. Obstet Gynecol. 2009;113(5):1075–1081. [DOI] [PubMed] [Google Scholar]
- 9.Molina RL, Pace LE. A renewed focus on maternal health in the United States. N Engl J Med. 2017;377(18):1705–1707. [DOI] [PubMed] [Google Scholar]
- 10.Robbins CL, Zapata LB, Farr SL, et al. Core state preconception health indicators -- Pregnancy Risk Assessment Monitoring System and Behavioral Risk Factor Surveillance System, 2009. MMWR. 2014;63(3). [PubMed] [Google Scholar]
- 11.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. Births: final data for 2016. Nat Vital Stat Rep. 2018;67(1). [PubMed] [Google Scholar]
- 12.Leonard SA, Main EK, Carmichael SL. The contribution of maternal characteristics and cesarean delivery to an increasing trend of severe maternal morbidity. BMC Pregnancy Childbirth. 2019;19(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robbins C, Boulet SL, Morgan I, et al. Disparities in preconception health indicators -- Behavioral Risk Factor Surveillance System, 2013-2015, and Pregnancy Risk Assessment Monitoring System, 2013-2014. MMWR. 2018;67(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. Am J Obstet Gynecol. 2010;202(4):335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mei Z, Cogswell ME, Looker AC, et al. Assessment of iron status in US pregnant women from the National Health and Nutrition Examination Survey (NHANES), 1999-2006. Am J Clin Nutr. 2011;93:1312–1320. [DOI] [PubMed] [Google Scholar]
- 16.Schatz M, Dombrowski MP, Wise R, et al. Asthma morbidity during pregnancy can be predicted by severity classification. J Allergy Clin Immunol. 2003;112(2):283–288. [DOI] [PubMed] [Google Scholar]
- 17.Grobman WA, Bailit JL, Rice MM, et al. Frequency of and factors associated with severe maternal morbidity. Obstet Gynecol. 2014;123(4):804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Main EK, Abreo A, McNulty J, et al. Measuring severe maternal morbidity: validation of potential measures. Am J Obstet Gynecol. 2016;214(5):643 e641–643 e610. [DOI] [PubMed] [Google Scholar]
- 19.Callaghan WM, Kuklina EV, Berg CJ. Trends in postpartum hemorrhage: United States, 1994-2006. Am J Obstet Gynecol. 2010;202(4):353 e351–356. [DOI] [PubMed] [Google Scholar]
- 20.Bateman BT, Mhyre JM, Hernandez-Diaz S, et al. Development of a comorbidity index for use in obstetric patients. Obstet Gynecol.. 2013;122(5):957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldman LE, Chu PW, Osmond D, Bindman A. The accuracy of present-on-admission reporting in adminstrative data. Health Serv Res. 2011;46(6pt1):1946–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grobman WA, Bailit JL, Rice MM, et al. Racial and ethnic disparities in maternal morbidity and obstetric care. Obstet Gynecol.. 2015;125(6):1460–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lyndon A, Lee HC, Gilbert WM, Gould JB, Lee KA. Maternal morbidity during childbirth hospitalization in California. J Matern Fetal Neonatal Med. 2012;25(12):2529–2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lisonkova S, Muraca GM, Potts J, et al. Association between prepregnancy body mass index and severe maternal morbidity. JAMA. 2017;318(18):1777–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanderWeele TJ, Robinson WR. On the causal interpretation of race in regressions adjusting for confounding and mediating variables. Epidemiology. 2014;25(4):473–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jain JA, Temming LA, D'Alton ME, et al. SMFM special report: putting the "M" back in MFM: reducing racial and ethnic disparities in maternal morbidity and mortality: a call to action. Am J Obstet Gynecol. 2018;218(2):B9–B17. [DOI] [PubMed] [Google Scholar]
- 27.Fingar KR, Hambrick MM, Heslin KC, Moore JE. Trends and disparities in delivery hospitalizations involving severe maternal morbidity, 2006-2015. Agency for Healthcare Research and Quality;2018. [PubMed] [Google Scholar]
- 28.Drukker L, Hants Y, Farkash R, Ruchlemer R, Samueloff A, Grisaru-Granovsky S. Iron deficiency anemia at admission for labor and delivery is associated with an increased risk for Cesarean section and adverse maternal and neonatal outcomes. Transfusion. 2015;55(12):2799–2806. [DOI] [PubMed] [Google Scholar]
- 29.Lydon-Rochelle MT, Holt VL, Nelson JC, et al. Accuracy of reporting maternal inhospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol. 2005;19(6):460–471. [DOI] [PubMed] [Google Scholar]
- 30.Howell EA, Egorova N, Balbierz A, Zeitlin J, Hebert PL. Black-white differences in severe maternal morbidity and site of care. Am J Obstet Gynecol. 2016;214(1):122 e121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Howell EA, Egorova NN, Balbierz A, Zeitlin J, Hebert PL. Site of delivery contribution to black-white severe maternal morbidity disparity. Am J Obstet Gynecol. 2016;215(2):143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Howell EA, Egorova NN, Janevic T, Balbierz A, Zeitlin J, Hebert PL. Severe maternal morbidity among Hispanic women in New York City: Investigation of health disparities. Obstet Gynecol. 2017;129(2):285–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dominguez TP. Race, racism, and racial disparities in adverse birth outcomes. Clin Obstet Gynecol. 2008;51(2):360–370. [DOI] [PubMed] [Google Scholar]
- 34.Jones CP. Levels of racism: a theoretic framework and a gardener's tale. Am J Public Health. 2000;90(8):1212–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fridman M, Korst LM, Chow J, Lawton E, Mitchell C, Gregory KD. Trends in maternal morbidity before and during pregnancy in California. Am J Public Health. 2014;104 Suppl 1:S49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Main EK, Markow C, Gould J. Addressing maternal mortality and morbidity in California through public-private partnerships. Health Affairs (Project Hope). 2018;37(9):1484–1493. [DOI] [PubMed] [Google Scholar]
- 37.Lydon-Rochelle MT, Holt VL, Cardenas V, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol.. 2005;193(1):125–134. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.