Abstract
Background
Reductions in the World Health Organization (WHO) risk drinking levels have been proposed as an alternative primary outcome for alcohol clinical trials. Yet, little is known about whether reductions in WHO risk drinking levels can be maintained over time. The current study examined whether reductions in WHO risk drinking levels were maintained for up to 1 year following treatment, and whether reductions over time were associated with improvements in functioning.
Methods
Secondary data analysis of individuals with alcohol dependence (n = 1,226) enrolled in the COMBINE study, a multisite, randomized, placebo‐controlled clinical trial. Logistic regression was used to examine the maintenance of end‐of‐treatment WHO risk level reductions and WHO risk level reductions at the 1‐year follow‐up. Repeated‐measures mixed models were used to examine the association between WHO risk level reductions and functional outcomes over time.
Results
Achieving at least a 1‐ or 2‐level reduction in risk by the end of treatment was significantly associated with WHO risk level reductions at the 1‐year follow‐up assessment (p < 0.001). Among individuals who achieved at least a 1‐level reduction by the end of treatment, 85.5% reported at least a 1‐level reduction at the 1‐year follow‐up. Among individuals who achieved at least a 2‐level reduction by the end of treatment, 77.8% reported at least a 2‐level reduction at the 1‐year follow‐up. WHO risk level reductions were associated with significantly lower alcohol consumption, better physical health (p < 0.01), and fewer alcohol‐related consequences (p < 0.001) up to 1 year following treatment.
Conclusions
One‐ and 2‐level reductions in WHO risk levels during alcohol treatment were maintained after treatment and associated with better functioning over time. These findings support the use of the WHO risk level reductions as an outcome measure that reflects clinically significant improvement in how individuals seeking treatment for alcohol use disorder feel and function.
Keywords: World Health Organization Risk Drinking Levels, Alcohol Use Disorder, Reduced Alcohol Consumption, Alcohol Treatment Outcomes, Low‐Risk Drinking, Alcohol Dependence
For individuals treated for alcohol use disorder (AUD), engaging in some level of drinking following treatment is common (Hunt et al., 1971; Maisto et al., 2018; Witkiewitz and Masyn, 2008). Sustained abstinence has long been considered the optimal outcome of AUD treatment (Betty Ford Institute Consensus Panel, 2007; Mann et al., 2017), and most research historically focused on abstinence as a primary measure of treatment outcomes (e.g., percent days abstinent from alcohol; Anton et al., 2006; Maisto et al., 2016). Yet, individuals seeking treatment for AUD are increasingly interested in drinking reduction goals (DeMartini et al., 2014; Haug et al., 2018; Ryan et al., 2017) and AUD treatment professionals have become more accepting of patients' drinking reduction goals (Davis and Rosenberg, 2013; Rosenberg and Davis, 1994). Given a growing interest in drinking reduction as a goal of AUD treatment (van Amsterdam and van den Brink, 2013; Mann et al., 2017), examining whether drinking reductions are maintained over time and associated with improvements in patients' functioning is an important question for AUD treatment outcomes research.
Primary Measures of AUD Treatment Outcomes
The Food and Drug Administration's (FDA's) draft guidance on the development of alcohol treatment medications (Food and Drug Administration, 2015) recommended 2 potential AUD treatment outcomes (i.e., end points) for medication development: (i) sustained abstinence; or (ii) no heavy drinking days, with heavy drinking days defined as more than 3 drinks in a day for women and 4 drinks in a day for men (National Institute on Alcohol Abuse and Alcoholism, 2005). The European Medicines Agency (EMA, 2010) recommends abstinence as a primary end point, but acknowledges the utility of drinking reduction end points for alcohol clinical trials, including reductions in total alcohol consumption, in heavy drinking days, or in the World Health Organization (WHO) risk drinking levels (WHO, 2000), which are defined by sex‐specific limits on the number of grams (g) of alcohol consumed per day. Specifically, as shown in Fig. 1, individuals can be abstinent (0 g males/females), low risk (1 to 40 g males/1 to 20 g females), medium risk (41 to 60 g males/21 to 40 g females), high risk (61 to 100 g males/41 to 60 g females), or very high risk (101+ g males/61+ g females). Importantly, guidance from both the FDA and the EMA highlights that end points for alcohol clinical trials should be associated with improvements in patient functioning.
Figure 1.
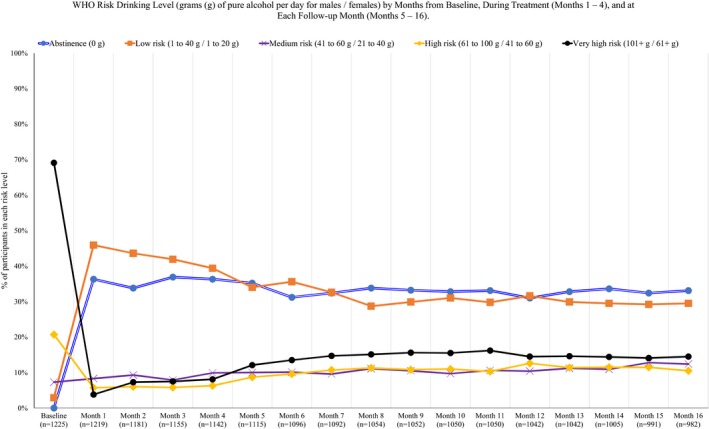
WHO risk drinking level (grams [g] of pure alcohol per day for males/females) by months from baseline, during treatment (months 1 to 4), and at each follow‐up month (months 5 to 16).
Reduction in the WHO risk levels as a potential outcome measure has recently been studied in both populations of individuals receiving treatment for AUD (Aubin et al., 2015; O'Malley et al., 2018; Witkiewitz et al., 2017a, 2018) and a general population sample of alcohol drinkers (Hasin et al., 2017; Knox et al., 2018). Findings across studies show that reductions in WHO risk drinking levels are associated with significant differences between active medication treatments and placebo (Aubin et al., 2015; Falk et al., 2019; O'Malley et al., 2018), improvements in physical health and the quality of life (Knox et al., 2018, 2019; Witkiewitz et al., 2018), reduced risk of alcohol dependence (Hasin et al., 2017), and reductions in drinking‐related consequences and improvements in mental health (Witkiewitz et al., 2017a).
Current Study: Examining the Maintenance of Drinking Reduction Outcomes
A major concern with nonabstinent drinking reduction outcomes is that they may not be maintained over time (Anton et al., 2012). For example, the FDA recommends that clinical trials be 6 months in duration based on the notion that “drinking patterns over shorter durations of time, such as 12 weeks, may not be stable or representative of future experience” (FDA, 2015, p. 5). Recent work in the field has supported the maintenance of low‐risk drinking outcomes, defined as not exceeding heavy drinking limits, over 1 year (Witkiewitz et al., 2017b), 3 years (Maisto et al., 2007), and up to 9 years following treatment (Kline‐Simon et al., 2017). However, the maintenance of the WHO risk drinking level reductions has not been studied extensively. Aubin and colleagues (2015) examined whether individuals who received nalmefene, compared to placebo, had a higher likelihood of achieving a 2‐level reduction in WHO risk drinking level over a 6‐month clinical trial, but did not examine the maintenance of the 2‐level reduction outcome beyond 6 months. Witkiewitz and colleagues (2017a, 2018) examined whether reductions in WHO risk levels during a 4‐month clinical trial, the COMBINE study, predicted functioning for up to 1 year following treatment, but did not examine whether reductions in WHO risk drinking levels themselves were maintained beyond the 4‐month trial period. To address this gap in the literature, the current study examined the maintenance of the WHO risk level reductions for up to 1 year following treatment. In line with FDA and EMA guidance, we also tested whether WHO risk level reductions were associated with functional improvement. We hypothesized that 1‐ and 2‐level reductions in WHO risk levels would be maintained over time and that reductions in WHO risk levels would be associated with better functional outcomes over time.
Materials and Methods
Participants and Procedures
The data for the current study were from the COMBINE study (Anton et al., 2006), a U.S. multisite, randomized, double‐blind, placebo‐controlled clinical trial that examined combinations of medications and behavioral interventions for treating alcohol dependence. All participants met the criteria for alcohol dependence based on DSM‐IV (American Psychiatric Association, 1994) and had at least 2 heavy drinking days (defined as more than 3 drinks for women and more than 4 drinks for men) in a consecutive 30‐day period within the 90 days prior to the baseline assessment. Exclusion criteria were a current substance use disorder (other than nicotine or cannabis), a psychiatric disorder requiring medication, or unstable medical conditions. Participants in the current analyses (n = 1,226) were randomized to receive: (i) active naltrexone (100 mg/d) or placebo naltrexone; (ii) active acamprosate (3,000 mg/d) or placebo acamprosate; or (iii) medication management (MM) or combined behavioral intervention with MM.
Follow‐up assessments were completed at the end of treatment (16 weeks after baseline) and at 3 assessments after treatment: 10 weeks posttreatment (26 weeks after baseline), 36 weeks posttreatment (52 weeks after baseline), and 1 year posttreatment (68 weeks after baseline).
Measures
Demographics
Demographics, including age, sex, and race/ethnicity, were assessed using a self‐report demographic questionnaire.
Alcohol Consumption
Daily standard drinks were measured using the Form‐90 (Miller, 1996) and Timeline Follow‐Back interview (Sobell and Sobell, 1992). We calculated WHO risk drinking levels based on the number of standard drinks (defined as 0.6 ounces of absolute alcohol = 14 g of pure alcohol). WHO risk levels were calculated based on the average number of grams of alcohol consumed per day (i.e., drinks per day) over a specific time period (in the current study, we averaged over 1‐month time periods). For the baseline period, we calculated the WHO risk drinking level using data from the month prior to the screening.
For all analyses, binary variables were included that reflected at least 1‐ or 2‐level reductions in the WHO risk drinking levels from baseline to each month of treatment (postbaseline months 1 through 4) and from baseline to each follow‐up month (postbaseline months 5 through 16). The reference group for the 1‐level reduction was no change or an increase in the WHO risk drinking level from baseline to the treatment/follow‐up months, and the reference group for the 2‐level reduction was the 1‐level reduction, no change, or increase in the WHO risk level from baseline to the treatment/follow‐up months.
Data from the Form‐90 (Miller, 1996) and Timeline Follow‐Back interview (Sobell and Sobell, 1992) were also used to calculate the alcohol consumption outcomes—percent heavy drinking days, percent drinking days, and drinks per drinking day—at the end of treatment (postbaseline week 16), 10 weeks posttreatment (post‐baseline week 26), 36 weeks posttreatment (postbaseline week 52), and 1 year posttreatment (postbaseline week 68).
Functioning Outcomes
Biological functioning was assessed at end of treatment (postbaseline week 16), 10 weeks posttreatment (postbaseline week 26), and 36 weeks posttreatment (postbaseline week 52), and included systolic blood pressure (SBP) and levels of the liver enzymes aspartate aminotransferase (AST), alanine aminotransferase (ALT), and γ‐glutamyltransferase (GGT). SBP was assessed at each clinic visit by clinical staff, and blood samples were sent to a central laboratory (Quintiles Laboratories, Marietta, GA) in which AST, ALT, and GGT clinical assays were performed utilizing automatic analyzer procedures. Lower levels of SBP, AST, ALT, and GGT are associated with better health outcomes (Kwo et al., 2017; Strandberg and Pitkala, 2003).
Alcohol‐related consequences were assessed with the Drinker Inventory of Consequences (DrInC; Miller et al., 1995), a 50‐item measure that uses a 4‐level response scale (0 = never and 3 = daily or almost daily). The DrInC total score (based on 45 drinking consequences, excluding the 5 control items) was used to assess alcohol‐related consequences at the 1‐year follow‐up (Cronbach's α = 0.97).
Statistical Analysis
Descriptive and inferential analyses were used to examine the maintenance of risk level reductions over time. Descriptive frequencies were used to determine the observed monthly prevalence of risk drinking level reductions. For inferential analyses, we used logistic regression and repeated‐measures mixed models to examine associations between WHO risk level reductions and functioning outcomes up to 1 year posttreatment. First, we examined the association between risk level reductions achieved in the last month of treatment and at 1 year posttreatment using logistic regression. Specifically, these analyses examined the odds of maintaining 1‐ and 2‐level reductions at the 1‐year follow‐up assessment as a function of achieving 1‐ and 2‐level reductions at the end of treatment, respectively. Second, to examine associations across all follow‐up months, general linear repeated‐measures mixed models with an identity link function were used to assess the association between WHO risk level reductions in each month over time and functional outcomes at each assessment over time. All mixed models were estimated using Mplus version 8 (Muthén and Muthén, 1998) using maximum likelihood estimation with robust estimation of standard errors to account for clustering within treatment sites (Yuan and Bentler, 2010). Missing data were accommodated via maximum likelihood estimation procedures, which provide an estimate of the variance–covariance matrix given all available data and allow for some missing data across months (Hallgren and Witkiewitz, 2013; Witkiewitz et al., 2014). Consistent with prior analyses examining the WHO risk level reductions in the COMBINE study data (Witkiewitz et al., 2017a, 2018), we controlled for the following covariates in all analyses: age, sex, body mass index, smoking status, and baseline WHO risk level. All covariates were grand‐mean centered.
Sensitivity Analyses
For all analyses, we performed 2 sets of sensitivity analyses. First, we reestimated all models with no change/increase imputed for individuals with missing drinking data, which was a small percent of the sample in each month (range of 1% in month 1 to 20% in month 16) given excellent retention in the COMBINE study (Anton et al., 2006). This method of imputing “failure” for missing outcomes has been shown to produce biased estimates (Hallgren et al., 2016); however, it is commonly recommended as a sensitivity analysis by regulatory agencies, including the FDA. Second, we examined the effect of excluding abstainers by conducting analyses among individuals who achieved 1‐ and 2‐level reductions and were not abstinent. These analyses provided a test of whether reductions in drinking, short of abstinence, were associated with maintenance of risk level reductions (short of abstinence) and improvements in functioning over time.
Results
Descriptive Analyses
Participants were mostly male (68.8%) and non‐Hispanic white (76.7%) (black/African American [7.9%], Asian [0.3%], Hispanic (11.2%), American Indian/Alaska Native [1.3%)], multiracial [1.3%], and other race [1.2%]), with an average age of 44.4 years (SD = 10.2). At baseline, the majority of individuals (69.1%) were in the “very high risk” category (drinking over 101/61 [males/females] grams of pure alcohol per day on average) and there were no abstainers. As shown in Fig. 1, more than half of the sample was categorized as abstinent or low risk in every month following baseline (note that 1 person was missing drinking data at baseline for a baseline sample size of n = 1,225).
The binary WHO risk level reduction variables were then created by calculating the reduction in risk drinking level from baseline to each month of treatment (months 1 to 4) and up to 12 months posttreatment (months 5 to 16). The majority of the sample reduced their drinking from baseline to the last month of treatment (month 4) by at least 1 level (n = 1,011, 88.5%) or at least 2 levels (n = 881, 77.1%). The percentage of individuals who achieved at least 1‐ and at least 2‐level reductions ranged from 79.0 to 84.2% and from 63.8 to 70.4%, respectively, across the follow‐up months (see Fig. 2).
Figure 2.
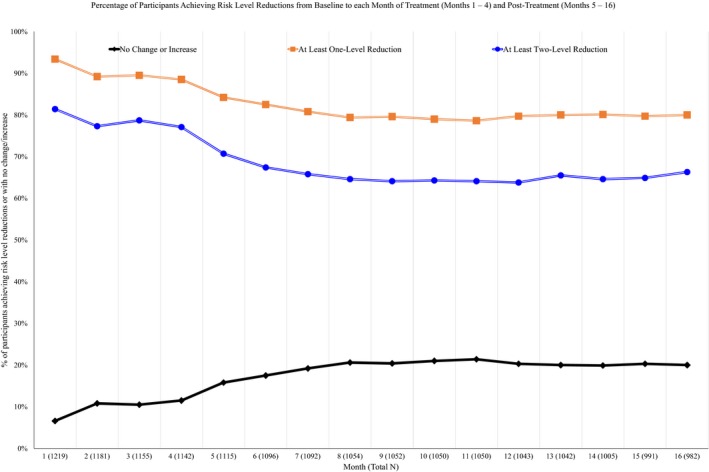
Percentage of participants achieving WHO risk level reductions from baseline to each month of treatment (months 1 to 4) and posttreatment (months 5 to 16).
Logistic Regression
Results from the logistic regression models indicated that reduction in WHO risk levels in the last month of treatment was significantly associated with at least 1‐ and 2‐level reductions in WHO risk levels at 1 year following treatment. Achieving at least a 1‐ or 2‐level reduction during the last month of treatment was associated with 9 to 10 times the odds of reporting at least a 1‐ or 2‐level reduction, respectively, at 1 year following treatment (1‐level reduction: Nagelkerke R 2 = 0.20; B[SE] = 2.33 [0.24], p < 0.001; odds ratio [OR] [95% confidence interval (CI)] = 10.25 [6.44, 16.29]; 2‐level reduction: Nagelkerke R 2 = 0.27; B[SE] = 2.24 [0.20], p < 0.001; OR [95% confidence interval (CI)] = 9.40 [6.42, 13.78]). Among individuals who achieved at least a 1‐level reduction by the end of treatment, 85.5% reported at least a 1‐level reduction at the 1‐year follow‐up. Similarly, among individuals who achieved a least a 2‐level reduction by the end of treatment, 77.8% reported at least a 2‐level reduction at the 1‐year follow‐up.
Linear Mixed Models
Next, we examined whether 1‐ and 2‐level reductions in WHO risk levels over time were associated with physical health, drinking consequences, and other measures of alcohol consumption over time. Descriptive statistics for functional outcomes by 1‐ and 2‐level reduction groups are provided in Table 1 (physical health outcomes) and Table 2 (drinking outcomes). Results from the linear mixed models are provided in Table 3. The reference group for the 1‐level reduction showed no change or an increase in the WHO risk drinking level, and the reference group for the 2‐level reduction showed a 1‐level reduction, no change, or an increase in the WHO risk level from baseline to the treatment/follow‐up months. Unstandardized coefficients can be interpreted as the decrease in outcomes over time based on achieving at least a 1‐ and 2‐level reduction over time, at the average level of covariates (covariate effects shown in Table S1). For example, at least a 1‐level reduction over time was associated with a 6.42 mm Hg reduction in SBP (p < 0.001), a 7.87 IU/l reduction in AST (p < 0.001), a 6.33 IU/l reduction in ALT (p < 0.001), a 26.92 IU/l reduction in GGT (p = 0.01), a reduction of 19.24 in DrInC total score (p < 0.001), and lower drinking intensity and drinking frequency over time (all p < 0.001). These findings reflect better functioning, on average over time, than in individuals with no change or an increase in the WHO risk drinking level over time. At least a 2‐level reduction was associated with a 6.00 mm Hg reduction in SBP (p < 0.001), a 7.19 IU/l reduction in AST (p < 0.001), a 6.00 IU/l reduction in ALT (p < 0.001), a 21.84 IU/l reduction in GGT (p = 0.005), a reduction of 17.40 in DrInC total score (p < 0.001), and lower drinking intensity and frequency (all p < 0.001) than in individuals with a 1‐level reduction, no change, or an increase in the WHO risk drinking level over time.
Table 1.
Means (Standard Deviations [SDs]) for Each Physical Health Outcome by at Least 1‐ and 2‐Level Reductions at Each Assessment Time Point
| Outcome | No change or increase mean (SD) | At least 1‐level reduction mean (SD) | 1‐level reduction, no change, or increase mean (SD) | At least 2‐level reduction mean (SD) |
|---|---|---|---|---|
| SBP (mm/Hg) | ||||
| Week 16 | 137.9 (17.6) | 129.8 (16.6) | 134.2 (17.1) | 129.5 (16.6) |
| Week 26 | 138.6 (17.5) | 130.3 (17.9) | 135.5 (19.2) | 129.9 (17.3) |
| Week 52 | 136.7 (16.9) | 130.6 (17.2) | 134.4 (17.7) | 130.3 (16.9) |
| AST (IU/l) | ||||
| Week 16 | 41.0 (35.7) | 28.2 (16.0) | 36.2 (29.1) | 27.7 (14.7) |
| Week 26 | 38.5 (34.9) | 29.6 (26.8) | 35.4 (29.8) | 29.2 (27.7) |
| Week 52 | 40.3 (37.5) | 31.2 (29.9) | 37.7 (33.8) | 30.5 (30.3) |
| ALT (IU/l) | ||||
| Week 16 | 40.8 (32.6) | 30.8 (23.5) | 38.0 (28.9) | 30.1 (23.1) |
| Week 26 | 40.8 (38.9) | 30.9 (22.2) | 36.7 (31.5) | 30.8 (22.9) |
| Week 52 | 41.3 (29.7) | 33.9 (33.9) | 40.4 (30.8) | 32.8 (34.3) |
| GGT (IU/l) | ||||
| Week 16 | 145.1 (467.1) | 43.5 (61.9) | 87.3 (303.6) | 42.9 (63.3) |
| Week 26 | 86.6 (223.3) | 47.2 (73.2) | 73.8 (172.6) | 44.9 (72.8) |
| Week 52 | 108.6 (245.4) | 53.4 (79.4) | 29.5 (197.3) | 51.2 (75.1) |
All numbers are observed (percentages are based on valid number of cases) with no imputation for missing data. Biomarker assessments were conducted at the end of treatment (week 16 after baseline) and at 2 assessments after treatment: 10 weeks posttreatment (postbaseline week 26) and 36 weeks posttreatment (postbaseline week 52). ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ‐glutamyltransferase; IU/l, international units per liter; SBP, systolic blood pressure.
Table 2.
Means (Standard Deviations SDs]) for Each Drinking Outcome by at Least 1‐ and 2‐Level Reductions at Each Assessment Time Point
| Outcome | No change or increase mean (SD) | At least 1‐level reduction mean (SD) | 1‐level reduction, no change, or increase mean (SD) | At least 2‐level reduction mean (SD) |
|---|---|---|---|---|
| DrInC | ||||
| Week 16 | 35.3 (14.3) | 11.0 (16.5) | 24.8 (22.9) | 10.1 (16.1) |
| Week 26 | 37.7 (25.2) | 13.6 (18.1) | 31.6 (24.2) | 11.3 (16.5) |
| Week 52 | 39.9 (23.2) | 16.8 (19.5) | 33.5 (22.8) | 14.8 (18.9) |
| Week 68 | 38.6 (22.9) | 15.7 (20.1) | 33.7 (22.0) | 13.2 (18.3) |
| PHDD | ||||
| Week 16 | 71.3% (32.9%) | 9.1% (17.5%) | 50.7% (38.2%) | 5.9% (11.3%) |
| Week 26 | 62.2% (35.4%) | 12.8% (21.9%) | 49.6% (36.6%) | 8.9% (15.6%) |
| Week 52 | 67.1% (33.1%) | 15.9% (24.5%) | 55.6% (35.8%) | 10.6% (18.3%) |
| Week 68 | 71.4% (32.4%) | 14.9% (23.6%) | 58.9% (35.7%) | 9.5% (16.8%) |
| PDD | ||||
| Week 16 | 77.6% (28.8%) | 18.9% (26.7%) | 59.9% (36.6%) | 15.4% (23.4%) |
| Week 26 | 70.5% (32.4%) | 23.9% (29.6%) | 61.2% (34.7%) | 18.2% (25.3%) |
| Week 52 | 73.7% (29.8%) | 27.7% (31.9%) | 65.8% (33.4%) | 21.6% (27.8%) |
| Week 68 | 78.4% (27.2%) | 27.1% (31.8%) | 69.9% (31.7%) | 20.7% (27.4%) |
| DPDD | ||||
| Week 16 | 12.2 (7.5) | 5.9 (4.1) | 9.9 (6.7) | 5.6 (4.0) |
| Week 26 | 11.8 (7.2) | 4.6 (5.4) | 9.6 (6.6) | 4.1 (5.6) |
| Week 52 | 11.7 (7.1) | 5.7 (6.2) | 9.7 (6.8) | 5.4 (6.4) |
| Week 68 | 11.6 (6.5) | 7.1 (5.7) | 10.1 (6.1) | 6.8 (5.9) |
All numbers are observed (percentages are based on valid number of cases) with no imputation for missing data. Alcohol consumption and consequences were measured at weeks 16, 26, 52, and 68. DPDD, drinks per drinking day; DrInC, Drinker Inventory of Consequences Total Score; PHDD, percent heavy drinking days; PDD, percent drinking days.
Table 3.
Linear Mixed Models Results for Functioning Outcomes Over Time Following Treatment as Predicted from 1‐ and 2‐Level Reductions Over Time (n = 1,226) and Sensitivity Analyses with Missing = Failure Imputation (n = 1,226) and Excluding Abstainers (n = 1,052)
| SBP (mm/Hg) | AST (IU/l) | ALT (IU/l) | GGT (IU/l) | DrInC | PHDD | PDD | DPDD | |
|---|---|---|---|---|---|---|---|---|
| B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | B (SE) | |
| Linear mixed models | ||||||||
| 1‐level reduction | −6.42 (1.11)*** | −7.87 (2.18)*** | −6.33 (2.09)*** | −26.92 (10.47)* | −19.24 (1.32)*** | −39.89 (1.76)*** | −32.69 (1.62)*** | −4.86 (0.36)*** |
| 2‐level reduction | −6.00 (0.84)*** | −7.19 (1.55)*** | −6.00 (1.53)*** | −21.84 (7.97)** | −17.40 (1.08)*** | −38.55 (1.45)*** | −33.65 (1.39)*** | −4.16 (0.28)*** |
| Missing = failure models | ||||||||
| 1‐level reduction | −5.69 (1.09)*** | −7.62 (2.09)*** | −6.12 (2.01) ** | −25.60 (10.07)* | −18.21 (1.27)*** | −29.71 (1.62)*** | −24.53 (1.49)*** | −3.23 (0.31)*** |
| 2‐level reduction | −5.56 (0.84)*** | −7.13 (1.53)*** | −5.86 (1.50)*** | −21.37 (7.67) ** | −16.84 (1.06)*** | −32.07 (1.41)*** | −28.37 (1.33)*** | −3.25 (0.26)*** |
| Excluding abstainers | ||||||||
| 1‐level reduction | −5.14 (1.13)*** | −6.36 (2.15)** | −4.17 (1.99)* | −12.64 (6.82) | −14.39 (1.22)*** | −38.27 (1.77)*** | −28.10 (1.63)*** | −3.54 (0.41)*** |
| 2‐level reduction | −4.26 (0.89)*** | −5.49 (1.60)** | −3.87 (1.56)* | −8.24 (5.21) | −13.14 (1.02)*** | −38.19 (1.54)*** | −29.34 (1.45)*** | −2.96 (0.33)*** |
B (SE) = unstandardized regression coefficients (standard error), which can be interpreted as the decrease in outcomes based on achieving at least a 1‐ and 2‐level reduction, at the average of all covariates (covariate effects reported in Table S1); AST, aspartate aminotransferase; ALT, alanine aminotransferase; DPDD, drinks per drinking day; DrInC, Drinker Inventory of Consequences Total Score; GGT, γ‐glutamyltransferase; IU/l, international units per liter; PDD, percent drinking days; PHDD, percent heavy drinking days; SBP, systolic blood pressure. The reference group for the 1‐level reduction was no change or an increase in the WHO risk drinking level from baseline to the treatment/follow‐up months, and the reference group for the 2‐level reduction was the 1‐level reduction, no change, or increase in the WHO risk level from baseline to the treatment/follow‐up months.
*p < 0.05, **p < 0.01, ***p < 0.001.
Sensitivity Analyses
Imputing Failure for Missing Data
All models were re‐estimated with failure imputed for missing data. For the 1‐level reduction outcome, failure was defined as no change or an increase in WHO risk level. For the 2‐level reduction outcome, failure was defined as a 1‐level reduction, no change, or an increase in WHO risk level. The logistic regression models were nearly identical. Achieving at least a 1‐ or 2‐level reduction during the last month of treatment was associated with 9 to 10 times the odds of reporting at least a 1‐ or 2‐level reduction, respectively, at 1 year posttreatment (1‐level reduction: B[SE] = 2.33 [0.19], p < 0.001; OR [95% CI] = 10.31 [7.01, 15.16]; 2‐level reduction: B[SE] = 2.26 [0.18], p < 0.001; OR [95% CI] = 9.55 [6.77, 13.47]). The results from the linear mixed models were substantively unchanged (see Table 3).
Excluding Abstainers
Sensitivity analyses included only individuals who did not achieve abstinence (n = 1,052). In this subgroup, the results of the logistic regression models with abstainers excluded were nearly identical to the prior models in which they were included. Achieving at least a 1‐ or 2‐level reduction during the last month of treatment (short of abstinence) was associated with greater than 8 times the odds of reporting at least a 1‐ or 2‐level reduction (short of abstinence), respectively, at 1 year posttreatment (1‐level reduction: B[SE] = 2.39 [0.29], p < 0.001; OR [95% CI] = 10.90 [6.14, 19.36]; 2‐level reduction: B[SE] = 2.16 [0.26], p < 0.001; OR [95% CI] = 8.69 [5.25, 14.39]). Of those who achieved at least a 1‐level reduction by the end of treatment and were not abstinent, 80.4% reported at least a 1‐level reduction by the 1‐year follow‐up. Similarly, of those who achieved a least a 2‐level reduction by the end of treatment and were not abstinent, 69.5% reported at least a 2‐level reduction at the 1‐year follow‐up. The results from the linear mixed models were similar (see Table 3), although effects on functional outcomes were smaller with abstainers excluded. In particular, the effects of 1‐ and 2‐level reductions on GGT were not significant with abstainers excluded.
Discussion
The current study examined whether 1‐ and 2‐level reductions in WHO risk levels were maintained over time in a large sample of individuals with alcohol dependence who received 4 months of treatment and were followed for 12 months after treatment. Consistent with study hypotheses, the 1‐ and 2‐level reductions were maintained over time and associated with significant improvements in functioning over time up to 1 year posttreatment. Results were robust to sensitivity analyses that imputed failure (e.g., no change or increase in WHO risk level) for missing data. The findings were also consistent when abstainers were excluded from the model, with 1 notable difference: At least 1‐ and 2‐level reductions were not significantly associated with lower GGT.
The results from the current study are consistent with prior work demonstrating that low‐risk drinking outcomes are maintained up to and beyond 1 year posttreatment (Kline‐Simon et al., 2017; Maisto et al., 2007; Witkiewitz et al., 2017b). The current study makes an important new contribution by specifically focusing on WHO risk level reductions, showing that they are maintained across time and associated with improvements in functional outcomes over time.
The current study was limited by the data available in the COMBINE study, a clinical trial that did not include measures of all outcomes over time. For example, biomarkers were measured through 9 months following treatment and were thus unavailable at the 1‐year follow‐up. More sensitive biomarkers, such as phosphatidylethanol and ethyl glucuronide, were not available in the COMBINE study data. Percent carbohydrate‐deficient transferrin, a biochemical marker that has previously been associated with the WHO risk level reductions (Witkiewitz et al., 2018), was not measured at follow‐up months in the COMBINE study. Also, all drinking data were obtained by verbal report. These results were also limited to a 1‐year follow‐up, and there is the possibility that reductions would not be maintained for longer follow‐ups, although there is evidence that reductions in drinking can be sustained over 3‐year and up to 9‐year follow‐ups (Kline‐Simon et al., 2017; Maisto et al., 2007). Future studies should extend the current analyses by examining the maintenance of WHO risk level reductions over longer periods of time and with other life functional assessments, such as medical outcomes and costs.
The current findings build on other recent studies that have provided support for the reduction in WHO risk drinking levels as primary outcomes in clinical trials (Falk et al., 2019; Hasin et al., 2017; Knox et al., 2018, 2019; Witkiewitz et al., 2017a, 2018). We found that 1‐ and 2‐level WHO risk drinking level reductions were maintained across a 1‐year follow‐up for most participants and reductions in WHO risk drinking levels over time corresponded to statistically significant and clinically meaningful differences in blood pressure, liver enzyme levels, alcohol consumption, and drinking‐related consequences, as compared to those among individuals who did not achieve reductions.
Sensitivity analyses provided further support for the maintenance of drinking reductions, even when abstainers were excluded from the analysis. These findings are particularly important because the majority of individuals with AUD who are seeking treatment prefer nonabstinence goals (DeMartini et al., 2014; Haug et al., 2018; Ryan et al., 2017). Moreover, many people with AUD do not seek treatment because they do not want to completely abstain from alcohol (Park‐Lee et al., 2017). More individuals with AUD may be interested in seeking treatment if they are aware of the possibility that drinking reduction goals are achievable, sustainable, and associated with improvements in functioning (Mann et al., 2017; van Amsterdam and van den Brink, 2013). The expansion of treatment options to be more inclusive of drinking reduction goals is critically important. The current findings show a high probability that such drinking reductions are maintained over time.
Disclosures
Dr. Kranzler is named as an inventor on PCT patent application #15/878640 entitled: “Genotype‐guided dosing of opioid agonists,” filed January 24, 2018. Dr. Mann received honoraria for consultancies from Pfizer. Dr. Hasin is Principal Investigator of a study funded by inVentiv Health Consulting that combines support from: Actavis, Inc.; Endo Pharmaceuticals; Janssen Pharmaceuticals, Inc.; Mallinckrodt, LLC; Pfizer, Inc.; Purdue Pharma, L.P.; Rhodes Pharmaceuticals, L.P.; Roxane Laboratories, Inc.; and Zogenix, Inc. Dr. O'Malley reports being a consultant or an advisory board member of Alkermes, Amygdala, Indivior, Mitsubishi Tanabe, and Opiant, and a NIDA Clinical Trials Network DSMB member with honorarium from the Emmes Corporation and donated study medications from Astra Zeneca and Novartis. Dr. Anton has been a consultant in recent past for Insys, Allergan, and Life Epigenetics, has received honorarium from Alkermes for grant reviews, and currently has grant funding from Laboratorio Farmaceutica CT. Drs. Witkiewitz, Kranzler, Mann, Hasin, O'Malley, and Anton are members of the American Society of Clinical Psychopharmacology's Alcohol Clinical Trials Initiative (ACTIVE Group) The ACTIVE Workgroup has been supported previously, but not in the past 36 months, by Abbott/Abbvie, Ethypharm, GSK, Janssen, Lilly, Pfizer, and Schering Plough, but in the past 36 months its activities were supported by Alkermes, Amygdala Neurosciences, Arbor Pharmaceuticals, Indivior, Lundbeck, Mitsubishi, and Otsuka. Drs. Falk and Litten have no disclosures.
Supporting information
Table S1. Full results for linear mixed models results for functioning outcomes over time following treatment as predicted from 1‐ and 2‐level reductions over time.
Acknowledgments
This study was funded by the U.S. National Institute on Alcohol Abuse and Alcoholism (R01AA022328). In addition to the authors, the following individuals are or were members of the Alcohol Clinical Trials Initiative (ACTIVE) Workgroup and provided intellectual input during attendance at Workgroup meetings: Joanne Fertig, PhD, and Megan Ryan, PhD, National Institute on Alcohol Abuse and Alcoholism; Tanya Ramey, MD, PhD, and David McCann, PhD, National Institute on Drug Abuse; Didier Meulien, MD, Lundbeck SAS; Anne Andorn, MD, and Jay Graham, MD, Indivior; Roger Meyer, MD, Best Practice Project Management, Inc.; Henri‐Jean Aubin, MD, Paris‐Sud Medical School; Charles O'Brien, MD, PhD, University of Pennsylvania; Bernard Silverman, MD, Alkermes, Inc.; Francoise Trinquet, MD, and Benjamin Zakine, MD, Ethypharm. Lindsay Snyder and Sarah Timm (ASCP) provided important administrative support to the ACTIVE Workgroup.
References
- American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders 4th ed. American Psychiatric Association, Washington, DC. [Google Scholar]
- Anton RF, Litten RZ, Falk DE, Palumbo JM, Bartus RT, Robinson RL, Kranzler HR, Kosten TR, Meyer RE, O'Brien CP, Mann K, Meulien D (2012) The Alcohol Clinical Trials Initiative (ACTIVE): purpose and goals for assessing important and salient issues for medications development in alcohol use disorders. Neuropsychopharmacology 37:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A (2006) Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. J Am Med Assoc 295:2003–2017. [DOI] [PubMed] [Google Scholar]
- Aubin H‐J, Reimer J, Nutt DJ, Bladström A, Torup L, François C, Chick J (2015) Clinical relevance of as‐needed treatment with nalmefene in alcohol‐dependent patients. Eur Addict Res 21:160–168. [DOI] [PubMed] [Google Scholar]
- Betty Ford Institute Consensus Panel (2007) What is recovery? A working definition from the Betty Ford Institute. J Subst Abuse Treat 33:221–228. [DOI] [PubMed] [Google Scholar]
- Davis AK, Rosenberg H (2013) Acceptance of non‐abstinence goals by addiction professionals in the United States. Psychol Addict Behav 27:1102–1109. [DOI] [PubMed] [Google Scholar]
- DeMartini KS, Devine EG, DiClemente CC, Martin DJ, Ray LA, O'Malley SS (2014) Predictors of pretreatment commitment to abstinence: results from the COMBINE study. J Stud Alcohol Drugs 75:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency (2010) Guideline on the Development of Medicinal Products for the Treatment of Alcohol Dependence European Medicines Agency, UK. [Google Scholar]
- Falk DE, O'Malley SS, Witkiewitz K, Anton RF, Litten RZ, Slater M, Kranzler HR, Mann KF, Hasin DS, Johnson B, Meulien D, Ryan M, Fertig J (2019) Evaluation of drinking risk levels as outcomes in alcohol pharmacotherapy trials: a secondary analysis of 3 randomized clinical trials. JAMA Psychiatry doi: 10.1001/jamapsychiatry.2018.3079 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Food and Drug Administration (2015) Alcoholism: Developing Drugs for Treatment (No. FDA D‐0152‐001). Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- Hallgren KA, Witkiewitz K (2013) Missing data in alcohol clinical trials: a comparison of methods. Alcohol Clin Exp Res 37:2152–2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA, Witkiewitz K, Kranzler HR, Falk DE, Litten RZ, O'Malley SS, Anton RF (2016) Missing data in alcohol clinical trials with binary outcomes. Alcohol Clin Exp Res 40:1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, Mann K, O'Malley SS, Scodes J, Robinson RL, Anton R, Fertig J, Isenberg K, McCann D, Meulien D, Meyer R, O'Brien C, Ryan M, Silverman B, Trinquet F, Wong C, Zakine B (2017) Change in non‐abstinent WHO drinking risk levels and alcohol dependence: a 3 year follow‐up study in the US general population. Lancet Psychiatry 4:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug S, Castro RP, Eggli P, Schaub MP (2018) Drinking goal trajectories and their association with client characteristics and outcomes among clients in outpatient alcohol treatment. Subst Use Misuse 53:2140–2151. [DOI] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG (1971) Relapse rates in addiction programs. J Clin Psychol 27:455–456. [DOI] [PubMed] [Google Scholar]
- Kline‐Simon AH, Litten RZ, Weisner CM, Falk DE (2017) Posttreatment low‐risk drinking as a predictor of future drinking and problem outcomes among individuals with alcohol use disorders: a 9‐year follow‐up. Alcohol Clin Exp Res 41:653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J, Scodes J, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, Mann K, O'Malley SS, Anton R, Hasin DS (2019) Reduction in non‐abstinent WHO drinking risk levels and depression/anxiety disorders: 3‐year follow‐up results in the US general population. Drug Alcohol Depend 197:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox J, Wall M, Witkiewitz K, Kranzler HR, Falk D, Litten R, Mann K, O'Malley SS, Scodes J, Anton R, Hasin DS; Alcohol Clinical Trials (ACTIVE) Workgroup (2018) Reduction in nonabstinent WHO drinking risk levels and change in risk for liver disease and positive AUDIT‐C scores: prospective 3‐year follow‐up results in the U.S. general population. Alcohol Clin Exp Res 42:2256–2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwo PY, Cohen SM, Lim JK (2017) ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol 112:18–35. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Clifford PR, Stout RL, Davis CM (2007) Moderate drinking in the first year after treatment as a predictor of three‐year outcomes. J Stud Alcohol Drugs 68:419–427. [DOI] [PubMed] [Google Scholar]
- Maisto SA, Hallgren KA, Roos CR, Witkiewitz K (2018) Course of remission from and relapse to heavy drinking following outpatient treatment of alcohol use disorder. Drug Alcohol Depend 187:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Witkiewitz K, Moskal D, Wilson AD (2016) Is the construct of relapse heuristic, and does it advance alcohol use disorder clinical practice? J Stud Alcohol Drugs 77:849–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Aubin H‐J, Witkiewitz K (2017) Reduced drinking in alcohol dependence treatment, what is the evidence? Eur Addict Res 23:219–230. [DOI] [PubMed] [Google Scholar]
- Miller WR (1996) Form 90: A Structured Assessment Interview for Drinking and Related Behaviors, Project MA. ed. National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD. [Google Scholar]
- Miller WR, Tonigan JS, Longabaugh R (1995) The Drinker Inventory of Consequences (DrInC), Project MA, ed. National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD. [Google Scholar]
- Muthén LK, Muthén BO (1998. ‐2017) Mplus User's Guide. 8th ed. Muthén & Muthén, Los Angeles, CA.
- National Institute on Alcohol Abuse and Alcoholism (2005) Helping Patients Who Drink Too Much: A Clinician's Guide National Institutes of Health, Bethesda, MD. [Google Scholar]
- O'Malley SS, Todtenkopf MS, Du Y, Ehrich E, Silverman BL (2018) Effects of the opioid system modulator, samidorphan, on measures of alcohol consumption and patient‐reported outcomes in adults with alcohol dependence. Alcohol Clin Exp Res 42:2011–2021. [DOI] [PubMed] [Google Scholar]
- Park‐Lee E, Lipari RN, Hedden SL, Kroutil LA, Porter JD (2017) Receipt of services for substance use and mental health issues among adults: results from the 2016 National Survey on Drug Use and Health. Available at: https://www.samhsa.gov/data/sites/default/files/NSDUH-DR-FFR2-2016/NSDUH-DR-FFR2-2016.htm#topofpage. Accessed March 22, 2019. [PubMed]
- Rosenberg H, Davis LA (1994) Acceptance of moderate drinking by alcohol treatment services in the United States. J Stud Alcohol 55:167–172. [DOI] [PubMed] [Google Scholar]
- Ryan ML, Falk DE, Fertig JB, Rendenbach‐Mueller B, Katz DA, Tracy KA, Strain EC, Dunn KE, Kampman K, Mahoney E, Ciraulo DA, Sickles‐Colaneri L, Ait‐Daoud N, Johnson BA, Ransom J, Scott C, Koob GF, Litten RZ (2017) A phase 2, double‐blind, placebo‐controlled randomized trial assessing the efficacy of ABT‐436, a novel v1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology 42:1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB (1992) back: a technique for assessing self‐reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biological Methods (Litten RZ, Allenl JP. eds), pp 41–72. Humana Press, Totowa, NJ. [Google Scholar]
- Strandberg T, Pitkala K (2003) What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens 12:293–297. [DOI] [PubMed] [Google Scholar]
- van Amsterdam J, van den Brink W (2013) Reduced‐risk drinking as a viable treatment goal in problematic alcohol use and alcohol dependence. J Psychopharmacol 27:987–997. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Falk DE, Kranzler HR, Litten RZ, Hallgren KA, O'Malley SS, Anton RF (2014) Methods to analyze treatment effects in the presence of missing data for a continuous heavy drinking outcome measure when participants drop out from treatment in alcohol clinical trials. Alcohol Clin Exp Res 38:2826–2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Hallgren KA, Kranzler HR, Mann KF, Hasin DS, Falk DE, Litten RZ, O'Malley SS, Anton RF (2017a) Clinical validation of reduced alcohol consumption after treatment for alcohol dependence using the World Health Organization risk drinking levels. Alcohol Clin Exp Res 41:179–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Kranzler HR, Hallgren KA, O'Malley SS, Falk DE, Litten RZ, Hasin DS, Mann KF, Anton RF (2018) Drinking risk level reductions associated with improvements in physical health and quality of life among individuals with alcohol use disorder. Alcohol Clin Exp Res 42:2453–2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Masyn KE (2008) Drinking trajectories following an initial lapse. Psychol Addict Behav 22:157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Wilson AD, Pearson MR, Hallgren KA, Falk DE, Litten RZ, Kranzler HR, Mann KF, Hasin DS, O'Malley SS, Anton RF (2017b) Temporal stability of heavy drinking days and drinking reductions among heavy drinkers in the COMBINE study. Alcohol Clin Exp Res 41:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2000) International Guide for Monitoring Alcohol Consumption and Related Harm World Health Organization, Geneva, Switzerland. [Google Scholar]
- Yuan K‐H, Bentler PM (2010) Finite normal mixture SEM analysis by fitting multiple conventional SEM models. Sociol Methodol 40:191–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Full results for linear mixed models results for functioning outcomes over time following treatment as predicted from 1‐ and 2‐level reductions over time.


