Abstract
Mild traumatic brain injury (mTBI) is a major public health concern that has generated considerable scientific interest as a complex brain disorder that is associated with long-term neural consequences. This paper reviews the literature on cerebrovascular dysfunction in chronic mTBI with a focus on the long-term neural implications of such dysfunction. Evidence is presented from human neuroimaging studies to support cerebrovascular involvement in long-term mTBI pathology. Additionally, a pathway between mTBI and neurodegeneration via cerebrovascular dysfunction is explored. Future work focused on identifying the neurobiological mechanisms underlying the neural consequences of mTBI will be important to guide therapeutic interventions and long-term care for mTBI patients.
Keywords: mTBI, cerebrovascular, neural integrity, neurodegeneration, cerebral blood flow, neuroinflammation
Introduction
Mild traumatic brain injury (mTBI) is a major public health concern, with an estimated 1.7 million people sustaining a TBI in the United States each year, of which 75% are classified as mild.1 Although most individuals exposed to mTBI recover to pre-injury functioning within months after the injury,2 mounting evidence suggests that there are long-term consequences of mTBI such as the development of neurodegenerative diseases including Alzheimer’s disease (AD), Chronic Traumatic Encephalopathy (CTE), and Parkinson’s disease.3–8 Moreover, mTBI has been linked to disruptions in brain structure and function years after the injury.9–20
Despite the body of evidence suggesting that mTBI is a potential risk factor for neurodegenerative processes, the underlying neurobiological mechanisms linking mTBI to neurodegeneration are still relatively unknown. One possibility is that mTBI influences neural integrity through disruptions in cerebrovascular function. This notion aligns with the neurovascular hypothesis of neurodegeneration in AD, which suggests that dysfunction of the cerebrovascular system causes detrimental effects on neuronal health via degraded blood brain barrier integrity and dysregulation of nutrient and oxygen neural transport systems.21
Substantial progress has been made in advancing the understanding of the molecular basis of neurovascular pathology in TBI and its associated long-term molecular changes in the brain (for review see Pop and Badaut22). Specifically, research on the pathophysiology of TBI suggests that TBI initiates an array of secondary cellular, metabolic, and inflammatory events that influence the cerebrovascular and neural systems.23–27 However, the global effects of these changes are relatively unknown, although neurodegenerative disease has been raised as a potential consequence.28 The goal of this paper was to synthesize the findings related to cerebrovascular dysfunction in mTBI and to discuss the possibility that there are long-term and large-scale neural consequences of cerebrovascular dysfunction. To that end, this paper reviews the pathophysiology of TBI and the role of the cerebrovascular system in such pathophysiology. It focuses on evidence from human neuroimaging studies of residual cerebrovascular dysfunction in mTBI to further support cerebrovascular involvement in long-term mTBI pathology. The review concludes by proposing that mTBI influences neural integrity through its effect on the cerebrovascular system. Moderating factors of this relationship are also explored.
Search terms for this review included various combinations of the following words in PubMed and Google Scholar, “cerebrovascular”, “arterial spin labeling”, “cerebral blood flow”, “mild traumatic brain injury”, “mTBI”, “fMRI”, “functional magnetic resonance imaging”, “single photon emission computerized tomography”, “SPECT”, “positron emission tomography”, “PET”, “neurovascular unit”, “neurodegeneration”, “white matter”, “cortical thickness”, and “cerebral metabolism.” Search terms were expanded to include words such as “posttraumatic stress disorder”, “PTSD”, “age”, “cardiovascular disease”, “genetic”, and “polygenic risk” for the moderating factors section. In addition, references of selected papers were searched for relevance to the current review.
Molecular Mechanisms of the Effects of mTBI on Cerebrovascular Function
Immediately after mTBI, there is an abrupt release of neurotransmitters and an ionic shift, with an efflux of potassium and influx of calcium from brain cells.24,25 The brain works to restore the neuronal membrane to normal ionic potential by activating and overworking the sodium-potassium pump, which triggers an increase in the required amount of adenosine triphosphate (ATP).23,24 This creates a dramatic increase in glucose metabolism, which occurs in a setting of diminished cerebral blood flow. The mismatch between supply and demand triggers a cellular energy crisis in the brain and a cerebral blood flow-metabolism uncoupling. After this initial state of acute hypermetabolism, the brain eventually goes into a state of depressed metabolism with elevated calcium levels that activate lipid peroxidases, proteseases, and phospholipases, which in turn increase the intracellular concentration of free fatty acids and free radicals.24 Over time, these events contribute to the development of oxidative stress, which is a process that occurs when reactive oxygen species exceed available antioxidants, loosening of the vascular and perivascular unit (i.e., increased blood brain barrier permeability), vascular and cellular membrane degradation, neurofilament and microtubule disruption, and possibly, cell death.23,24,29 mTBI also induces an array of inflammatory tissue responses through the release of pro-inflammatory cytokines, prostaglandins, and free radicals, which in turn mobilize glial and immune cells that infiltrate injured tissue to repair it after injury.23 However, if uncontrolled and unresolved, chronic neuroinflammation can lead to disruptions in blood-brain barrier permeability and edema formation, further reducing tissue perfusion and contributing to depressed metabolism.23 Importantly, these molecular alterations are not restricted to civilian-related mTBI, as recent work suggests that blast-related mTBI is also associated with alterations in proteins related to metabolic, vascular, and inflammatory processes important for cerebrovascular function.30
Reduced cerebral blood flow coincides with the cascade of metabolic and inflammatory events after mTBI. Alterations to the neurovascular unit, which is composed of endothelial cells, pericytes, smooth muscle cells, astrocytes, and neurons, including disruptions to the blood-brain barrier, contribute to this marked reduction in cerebral blood flow after mTBI.22,26 Specifically, one mechanism for cerebral blood flow disruption in mTBI is disrupted cerebrovascular autoregulation, i.e., the dilation/constriction of the cerebral vasculature to manage cerebral perfusion pressure. Normally, the brain autoregulates blood flow to provide constant flow regardless of blood pressure. However, after TBI, changes in cerebrovascular autoregulation via alterations in the molecular neurovascular unit such as alterations to endothelin-1, nitric oxide levels, and cyclic adenosine monophosphats, that determine the tone of the cerebral vasculature, render the brain susceptible to changes in cerebral pressure, reduced blood flow, and increased risk for ischemia.22,31–33
Another mechanism by which cerebral blood flow is altered is through disruptions in aquaporin-4 (AQP-4), a water channel protein located on perivascular astrocytic end-feed that is in contact with cerebral vessels. Studies have shown that AQP-4 plays a role in brain homeostasis and central plasma osmolality regulation 34,35 and that the distribution of AQP-4 in the perivascular space may be related to perivascular volume, which is crucial for cerebral blood flow.36 Importantly, animal models have shown that AQP-4 is upregulated after brain injury and co-localized with glial fibrillary acidic protein (GFAP), an astrocytic marker, in close proximity to intracerebral vessels in mTBI brain tissue,37,38 suggesting a possible role of AQP-4 in disruptions to the cerebrovascular system in mTBI.
Overall, evidence from these experimental studies supports the notion that mTBI is associated with cerebrovascular dysfunction and that reduced cerebral blood flow may be an observable in-vivo marker of cerebrovascular changes associated with delayed cell death and neuronal membrane disruption in mTBI.
Neuroimaging Evidence of Cerebrovascular Dysfunction in mTBI
Although the majority of molecular disruptions caused by mTBI normalize to pre-injury levels within weeks after the injury, evidence from neuroimaging studies of long-lasting neural changes after mTBI suggest that other processes are not short-lived but instead may stabilize to a new substandard level. Specifically, there is growing evidence to suggest residual alterations in cerebral blood flow in the human brain following mTBI (Table 1, but see Peng et al.39). For example, Grossman et al.40 used arterial spin labeling (ASL), which is a non-invasive neuroimaging technique that uses magnetically labeled blood water as a tracer for cerebral blood flow, to examine a group of mTBI patients one month after injury and then again more than 9 months after injury. They found reduced cerebral blood flow in the thalamus both at one month and at the follow up time point compared to controls, suggesting persistent residual cerebral blood flow disruptions. Similarly, Ge et al.41 found reduced cerebral blood flow in deep gray matter and white matter brain regions in a group of 21 mTBI patients that were on average 26.5 months post-injury compared to 18 healthy age-matched controls. These findings are consistent with results from a study using single photon emission computerized tomography (SPECT) imaging which found hypometabolism in frontal, prefrontal, and temporal cortices and subcortical structures in adult mTBI patients who were on average 5 years post injury compared to controls.42 In another study using ASL to explore perfusion in resting state networks, Sours and colleagues43 found that patients in the chronic stage of mTBI failed to maintain a balance of cerebral blood flow between two resting state networks compared to the control group.
Table 1.
Summary of Human Neuroimaging Studies Evaluating Residual Cerebrovascular Dysfunction in mTBI.
| Author | Sample Size & Description | Type of mTBI | Average Time Since mTBI | Neuroimaging Method | Cerebrovascular Findings |
|---|---|---|---|---|---|
| Bartnik-Olson et al. 201446 | 15 pediatric mTBI patients with persistent post-concussive symptoms and 15 demographically similar controls | Pediatric, sports-related | 5.8 months | Perfusion Weighted Imaging | ↓ CBF and CBV in bilateral thalami in mTBI patients compared to controls. All other regions showed non-significant decreases. |
| Bonne et al. 200342 | 28 symptomatic males with mTBI and 20 matched controls | 20 MVA, 4 work-related, 4 combat-related | 5 years | SPECT | ↓ perfusion in mTBI compared to controls in (1) left pre-central gyrus extending into inferior frontal gyrus and backwards to the left superior and mid-temporal gyrus and upwards to the lateral sulcus and (2) right cingulate gyrus and medial frontal lobe extending back through striatal structures and longitudinal fasciculi to right temporal gyrus and sub-cortical structures. |
| Ge et al. 200941 | 21 patients with mTBI and 18 healthy age-matched controls | ND | 26.5 months | ASL | ↓ CBF in bilateral thalami and head of caudate nuclei in mTBI compared to controls. The ↓ CBF was significantly correlated with neuropsychological performance. |
| Gross et al. 199647 | 20 patients with mTBI, no controls | 13 MVA, 5 hit on head, 2 falls | 43 months | F-2-deoxyglucose PET | ↓ (more than 2SD) and ↑ (more than 2SD) metabolic activity in midtemporal region, anterior cingulate, precuneus, anterior temporal region, white matter of frontal region, corpus callosum, mid and post cingulate, superior lateral frontal, medial frontal, gyrus rectus, hippocampus, and uncus. The increases and decreases varied with each patient. Deficits in cognitive domains of memory, executive functioning, language, and perception were associated with alterations in temporal, frontal, and parietal regions. |
| Grossman et al. 201340 | 20 patients with mTBI (10 returned for follow-up) and 16 controls | 6 assault, 4 MVA, 8 falls, 2 sports-related | 1 month and a follow-up of >9 months later | ASL | ↓ CBF at baseline (1 month) and at follow-up (>9 months) in thalamus in mTBI patients compared to controls. CBF at baseline was associated with white matter integrity. |
| Ponto et al. 201544 | 8 OEF/OIF veterans with TBI (5 mild, 3 moderate), 11 veteran controls | 5 mTBI were blast-related, 3 moderate included (1) MVA, (2) helicopter shot down, (3) blast-related MVA | 39 months | Quantitative [15O] water imaging | ↓ global CBF in TBI compared to controls. Groups did not significantly differ in regional CBF. |
| Sours et al. 201543 | 28 patients with mTBI and 28 matched controls. mTBI was further divided into those with chronic post-concussive symptoms (12 with, 16 without). | ND | Evaluated at three time points: 11 days, 1 month, and >4 months | Pulsed ASL | mTBI patients at the chronic stage (>4 months) had an imbalance in CBF ratio between nodes of two resting state networks (default mode network and task positive network) compared to controls. mTBI patients with chronic post-concussive symptoms had this imbalance compared to those without across multiple stages of recovery. |
| Wang et al. 201545 | 14 adolescents with mTBI and 15 healthy control adolescents | Adolescent, sports-related/recreational | 7.4 months | Pulsed ASL | ↓ CBF in bilateral frontotemporal regions in mTBI compared to controls. Individuals with multiple mTBIs did not differ in CBF from those with only one mTBI. |
Note: ASL=arterial spin labelling; CBF=cerebral blood flow; CBV=cerebral blood volume; mTBI=mild traumatic brain injury; MVA=motor vehicle accidents; ND=not discussed; OEF/OIF=Operation Enduring Freedom/Operation Iraqi Freedom; SD=standard deviation.
This notion, that mTBI is associated with a disrupted cerebrovascular system, also extends to blast-related mTBI. Recent work by Ponto et al44 showed reduced global cerebral blood flow in blast-related mild and moderate TBI patients who were on average 3 years removed from their injury compared to veteran controls. In addition, residual cerebrovascular impairments have also been reported in pediatric mTBI, with reduced cerebral blood flow in bilateral frontotemporal regions45 and bilateral thalami46 in children who were 3–12 months removed from their injury compared to controls. Importantly, evidence suggests that cerebrovascular dysfunction in mTBI may have implications for cognition, as reductions in cerebral metabolism is associated with poorer performance on neuropsychological tests in chronic mTBI.47 Collectively, these findings raise the possibility that cerebrovascular dysfunction plays a role in residual mTBI pathophysiology, with potential long-term cognitive consequences.
Cerebrovascular Function and Neural Integrity
The cerebrovascular system is critically involved in the maintenance of neural integrity. In particular, cerebral blood flow is especially important in the preservation of brain health.48–50 Studies in older adults have consistently demonstrated that reduced cerebral blood flow contributes to deleterious structural integrity.51–55 Further, recent work has demonstrated an association between reduced cerebral blood flow and faster rates of cognitive decline in healthy older adults.56 Work in pathological aging has shown that cerebral blood flow decreases in association with increased AD severity57 and with accelerated cognitive decline in mild cognitive impairment patients.58,59 Interestingly, in work by Wang and colleagues,57 hippocampal volume was only reduced in later stages of AD, suggesting that reductions in cerebral blood flow may be an early biomarker of AD-related neurodegeneration. Together, these findings suggest that cerebral blood flow is an important indicator of neural integrity and that reductions in cerebral blood flow may alter structural brain integrity.
Although this relationship between cerebrovascular dysfunction and neural integrity has been well-studied in normal and pathological aging, few studies have explored this link in mTBI. Nonetheless, there is ample evidence suggesting that mTBI is associated with alterations in the structural integrity of the brain.10,14,16,17,60,61 However, the mechanisms linking mTBI to these neural consequences remain an active area of investigation. Given that mTBI is implicated as a risk factor for the development of neurodegenerative diseases such as AD and CTE, 3,4,62 both of which are associated with widespread vascular pathology and perivascular disruptions,4,5,63 it is possible that disruptions in the cerebrovascular system contribute to the degradation of the brain’s structural integrity in mTBI and thus, may serve as a pathological link between mTBI and neurodegenerative disease (see Figure 1). This hypothesis is well supported by a recent review examining the pathophysiology of TBI and AD, suggesting that vascular damage after TBI may propagate molecular AD processes.28 Moreover, work in veterans examining mild and moderate TBI has explicitly shown that disruptions in cerebral blood flow have direct neural consequences over time. In particular, this study demonstrated that reduced cerebral blood flow in the cingulate cortex was significantly associated with reduced white matter integrity in the cingulum bundle in veterans who were further removed from their brain injury, suggesting that alterations in cerebral blood flow may contribute to long-term microstructural tissue degradation in TBI.64 Despite these advances in the literature, this work is largely cross-sectional. Longitudinal studies will be essential in furthering our understanding of the mechanisms underlying neurodegeneration in mTBI years after the injury.
Figure 1.
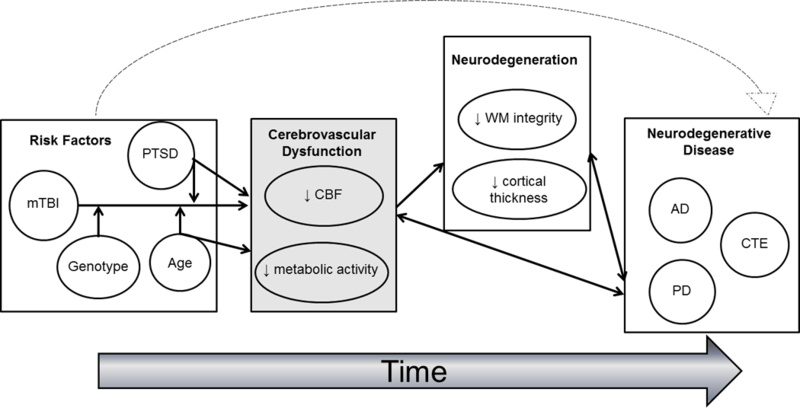
This figure shows the conceptual framework of the hypothesized associations between mTBI, genetic risk, PTSD, age, cerebrovascular dysfunction, neurodegeneration, and neurodegenerative disease. This figure shows that mTBI is associated with residual disruptions in cerebrovascular function, a relationship that may also be moderated by other environmental (e.g., PTSD), age, and genetic risk factors. In turn, these residual disruptions in the cerebrovascular system may contribute to compromised brain integrity. Lastly, this figure shows that this association between mTBI, cerebrovascular dysfunction, and neurodegeneration may propagate neurodegenerative disease processes and thus serve as a pathological link between mTBI and neurodegenerative disease. AD=Alzheimer’s disease; CBF=cerebral blood flow; CTE=chronic traumatic encephalopathy; PD=Parkinson’s disease; PTSD=posttraumatic stress disorder; WM=white matter
Moderating Factors
Genetic Factors Relevant to the Neural Health of Patients with mTBI
Given that the pathophysiology of mTBI, including cerebrovascular dysfunction, is influenced by molecular processes such as oxidative stress and neuroinflammation, individual differences in genes that are related to these processes may play a modulatory role on residual reductions in cerebral blood flow and hypometabolism. One of the consequences of the molecular cascade of events after mTBI is oxidative stress, which is a cellular process that occurs when pro-oxidant molecules such as reactive oxygen species exceed the capacity of available antioxidants to counteract their effects. Previous studies have shown that oxidative stress may exacerbate neural consequences of mTBI. For example, in vitro studies have shown that oxidative stress promotes tau hyperphosphorylation and aggregation65,66 and may be involved in the development of CTE, which is characterized by widespread deposits of hyperposphorylated tau.5,67 Similarly, evidence suggests that neuroinflammation may also be linked to the neural consequences of TBI. For example, in an animal study, Scherbel and colleagues68 found that mice deficient in tumor necrosis factor (TNF), which plays a role in cell growth regulation, inflammation, and autoimmune processes, had significantly more cortical tissue loss than their wild-type littermates (controls) four weeks after brain injury. Both injured mice groups (TNF deficient and wild-type) displayed significant deficits in memory at one week post injury compared to uninjured groups, but deficits in the brain-injured TNF deficient mice were significantly less severe than in brain-injured wild-type mice. These results demonstrate that while TNF in the acute posttraumatic period may be deleterious it may also play a role in facilitating long-term repair, suggesting that inflammatory processes may be involved in neurotoxic as well as neurotrophic effects on the brain after TBI. Collectively, these studies illustrate the possibility that individual differences in genes encoding oxidative stress and inflammatory processes may be important in mediating mTBI long-term neural recovery.
This notion, that genetic factors may modulate (i.e., exacerbate or potentiate) the relationship between mTBI and neural integrity, is consistent with recent work suggesting that genetic factors influence the association between mTBI and cortical thickness.13 Using polygenic risk scores that are derived from large-scale consortia-based genome wide association studies (GWAS) and are computed to provide an index of an individual’s genetic risk across the genome of a particular trait, Hayes and colleagues showed that individuals who reported a mTBI and had greater polygenic risk for AD had reduced cortical thickness in AD-vulnerable brain regions. Importantly, this study also demonstrated that the effect on cortical thickness was accelerated in males further removed from their injury, providing preliminary evidence that genetic risk may moderate the association between mTBI and neurodegeneration.
PTSD and Cerebrovascular Function in Patients with mTBI
Posttraumatic stress disorder (PTSD) is a common comorbid condition affecting many veterans with mTBI. PTSD is a debilitating mental illness defined by symptoms of re-experiencing of a traumatic event (e.g., flashbacks), avoidance (e.g., avoiding trauma provoking situations or stimuli), negative cognitions and mood, and hyperarousal. Estimates suggest that as many as 40% of Operation Enduring Freedom/Iraqi Freedom/New Dawn (OEF/OIF/OND) veterans who suffered a loss of consciousness from mTBI also meet criteria for PTSD69 and approximately 30% of individuals who initially develop PTSD following a psychologically traumatic event suffer from a chronic form of the condition that lasts for years,70 among whom psychiatric and medical comorbidities are common.71
Documented physical co-morbidities of PTSD include early onset of age-related conditions such as cardiovascular disease and peripheral metabolic dysfunction.72–74 For example, in a study of over 18,000 veterans, Boscarino75 found that PTSD was associated with early-age heart disease compared to controls, even after controlling for cardiovascular risk factors (e.g., body mass index, number of years smoking). Similarly, in a U.S population study of older adults, Pietrzak and colleagues76 found that lifetime PTSD was associated with increased odds of hypertension, angina, tachycardia, and other related conditions, even when accounting for significant comorbidities such as substance abuse and mood and anxiety disorders. Other research points to an association between PTSD and cardiac dysfunction, with one twin study showing that the incidence of coronary heart disease was more than double in twins with PTSD than in those without PTSD77 and another study finding that veterans with PTSD were at increased risk for developing heart failure over a seven year follow-up time period.78 Furthermore, recent research has demonstrated that these PTSD-related peripheral disruptions in metabolic health are associated with reductions in neural integrity, with alterations in the cortical mantle79 and white matter tracts.80 However, all of the studies conducted to date have focused on peripheral metabolic dysfunction and associated consequences; it remains unknown whether these peripheral alterations are also manifested in disruptions in cerebrovascular function. Given that PTSD has also been associated with molecular disease processes such as oxidative stress and neuroinflammation,81–84 which have also been linked to disruptions in the cerebrovascular system in mTBI, it is conceivable that PTSD may have similar associations with cerebrovascular health. Moreover, as recent findings suggest that PTSD and mTBI may interact to influence neural integrity,85 it is possible that a similar relationship exists relative to cerebrovascular function. Specifically, it is possible that through the mechanisms of inflammation and oxidative stress, PTSD produces a cellular state in the brain that exacerbates the effects of mTBI on cerebrovascular function and neurodegeneration. It will be important for future work to consider this possibility.
Age as a Factor Relevant to Cerebrovascular Function in Patients with mTBI
Chronological age is a well-established risk factor for vascular disease. Work has shown that aging is associated with morphological changes to the vasculature, including arterial wall thickening and vascular stiffening.86–89 These changes result in increased blood pressure and reduced perfusion and contribute to the increased rate of vascular risk factors such as hypertension and diabetes.86,90,91
In the brain, studies in neurologically healthy older adults have shown a link between vascular health and structural integrity.51−55 For example, in a group of older adults, Brickman and colleagues51 found that regions with reduced cerebral blood flow were more likely to be classified as white matter hyperintensities, which in turn have been associated with cognitive impairment.91 Consistent with these findings, Promjunyakul et al.52 examined cognitively intact elderly subjects over an 18 month period and found that brain regions with white matter hyperintensities at follow-up had significantly lower cerebral blood flow at baseline, suggesting that baseline cerebral blood flow may be related to the development of white matter lesions over time. Other work has shown that reduced cortical cerebral blood flow was associated with disrupted white matter integrity in older adults.48 Together, these findings suggest that overall blood supply to the brain is an important indicator of neural integrity and that alterations in the cerebrovasculature with advancing age may potentially exacerbate structural deterioration in the brain. Thus, it is concievable that age may act as a moderating factor in the relationship between mTBI and cerebrovascular dysfunction. Specifically, increasing age may initiate cellular mechanisms of vascular disruption that may then excerbate the effect of mTBI on cerebrovascular health. It will be important for studies examining the association between mTBI and cerebrovascular dysfunction to also consider age as a potential moderating factor of that relationship.
Limitations
It is worth noting a few limitations of the work reviewed here. First, the work to date is largely cross-sectional. Future longtiduinal work is needed to confirm the current hypothesis that disruptions in the cerebrovascular system contribute to neural integrity loss in mTBI. Second, cerebral blood flow is tightly coupled to neural activity (i.e., neurovascular coupling), which may potentially limit interpretation of existing work. Specifically, it is possible that neural activity rather than vascular function may underlie the relationship between mTBI and neurodegeneration. However, longitudinal evidence in older adults supports the hypothesis proposed in this review, with results showing an association between cerebrovascular dysfunction and long-term structural changes.52 Nonetheless, it will be important for future work to simultaneously examine neural activity and vascular function and their interactions in the overarching relationship between mTBI and structural integrity. Lastly, it should be noted that cerebral blood flow is one of many indices of cerebrovascular health. Future research should explore other metrics of vascular reactivity to garner a more complete picture of the relationship between cerebrovascular dysfunction and mTBI.
Conclusions
The work discussed in this article underscores the importance of studying cerebrovascular health in mTBI and suggests that disruptions in the cerebrovascular system may have deleterious effects on neural integrity. Nonetheless, it remains an open question whether cerebrovascular disruptions act as a pathological link between mTBI and neurodegenerative disease. Ultimately, longitudinal studies are needed to fully evaluate this possibility. Research studies collecting detailed longitudinal biological, neuroimaging, and psychiatric data such as the Translational Research Center for TBI and Stress Disorders (TRACTS), the Chronic Effects of Neurotrauma Consortium (CENC), and the Alzheimer’s Disease Neuroimaging Initiative-Department of Defense (ADNI-DOD) are currently ongoing and offer promising insight into this question. The continued efforts of these large-scale longitudinal studies are critical toward a more comprehensive understanding of chronic mTBI. Moving into the future, research focused on the cerebrovascular system will be important to advance our understanding of the neurobiological mechanisms underlying the long-term consequences of mTBI and to guide long-term health strategies for mTBI patients. These advances will help to propel the field forward and may offer unique therapeutic interventions and targets.
Acknowledgements:
This work was supported by VA Clinical Science Research and Development Career Development Award (1IK2CX001772-01) awarded to DRS, National Institutes of Mental Health (NIMH) training grant (T32MH019836-01) awarded to Terence Keane, Ph.D. supporting DRS, and the National Center for PTSD. The contents of this article do not represent the views of the U.S. Department of Veterans Affairs, the National Institutes of Health, or the United States Government.
Footnotes
Conflicts of Interest: The author declares no conflict of interest.
References
- 1.Cassidy JD, Carroll LJ, Peloso PM, et al. Incidence, risk factors and prevention of mild traumatic brain injury: Results of the WHO collaborating centre task force on mild traumatic brain injury. J rehabil med. 2004(43 Suppl):28–60. [DOI] [PubMed] [Google Scholar]
- 2.Carroll LJ, Cassidy JD, Peloso PM, et al. Prognosis for mild traumatic brain injury: Results of the WHO collaborating centre task force on mild traumatic brain injury. J rehabil med. 2004;36(43 Suppl):84–105. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci transl med. 2012;4(134):134ra160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKee AC, Cantu RC, Nowinski CJ, et al. Chronic traumatic encephalopathy in athletes: Progressive tauopathy after repetitive head injury. J neuropathol exp neurol. 2009;68(7):709–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McKee AC, Stein TD, Nowinski CJ, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(1):43–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH. Chronic traumatic encephalopathy in a National Football League player. Neurosurgery. 2005;57(1):128–134. [DOI] [PubMed] [Google Scholar]
- 7.Gardner RC, Burke JF, Nettiksimmons J, Kaup A, Barnes DE, Yaffe K. Dementia risk after traumatic brain injury vs nonbrain trauma: The role of age and severity. JAMA neurology. 2014;71(12):1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gardner RC, Byers AL, Barnes DE, Li Y, Boscardin J, Yaffe K. Mild TBI and risk of Parkinson disease. Neurology. 2018; epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Human brain mapping. 2016;37(1):220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes JP, Miller DR, Lafleche G, Salat DH, Verfaellie M. The nature of white matter abnormalities in blast-related mild traumatic brain injury. Neuroimage: Clinical. 2015;8(0):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with overall cognitive status in blast-related mTBI. Brain imaging and behavior. 2017; 11(4): 1129–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vakhtin AA, Calhoun VD, Jung RE, Prestopnik JL, Taylor PA, Ford CC. Changes in intrinsic functional brain networks following blast-induced mild traumatic brain injury. Brain inj. 2013;27(11):1304–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes JP, Logue MW, Sadeh N, et al. Mild traumatic brain injury is associated with reduced cortical thickness in those at risk for Alzheimer’s disease. Brain. 2017; 140(3):813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davenport ND, Lim KO, Armstrong MT, Sponheim SR. Diffuse and spatially variable white matter disruptions are associated with blast-related mild traumatic brain injury. NeuroImage. 2011;59(3):2017–2024. [DOI] [PubMed] [Google Scholar]
- 15.Jorge RE, Acion L, White T, et al. White matter abnormalities in veterans with mild traumatic brain injury. The american journal of psychiatry. 2012;169(12):1284–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mac Donald CL, Johnson AM, Cooper D, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. New england journal of medicine. 2011;364(22):2091–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lipton ML, Gellella E, Lo C, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel-wise analysis of diffusion tensor imaging. Journal of neurotrauma. 2008;25(11):1335–1342. [DOI] [PubMed] [Google Scholar]
- 18.Lo C, Shifteh K, Gold T, Bello JA, Lipton ML. Diffusion tensor imaging abnormalities in patients with mild traumatic brain injury and neurocognitive impairment. J comput assist tomogr. 2009;33(2):293–297. [DOI] [PubMed] [Google Scholar]
- 19.Mayer AR, Yang Z, Yeo RA, et al. A functional MRI study of multimodal selective attention following mild traumatic brain injury. Brain imaging and behavior. 2012;6(2):343–354. [DOI] [PubMed] [Google Scholar]
- 20.Scheibel RS, Newsome MR, Troyanskaya M, et al. Altered brain activation in military personnel with one or more traumatic brain injuries following blast. The journal of the international neuropsychological society. 2012;18(1):89–100. [DOI] [PubMed] [Google Scholar]
- 21.Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends in neurosciences. 2005;28(4):202–208. [DOI] [PubMed] [Google Scholar]
- 22.Pop V, Badaut J. A Neurovascular perspective for long-term changes after brain trauma. Translational stroke research. 2011;2(4):533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdul-muneer PM, Chandra N, Haorah J. Interactions of oxidative stress and neurovascular inflammation in the pathogenesis of traumatic brain injury. Molecular neurobiology. 2015;51(3):966–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giza CC, Hovda DA. The neurometabolic cascade of concussion. Journal of athletic training. 2001;36(3):228–235. [PMC free article] [PubMed] [Google Scholar]
- 25.Barkhoudarian G, Hovda DA, Giza CC. The molecular pathophysiology of concussive brain injury. Clinics in sports medicine. 2011;30(1):33–48. [DOI] [PubMed] [Google Scholar]
- 26.Badaut J, Bix GJ. Vascular neural network phenotypic transformation after traumatic injury: Potential role in long-term sequelae. Translational stroke research. 2014;5(3):394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75(suppl_4):S24–S33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franzblau M, Gonzales-Portillo C, Gonzales-Portillo GS, et al. Vascular damage: A persisting pathology common to Alzheimer’s disease and traumatic brain injury. Medical hypotheses. 2013;81(5):842–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Readnower RD, Chavko M, Adeeb S, et al. Increase in blood brain barrier permeability, oxidative stress, and activated microglia in a rat model of blast induced traumatic brain injury. Journal of neuroscience research. 2010;88(16):3530–3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmed F, Plantman S, Cernak I, Agoston DV. The temporal pattern of changes in serum biomarker levels reveals complex and dynamically changing pathologies after exposure to a single low-intensity blast in mice. Frontiers in neurology. 2015;6(114):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Werner C, Engelhard K. Pathophysiology of traumatic brain injury. British journal of anaesthesia. 2007;99(1):4–9. [DOI] [PubMed] [Google Scholar]
- 32.Junger EC, Newell DW, Grant GA, et al. Cerebral autoregulation following minor head injury. Journal of neurosurgery. 1997;86(3):425–432. [DOI] [PubMed] [Google Scholar]
- 33.Strebel S, Lam AM, Matta BF, Newell DW. Impaired cerebral autoregulation after mild brain injury. Surgical neurology. 1997;47(2):128–131. [DOI] [PubMed] [Google Scholar]
- 34.Badaut J, Lasbennes F, Magistretti PJ, Regli L. Aquaporins in brain: Distribution, physiology, and pathophysiology. Journal of cerebral blood flow & metabolism. 2002;22(4):367–378. [DOI] [PubMed] [Google Scholar]
- 35.Papadopoulos MC, Verkman AS. Aquaporin-4 and brain edema. Pediatric nephrology. 2007;22(6):778–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Badaut J, Verbavatz JM, Freund-Mercier MJ, Lasbennes F. Presence of aquaporin-4 and muscarinic receptors in astrocytes and ependymal cells in rat brain: A clue to a common function? Neuroscience Letters. 2000;292(2):75–78. [DOI] [PubMed] [Google Scholar]
- 37.Abdul-Muneer PM, Schuetz H, Wang F, et al. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in animal model of mild traumatic brain iInjury induced by primary blast. Free radical biology & medicine. 2013;60:282–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sun MC, Honey CR, Berk C, Wong NLM, Tsui JKC. Regulation of aquaporin-4 in a traumatic brain injury model in rats. Journal of neurosurgery. 2003;98(3):565–569. [DOI] [PubMed] [Google Scholar]
- 39.Peng SP, Li YN, Liu J, et al. Pulsed arterial spin labeling effectively and dynamically observes changes in cerebral blood flow after mild traumatic brain injury. Neural Regeneration Research. 2016;11(2):257–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grossman EJ, Jensen JH, Babb JS, et al. Cognitive impairment in mild traumatic brain injury: A longitudinal diffusional kurtosis and perfusion imaging study. American journal of neuroradiology. 2013;34(5):951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ge Y, Patel MB, Chen Q, et al. Assessment of thalamic perfusion in patients with mild traumatic brain injury by true FISP arterial spin labeling MR imaging at 3T. Brain injury. 2009;23(7–8):666–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonne O, Gilboa A, Louzoun Y, et al. Cerebral blood flow in chronic symptomatic mild traumatic brain injury. Psychiatry research: Neuroimaging. 2003;124(3):141–152. [DOI] [PubMed] [Google Scholar]
- 43.Sours C, Zhuo J, Roys S, Shanmuganathan K, Gullapalli RP. Disruptions in resting state functional connectivity and cerebral blood flow in mild traumatic brain injury patients. PLOS ONE. 2015;10(8):e0134019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponto LLB, Brashers-Krug TM, Pierson RK, et al. Preliminary investigation of cerebral blood flow and amyloid burden in veterans with and without combat-related traumatic brain injury. The journal of neuropsychiatry and clinical neurosciences. 2015;28(2):89–96. [DOI] [PubMed] [Google Scholar]
- 45.Wang Y, West JD, Bailey JN, et al. Decreased cerebral blood flow in chronic pediatric mild TBI: An MRI perfusion study. Developmental neuropsychology. 2015;40(1):40–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bartnik-Olson BL, Holshouser B, Wang H, et al. Impaired neurovascular unit function contributes to persistent symptoms after concussion: A pilot study. Journal of neurotrauma. 2014;31(17):1497–1506. [DOI] [PubMed] [Google Scholar]
- 47.Gross H, Kling A, Henry G, Herndon C, Lavretsky H. Local cerebral glucose metabolism in patients with long-term behavioral and cognitive deficits following mild traumatic brain injury. The journal of neuropsychiatry and clinical neurosciences. 1996;8(3):324–334. [DOI] [PubMed] [Google Scholar]
- 48.Chen JJ, Rosas HD, Salat DH. The relationship between cortical blood flow and sub-cortical white-matter health across the adult age span. PloS one. 2013;8(2):e56733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salat DH. Imaging small vessel-associated white matter changes in aging. Neuroscience. 2014;276:174–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Steketee RME, Meijboom R, de Groot M, et al. Concurrent white and gray matter degeneration of disease-specific networks in early-stage Alzheimer’s disease and behavioral variant frontotemporal dementia. Neurobiology of aging. 2016;43:119–128. [DOI] [PubMed] [Google Scholar]
- 51.Brickman AM, Zahra A, Muraskin J, et al. Reduction in cerebral blood flow in areas appearing as white matter hyperintensities on magnetic resonance imaging. Psychiatry research. 2009;172(2):117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Promjunyakul N, Lahna D, Kaye JA, et al. Characterizing the white matter hyperintensity penumbra with cerebral blood flow measures. NeuroImage: Clinical. 2015;8:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Promjunyakul N, Lahna DL, Kaye JK, et al. Comparison of cerebral blood flow and structural penumbras in relation to white matter hyperintensities: A multi-modal magnetic resonance imaging study. Journal of cerebral blood flow & metabolism. 2016;36(9):1528–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shi Y, Thrippleton MJ, Makin SD, et al. Cerebral blood flow in small vessel disease: A systematic review and meta-analysis. Journal of cerebral blood flow & metabolism. 2016;36(10):1653–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernbaum M, Menon BK, Fick G, et al. Reduced blood flow in normal white matter predicts development of leukoaraiosis. Journal of cerebral blood flow & metabolism. 2015;35(10):1610–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xekardaki A, Rodriguez C, Montandon M-L, et al. Arterial spin labeling may contribute to the prediction of cognitive deterioration in healthy elderly individuals. Radiology. 2014;274(2):490–499. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z, Das SR, Xie SX, Arnold SE, Detre JA, Wolk DA. Arterial spin labeled MRI in prodromal Alzheimer’s disease: A multi-site study. NeuroImage: Clinical. 2013;2:630–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benedictus MR, Leeuwis AE, Binnewijzend MAA, et al. Lower cerebral blood flow is associated with faster cognitive decline in Alzheimer’s disease. European radiology. 2017;27(3):1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chao LL, Buckley ST, Kornak J, et al. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer disease and associated disorders. 2010;24(1):19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morey RA, Haswell CC, Selgrade ES, et al. Effects of chronic mild traumatic brain injury on white matter integrity in Iraq and Afghanistan war veterans. Hum brain mapp. 2012;34(11):2986–2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mac Donald C, Johnson A, Cooper D, et al. Cerebellar white matter abnormalities following primary blast injury in US military personnel. PLoS One. 2013;8(2):e55823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fleminger S, Oliver D, Lovestone S, Rabe-Hesketh S, Giora A. Head injury as a risk factor for Alzheimer’s disease: The evidence 10 years on; a partial replication. Journal of neurology, neurosurgery, and psychiatry. 2003;74(7):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nature reviews neuroscience. 2011;12(12):723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clark AL, Bangen KJ, Sorg SF, et al. Dynamic association between perfusion and white matter integrity across time since injury in Veterans with history of TBI. NeuroImage: Clinical. 2017;14:308–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gómez‐Ramos A, Díaz‐Nido J, Smith Mark A, Perry G, Avila J. Effect of the lipid peroxidation product acrolein on tau phosphorylation in neural cells. Journal of neuroscience research. 2002;71(6):863–870. [DOI] [PubMed] [Google Scholar]
- 66.Su B, Wang X, Lee H-g, et al. Chronic oxidative stress causes increased tau phosphorylation in M17 neuroblastoma cells. Neuroscience letters. 2010;468(3):267–271. [DOI] [PubMed] [Google Scholar]
- 67.Dias-Santagata D, Fulga TA, Duttaroy A, Feany MB. Oxidative stress mediates tau-induced neurodegeneration in Drosophila. The journal of clinical investigation. 2007;117(1):236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scherbel U, Raghupathi R, Nakamura M, et al. Differential acute and chronic responses of tumor necrosis factor-deficient mice to experimental brain injury. Proceedings of the national academy of sciences. 1999;96(15):8721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. New england journal of medicine. 2008;358(5):453–463. [DOI] [PubMed] [Google Scholar]
- 70.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52(12):1048–1060. [DOI] [PubMed] [Google Scholar]
- 71.Pietrzak RH, Goldstein RB, Southwick SM, Grant BF. Medical comorbidity of full and partial posttraumatic stress disorder in United States adults: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Psychosomatic medicine. 2011;73(8):697–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lohr JB, Palmer BW, Eidt CA, et al. Is Post-traumatic stress disorder associated with premature senescence? A review of the literature. The american journal of geriatric psychiatry. 2015;23(7):709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schlenger WE, Corry NH, Williams CS, et al. A prospective study of mortality and trauma-related risk factors among a nationally representative sample of Vietnam veterans. American journal of epidemiology. 2015;182(12):980–990. [DOI] [PubMed] [Google Scholar]
- 74.Wolf EJ, Schnurr PP. Posttraumatic stress disorder-related cardiovascular disease and accelerated cellular aging. Psychiatric annals. 2016;46(9):527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: Implications for surveillance and prevention. Psychosomatic medicine. 2008;70(6):668–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pietrzak Robert H, Goldstein Risë B, Southwick Steven M, Grant Bridget F. Physical health conditions associated with posttraumatic stress disorder in U.S. older adults: Results from wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of the american geriatrics society. 2012;60(2):296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vaccarino V, Goldberg J, Rooks C, et al. Post-traumatic stress disorder and incidence of coronary heart disease: A twin study. Journal of the american college of cardiology. 2013;62(11):970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Roy SS, Foraker RE, Girton RA, Mansfield AJ. Posttraumatic stress disorder and incident heart failure among a community-based sample of US veterans. American journal of public health. 2015;105(4):757–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf EJ, Sadeh N, Leritz EC, et al. Posttraumatic stress disorder as a catalyst for the association between metabolic syndrome and reduced cortical thickness. Biological psychiatry. 2016;80(5):363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wolf EJ, Logue MW, Hayes JP, et al. Accelerated DNA methylation age: Associations with PTSD and neural integrity. Psychoneuroendocrinology. 2016;63:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ceprnja M, Derek L, Unić A, et al. Oxidative stress markers in patients with post-traumatic stress disorder. Collegium antropologicum. 2011;35(4):1155–1160. [PubMed] [Google Scholar]
- 82.Lindqvist D, Wolkowitz OM, Mellon S, et al. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain, behavior, and immunity. 2014;42:81–88. [DOI] [PubMed] [Google Scholar]
- 83.Miller MW, Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: Neurodegeneration and the accelerated-aging hypothesis. Molecular psychiatry. 2014;19:1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Logue MW, Baldwin C, Guffanti G, et al. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Molecular psychiatry. 2013;18(8):937–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lindemer ER, Salat DH, Leritz EC, McGlinchey RE, Milberg WP. Reduced cortical thickness with increased lifetime burden of PTSD in OEF/OIF Veterans and the impact of comorbid TBI. NeuroImage: Clinical. 2013;2:601–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Barodka VM, Joshi BL, Berkowitz DE, Hougue CW, Nyhan D. Implications of vascular aging. Anesth Analg. 2011; 112(5):1048–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee HY, Oh BH. Aging and arterial stiffness. Circulation Journal. 2010; 74(11):2257–2262. [DOI] [PubMed] [Google Scholar]
- 88.Lakatta EG, Wang M, Najjar SS. Arterial aging and subclinical arterial disease are fundamentally intertwined at macroscopic and molecular levels. Med Clin North Am. 2009; 93(3):583–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fleg JL, Strait J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Failure Reviews. 2012; 17(4–5):545–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burke GL, Evans GW, Riley WA, Sharrett AR, Howard G, Barnes RW, Rosamond W, Crow RS, Rautaharju P, Heiss G. Arterial wall thickness is associated with prevalent cardiovascular disease in middle-aged adults: The atherosclerosis risk in communities (ARIC) study. Stroke A Journal of Cerebral Circulation. 1995; 26(3):386–391. [DOI] [PubMed] [Google Scholar]
- 91.Gunning-Dixon FM, Raz N. The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology. 2000;14:224–232. [DOI] [PubMed] [Google Scholar]


