Abstract
Aims:
The FDA-approved histone deacetylase (HDAC) inhibitor, suberoylanilide hydroxamic acid (SAHA, Vorinostat) has been shown to induce cardiomyocyte autophagy and blunt ischemia/reperfusion (I/R) injury when administered at the time of reperfusion. However, the precise mechanisms underlying the cardioprotective activity of SAHA are unknown. Mitochondrial dysfunction and oxidative damage are major contributors to myocardial apoptosis during I/R injury. We hypothesize that SAHA protects the myocardium by maintaining mitochondrial homeostasis and reducing reactive oxygen species (ROS) production during I/R injury.
Methods:
Mouse and cultured cardiomyocyte (neonatal rat ventricular myocytes and human embryonic stem cell-derived cardiomyocytes) I/R models were used to investigate the effects of SAHA on mitochondria. ATG7 knockout mice, ATG7 knockdown by siRNA in cardiomyocytes and PGC-1α knockdown by adenovirus were used to test the dependency of autophagy and PGC-1α-mediated mitochondrial biogenesis respectively.
Results:
Intact and total mitochondrial DNA (mtDNA) content and mitochondrial mass were significantly increased in cardiomyocytes by SAHA pretreatment before simulated I/R. In vivo, I/R induced >50% loss of mtDNA content in the border zones of mouse hearts, but SAHA pretreatment and reperfusion treatment alone reverted mtDNA content and mitochondrial mass to control levels. Moreover, pretreatment of cardiomyocytes with SAHA resulted in a 4-fold decrease in I/R-induced loss of mitochondrial membrane potential and a 25%−40% reduction in cytosolic ROS levels. However, loss-of-function of ATG7 in cardiomyocytes or mouse myocardium abolished the protective effects of SAHA on ROS levels, mitochondrial membrane potential, mtDNA levels, and mitochondrial mass. Lastly, PGC-1α gene expression was induced by SAHA in NRVMs and mouse heart subjected to I/R, and loss of PGC-1α abrogated SAHA’s mitochondrial protective effects in cardiomyocytes.
Conclusions:
SAHA prevents I/R induced-mitochondrial dysfunction and loss, and reduces myocardial ROS production when given before or after the ischemia. The protective effects of SAHA on mitochondria are dependent on autophagy and PGC-1α-mediated mitochondrial biogenesis.
Keywords: Autophagy, Mitochondrial homeostasis, HDAC inhibition, ROS, Myocardial ischemia/reperfusion injury
1. Introduction
Acute myocardial infarction (MI) is a major cause of morbidity and mortality worldwide, and myocardial ischemia/reperfusion (I/R) injury is a key factor in determining infarct size [1]. I/R injury drives a number of pathological conditions that correlate with the final infarct size, including metabolic disorders, inflammatory responses, and cardiac myocyte apoptosis and subsequent heart failure [2]. As a result, reperfusion injury has been estimated to cause approximately half of the final infarct size [3]. Because no standard therapy is currently available to treat I/R injury, a better understanding of the underlying processes and mechanisms is critical for the development of effective therapies for MI patients.
Cardiac mitochondria are responsible for energy generation, as well as many other metabolic reactions crucial for cardiac function [4]. As a result, mitochondrial dysfunction is a key contributor to myocardial injury during I/R. Signs of mitochondrial dysfunction are observed soon after ischemia, including mitochondrial calcium overload and the opening of mitochondrial permeability transition pore (mPTP); these changes lead to mitochondrial membrane depolarization, the release of pro-apoptotic proteins, and eventually cardiomyocyte death [5]. Mitochondria are the primary source of reactive oxygen species (ROS), which contribute to myocardial I/R injury [6], as well as cardiomyocyte death and heart failure [7]. These damaging ROS can also target the mitochondria themselves [8], resulting in mitochondrial DNA (mtDNA) damage, diminished mitochondrial protein synthesis, loss of mitochondrial membrane potential, and decreased energy production [5, 9]. Thus, maintenance of mitochondrial homeostasis is crucial for cardiomyocyte protection during I/R injury.
Autophagy is an intracellular pathway that regulates the turnover of cellular components [4]. During I/R injury, activation of autophagy helps to maintain the energetic balance by promoting ATP generation during ischemia, then subsequently switches to clearance of damaged organelles and proteins during the reperfusion phase [8]. And maintaining autophagic flux during reperfusion reduces infarct size and protects the heart from I/R Injury [10]. Mitophagy, the specific autophagic elimination of mitochondria, removes specifically damaged mitochondria to maintain mitochondrial homeostasis. Enhanced mitochondrial clearance in T lymphocytes, maintaining mitochondrial mass in skeletal muscle and mitochondrial integrity are mediated by autophagy [11–13]. Moreover, mitochondrial biogenesis is regulated by the transcriptional coactivator peroxisome proliferator co-activator 1 alpha (PGC-1α) [14]. However, the direct role of PGC-1α in mitochondrial biogenesis during I/R injury is currently unknown.
A recent series of preclinical studies have demonstrated the potent cardioprotective benefits of histone deacetylase (HDAC) inhibitors in murine and rabbit models of I/R injury [15, 16]. In particular, suberoylanilide hydroxamic acid (SAHA, Vorinostat, Zolinza®-Merck), an FDA-approved HDAC inhibitor for T cell lymphoma treatment, has been shown to blunt I/R injury by inducing cardiomyocyte autophagy [17]. However, the molecular mechanisms underlying the cardioprotective effects of SAHA have not yet been elucidated. Due to the previously described link between autophagic flux and turnover of damaged mitochondria in I/R injury, we hypothesized that SAHA protects the myocardium by maintaining mitochondrial homeostasis and reducing ROS levels during reperfusion injury. To test this hypothesis, we evaluated the effects of SAHA on ROS levels, mtDNA copy number, and mitochondrial membrane potential in cardiomyocytes subjected to I/R injury in vitro and in vivo.
2. Materials and Methods
2.1. Animals Care
All animals handled in this study were in accordance with the standards established in the Guide for the Care and Use of Laboratory Animals published by the Institute of Laboratory Animal Resources of the National Research Council (United States) and approved by the Animal Care Committee of the University of Alabama at Birmingham. All mice used in this study were housed under identical conditions in a pathogen-free environment with a 12:12h light/dark cycle and free access to laboratory chow and water.
2.2. Generation of Time-specific and Cardiomyocyte-specific ATG7 Knockout Mice
The ATG7F/F mouse was provided by Dr. Massaki Komatsu [18], and αMHC-merCremer mouse was obtained from Jackson lab [19]. Tamoxifen dissolved in peanut oil was injected IP at the concentration of 20mg/kg for 5 days at the age of 8–12 weeks old according to prior protocols to avoid cardiac toxicity [20]. One week after the last injection, mice were subjected to I/R surgeries.
2.3. Mouse Model of I/R
For I/R surgeries, 8 to 12-week-old C57BL/6 wild-type mice and ATG7 KO mice were used. All mice were anesthetized with 2–4% isoflurane and placed in a supine position on a heating pad (37°C). Animals were intubated with a 19G stump needle and ventilated with room air using a MiniVent mouse ventilator (Hugo Sachs Elektronik; stroke volume 250 μL, respiratory rate 150–200 breaths per minute). Following left thoracotomy between the 2nd and 3rd ribs, the LAD (Left Anterior Descending coronary artery) was visualized under a microscope and ligated using a 6–0 prolene suture. Regional ischemia was confirmed by visual inspection under a dissecting microscope (Leica) by discoloration of the occluded distal myocardium. For reperfusion, the ligation was released after 45 minutes of ischemia and the tissue allowed to re-perfuse as confirmed by visual inspection. (Figure S1). For the pretreatment group, mice received 4 dose of SAHA 50mg/kg (one day before the surgery q12h, on the day of surgery, one dose before the surgery and at the time of reperfusion. For the reperfusion only group, mice received one dose of SAHA 100mg/kg (dissolved in DMSO at the concentration of 50mg/ml, 2μl DMSO/g of body weight with SAHA injection) at the time of reperfusion and the control group received 2μl DMSO/g of body weight. 24 hours of reperfusion was performed after 45 minutes ischemia. Then the mice were sacrificed, the heart was divided into three tissue zones visually under microscopes: ischemic, border and remote zones, which were used for subsequent Western blot analysis, DNA isolation and EM (electron microscopy) analyses.
2.4. Neonatal Rat Cardiomyocytes Isolation and Differentiation of Human Embryonic Stem Cell-derived Cardiomyocytes
Neonatal ventricular myocytes (NRVMs) were isolated from Sprague-Dawley rats that are one day old according to the methods described previously [17]. These cells were plated to enrich for cardiac myocytes approximately 1×106 plated cells per dish and cultured for 24 hours in DMEM/M199 (3:1 ratio) containing 10% FBS and 100 μM BrdU (Sigma Aldrich, St Louis, MO, USA). Human Embryonic Stem Cell (hESCs)-derived Cardiomyocytes were differentiated into cardiomyocytes using a previously reported directed differentiation protocol from Dr. Jianyi Zhang [21]. The differentiation efficiency is >80%.
2.5. siRNA Knockdown
NRVMs were isolated and seeded at a density of 1.2 million/well in a 6-well dish. The purity of the cardiomyocytes is >85%. 24 hours after plating, cardiomyocytes were incubated with siRNA negative control (Neg, SIC001), siRNAs targeting ATG7 (SASI_Rn01_00050326), and siRNAs targeting ATG5 (SASI_Rn01_00094887), each from Sigma and used according to the manufacturer’s recommended protocols. Briefly, siRNAs were reconstituted into a 40 μM stock solution. 3μL of the siRNA stock and 3μL of RNAiMax transfectant were mixed together in 1 mL Optima medium. Cardiomyocytes were incubated with the RNAiMax for 6 hours, followed by addition of 1 mL of culture medium containing 20% serum. 24 hours after the siRNA incubation, the cardiomyocytes were treated with SAHA at 2μM (overnight). Then, the cells were subjected to I/R experiment.
2.6. PGC-1α Knockdown by adenovirus
Adenovirus for PGC-1α Knockdown were kindly provided by Dr. Glenn Rowe, and the details were described in a previous publication [22]. 24 hours after plating, NRVMs were infected with adenovirus expressing GFP, PGC-1α and PGC-1α siRNA at the MOI of 10 for 24 hours. The infection efficiency is >95% based on fluorescent microscopy. Then the cardiomyocytes were treated with SAHA (overnight) and subjected to I/R experiment.
2.7. Fluorescence Microscopy and Flow Cytometry Analysis
Dichlorofluorescein (H2DCFDA), tetramethylrhodamine methyl ester (TMRM) and MitoTracker Green (Invitrogen Molecular Probe, OR, USA) were used to measure total cellular ROS levels, mitochondrial membrane potential, and mitochondrial mass, respectively. Following previously described protocols [23, 24], NRVMs and hESC-CMs were incubated in H2DCFDA, TMRM or MitoTracker Green respectively for 20 min and imaged immediately using a Nikon Eclipse Ti fluorescent microscope and quantified using ImageJ version 1.48v software (NIH, Bethesda USA). Furthermore, the average fluorescence intensity of H2DCFDA and MitoTracker Green in each group was determined by FACS Calibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, USA), and FlowJo 7.6.1 software (TreeStar, Ashland, OR, USA) were used to analyze the data.
2.8. Western Blots Analysis
Protein estimation of whole cell lysate was performed using the BCA Protein Assay Kit (Thermo Fisher Scientific, Rockford, IL, USA). An equal amount of protein from each sample was separated on SDS-PAGE, trans-blotted onto PVDF membrane and subjected to immunoblot assay by primary antibodies followed by secondary antibodies. Antibodies of VDAC (#12454), ATG5 (#12994), ATG7 (#8558) and GAPDH (#5174) were purchased from Cell Signaling Technology, and LC3-II (Rabbit anti-LC3 polyclonal antibody) is a gift from Dr. Hill’s Laboratory at UT Southwestern Medical Center.
2.9. DNA Isolation, mtDNA Copy Number and mtDNA Damage Measurement
Total DNA and RNA were isolated from NRVMs, hESC-CMs and three zones of hearts with I/R injury using DNA and RNA extraction kits (Qiagen, Valencia, CA, USA). Intact and mitochondrial DNA (mtDNA) content were measured by both semi-quantitative PCR and qPCR [25]. Briefly, intact mtDNA (16.2 kb) and total mtDNA including fragments ( 0.22 kb) and nuclear β-actin gene expression are amplified using primers listed in Table S1 [26]. Semi-quantitative PCR products were quantified by densitometry analysis using ImageQuant (GE Healthcare). For mtDNA qPCR, primers for mtDNA specific gene COXII, D-Loop and ATP6 are used.
2.10. Statistical analysis
Statistical analysis of the differences among groups was evaluated with a one-way ANOVA followed by Duncan’s multiple-comparison test and student’s t-test for paired data using SPSS software (version 19.0, SPSS Inc., Chicago, IL, USA). Significant differences were established at the level of p < 0.05. Data are expressed as means ± SEM.
3. Results
3.1. SAHA Reduces Mitochondrial DNA Damage and Promotes Mitochondrial Biogenesis in Cardiomyocytes and Mouse Heart Tissue Subjected to I/R Injury
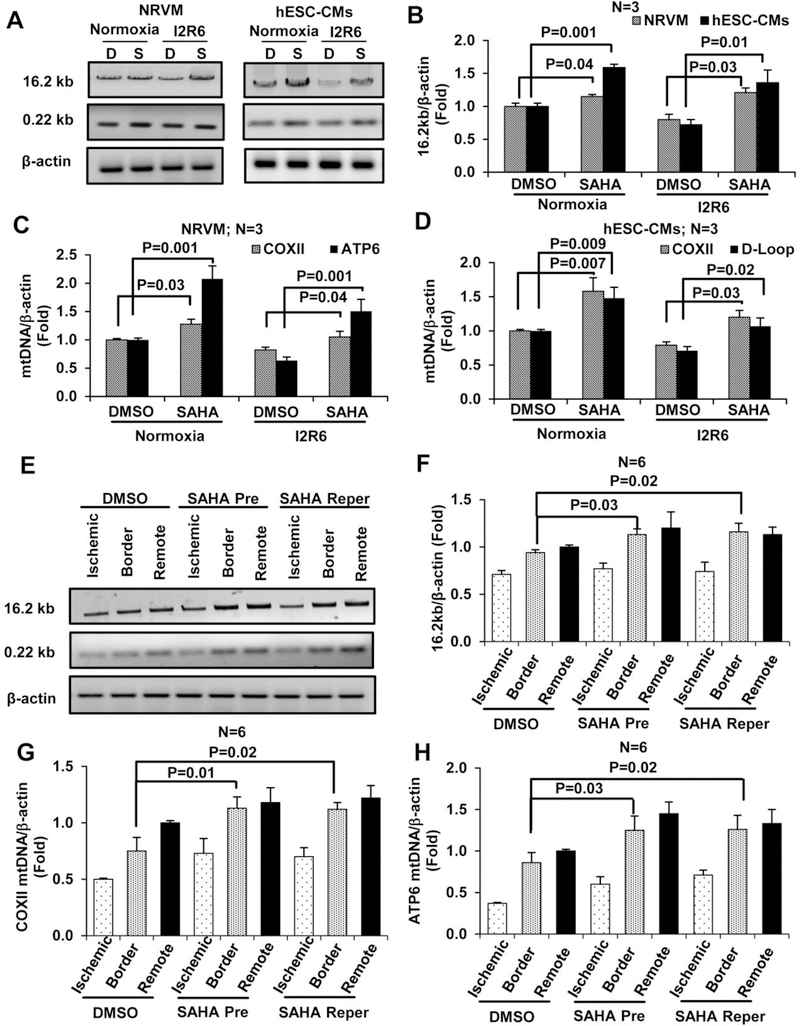
Mitochondria-dependent pathways are key mediators of myocardial injury and cell death during I/R [27]. Cardiac I/R injury generates ROS that cause oxidative damage, oxidizing the mtDNA and other macromolecules [9]. Thus, we assessed the protective effects of SAHA on mtDNA during I/R injury. Semi-quantitative PCR (qPCR) analysis of intact and total mtDNA (16.2 kb and 0.22 kb) was used to detect mtDNA damage in cardiomyocytes [25]. Results from these experiments showed that SAHA pretreatment increased levels of intact mtDNA after 6 hours of reperfusion in NRVMs compared with vehicle-treated cells (DMSO) (Figure 1 A–B). With respect to mtDNA content, qPCR analysis revealed that SAHA treatment increased the mtDNA copy numbers of COXII and ATP6 by 20% and 50%, respectively, in NRVMs compared with the DMSO group (Figure 1 C). Analysis of COXII and D-Loop levels revealed similar results in hESC-CMs (Figure 1 D) and AC16 (a human cardiomyocyte cell line) (Figure S2 A–D).
Figure 1. SAHA treatment increases mitochondrial DNA levels in cardiomyocytes and mouse heart subjected to I/R injury.
Cardiomyocytes (NRVMs and hESC-CMs) were subjected to simulated I/R injury and treatment with DMSO or SAHA. After 2 hours of ischemia and 6 hours of reperfusion (I2R6), DNA was isolated and amplified using species-specific primers to assess the degree of mtDNA damage and integrity. A-B, Representative images of the 16.2 kb and 0.22 kb products from semi-qPCR and quantification of the 16.2 kb product in NRVMs and hESC-CMs. C, mtDNA copy number in NRVMs was analyzed by qPCR using primers specific for COXII and ATP6. D, mtDNA copy number was analyzed in hESC-CMs by qPCR using primers specific for COXII and D-Loop. After cardiac I/R injury (45 min/24 hour) in mice, DNA was isolated from three zones of the heart (ischemic, border, and remote). E-F, Representative images and quantification of the 16.2 kb and 0.22 kb products from semi-qPCR. G-H, mtDNA copy number in three zones of mouse heart subjected to I/R analyzed by qPCR using COXII and ATP6 primers. I2R6, Ischemia 2 hours and reperfusion 6 hours; D, DMSO; S, SAHA; Pre, Pretreatment; Reper, Reperfusion only treatment.
In wildtype C57BL6 mice subjected to I/R surgery, SAHA reperfusion only treatment reduced infarct size by approximately 50% after normalization to the area at risk (Figure S3). The ischemic, border and remote zones of the left ventricle were dissected 24 hours after I/R injury. Compared with DMSO-treated animals, animals with SAHA pretreatment or SAHA only reperfusion treatment exhibited increased levels of intact mtDNA in the border zone (Figure 1 E–F). mtDNA copy number was also measured using primers specific to COXII and ATP6, demonstrating that SAHA pretreatment and reperfusion only treatment restored mtDNA copy number to normal levels in the border zone (Figure 1 G–H).
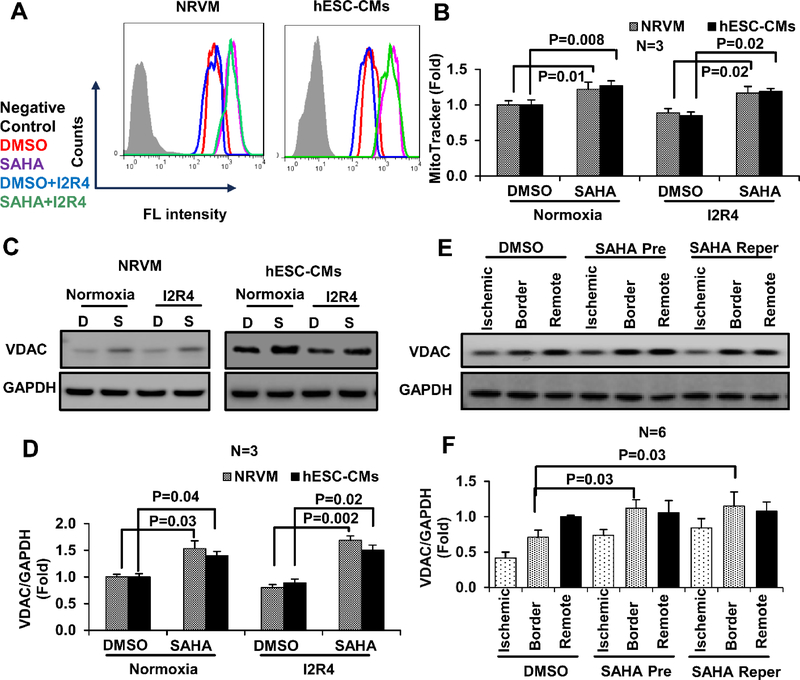
To further explore changes in mitochondria with SAHA, mice hearts subjected to I/R and SAHA treatments were examined by EM. Both SAHA pretreatment and reperfusion only treatments significantly increased mitochondrial size in the border zone (see images in Figure S4 A and quantification in Figure S4 B). A trend of increased mitochondrial number was observed with SAHA treatment, but the trend did not reach statistical significance (Figure S4 C). Moreover, SAHA pretreatment did increase mitochondrial mass in NRVMs, as measured by MitoTracker (Figure 2 A–B). To confirm the observed increase in mitochondrial mass, expression of voltage-dependent anion channel (VDAC), the most abundant protein of the outer mitochondrial membrane, was analyzed by Western blot. SAHA pretreatment increased VDAC levels by approximately 50% in both NRVMs and hESC-CMs subjected to I/R injury compared with DMSO-treated cells (Figure 2 C–D). VDAC expression was also increased in the border zone of SAHA-treated mouse hearts subjected to I/R injury (Figure 2 E–F). Collectively, these results suggest that SAHA treatment reduces mtDNA damage and promotes mitochondrial mass in cardiomyocytes subjected to I/R injury.
Figure 2. SAHA increases mitochondrial mass in cardiomyocytes and mouse heart tissue subjected to I/R injury.
Mitochondria were stained with MitoTracker Green and analyzed by flow cytometry. A, Mitochondria mass detected by flow cytometry in NRVMs and hESC-CMs subjected to simulated I/R injury and treated with DMSO or SAHA (representative histogram). B, Quantification of MitoTracker Green fluorescence intensity in NRVMs and hESC-CMs. C-D, Representative western blots and quantification of VDAC expression in cell lysate samples from NRVMs and hESC-CMs subjected to simulated I/R injury. E-F, Representative western blots and quantification of VDAC expression in three zones of mouse heart subjected to I/R injury. I2R4, Ischemic 2 hours and reperfusion 4 hours; D, DMSO; S, SAHA; Pre, Pretreatment; Reper, Reperfusion only treatment.
3.2. SAHA Reduces ROS Production in Cardiomyocytes Subjected to I/R Injury
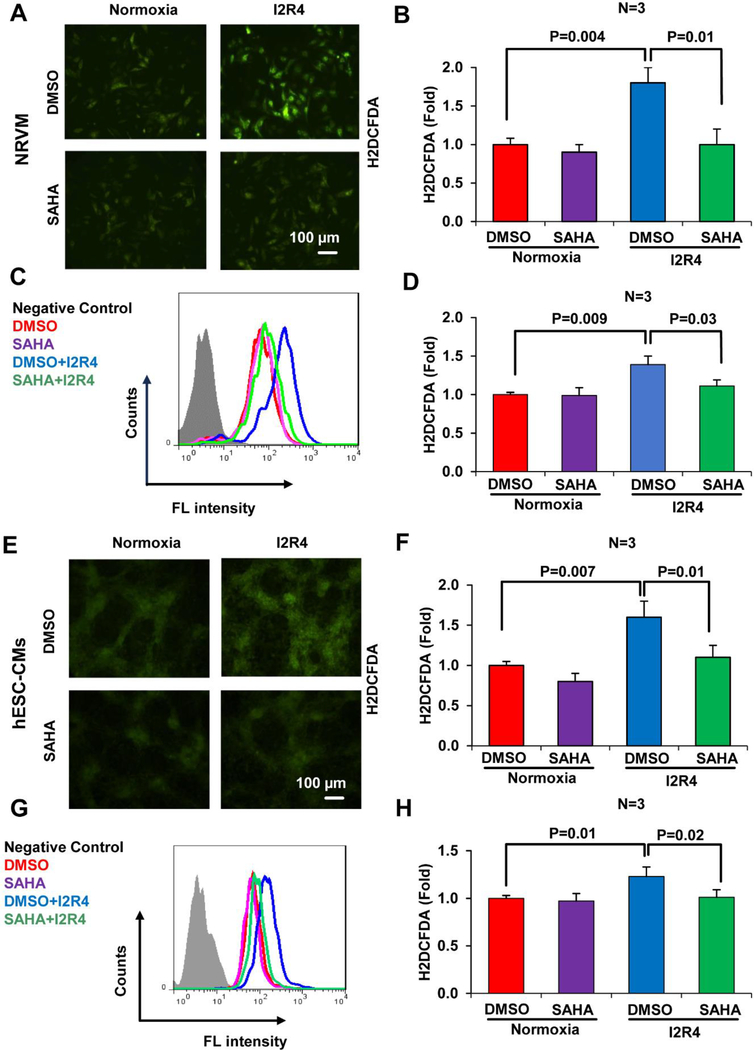
Excessive ROS generation from mitochondria is a potential mediator of reperfusion injury in the ischemic myocardium [6]. Attenuation of ROS levels has been shown to reduce infarct size and protect the heart against I/R injury [28]. Thus, we analyzed the effects of SAHA on ROS levels in cardiomyocytes subjected to I/R injury. H2DCFDA was used to quantify cellular ROS levels in NRVMs and hESC-CMs by microscopy and flow cytometry. During reperfusion after ischemia, ROS levels were significantly increased in control DMSO-treated cells with increasing time of reperfusion. In contrast, SAHA pretreatment for 16 hours prior to ischemia remarkably reduced ROS levels in NRVMs (Figure 3 A–D, Figure S5 A for time course) and hESC-CMs (Figure 3 E–H, Figure S5 B for time course). Similarly, SAHA pretreatment reduced cytosolic ROS levels in AC16 cells subjected to I/R (Figure S5 C). Consistent with reduced ROS levels, SAHA pretreatment also reduced I/R injury-induced cell death by approximately 20% in NRVMs and hESC-CMs (Figure S6 A–B). Together, these results suggest that SAHA pretreatment decreases oxidative stress and cell death in cardiomyocytes subjected to I/R injury.
Figure 3. SAHA treatment decreases oxidative stress in cardiomyocytes subjected to I/R injury.
Cardiomyocytes (NRVMs and hESC-CMs) subjected to simulated I/R injury and treated with DMSO or SAHA were stained with H2DCFDA to detect ROS. Staining intensity was quantified by ImageJ software and flow cytometry. A-B, Representative fluorescence microscopy images of H2DCFDA staining and fluorescence intensity quantification in NRVMs. C-D, Representative histograms and quantification of H2DCFDA fluorescence intensity in NRVMs. E-F, Representative fluorescence microscopy images of H2DCFDA staining and fluorescence intensity quantification in hESC-CMs. G-H, Representative histograms and quantification of H2DCFDA fluorescence intensity in hESC-CMs. I2R4, Ischemic 2 hours and reperfusion 4 hours.
3.3. SAHA Preserves Mitochondrial Membrane Potential in Cardiomyocytes Subjected to I/R Injury
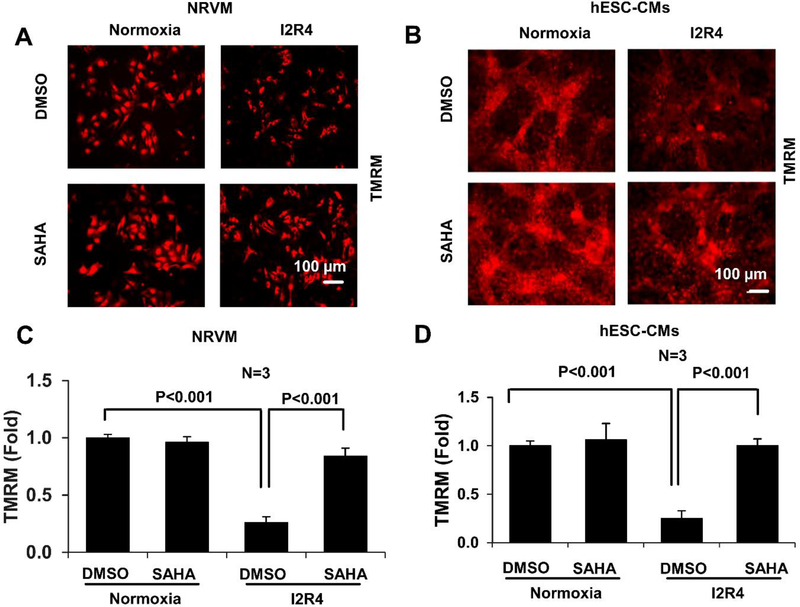
ROS generation during ischemia contributes to the loss of mitochondrial membrane potential, which plays a causal role in cardiomyocyte death and heart failure [7]. To analyze mitochondrial membrane potential, NRVMs and hESC-CMs were stained with TMRM and visualized by fluorescence microscopy. During reperfusion after ischemia, a significant decrease in TMRM fluorescence was observed in both NRVMs and hESC-CMs, which was almost completely ablated by SAHA treatment (Figure 4 A–D, Figure S7 A–B for time course). SAHA-induced preservation of mitochondrial membrane potential was also observed in AC16 cells subjected to I/R injury (Figure S7 C). In mouse hearts subjected to I/R (I 45min and R 3h), SAHA treatment at reperfusion significantly protected the function if mitochondrial complex I (most affected complexes during I/R injury [29]) and IV in the ischemic zone. SAHA also improved complex IV activity in the remote zone. There is a trend of increased complex I activity in the remote zone (Figure S8).These results indicate that SAHA treatment protects cardiomyocytes subjected to I/R injury against loss of mitochondrial membrane potential and preserve mitochondrial complex function.
Figure 4. SAHA preserves mitochondrial membrane potential in cardiomyocytes subjected to I/R injury.
Cardiomyocytes (NRVMs and hESC-CMs) subjected to simulated I/R injury and treated with DMSO or SAHA were stained with TMRM to assess mitochondrial membrane potential. Images were obtained by fluorescence microscopy and quantified using ImageJ software. A-B. Representative images of TMRM staining in NRVMs and hESC-CMs subjected to simulated I/R injury. C-D, Quantification of TMRM fluorescence intensity in NRVMs and hESC-CMs subjected to simulated I/R injury. I2R4, Ischemic 2 hours and reperfusion 4 hours.
3.4. SAHA-induced Preservation of Mitochondrial Homeostasis in Cardiomyocytes Depends on Autophagy
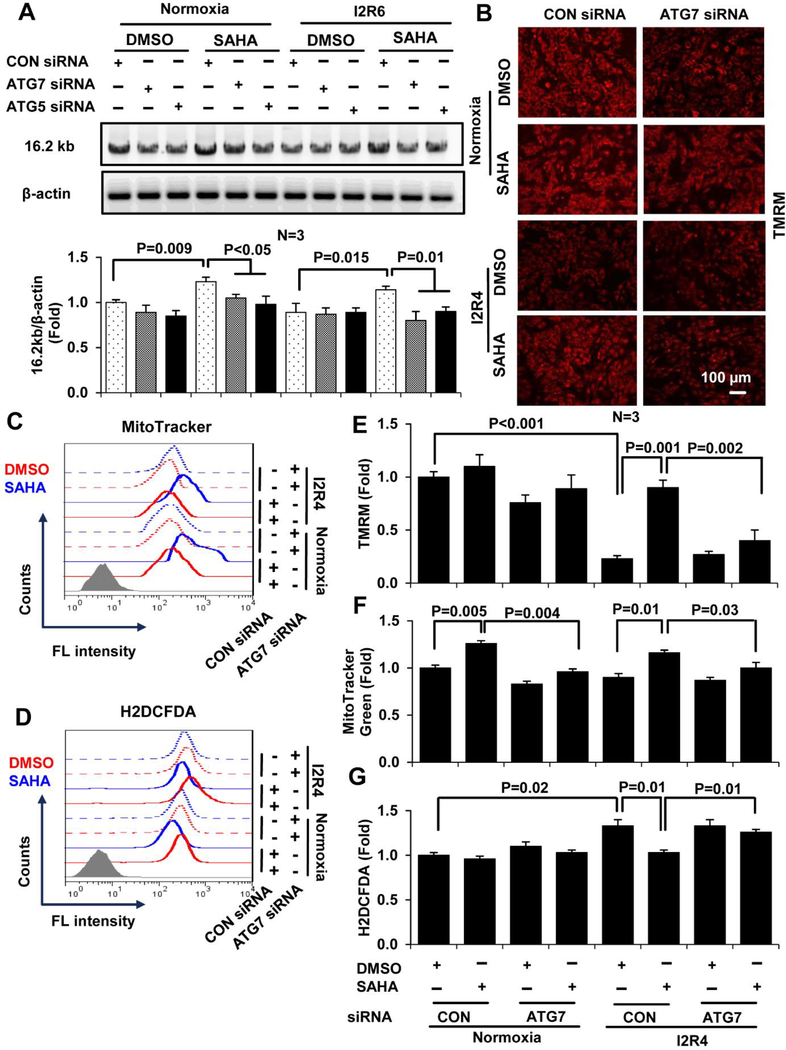
HDAC inhibition may have pleiotropic effects on cardiomyocytes during I/R injury, such as inducing autophagy [17], reducing apoptosis [30], directly modulating mitochondria function [31] and stem cell activation [32]. Our previous work has shown that SAHA blunts I/R injury in the heart through induction of autophagy, and this SAHA-reduced cell death during I/R injury is abolished by siRNA-knock down essential autophagy proteins ATG7 [17]. Since SAHA works so well and so quickly, we postulate that SAHA-induced autophagy might be the most important biological process that dictates the mitochondrial homeostasis and the survival of cardiomyocytes right after reperfusion. Thus, we next examined whether induction/maintain of basal autophagy is required for the protective effects of SAHA on mitochondria homeostasis after I/R injury. We used siRNA to knock down two essential autophagy proteins, ATG5 and ATG7, in order to block autophagic flux. Knockdown of ATG5 or ATG7 in NRVMs abolished SAHA-dependent increases in intact mtDNA during I/R injury (Figure 5 A). Consistent with this observation, ATG7 knockdown prevented SAHA-induced preservation of mitochondrial membrane potential in NRVMs subjected to I/R (Figure 5 B, images; Figure 5 E, quantification). Similarly, flow cytometry revealed that SAHA-induced increases of mitochondrial mass (Figure 5 C, flow cytometry; Figure 5 F, quantification) and decreases of ROS levels (Figure 5 D, flow cytometry; Figure 5 G, quantification) were abrogated by ATG7 knockdown. In addition, bafilomycin A has been proposed to prevent the fusion of autophagosomes with lysosomes to block the autophagic flux [33]. We used bafilomycin A to block the autophagy pharmaceutically at the time of the reperfusion to avoid the possible confounding effect of long-term treatment for the loss of ATG7. We showed that autophagic flux is indeed needed for the protection of SAHA on mtDNA (Figure S9). Together, these data indicate that the beneficial effects of SAHA on mitochondrial homeostasis in cardiomyocytes subjected to I/R injury are dependent on autophagy.
Figure 5. The protective effects of SAHA on mitochondria in cardiomyocytes depend on autophagy.
NRVMs were treated with siRNAs targeting ATG5 and ATG7 and then subjected to simulated I/R injury and treatment with DMSO or SAHA. A, Representative images and quantification of the 16.2 kb product amplified by semi-qPCR from ATG5/7 and control siRNA-treated NRVMs treated with DMSO or SAHA during I/R injury. B, Representative fluorescence microscopy images of siRNA-treated NRVMs stained with TMRM to analyze mitochondrial membrane potential. C-D, Representative histograms quantifying MitoTracker Green (mitochondrial mass) fluorescence intensity (C) and H2DCFDA (ROS) fluorescence intensity (D) in siRNA-treated NRVMs. E-G, Quantification of TMRM fluorescence intensity in ATG7 siRNA-treated NRVMs using ImageJ Software (E) and quantification of MitoTracker Green (F) and H2DCFDA fluorescence intensity by flow cytometry (G). I2R4, Ischemic 2 hours and reperfusion 4 hours.
3.5. Protective Effects of SAHA on Mitochondrial Homeostasis in Mouse Heart Depend on Autophagy
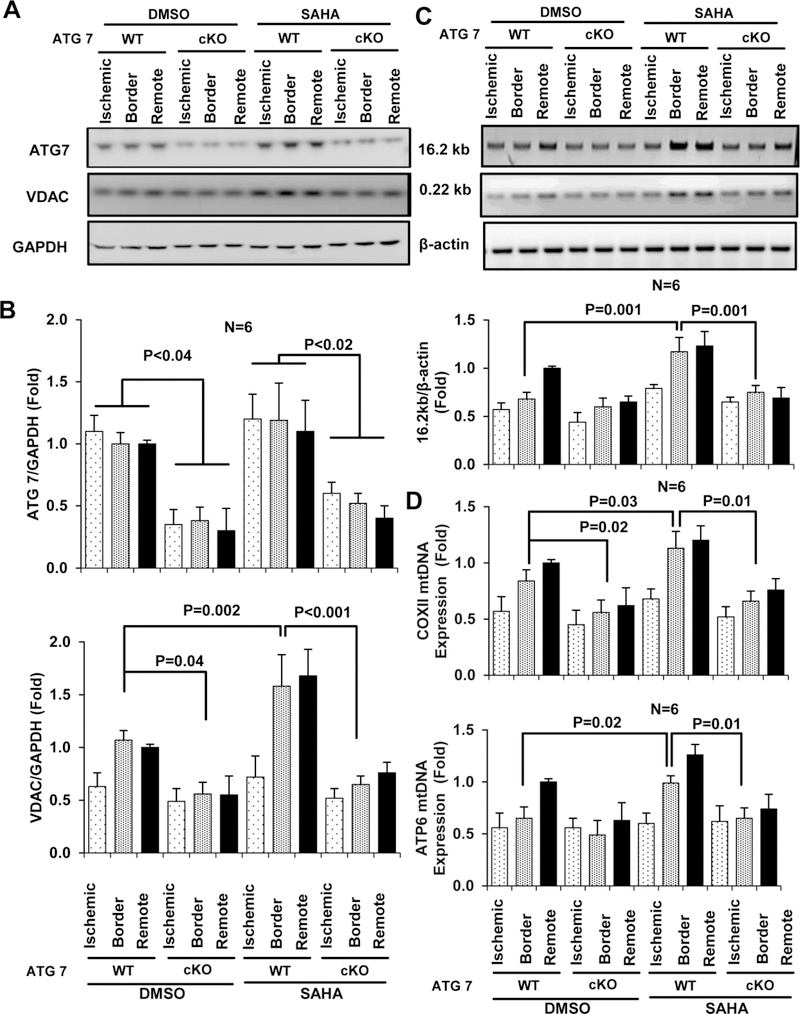
For in vivo studies, we subjected time and myocardium-specific ATG7 knockout mice (αMHC-merCremer+; Atg7f/f with tamoxifen injection, ATG7 cKO) to I/R surgery. ATG7 cKO mice were divided into control (DMSO) and SAHA reperfusion only treatment groups. ATG7 expression was successfully downregulated (approximately 70%) in mouse myocardial tissue, and residual ATG7 protein expression was most likely from non-myocytes present in the heart. After I/R injury, SAHA-induced increases in VDAC expression were abolished in ATG7 cKO mice (Figure 6 A). Similarly, EM analysis demonstrated that SAHA treatment failed to preserve mitochondrial mass in ATG7 cKO mice (Figures S10). In ATG7 cKO group mice, SAHA failed to prevent damage to intact mtDNA and maintain mtDNA content in the border zone in comparison to wild-type littermate controls, where SAHA prevented damage and preserved mtDNA content to almost normal levels (Figures 6 C–D). Thus, these results demonstrate that autophagy is required for SAHA to maintain mitochondrial homeostasis in the myocardium during I/R injury.
Figure 6. The protective effects of SAHA protective effect on mouse heart mitochondria depend on autophagy.
After cardiac I/R injury (45 min/24 hour), heart tissue was isolated from ATG7 WT (ATG7 f/f treated with Tamoxifen IP) and ATG7 cKO (αMHC-merCremer+; Atg7f/f treated with Tamoxifen IP) mice and separated by zone (ischemic, border, and remote) for DNA and protein extraction. A-B, Representative images and quantification of western blot analysis of ATG7 and VDAC expression in the three zones. C, Representative images (upper) and quantification (lower) of the 16.2 kb and 0.22 kb products amplified by semi-qPCR using samples from the three zones of heart tissue. D, mtDNA copy number analyzed by qPCR using primers specific for COXII (upper) and ATP6 (lower).
3.6. SAHA Enhances Mitochondrial Homeostasis via PGC-1α Activity
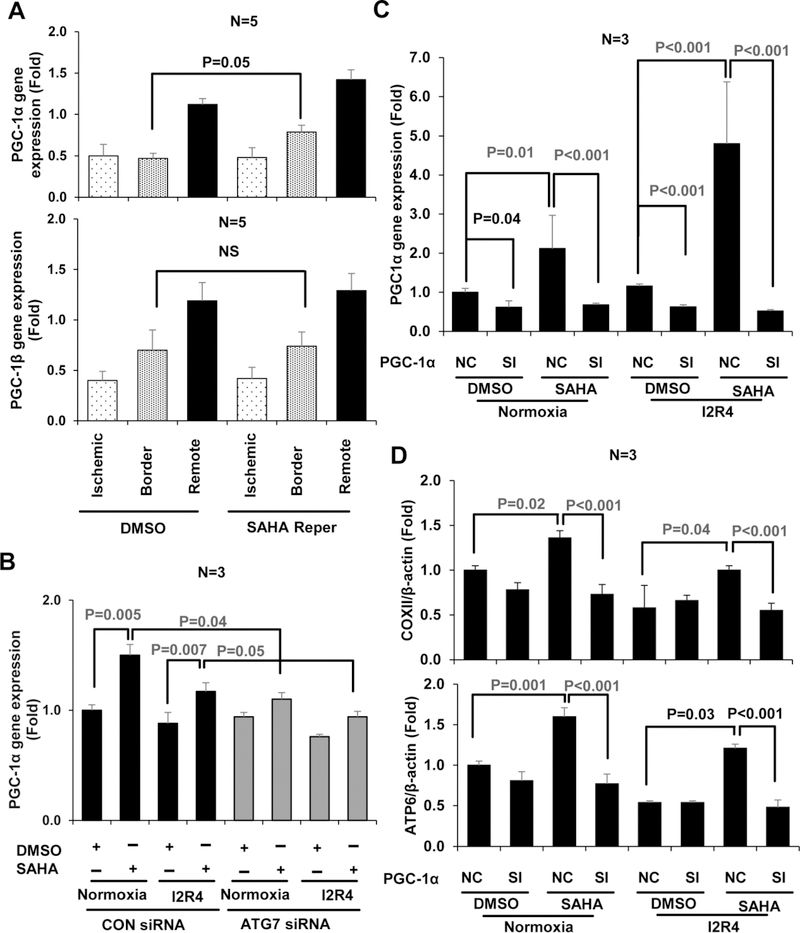
The intact mtDNA (16.2 kb) and mtDNA content (COXII and ATP6/D-Loop) were both increased by SAHA treatment, which suggests that SAHA mainly promotes mtDNA synthesis via mitochondrial biogenesis. Mitochondrial biogenesis is largely governed by PGC-1α [14]. However, it is unclear that PGC-1α plays a direct role in the cardiac protective effects of SAHA. We used qRT-PCR to detect the PGC-1α expression since there are no antibodies that can detect the endogenous PGC-1α protein reliably [34]. In our study, gene expression of PGC-1α was significantly increased by SAHA both in vitro and in vivo, (Figure 7 A, 7 B normoxia) but not PGC-1β (Figure 7 A, and S11). Moreover, the SAHA-induced PGC-1α gene expression in NRVMs was abolished by ATG7 siRNA treatment (Figure 7 B).
Figure 7. SAHA induces PGC-1α-mediated mitochondrial biogenesis in cardiomyocytes subjected to I/R injury.
At the end of I/R injury in heart and cardiomyocytes, samples were collected for RNA extraction. Heart tissue was isolated and separated into three zones (Ischemic, Border and Remote). A: PGC-1α/β mRNA expression level by real-time qRT-PCR in mouse hearts subjected to I/R injury. B: PGC-1α mRNA expression level in NRVMs treated with ATG7 siRNA and subjected to simulated I/R injury. C: Real-time qRT-PCR assay for PGC-1α mRNA relative expression in NRVM treated with adenovirus for PGC-1α knockdown during I/R injury. D: mtDNA copy number by qPCR using primers of COXII and ATP6 in NRVM treated with adenovirus hosting PGC-1α siRNA during I/R injury. SI, siRNA knockdown of PGC-1α; NC, negative control, adenoviruses hosting only GFP.
Then we investigate the direct role of PGC-1α on mtDNA in cardiomyocytes. We used adenoviruses hosting PGC-1α siRNA to achieve knockdown in NRVMs (Figure 7 C). PGC-1α siRNA reduced baseline PGC-1α expression by around 50%, and it abolished almost all of SAHA-induced PGC-1α expression. Moreover, the SAHA-increased mtDNA level (COXII and ATP6) was completely abolished by knockdown of PGC-1α (Figure 7 D). These data indicate that SAHA-induced autophagy is essential for maintaining mtDNA content during I/R, and is likely via PGC-1α-mediated mitochondrial biogenesis. There is another possibility that loss of PGC-1α affects mitochondrial biogenesis through altering autophagic flux. We found that the expressions of ATG7 and LC3 levels were not significantly changed by PGC-1α siRNA, compared with siRNA control group (Figure S12 A, B). The loss of PGC-1α did not affect autophagic flux in NRVMs (Figure S12 C,D). These data indicate that PGC-1α does not regulate autophagic flux in cardiac I/R and probably maintains mitochondrial homeostasis mainly through mitochondrial biogenesis.
4. Discussion
Myocardium reperfusion injury contributes almost half of myocardial infarct size in myocardial infarction patients, and infarct size is closely correlated with the probability of developing heart failure [1]. Despite this knowledge, myocardial reperfusion injury is largely a missed therapeutic target. Although numerous therapeutic strategies have been developed to mitigate reperfusion injury, no standard therapies are currently available [3]. Enhancing autophagy protects against I/R injury in cardiac myocytes in vitro and in vivo [10]. Furthermore, a recent report shows that cardiomyocyte-specific disruption of autophagy by conditional knockout of ATG7, an essential autophagy-related gene, leads to severe myocardial dysfunction during I/R injury [35]. Mitochondrial dysfunction has also been shown to play a significant role in inducing myocardial apoptosis during I/R injury [5]. However, it is unclear whether autophagy plays a protective role on mitochondrial homeostasis in cardiomyocytes during I/R injury. Administration of SAHA (HDAC inhibitor) during reperfusion reduces myocardial infarct size through maintaining autophagic flux in a large animal model [17]. Therefore, using SAHA and ATG loss of function as tools, we investigated the role of autophagy in mitochondrial homeostasis in cardiomyocytes during I/R injury.
HDAC Inhibition Reduces ROS Production and Increases mtDNA Levels.
Mitochondria regulate a myriad of cellular and metabolic processes in addition to serving as the primary site of ATP production. Oxidative stress arising from the mitochondria during myocardial I/R promotes cardiomyocyte apoptosis [36]. In fact, I/R-induced cell death is closely associated with known indicators of mitochondrial dysfunction, including loss of mitochondrial electron transport chain complex activity, decreased ATP production, mPTP opening, increased ROS production, and mitochondrial membrane permeability loss [5]. In our study in vivo, mitochondrial complex I and IV activities in ischemic zones were protected by SAHA (Figure S8). Our results in vitro also show that reperfusion (within 6 hours) after ischemia leads to an increase in ROS levels, reduction of mitochondrial membrane permeability in cardiomyocytes, and induction of cell death. These results are similar to those previously observed in hepatocytes and other cell types [37, 38]. The mechanisms underlying reduced ROS production in SAHA-treated cells are not clear. HDAC1 (class I HDACs), which is located in cardiomyocyte mitochondria, has been recently reported that HDAC1 activity regulates metabolic ROS production and contributes to I/R injury in isolated rat hearts and cardiac myocytes within one hour of reperfusion [31]. SAHA is a class I/II HDAC inhibitor; it is possible that SAHA solely affects directly on mitochondria to alleviate I/R injury in the heart. However, decreased ROS levels may be also due to the clearance of damaged mitochondria by autophagy since the loss of ATG7 abolished these effects in SAHA-treated cells. It will be interesting to see whether blocking mitochondrial HADC1 induces autophagy and mitochondrial biogenesis in a whole animal model and cardiomyocytes [31].
Although ROS serve as an important signal for normal cellular function, excess intracellular ROS cause mitochondrial DNA injury [36]. The mitochondrial genome consists of a short, double-stranded, circular DNA molecule (16.5 kb) that encodes 13 proteins comprising part of the machinery of the electron transport system located in the mitochondrial inner membrane [25]. Therefore, damage to mtDNA can induce mitochondrial dysfunction and decreases in oxidative phosphorylation. Consistent with this model, aging and tobacco use have been shown to damage cellular mtDNA and cause mitochondrial dysfunction [25]. Our results suggest that mtDNA damage occurs during I/R injury, which is in agreement with the excessive cellular ROS production observed in cardiomyocytes subjected to I/R in vitro. However, there is only a trend of reduced mtDNA in cardiomyocytes after 6 hours of reperfusion, but significant reduction after 24hours of reperfusion (Figure S11), which indicate that decreased of mtDNA might be a later event compared with the loss of MMP and increase of ROS. Prior to this study, the effects of HDAC inhibitors on nuclear DNA have been well investigated, but little was known about its effects on mtDNA [39]. More importantly, we observed that levels of intact and total mtDNA were increased by SAHA treatment in cardiomyocytes subjected to I/R injury, which indicating the mitochondrial biogenesis is upregulated by SAHA.
HDAC Inhibition and Mitochondrial Homeostasis in Different Cell Types
HDACs inhibition plays an important role in inducing tumor cell apoptosis through transcriptional regulation. The HDAC inhibitor suberic bishydroxamate (SBHA) induces apoptosis in melanoma cells by promoting changes in mitochondrial membrane permeability [40], and the HDAC inhibitors MS-275 and SAHA promote mitochondria-dependent apoptosis in human leukemia cells through induction of ROS generation [41, 42]. These data indicate that HDAC inhibition kills cancer cells. In contrast, in cardiac tissue, HDAC inhibitors have been shown to suppress cardiac hypertrophy and I/R injury and protect against cell death [17, 43]. Furthermore, inhibitors of Class I HDACs enhance mitochondrial biogenesis and oxidative metabolism in skeletal muscle and adipose tissue [44]. Collectively, these results suggest that responses to HDAC inhibition differ between actively proliferating cancer cells and quiescent cardiomyocytes. In our study, we show that SAHA treatment prevented I/R-induced reductions in total mtDNA (COXII, D-Loop and ATP6) and increased mitochondrial mass. Our findings suggest that SAHA treatment improves not only mitochondrial function, but also enhances mitochondrial biogenesis during cardiac I/R injury. Because there are no histones in the mitochondria, the interaction between HDAC inhibitors and mitochondrial homeostasis are likely mediated through indirect effects on autophagy and mitochondrial biogenesis.
Mitochondrial Homeostasis is Dependent on Autophagy at Baseline and during I/R
To protect against stress, cells have developed quality control mechanisms to maintain the overall health of mitochondria, including fusion, fission, mitochondrial autophagy, and mitochondrial biogenesis [45]. Autophagy in T lymphocytes shows an essential role in the mitochondrial clearance [11]. Stimulation of autophagy induces mitochondrial biogenesis, mitochondrial remodeling and activity in skeletal muscle [12, 46]. Moreover, the mitochondria-specific type of autophagy, mitophagy, removes damaged mitochondria which are toxic to cells and cause immune responses [5]. On the other hand, the lack of autophagy leads to mitochondrial dysfunction [47]. Under the condition without I/R injury, it is reported that muscle-specific deletion of ATG7 resulted in the accumulation of abnormal mitochondria and defection of mitochondrial respiration [48, 49]. Atg7 knockout erythrocytes accumulate damaged mitochondria with altered membrane potential which leads to cell death [50]. All these finds indicate that autophagy plays the basic role for mitochondrial homeostasis. Enhancing autophagy also protects cardiac myocytes from I/R injury in vitro and in vivo [10]. Thus, autophagy-dependent mitochondrial homeostasis is emerging as an important mediator of responses to I/R injury.
In our study, knockdown of ATG7 in cardiomyocytes abolished SAHA’s protective effects during I/R injury (including reductions in ROS levels; increases in intact/total mtDNA content; and preservation of mitochondrial mass). We then tested the cardioprotective effects of SAHA in time- and cardiomyocyte-specific ATG7 knockouts. As expected, this ATG7 cKO reduced mtDNA and mitochondrial mass at baseline, indicating that autophagy is needed to maintain a healthy mitochondria population. These observations are consistent with the heart failure phenotype observed in ATG5 and ATG7 acute cardiomyocyte knockouts generated using tamoxifen and αMHC-merCremer [51]. However, these results also complicate the interpretation of the in vivo data on the cardioprotection of SAHA in cardiac ATG7 cKO mice, which may be too sick to respond to the cardioprotective effects of SAHA. ATG7 knockdown had little effect on mitochondria at baseline in NRVMs, but still abolished SAHA-induced mitochondrial protection, suggesting that the protective effects of SAHA are dependent on ATG7. Furthermore, we have shown that block autophagic flux by adding bafilomycin A in the culture media at reperfusion abolished the mtDNA increase induced by SAHA (Figure S9). These results indicate that the protective effects of SAHA on mitochondria are depended on autophagy, and suggest that the removal of damaged mitochondria by autophagy may be essential for mitochondrial biogenesis.
Additional Mechanisms that May Contribute to HDAC Inhibition-induced Autophagy and Mitochondrial Homeostasis
Mechanistic target of rapamycin (mTOR) and AMPK pathway have critical roles in regulating growth and reprogramming metabolism, such as mitochondrial biogenesis and autophagy [52–54]. HDAC inhibitors-induced apoptosis and autophagy through the FOXO1-mTOR signaling and transcriptional regulation of autophagy genes in human cancer cells [55]. And HDAC inhibitors blunt cardiac hypertrophy also through mTOR dependent pathways [56]. These results suggest that combining modulating mTOR activity and HDAC inhibitors is a potential strategy to heart disease.
The relationship between autophagy and mitochondrial homeostasis in different cell types is unclear. ATG3-dependent autophagy mediates mitochondrial homeostasis in stem cells.[23] ATG12, the autophagy ubiquitin-like modifier, is conjugated to ATG3 to reduce mitochondrial mass and induces cell death in mammalian cells [57]. In addition to autophagy, mitochondrial homeostasis is controlled by PGC-1α-mediated mitochondrial biogenesis and mitochondrial dynamics (fission and fusion) [14]. In skeletal muscle, PGC-1α promotes exercise-induced autophagy and denervation-induced mitophagy [58]. In neurons, mitochondrial density was increased in PGC-1α/β overexpression through autophagy [59]. In our study, the expression of PGC-1α was significantly increased by SAHA both in vitro and in vivo. These results suggest that SAHA may play an important role in the induction of PGC-1α-mediated mitochondrial biogenesis. Indeed, stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide has been shown to protect cardiomyocytes from programmed cell death [24]. However, mitochondria number is not increased even when only PGC-1α is induced by I/R injury in the kidney and brain [60, 61]. Interestingly, we found that the SAHA-induced PGC-1α gene expression was dependent on autophagy gene ATG7, which indicates that autophagy is an essential process that maintains PGC-1α-mediated mitochondrial biogenesis. This is consistent with prior study showing that mitochondrial dysfunction in cardiac-specific ATG3 deficient mice was accompanied with mitochondrial content loss and reduced PGC1α expression [62]. Thus, autophagy/mitophagy is essential for generating signals that activate mitochondrial biogenesis during I/R for maintaining mitochondrial homeostasis.
In addition, the most important mitochondrial fission protein is Drp1, which mediates mitochondrial autophagy and plays a protective role against pressure-overload-induced mitochondrial dysfunction and heart failure [63]. Disruption of Drp1 has been shown to reduce mitochondrial autophagy and exacerbate the development of mitochondrial dysfunction in cardiomyocytes [64]. Our results demonstrate that SAHA treatment increases mitochondrial mass and maintains mitochondrial membrane potential, ultimately resulting in cardiomyocyte survival after I/R. These beneficial effects are dependent on the essential autophagy gene ATG7, but it will be interesting to investigate whether SAHA also upregulates mitophagy or mitochondrial fission/fusion in our future study.
HDAC inhibition has a broad effect on cellular function; it likely induces a full array of protective effects during the reperfusion injury [15, 17, 31, 65–67]. More importantly, it was shown recently that by only inhibition of mitochondrial HDAC1 reduced the infarct size in isolated rat hearts one hour after reperfusion [31]. Whether this mitochondrial HDAC1 inhibition will translate into long-term infarct reduction or in a whole animal model is still unknown. Furthermore, whether SAHA and MS-275 have the same effects is also not clear. It will be interesting to see whether blocking mitochondrial HADC1 induces autophagy and mitochondrial biogenesis in a whole animal model [31]. SAHA is the only FDA approved cancer medication that is shown to reduce infarct size in a large animal model when given at reperfusion [17]. Understanding the mechanisms of its action is important and may lead to new therapeutic targets.
Conclusion and perspective
Results from our study show that the FDA-approved HDAC inhibitor SAHA enhances autophagy-dependent mitochondrial homeostasis, which are essential for reducing cardiac I/R injury. The beneficial effects of SAHA involve clearing damaged mitochondria and stimulating mitochondrial biogenesis, which provides new therapeutic targets for mitigating reperfusion injury while avoiding the potential toxic effects of non-selective HDAC inhibition.
Supplementary Material
Acknowledgments
We would like to thank Dr. Joseph Hill for providing reagents for analysis of autophagy.
Sources of Funding
This work was supported by a grant from the National Institutes of Health (K08HL127305).
Footnotes
Competing interests
The authors report no commercial or proprietary interest in any product or concept discussed in this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Yellon DM, Hausenloy DJ, Myocardial reperfusion injury, N Engl J Med 357(11) (2007) 1121–35. [DOI] [PubMed] [Google Scholar]
- [2].Turer AT, Hill JA, Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy, Am J Cardiol 106(3) (2010) 360–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Longacre L. Schwartz, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping P, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM, Heart L. National, N.I.o.H. Blood Institute, New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop, Circulation 124(10) (2011) 1172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lee MS, Role of mitochondrial function in cell death and body metabolism, Front Biosci (Landmark Ed) 21 (2016) 1233–44. [DOI] [PubMed] [Google Scholar]
- [5].Shires SE, Gustafsson AB, Mitophagy and heart failure, J Mol Med (Berl) 93(3) (2015) 253–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Murphy E, Steenbergen C, Mechanisms underlying acute protection from cardiac ischemia-reperfusion injury, Physiol Rev 88(2) (2008) 581–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nazari A, Sadr SS, Faghihi M, Azizi Y, Hosseini MJ, Mobarra N, Tavakoli A, Imani A, Vasopressin attenuates ischemia-reperfusion injury via reduction of oxidative stress and inhibition of mitochondrial permeability transition pore opening in rat hearts, Eur J Pharmacol 760 (2015) 96–102. [DOI] [PubMed] [Google Scholar]
- [8].Saito T, Sadoshima J, Molecular mechanisms of mitochondrial autophagy/mitophagy in the heart, Circ Res 116(8) (2015) 1477–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Scarffe LA, Stevens DA, Dawson VL, Dawson TM, Parkin and PINK1: much more than mitophagy, Trends Neurosci 37(6) (2014) 315–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zheng Y, Li X, Wang J, Tan J, Zhang C, Yang H, Berbamine Postconditioning Protects the Heart From Ischemia/Reperfusion Injury Through the Regulation of Autophagy During Reperfusion, Circulation Research 117(Suppl 1) (2015) A257–A257. [Google Scholar]
- [11].Pua HH, Guo J, Komatsu M, He YW, Autophagy is essential for mitochondrial clearance in mature T lymphocytes, J Immunol 182(7) (2009) 4046–55. [DOI] [PubMed] [Google Scholar]
- [12].Lesmana R, Sinha RA, Singh BK, Zhou J, Ohba K, Wu Y, Yau WW, Bay BH, Yen PM, Thyroid Hormone Stimulation of Autophagy Is Essential for Mitochondrial Biogenesis and Activity in Skeletal Muscle, Endocrinology 157(1) (2016) 23–38. [DOI] [PubMed] [Google Scholar]
- [13].Besteiro S, Brooks CF, Striepen B, Dubremetz JF, Autophagy protein Atg3 is essential for maintaining mitochondrial integrity and for normal intracellular development of Toxoplasma gondii tachyzoites, PLoS Pathog 7(12) (2011) e1002416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Xu X, Duan S, Yi F, Ocampo A, Liu G-H, Belmonte JCI, Mitochondrial regulation in pluripotent stem cells, Cell metabolism 18(3) (2013) 325–332. [DOI] [PubMed] [Google Scholar]
- [15].Granger A, Abdullah I, Huebner F, Stout A, Wang T, Huebner T, Epstein JA, Gruber PJ, Histone deacetylase inhibition reduces myocardial ischemia-reperfusion injury in mice, FASEB J 22(10) (2008) 3549–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cao DJ, Wang ZV, Battiprolu PK, Jiang N, Morales CR, Kong Y, Rothermel BA, Gillette TG, Hill JA, Histone deacetylase (HDAC) inhibitors attenuate cardiac hypertrophy by suppressing autophagy, Proc Natl Acad Sci U S A 108(10) (2011) 4123–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xie M, Kong Y, Tan W, May H, Battiprolu PK, Pedrozo Z, Wang ZV, Morales C, Luo X, Cho G, Jiang N, Jessen ME, Warner JJ, Lavandero S, Gillette TG, Turer AT, Hill JA, Histone deacetylase inhibition blunts ischemia/reperfusion injury by inducing cardiomyocyte autophagy, Circulation 129(10) (2014) 1139–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S, Discovery of Atg5/Atg7-independent alternative macroautophagy, Nature 461(7264) (2009) 654–8. [DOI] [PubMed] [Google Scholar]
- [19].Sohal DS, Nghiem M, Crackower MA, Witt SA, Kimball TR, Tymitz KM, Penninger JM, Molkentin JD, Temporally regulated and tissue-specific gene manipulations in the adult and embryonic heart using a tamoxifen-inducible Cre protein, Circ Res 89(1) (2001) 20–5. [DOI] [PubMed] [Google Scholar]
- [20].Louch WE, Sheehan KA, Wolska BM, Methods in cardiomyocyte isolation, culture, and gene transfer, J Mol Cell Cardiol 51(3) (2011) 288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Zhang L, Guo J, Zhang P, Xiong Q, Wu SC, Xia L, Roy SS, Tolar J, O’Connell TD, Kyba M, Liao K, Zhang J, Derivation and high engraftment of patient-specific cardiomyocyte sheet using induced pluripotent stem cells generated from adult cardiac fibroblast, Circ Heart Fail 8(1) (2015) 156–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Thom R, Rowe GC, Jang C, Safdar A, Arany Z, Truncated PGC-‐1α and angiogenesis in skeletal muscle, (2014). [Google Scholar]
- [23].Liu K, Zhao Q, Liu P, Cao J, Gong J, Wang C, Wang W, Li X, Sun H, Zhang C, Li Y, Jiang M, Zhu S, Sun Q, Jiao J, Hu B, Zhao X, Li W, Chen Q, Zhou Q, Zhao T, ATG3-dependent autophagy mediates mitochondrial homeostasis in pluripotency acquirement and maintenance, Autophagy 12(11) (2016) 2000–2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hickson-Bick DL, Jones C, Buja LM, Stimulation of mitochondrial biogenesis and autophagy by lipopolysaccharide in the neonatal rat cardiomyocyte protects against programmed cell death, Journal of molecular and cellular cardiology 44(2) (2008) 411–418. [DOI] [PubMed] [Google Scholar]
- [25].Ballinger SW, Patterson C, Knight-Lozano CA, Burow DL, Conklin CA, Hu Z, Reuf J, Horaist C, Lebovitz R, Hunter GC, McIntyre K, Runge MS, Mitochondrial integrity and function in atherogenesis, Circulation 106(5) (2002) 544–9. [DOI] [PubMed] [Google Scholar]
- [26].Braun M, Hettinger N, Koentges C, Pfeil K, Cimolai MC, Hoffmann MM, Osterholt M, Doenst T, Bode C, Bugger H, Myocardial mitochondrial and contractile function are preserved in mice lacking adiponectin, PLoS One 10(3) (2015) e0119416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lesnefsky EJ, Moghaddas S, Tandler B, Kerner J, Hoppel CL, Mitochondrial dysfunction in cardiac disease: ischemia--reperfusion, aging, and heart failure, J Mol Cell Cardiol 33(6) (2001) 1065–89. [DOI] [PubMed] [Google Scholar]
- [28].Hendgen-Cotta UB, Merx MW, Shiva S, Schmitz J, Becher S, Klare JP, Steinhoff HJ, Goedecke A, Schrader J, Gladwin MT, Kelm M, Rassaf T, Nitrite reductase activity of myoglobin regulates respiration and cellular viability in myocardial ischemia-reperfusion injury, Proc Natl Acad Sci U S A 105(29) (2008) 10256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gorenkova N, Robinson E, Grieve DJ, Galkin A, Conformational change of mitochondrial complex I increases ROS sensitivity during ischemia, Antioxidants & redox signaling 19(13) (2013) 1459–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Chen M, Liu Q, Chen L, Zhang L, Gu E, Remifentanil postconditioning ameliorates histone H3 acetylation modification in H9c2 cardiomyoblasts after hypoxia/reoxygenation via attenuating endopl asmic reticulum stress, Apoptosis 22(5) (2017) 662–671. [DOI] [PubMed] [Google Scholar]
- [31].Herr DJ, Baarine M, Aune SE, Li X, Ball LE, Lemasters JJ, Beeson CC, Chou JC, Menick DR, HDAC1 localizes to the mitochondria of cardiac myocytes and contributes to early cardiac reperfusion injury, J Mol Cell Cardiol 114 (2018) 309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang L, Chen B, Zhao Y, Dubielecka PM, Wei L, Qin GJ, Chin YE, Wang Y, Zhao TC, Inhibition of histone deacetylase-induced myocardial repair is mediated by c-kit in infarcted hearts, The Journal of biological chemistry 287(47) (2012) 39338–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Shacka JJ, Klocke BJ, Shibata M, Uchiyama Y, Datta G, Schmidt RE, Roth KA, Bafilomycin A1 inhibits chloroquine-induced death of cerebellar granule neurons, Mol Pharmacol 69(4) (2006) 1125–36. [DOI] [PubMed] [Google Scholar]
- [34].Rowe GC, El-Khoury R, Patten IS, Rustin P, Arany Z, PGC-1α is dispensable for exercise-induced mitochondrial biogenesis in skeletal muscle, PloS one 7(7) (2012) e41817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Li S, Liu C, Gu L, Wang L, Shang Y, Liu Q, Wan J, Shi J, Wang F, Xu Z, Autophagy protects cardiomyocytes from the myocardial ischaemia-reperfusion injury through the clearance of CLP36, Open biology 6(8) (2016) 160177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Bliksoen M, Baysa A, Eide L, Bjoras M, Suganthan R, Vaage J, Stenslokken KO, Valen G, Mitochondrial DNA damage and repair during ischemia-reperfusion injury of the heart, J Mol Cell Cardiol 78 (2015) 9–22. [DOI] [PubMed] [Google Scholar]
- [37].Zhang J, Nadtochiy SM, Urciuoli WR, Brookes PS, The cardioprotective compound cloxyquin uncouples mitochondria and induces autophagy, Am J Physiol Heart Circ Physiol 310(1) (2016) H29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Serracino-Inglott F, Habib NA, Mathie RT, Hepatic ischemia-reperfusion injury, Am J Surg 181(2) (2001) 160–6. [DOI] [PubMed] [Google Scholar]
- [39].Chen H, Dzitoyeva S, Manev H, Effect of valproic acid on mitochondrial epigenetics, Eur J Pharmacol 690(1–3) (2012) 51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Zhang XD, Gillespie SK, Borrow JM, Hersey P, The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells, Mol Cancer Ther 3(4) (2004) 425–35. [PubMed] [Google Scholar]
- [41].Nebbioso A, Clarke N, Voltz E, Germain E, Ambrosino C, Bontempo P, Alvarez R, Schiavone EM, Ferrara F, Bresciani F, Weisz A, de Lera AR, Gronemeyer H, Altucci L, Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells, Nat Med 11(1) (2005) 77–84. [DOI] [PubMed] [Google Scholar]
- [42].Carew JS, Nawrocki ST, Kahue CN, Zhang H, Yang C, Chung L, Houghton JA, Huang P, Giles FJ, Cleveland JL, Targeting autophagy augments the anticancer activity of the histone deacetylase inhibitor SAHA to overcome Bcr-Abl-mediated drug resistance, Blood 110(1) (2007) 313–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Antos CL, McKinsey TA, Dreitz M, Hollingsworth LM, Zhang CL, Schreiber K, Rindt H, Gorczynski RJ, Olson EN, Dose-dependent blockade to cardiomyocyte hypertrophy by histone deacetylase inhibitors, J Biol Chem 278(31) (2003) 28930–7. [DOI] [PubMed] [Google Scholar]
- [44].Galmozzi A, Mitro N, Ferrari A, Gers E, Gilardi F, Godio C, Cermenati G, Gualerzi A, Donetti E, Rotili D, Valente S, Guerrini U, Caruso D, Mai A, Saez E, De Fabiani E, Crestani M, Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue, Diabetes 62(3) (2013) 732–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tong M, Sadoshima J, Mitochondrial autophagy in cardiomyopathy, Curr Opin Genet Dev 38 (2016) 8–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Call JA, Wilson RJ, Laker RC, Zhang M, Kundu M, Yan Z, Ulk1-mediated autophagy plays an essential role in mitochondrial remodeling and functional regeneration of skeletal muscle, Am J Physiol Cell Physiol 312(6) (2017) C724–C732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Williams RA, Smith TK, Cull B, Mottram JC, Coombs GH, ATG5 is essential for ATG8-dependent autophagy and mitochondrial homeostasis in Leishmania major, PLoS Pathog 8(5) (2012) e1002695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Masiero E, Agatea L, Mammucari C, Blaauw B, Loro E, Komatsu M, Metzger D, Reggiani C, Schiaffino S, Sandri M, Autophagy is required to maintain muscle mass, Cell metabolism 10(6) (2009) 507–15. [DOI] [PubMed] [Google Scholar]
- [49].Wu JJ, Quijano C, Chen E, Liu H, Cao L, Fergusson MM, Rovira II, Gutkind S, Daniels MP, Komatsu M, Finkel T, Mitochondrial dysfunction and oxidative stress mediate the physiological impairment induced by the disruption of autophagy, Aging (Albany NY) 1(4) (2009) 425–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mortensen M, Ferguson DJ, Edelmann M, Kessler B, Morten KJ, Komatsu M, Simon AK, Loss of autophagy in erythroid cells leads to defective removal of mitochondria and severe anemia in vivo, Proceedings of the National Academy of Sciences of the United States of America 107(2) (2010) 832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nakai A, Yamaguchi O, Takeda T, Higuchi Y, Hikoso S, Taniike M, Omiya S, Mizote I, Matsumura Y, Asahi M, Nishida K, Hori M, Mizushima N, Otsu K, The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress, Nat Med 13(5) (2007) 619–24. [DOI] [PubMed] [Google Scholar]
- [52].Scarpulla RC, Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network, Biochimica et biophysica acta 1813(7) (2011) 1269–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Mihaylova MM, Shaw RJ, The AMPK signalling pathway coordinates cell growth, autophagy and metabolism, Nat Cell Biol 13(9) (2011) 1016–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kim J, Kundu M, Viollet B, Guan KL, AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1, Nat Cell Biol 13(2) (2011) 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang J, Ng S, Wang J, Zhou J, Tan SH, Yang N, Lin Q, Xia D, Shen HM, Histone deacetylase inhibitors induce autophagy through FOXO1-dependent pathways, Autophagy 11(4) (2015) 629–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Morales CR, Li DL, Pedrozo Z, May HI, Jiang N, Kyrychenko V, Cho GW, Kim SY, Wang ZV, Rotter D, Rothermel BA, Schneider JW, Lavandero S, Gillette TG, Hill JA, Inhibition of class I histone deacetylases blunts cardiac hypertrophy through TSC2-dependent mTOR repression, Science signaling 9(422) (2016) ra34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Radoshevich L, Murrow L, Chen N, Fernandez E, Roy S, Fung C, Debnath J, ATG12 conjugation to ATG3 regulates mitochondrial homeostasis and cell death, Cell 142(4) (2010) 590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Halling JF, Ringholm S, Nielsen MM, Overby P, Pilegaard H, PGC‐1α promotes exercise‐induced autophagy in mouse skeletal muscle, Physiological reports 4(3) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J, Kuum M, Kaasik A, PGC-1α and PGC-1β regulate mitochondrial density in neurons, Journal of biological chemistry 284(32) (2009) 21379–21385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Chen SD, Yang DI, Lin TK, Shaw FZ, Liou CW, Chuang YC, Roles of oxidative stress, apoptosis, PGC-1alpha and mitochondrial biogenesis in cerebral ischemia, Int J Mol Sci 12(10) (2011) 7199–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Funk JA, Schnellmann RG, Accelerated recovery of renal mitochondrial and tubule homeostasis with SIRT1/PGC-1alpha activation following ischemia-reperfusion injury, Toxicol Appl Pharmacol 273(2) (2013) 345–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Li Z, Zhang Q, Pires K, Abel ED, Autophagy is Required for Mitochondrial Biogenesis in the Heart, Am Heart Assoc, 2014. [Google Scholar]
- [63].Shirakabe A, Zhai P, Ikeda Y, Saito T, Maejima Y, Hsu CP, Nomura M, Egashira K, Levine B, Sadoshima J, Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure, Circulation 133(13) (2016) 1249–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, Sadoshima J, Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress, Circ Res 116(2) (2015) 264–78. [DOI] [PubMed] [Google Scholar]
- [65].Zhao TC, Cheng G, Zhang LX, Tseng YT, Padbury JF, Inhibition of histone deacetylases triggers pharmacologic preconditioning effects against myocardial ischemic injury, Cardiovascular res earch 76(3) (2007) 473–81. [DOI] [PubMed] [Google Scholar]
- [66].Aune SE, Herr DJ, Mani SK, Menick DR, Selective inhibition of class I but not class IIb histone deacetylases exerts cardiac protection from ischemia reperfusion, J Mol Cell Cardiol 72 (2014) 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Zhang L, Wang H, Zhao Y, Wang J, Dubielecka PM, Zhuang S, Qin G, Chin YE, Kao RL, Zhao TC, Myocyte-specific overexpressing HDAC4 promotes myocardial ischemia/reperfusion injury, Mol Med 24(1) (2018) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.