Abstract
Mood disorders, including major depression, bipolar disorder, and seasonal affective disorder, are debilitating disorders that affect a significant portion of the global population. Individuals suffering from mood disorders often show significant disturbances in circadian rhythms and sleep. Moreover, environmental disruptions to circadian rhythms can precipitate or exacerbate mood symptoms in vulnerable individuals. Circadian clocks exist throughout the central nervous system and periphery, where they regulate a wide variety of physiological processes implicated in mood regulation. These processes include monoaminergic and glutamatergic transmission, hypothalamic-pituitary-adrenal axis function, metabolism, and immune function. While there seems to be a clear link between circadian rhythm disruption and mood regulation, the mechanisms that underlie this association remain unclear. This review will touch on the interactions between the circadian system and each of these processes and discuss their potential role in the development of mood disorders. While clinical studies are presented, much of the review will focus on studies in animal models, which are attempting to elucidate the molecular and cellular mechanisms in which circadian genes regulate mood.
Keywords: bipolar disorder, circadian, depression, inflammation, metabolism, microbiome, stress
1 |. INTRODUCTION
Mood disorders, such as major depression, bipolar disorder, and seasonal affective disorder, are highly prevalent and debilitating disorders. Circadian rhythm and sleep disturbances are one of the major diagnostic criteria for these disorders. Individuals with mood disorders display altered rhythms in activity, sleep/wake, blood pressure, and hormone secretion (McClung, 2007). Furthermore, many drugs used for the treatment of mood disorders shift or stabilize circadian rhythms, which may be important for their therapeutic efficacy (McClung, 2011). According to the “social zeitgeber theory” of mood disorders, stressful life events disrupt social routines that may lead to altered biological rhythms and increased vulnerability for the development of mood disorders (Ehlers, Frank, & Kupfer, 1988). In support of this theory, circadian rhythm disturbances, such as jet lag or shift work, have been shown to precipitate or exacerbate mood symptoms (Asaoka et al., 2013; Inder, Crowe, & Porter, 2016; Kalmbach, Pillai, Cheng, Arnedt, & Drake, 2015). Several human genetic studies have implicated circadian genes in mood disorders including genome-wide association studies (GWAS), which take into account the entire clock gene network (Etain, Milhiet, Bellivier, & Leboyer, 2011; McCarthy & Welsh, 2012). Reduced amplitude in circadian gene expression is found in fibroblast cultures taken from subjects with bipolar disorder compared to controls (Yang, Van Dongen, Wang, Berrettini, & Bucan, 2009). Moreover, a study in human postmortem brain found that subjects with major depressive disorder (MDD) have much weaker 24-hr rhythms in gene expression compared to healthy controls in a number of mood-associated brain regions, including the dorsolateral prefrontal cortex, hippocampus, nucleus accumbens, and amygdala (Li et al., 2013). These changes suggest shifts in peak timing and disruptions in phase relationships in clock-regulated genes across multiple regions of the brain. Thus, it has been hypothesized that abnormalities in the circadian system may play a role in the development and maintenance of mood disorders.
The master circadian clock resides in the suprachias- matic nucleus (SCN) of the hypothalamus and coordinates rhythms throughout the brain and the periphery. The SCN is composed of self-sustaining oscillators that are entrained to the external environment through both photic and non- photic cues, also known as zeitgebers. Circadian rhythms in individual cells throughout the SCN and the rest of the body are generated by a molecular clock composed of multiple transcriptional-translational feedback loops. Within the core molecular clock, the transcription factor Circadian Locomotor Output Cycles Kaput (CLOCK) or the homologous protein Neuronal PAS Domain Protein 2 (NPAS2) heterodimerize with the transcription factor Brain and Muscle Arnt-like Protein 1 (BMAL1) and bind to enhancer box (E-box) sequences to activate the transcription of Period (Per1, Per2, and Per3) and Cryptochrome (Cry1 and Cry2) genes. PER and CRY are translated in the cytoplasm, where they dimerize and shuttle back into the nucleus to inhibit their own transcription, forming a negative feedback loop that cycles every 24 hr. CLOCK/BMAL1 also regulate the expression of the nuclear hormone receptors, Rev-erbα and Rorα, which repress or activate Bmal1 transcription, respectively, to form an auxiliary feedback loop that stabilizes the core loop.
Circadian rhythms in the brain are incredibly important for maximizing energy efficiency and neuronal health. The brain requires a large amount of energy to function on a daily basis. In fact, the brain uses approximately 20% of the energy of the entire body and it needs to do so in an efficient manner (Du et al., 2008). During wake, neurons are actively firing in response to activity and environmental stimuli, creating the buildup of reactive oxygen species (ROS) and other by-products (Albrecht & Ripperger, 2018; Musiek & Holtzman, 2016). During sleep, antioxidants remove excess ROS and misfolded and aggregated proteins are cleared via a glymphatic system (Musiek et al., 2013; Xie et al., 2013). Disruptions to the timing and duration of sleep and wake will disrupt these processes. In fact, a recent study found that even one night of sleep deprivation can result in accumulation of amyloid-β (one of the proteins that accumulates in the brain in Alzheimer’s disease) in human brain (Shokri-Kojori et al., 2018). Moreover, the antiphasic circadian activity patterns between astrocyte support cells (which are involved in antioxidant responses and provide an energy supply to neurons) and neurons, is key to optimal neuronal function. In the rodent SCN for example, glial cells become active during circadian nighttime, suppressing the activity of neurons, and neurons are active during the circadian day (Brancaccio, Patton, Chesham, Maywood, & Hastings, 2017). Thus, optimal brain function depends upon consistent sleep/wake timing and synchronization across various cell types in the brain.
A number of hypotheses have been proposed to explain the underlying mechanisms by which circadian rhythms may influence mood (McClung, 2011, 2013). Mood disorders are highly heterogenous and are thought to develop due to a combination of various factors. Mood episodes and symptoms can be frequent (daily or weekly symptoms or monthly episodes) or can be separated by many years. Some people have a seasonal pattern of episodes, while others do not. In addition, depression can involve too little sleep or too much sleep, too much eating, or starvation. Bipolar disorder includes not only depressive episodes, but episodes of mania and periods of euthymia or “stabilized mood” in between. Thus, a wide range of physiological processes have been implicated in mood regulation, including monoamine signaling, glutama- tergic transmission, hypothalamic-pituitary-adrenal (HPA) axis function, metabolic peptide signaling, microbiome, neuroinflammation, and mitochondrial function. The circadian system has been shown to interact with each of these systems. Here, we highlight these interactions and discuss their potential role in mood disorders.
2 |. MONOAMINE SIGNALING
Alterations in monoamine (i.e., serotonin, dopamine, and norepinephrine) neurotransmitter systems have been observed in mood disorders, such as MDD (Meyer, 2008). Furthermore, antidepressants, antipsychotics, and mood-stabilizing drugs used in the treatment of these disorders all affect monoam- inergic signaling. Serotonin, dopamine, and norepinephrine show circadian rhythms in expression and release (McClung, 2007). Furthermore, the receptors and enzymes involved in the synthesis of these monoamines show rhythmic expression (McClung, 2007). On a circuit level, these rhythms arise, in part, due to indirect projections from the SCN to brain regions responsible for the synthesis of these neurotransmitters, including the dorsal raphe (serotonin), ventral tegmental area (VTA; dopamine), and locos coeruleus (norepinephrine; Parekh & McClung, 2015).
Studies from our laboratory using the Clock∆19 mouse have revealed an important role for the dopamine system in the circadian regulation of mood and reward. Clock∆19 mice contain a single base mutation in the Clock gene, which leads to a loss of exon 19 and a protein with dominate-negative function (King et al., 1997). The phenotype of the Clock∆19 mice consists of circadian and metabolic abnormalities, as well as a manic-like phenotype (Kristensen, Nierenberg, & Ostergaard, 2018; Logan & McClung, 2016). While no animal model will ever fully recapitulate the complex phenotypes that characterize bipolar disorder, and in particular the phenomenon of mood cycling, Clock∆19 mice display primarily manic-1 ike behavior during the day and euthymia-like behavior at night, which at least suggests some sort of spontaneous cycling behavior in these animals (McClung et al., 2005; Roybal et al., 2007; Sidor et al., 2015). This manic-like phenotype consists of enhanced hyperactivity and reward-r elated behaviors, as well as a reduction in anxiety- and depressive-l ike behaviors, and the majority of these behaviors can be reversed with lithium, a first-line treatment for bipolar disorder (Arey et al., 2014; McClung et al., 2005; Roybal et al., 2007; Sidor et al., 2015). Many studies have established that the VTA is a primary site of action for the manic-like phenotype of the ClockA19 mouse (Coque et al., 2011; McClung et al., 2005; Sidor et al., 2015). The manic-like phenotype coincides with daytime increases in VTA dopamine neuron activity, tyrosine hydroxylase (TH) expression, and dopamine synthesis in the VTA (Logan et al., 2018; Sidor et al., 2015). Notably, the manic-like phenotype can be reversed by daytime-specific administration of a TH inhibitor. Moreover, chronic stimulation of VTA dopamine neurons through optogenetics recapitulates the manic-like phenotype (Sidor et al., 2015). Interestingly, site-specific knockdown of Clock in the VTA results in a mixed manic/depressive state, with hyperactivity and decreased anxiety-like behavior, but also an increase in depressive-like behavior (Mukherjee et al., 2010).
Other studies have investigated the molecular mechanisms underlying the manic-like phenotype of the Clock∆19 mouse and have found that CLOCK negatively regulates TH expression by binding to E-box sequences at promoter regions of the gene (Figure 1; Sidor et al., 2015). The expression of TH is also regulated by cAMP response element-binding protein (CREB)-mediated binding to CRE sites in the TH promoter (Lazaroff, Patankar, Yoon, & Chikaraishi, 1995; Piech-Dumas & Tank, 1999). A recent study from our laboratory demonstrated that CLOCK activity is sensitive to cellular redox state and represses CREB-induced TH transcription through diurnal interactions with the histone and protein deacetylase, Sirtuin 1 (SIRT1), at the TH promoter (Logan et al., 2018). This repression of CREB-induced TH transcription is eliminated in Clock∆19 mice, resulting in increased TH expression during the day, during which the mice exhibit manic-like behavior. Interestingly, cocaine exposure abolishes SIRT1 rhythms in the VTA and pharmacological activation of SIRT1 reduces both dopamine signaling and cocaine-conditioned place preference in Clock ∆19 mice, suggesting a role for this mechanism in reward and addiction (Logan et al., 2018). Other clock genes, such as REV-ERBα, also play an important role in mood regulation. A study by Chung et al. (2014) showed that Rev-erbα knockout mice display a hyperdopa- minergic state that is accompanied by manic-like behavior. Similar effects were induced through pharmacological inhibition of REV-ERBα in the ventral midbrain. Furthermore, they demonstrated that TH expression is directly repressed by REV-ERBα, providing a mechanism through which this effect may occur (Chung et al., 2014). Taken together, these data strongly implicate VTA dopaminergic activity in the circadian regulation of mood and reward.
FIGURE 1.
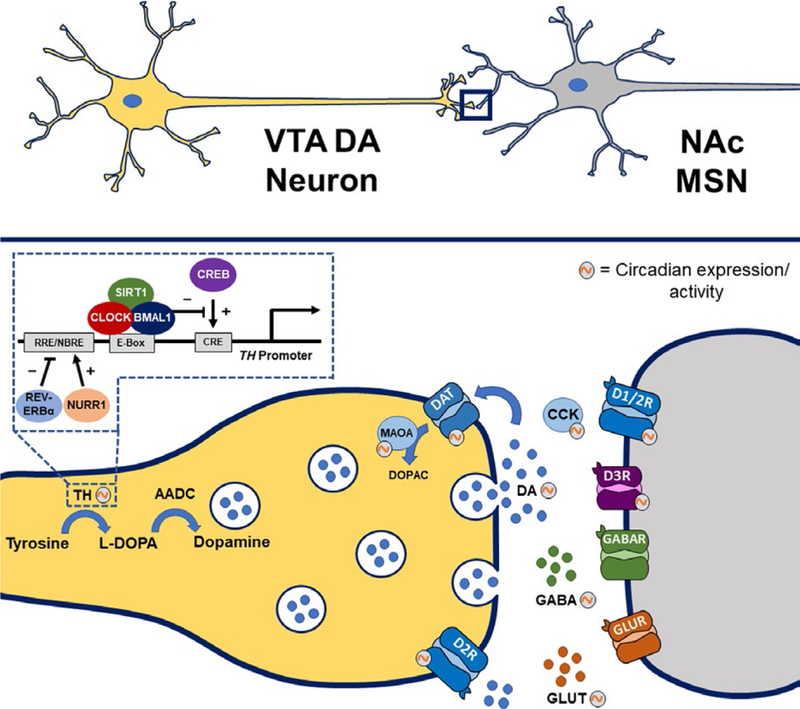
Clock genes regulate components of dopaminergic transmission within the ventral tegmental area (VTA)-nucleus accumbens (NAc) circuitry. Components of the dopamine synapse involved in the synthesis, uptake, and degradation of dopamine show circadian rhythms in expression or activity. These rhythmic components include tyrosine hydroxylase (TH), monoamine oxidase A (MAOA), dopamine transporter (DAT), and dopamine receptors type 1 (D1R), type 2 (D2R), and type 3 (D3R). Tyrosine hydroxylase transcription is activated by cAMP response element-binding protein (CREB)-mediated binding to CRE sites in the TH promoter. The CLOCK/BMAL1 complex interacts with the histone and protein deacetylase, Sirtuin 1 (SIRT1), to repress CREB-induced TH transcription in a diurnal-dependent manner. There are also circadian rhythms in various neurotransmitters and neuropeptides, including dopamine (DA), glutamate (GLUT), γ-aminobutyric acid (GABA), and cholecystokinin (CCK)
In addition to the dopamine system, several studies have implicated a role for serotonin in the circadian regulation of mood and reward. The circadian and serotonergic systems are reciprocally connected and likely interact to influence mood regulation (Ciarleglio, Resuehr, & McMahon, 2011). Studies have shown serotonin manipulation can influence circadian rhythms in both the SCN and locomotor activity (Vadnie & McClung, 2017). For example, SSRIs have been shown to phase advance SCN neural activity, as well as locomotor activity (Cuesta, Clesse, Pevet, & Challet, 2009; Cuesta, Mendoza, Clesse, Pevet, & Challet, 2008; Prosser, Lee, & Wehner, 2006; Sprouse, Braselton, & Reynolds, 2006), which could play a role in improving rhythm disturbances observed in mood disorders. Interestingly, the influence of circadian and monoaminergic systems on mood has also been demonstrated through manipulations of the melatonin system. Melatonin is a hormone produced by the pineal gland that plays an important role in sleep regulation. The antidepressant effects of the drug, agomelatine, are mediated through its interactions with both melatonin receptors and serotonin 5-HT2C receptors (Guardiola-Lemaitre et al., 2014). Agomelatine is a melatonin receptor agonist and 5-HT2c antagonist and has been shown to modulate monoaminergic neuronal activity (Chenu, El Mansari, & Blier, 2013). Furthermore, melatonin MT1 receptor knockout mice show increased depressive-like behavior, which is reversed by chronic treatment with the tricyclic antidepressant desipra- mine (Comai, Ochoa-Sanchez, Dominguez-Lopez, Bambico, & Gobbi, 2015). Another study demonstrated the anxiolytic effects of agomelatine after social defeat require an intact SCN (Tuma, Strubbe, Mocaer, & Koolhaas, 2005). Furthermore, serotonergic signaling in the amygdala can modulate the anti-depressant actions of melatonin (Micale, Arezzi, Rampello, & Drago, 2006). These studies highlight a role for the interaction of circadian and monoamine systems in regulating mood.
Lastly, norepinephrine may also be involved in the circadian regulation of mood. A study by Ben-Hamo et al. (2016) found that rats exposed to a short light-dark cycle (22 hr) showed desynchronized oscillations in the SCN and a depressive phenotype during the active phase (Ben-Hamo et al., 2016). These changes are paralleled by increased norepinephrine and dopamine levels and serotonin turnover in the prefron- tal cortex (PFC; Ben-Hamo et al., 2016). These changes may be stress-induced, as norepinephrine and dopamine release are enhanced in the PFC in response to stress (Di Chiara, Loddo, & Tanda, 1999; Finlay, Zigmond, & Abercrombie, 1995). Interestingly, deletion of Bmal1 in the cerebral cortex induces depressive-l ike behavior in the tail suspension test and a reduction in cortical norepinephrine levels, suggesting cortical clock regulation of mood may involve norepinephrine function (Bering, Carstensen, Wortwein, Weikop, & Rath, 2018). Lastly, studies have shown that constant darkness can induce a depressive-like state and apoptosis of monoaminergic neurons, particularly noradrenergic neurons in the locos coeruleus, and these effects are reversed with the norepinephrine reuptake inhibitor, desipramine (Gonzalez & Aston-Jones, 2008).
Together, these studies reveal a role for monoamine- related neuronal activity in the circadian regulation of mood. A more thorough understanding of the mechanisms underlying this relationship may lead to novel therapeutic targets for the treatment of mood disorders, as well as the circadian rhythm alterations associated with these disorders.
3 |. GLUTAMATERGIC TRANSMISSION
The discovery that ketamine, a glutamate NMDA receptor channel blocker, can produce rapid antidepressant effects has generated a great deal of interest in the role of glutamatergic transmission in mood regulation (Duman, 2018). As opposed to most antidepressant medications which require weeks to months before there are therapeutic effects, a single, subanesthetic dose of ketamine can produce an antidepressant response within hours that can last for up to a week (Zarate et al., 2006). The exact mechanisms by which ketamine produces these effects are still unclear; however, it seems likely that at least initially ketamine increases glutamatergic transmission in the mPFC, hippocampus, and other regions of the brain. This increase in glutamatergic transmission is potentially mediated through blockage of NMDA receptors on GABA interneurons that normally inhibit this transmission (Duman, 2018). Another recent study found that ketamine blocks NMDA receptors in the lateral habenula, leading to a reduction in burst firing normally driven by NMDA receptor activity (Yang et al., 2018). Since the lateral habenula is involved in inhibiting monoaminergic reward circuitry, this reduction in burst firing results in activation of dopamine and serotonin neurons, potentially leading to the rapid (but perhaps not sustained) antidepressant response. Interestingly, studies over the last 30 years have found that one night of sleep deprivation (SD) can also produce similar rapid antidepressant effects in about 50% of subjects (Borbely & Wirz-Justice, 1982; Wu & Bunney, 1990), leading people to question whether these rapid antidepressant effects of keta- mine and SD share common mechanisms of action, and if they involve the modulation of the molecular clock. In cell culture (NG108–15 neuronal cells), ketamine can inhibit CLOCK:BMAL1-mediated transcriptional activation and this is attenuated by treatment with a GSK3β antagonist (Bellet, Vawter, Bunney, Bunney, & Sassone-Corsi, 2011). This suggests that ketamine alters CLOCK:BMAL1 function perhaps via activation of GSK3β, a key modulator of the molecular clock. Another study from the same group treated mice with either ketamine or SD and subjected the mice to the forced swim test, a measure of antidepressant efficacy. They then took tissue samples from the anterior cingulate cortex and compared the transcriptional response between treatments. They found 64 genes which were commonly altered by both treatments (representing 5% of genes changed by ketamine and 11% of genes changed by SD, p < 0.001), including a number of downregulated circadian genes, such as Per2, Npas4, Rorb, Dbp, and Ciart. The authors speculate that both ketamine and SD alter common molecular clock components, resulting in changes in neuronal plasticity and an antidepressant effect (Orozco-Solis et al., 2017). The longer lasting antidepressant effects of ketamine (up to 2 weeks) involve activation of mTOR signaling pathways, brain-derived neurotrophic factor (BDNF) release, and changes in dendritic spines (Autry et al., 2011; Li et al., 2010). These changes may occur primarily during sleep following ketamine treatment. A study of 30 subjects with treatment-resistant depression found a positive correlation between the baseline delta sleep ratio (SWA(NREM1)/SWA(NREM2)) the night before keta- mine treatment and the response to ketamine the following day, suggesting that a low baseline delta sleep ratio, indicative of deficient production of slow wave sleep, may be a predictor of ketamine response (Duncan, Selter, Brutsche, Sarasso, & Zarate, 2013). Moreover, following ketamine treatment, there was an increase in slow wave activity in the first non-REM sleep episode, along with increased plasma BDNF (Duncan, Sarasso, et al., 2013). Furthermore, the response included an increase in high amplitude waves during early sleep, along with an increase in slow wave slope, suggesting increased synaptic strength. Changes in BDNF were proportional to changes in EEG measures and these changes were only found in subjects who experienced an antidepressant response to ketamine. These data suggest that ketamine is modulating synaptic connections during the first sleep episode following treatment, and that this first sleep episode may be crucial to the sustained antidepressant response.
Changes in sleep/wake activity rhythms can also be predictive of a response to ketamine. In a study of 51 subjects with treatment-r esistant depression, activity rhythms were measured using actigraphy (Duncan, Slonena, et al., 2017). The study found that those who would go on to respond to ket- amine had lower central value (mesor) and earlier acrophase measures at baseline. They also showed advanced timing on day 1 after ketamine and importantly, increased rhythm amplitude on day 3. In contrast, ketamine non-responders had a lower mesor and blunted amplitude in response to ketamine on day 1. These findings are exciting in that they demonstrate an association between the clinical response to ketamine and sleep/wake rhythms along with a potential modulation of the internal circadian system. They show not only that treatment response can be predicted by certain diurnal patterns of activity, but also that ketamine may produce lasting effects in these individuals, at least in part, through amplification of diurnal rhythms. It is also worth noting that depression can be the result of many different factors, disrupted circadian rhythms being only one of them, and thus other types of treatments may be more advantageous for those individuals without these particular circadian phenotypes, while ketamine may be therapeutic only for a particular population with these specific circadian phenotypes. Interestingly, both lithium and valproic acid (mood-stabilizing medications) also increase molecular rhythm amplitude in fibroblast cultures and this effect may underlie their ability to stabilize rhythms and prevent the precipitation of mood episodes (Johansson, Brask, Owe-Larsson, Hetta, & Lundkvist, 2011; Li, Lu, Beesley, Loudon, & Meng, 2012). While we know that glutamatergic activity in the SCN is involved in setting the central clock, future studies are needed to determine if and how ketamine impacts the central clock in the SCN, as well as molecular clocks in other brain regions. With this knowledge, we may be able to use additional chronotherapies, like bright light therapy, to extend and sustain therapeutic effects of ketamine or SD (Duncan, Ballard, & Zarate, 2017).
4 |. HPA AXIS
The neuroendocrine component of the stress response is mediated by the HPA axis. In response to stress, corticotropin- releasing factor (CRF) is released from neurons in the paraventricular nucleus of the hypothalamus (PVN) into the hypophyseal portal system, where it travels to the anterior pituitary and stimulates the release of adrenocorticotropic hormone (ACTH) into the circulation. ACTH stimulates the release of glucocorticoids (corticosterone or cortisol) from the adrenal gland, which then mediates the stress response. Glucocorticoids inhibit the HPA axis through negative feedback at the PVN and anterior pituitary. HPA axis and glucocorticoid dysregulation has been implicated in mood disorders, such as depression (Pariante & Lightman, 2008). Hypercortisolism is often observed in patients with depression (Hinkelmann et al., 2012; Linkowski et al., 1987; Rubin, Poland, Lesser, Winston, & Blodgett, 1987). However, atypical depression, a subtype of major depression characterized by symptoms such as lethargy, fatigue, hyperphagia, and weight gain, is associated with lower levels of cortisol (Gold & Chrousos, 2002). Furthermore, in depressed patients with increased cortisol levels, antidepressant treatment has been shown to return cortisol to control levels (Hinkelmann et al., 2012; Linkowski et al., 1987).
The HPA axis is under circadian control by the SCN (Moore & Eichler, 1972). Glucocorticoids show robust circadian rhythms in expression, with peak levels occurring at the beginning of the active phase. This temporal rhythmicity is controlled by diurnal variations in CRF and ACTH activity, as well as a peripheral clock in the adrenal glands (Ishida et al., 2005; Nader, Chrousos, & Kino, 2010; Oster et al., 2006; Son, Chung, & Kim, 2011). In addition, molecular clock proteins interact with the glucocorticoid receptor (GR) to produce diurnal variations in its expression and sensitivity. For example, CRY proteins interact with the GR to rhythmically repress its transcriptional activity (Lamia et al., 2011). The CLOCK/BMAL1 complex also modulates the circadian sensitivity of GR through CLOCK-mediated acetylation (Charmandari et al., 2011; Kino & Chrousos, 2011; Nader, Chrousos, & Kino, 2009). The function of GR is also modulated through interactions with other clock proteins, such as REV-ERBa (Okabe et al., 2016) and CHRONO, a novel circadian protein that negatively regulates the molecular clock and interacts with GR to decrease its transcription activity (Anafi et al., 2014; Goriki et al., 2014). Notably, the interaction between the circadian system and the HPA axis is reciprocal, as glucocorticoids provide feedback on the clock by binding to glucocorticoid response elements and altering the transcription of circadian clock genes, such as Per1 and Per2, allowing for synchronization of peripheral clocks and extra- SCN brain clocks (Koch, Leinweber, Drengberg, Blaum, & Oster, 2017; Oster et al., 2017; Spencer, Chun, Hartsock, & Woodruff, 2018).
A number of studies suggest the circadian system interacts with the stress system to influence mood regulation (Landgraf, McCarthy, & Welsh, 2014). Circadian clock regulation of HPA axis function has been demonstrated through studies utilizing genetic mouse models containing mutations in circadian clock genes. For example, Bmal1 mutant mice show reduced glucocorticoid levels, adrenal sensitivity to ACTH, and a downregulation in gene expression related to cholesterol synthesis in adrenal cells (Leliavski, Shostak, Husse, & Oster, 2014). These mice also show an attenuation in stress-induced glucocorticoid levels and reduced depressive- like behavior in the forced swim test (Leliavski et al., 2014). Mutations in Clock, Cry, and Per are also associated with alterations in glucocorticoid levels and rhythmicity (Becker- Krail & McClung, 2016; Koch et al., 2017). The relationship between the circadian and stress system is bidirectional, with stress also affecting function of the molecular clock. For example, stress increases Per1 expression in the brain and peripheral tissues (Al-Safadi, Branchaud, Rutherford, & Amir, 2015; Al-Safadi et al., 2014; Takahashi et al., 2001, 2013; Yamamoto et al., 2005). In addition, Per2 rhythms are stimulated by glucocorticoids and these rhythms are altered in the bed nucleus of the stria terminalis and the amygdala following inactivation of the GR (Segall, Milet, Tronche, & Amir, 2009; So, Bernal, Pillsbury, Yamamoto, & Feldman, 2009). A study from our laboratory explored the effects of unpredictable chronic mild stress, a rodent model of depression, on Per2 rhythms in the mouse brain using Per2 lucifer-ase reporter (Per2::luc) mice (Logan et al., 2015). Chronic stress decreased rhythm amplitude in the SCN and increased rhythm amplitude in the nucleus accumbens (NAc), with these changes directly correlating to depressive-like behavior (Logan et al., 2015). These changes in amplitude may be due to differences in GR expression, as the NAc expresses high levels of GR and the SCN is largely absent of GR expression (Balsalobre et al., 2000; Barik et al., 2010; Der-Avakian et al., 2006). A study by Landgraf et al. (2016) investigated the role of the circadian clock in the SCN in regulating mood. The authors found that site-specific knockdown of Bmal1 in the SCN of Per2::luc mice significantly suppressed Per2 rhythms and induced depressive- and anxiety-l ike behavior, demonstrating a causal role for SCN rhythms in mood regulation. The authors speculate that disruption of the central clock may affect mood through downstream systems that are more directly involved in mood regulation. In support of this, they find that disrupting rhythms in the SCN altered HPA axis function, as the mice displayed altered corticosterone rhythms and an attenuated stress-induced corticosterone response (Landgraf et al., 2016). Of note, the mood-related findings from this study contrast with the study described above by Leliavski et al. (2014), in which Bmall mutant mice showed a reduction in depressive-rike behavior. However, these discrepancies may be explained, in part, by the method of Bmall knockdown. Landgraf et al. (2016) site-specifically knocked down Bmall in the SCN, whereas Leliavski et al. (2014) used mutant mice that lack Bmall in both the brain and periphery.
A variety of studies have utilized inappropriate light exposure and extreme photoperiod changes to investigate the interaction of stress and circadian systems in mood regulation. For example, inappropriate light exposure (i.e., light at night) is associated with increased corticosterone levels in rodents (Koch et al., 2017). In addition, short photoperiods induce anxiety- and depressive-like behavior in diurnal rodents, which are reduced after bright light treatment (Ashkenazy, Einat, & Kronfeld-Schor, 2009a,b; Einat, Kronfeld-Schor, & Eilam, 2006). A study by Dulcis, Jamshidi, Leutgeb, and Spitzer (2013) found that long-day (i.e., short-active) photoperiods elevate corticosterone levels and enhance anxiety- and depressive-like behaviors in nocturnal rats, while short-day (i.e., long-active) photoperiods produce the opposite effect. Interestingly, photoperiod changes caused individual interneurons in the PVN to switch between dopamine and somatostatin expression, with increased switching from somatostatin to dopamine for short-day periods and dopamine to somatostatin for long-day periods. Moreover, ablation of dopamine neurons in the PVN produced anxiety- and depressive-like behavior, which was rescued through short-day photoperiod induction of new dopaminergic neurons (Dulcis et al., 2013). The authors also revealed that somatostatin (SST2/4) and dopamine (D2) receptors colocalize with CRF in the hypothalamus and observed an increase in CRF and corticosterone levels in the cerebrospinal fluid and plasma, respectively, following long-day photoperiod. This may represent a potential mechanism by which long-day photoperiod induces depressive- and anxiety-like behavior in mice (Dulcis et al., 2013). While the PVN receives direct input from the SCN, the mechanisms by which the central clock may regulate neurotransmitter switching remain to be determined.
A study by Young et al. (2018) utilized similar short-day and long-day photoperiod changes in mice to model season- induced switching between mood states. The authors found that a short-day (i.e., long-active) period induced a manic state, while a long-day (i.e., short-active) period induced a depressive state, consistent with season-induced switching between mood states observed in patients with bipolar disorder (Wang & Chen, 2013; Young & Dulcis, 2015). Interestingly, similar to the Dulcis et al. (2013) study, switches in mood states were correlated with neurotransmitter switching from dopamine to somatostatin in the PVN. Mice with a reduction in dopamine transporter (DAT) expression showed exacerbated manic and depressive-like states in response to their respective photoperiods, revealing an important role for dopamine function in season-induced switching between mood states (Young et al., 2018). Together, these studies suggest that the interaction of circadian and stress systems is important for regulation of mood. However, it is worth noting that studies from the Hattar laboratory have identified specific projections from the intrinsically photosensitive retinal ganglion cells (ipRGCs) and the perihabenular nucleus that may potentially mediate the depression-inducing effects of aberrant light cycles independent from the central clock in the SCN (Fernandez et al., 2018; LeGates et al., 2012). Thus, future studies will be necessary to determine the exact role of these interactions in the development of mood disorders.
5 |. METABOLIC PEPTIDES
Mood disorders and metabolic disorders show a high rate of comorbidity (Amare, Schubert, Klingler-Hoffmann, CohenWoods, & Baune, 2017; Mansur, Brietzke, & McIntyre, 2015; Milaneschi, Simmons, van Rossum, & Penninx, 2018). For example, depression is highly comorbid with obesity, and the relationship between the two is bidirectional (Milaneschi et al., 2018). This association is particularly strong among obese individuals with an adverse metabolic profile, including dyslipidemia or insulin resistance (Jokela, Hamer, Singh-Manoux, Batty, & Kivimaki, 2014). Recent evidence indicates environmental and genetic disruptions of circadian rhythms may play a role in the link between metabolic and mood disorders (Barandas, Landgraf, McCarthy, & Welsh, 2015). Peripheral tissues, including the liver, pancreas, and gut, express local self-sustaining clocks that regulate metabolic function. Moreover, peptides and hormones that regulate metabolism and feeding, such as leptin, ghrelin, orexin, and cholecystokinin (CCK), display circadian rhythms in expression (Feillet, 2010; Schade et al., 1993; Turek et al., 2005).
Food intake is largely regulated by the hormones leptin and ghrelin, which are released from the adipose tissue and gut, respectively. These hormones act within the arcuate nucleus of the hypothalamus to regulate food intake and energy homeostasis. Ghrelin is released from the gut in response to hunger and stimulates food intake, while leptin is released from adipose tissue and reduces feeding (Sobrino Crespo, Perianes Cachero, Puebla Jimenez, Barrios, & Arilla Ferreiro, 2014). Both leptin and ghrelin show circadian rhythms in expression, and their levels in the plasma are sensitive to food availability (Feillet, 2010). In addition to their role in obesity (Cui, Lopez, & Rahmouni, 2017), ghrelin and leptin have been implicated in the regulation of mood and reward (Lu, 2007; Milaneschi et al., 2018; Morris, Voon, & Leggio, 2018; Spencer, Emmerzaal, Kozicz, & Andrews, 2015). A number of rodent studies have revealed an antidepressant effect of leptin, with the hippocampus being a primary site of action (Garza, Guo, Zhang, & Lu, 2012; Lu, Kim, Frazer, & Zhang, 2006; Yamada et al., 2011). Furthermore, global and site-specific (i.e., hippocampus and cortex) knockout of the leptin receptor induces depressive-like behavior (Guo, Huang, Garza, Chua, & Lu, 2013; Guo et al., 2012; Liu et al., 2017; Sharma, Elased, Garrett, & Lucot, 2010). In humans, high levels of leptin are associated with atypical depression, with the association being more robust for increased adiposity, appetite, and weight (Milaneschi, Lamers, Bot, Drent, & Penninx, 2017). These data implicate a role for leptin dysregulation in the comorbidity between obesity and depression. However, the exact role of ghrelin in mood regulation is a little less clear, as some studies show that ghrelin produces anxiety-like behavior, while others show anxiolytic and antidepressant effects (Morris et al., 2018; Spencer et al., 2015; Wittekind & Kluge, 2015). Ghrelin and leptin may modulate mood through their interaction with the HPA axis (Barandas et al., 2015; Morris et al., 2018; Roubos, Dahmen, Kozicz, & Xu, 2012; Spencer et al., 2015). Furthermore, chronic restraint stress disrupts diurnal rhythms in leptin expression (de Oliveira et al., 2014). Given their important role in mood and metabolism, circadian disruptions in leptin and ghrelin expression may have an impact on mood regulation and its relationship to obesity.
Ghrelin and leptin also interact with orexin neurons in the lateral hypothalamus, which promote arousal and feeding when activated by ghrelin and induce sleep when inhibited by leptin (Adamantidis & de Lecea, 2008). Orexin is a peptide that simulates food intake and is known for its role in regulating arousal, energy, as well as mood and reward. Orexin also plays a role in the stress response and the interactions between these systems are bidirectional (Blasiak, Gundlach, Hess, & Lewandowski, 2017; Grafe & Bhatnagar, 2018; James, Campbell, & Dayas, 2017). Interestingly, orexin modulation of the stress response is mediated by leptin through leptin-sensitive neurons in the lateral hypothalamus, suggesting an interaction between these two systems in the stress response (Bonnavion, Jackson, Carter, & de Lecea, 2015). Depression is linked to both hyperactivity and hypoactivity of the orexin system (Nollet & Leman, 2013). In addition, individuals with depression display altered rhythms in activity, sleep/wake, body temperature, and hormone secretion (Germain & Kupfer, 2008; McClung, 2007; Souetre et al., 1989). Interestingly, depressed subjects also exhibit a reduction in diurnal rhythms of orexin expression (Salomon et al., 2003). Circadian rhythms of orexin expression are regulated by the SCN (Blasiak et al., 2017). Furthermore, Clock mutant mice show a decrease in orexin expression, as well as a loss of rhythmicity (Turek et al., 2005). These mice also lose circadian rhythmicity in other peptides, such as ghrelin and cocaine- and amphetamine-regulated transcript (Turek et al., 2005). Moreover, microarray analysis of VTA tissue from Clock∆19 mice identified altered transcription of a number of genes involved in dopaminergic transmission (McClung et al., 2005). One of the genes significantly downregulated was Cck, a peptide that shows robust circadian rhythms in expression in the brain and plays a role in feeding and regulation of mood and anxiety-related behavior (Arey et al., 2014; McClung et al., 2005; Schade et al., 1993; Weber, Lauterburg, Tobler, & Burgunder, 2004). CCK is a direct transcriptional target of CLOCK in the VTA and Cck levels are decreased in this region in Clock∆19 mice (Arey et al., 2014; McClung et al., 2005). Furthermore, site-specific knockdown of Cck in the VTA of wild-type mice produces a manic-like phenotype similar to the Clock∆19 mice. Chronic lithium treatment increases Cck levels in Clock∆19 mice, which is necessary for the therapeutic actions of the drug in this mouse model (Arey et al., 2014). These data suggest a critical role for CCK in the manic-like phenotype of the Clock∆19 mice. Interestingly, Clock mutant mice become obese on a high-fat diet and develop a metabolic syndrome (Turek et al., 2005). Moreover, mutations in other clock genes, such as PER and CRY, also produce both metabolic and mood phenotypes, further demonstrating the important role of the core circadian clock in regulating both metabolism and mood (Barandas et al., 2015). Future studies are necessary for a more comprehensive understanding of the molecular mechanisms that underlie this relationship.
6 |. MICROBIOME
A high comorbidity exists between gastrointestinal disorders and stress-related psychiatric disorders. For example, over 50% of patients with irritable bowel syndrome have depression or anxiety (Kelly et al., 2015; Kennedy, Cryan, Dinan, & Clarke, 2014). This comorbidity highlights a potentially important role of the gut-brain axis in mood regulation. The microbiome is a key component of the gut-brain axis, which comprises the microbiota-gut-brain axis, a bidirectional pathway that influences metabolism, behavior, and mood (Cussotto, Sandhu, Dinan, & Cryan, 2018; Dinan & Cryan, 2017; Kelly et al., 2015; Rogers et al., 2016). The mammalian microbiome consists of trillions of bacteria that reside in the intestine of the host. Recent evidence suggests the gut microbiome may play a role in mood regulation and stress-related psychiatric disorders, such as depression. For example, germ-free mice show alterations in the stress response and exhibit alterations in monoaminergic signaling in limbic brain regions (Cussotto et al., 2018; Rogers et al., 2016). Interestingly, prebiotics and probiotics, which stimulate the growth of healthy bacteria in the gut, can produce anxiolytic effects and normalization of the HPA axis in response to stress (Cussotto et al., 2018). Alterations in gut microbiome composition and diversity have been observed in patients with MDD (Jiang et al., 2015; Kelly et al., 2016; Lin et al., 2017; Zheng et al., 2016). Notably, a study by Zheng et al. (2016) showed that fecal transplantation of samples from patients with MDD into germ-free mice resulted in depressive-like behavior in the recipient mouse compared to those that received transplants from healthy controls. Mice that received transplants from depressed patients also displayed alterations in microbial gene expression and fecal and serum metabolites related to carbohydrate and amino acid metabolism, suggesting that alterations in host metabolism may be a potential mechanism by which the microbiome influences mood (Zheng et al., 2016). A similar fecal transplant study was performed by Kelly et al. (2016), but instead of using germ- free mice, rats treated with a cocktail of antibiotics were utilized. These microbiota-deficient rats that received a fecal transplantation from patients with MDD showed depressive-and anxiety-like behaviors, as well as alterations in tryptophan metabolism, which could influence serotonin production (Kelly et al., 2016). Taken together, these data suggest a potential role for the microbiome in the development of depressive-like behavior.
Several recent studies have demonstrated a significant interaction between circadian rhythms and the gut microbiome. A study by Thaiss et al. (2014) showed the intestinal microbiota in rodents and humans displays diurnal rhythms in abundance and metabolic function that are highly regulated by feeding rhythms. Furthermore, diurnal oscillations in microbial localization and metabolite production regulate circadian activity in host metabolism, including systemic metabolite oscillations and transcriptional oscillations in peripheral tissues (Thaiss et al., 2016). Microbiota rhythmicity is also sensitive to circadian disruptions in the host. For example, mice exposed to a jet lag paradigm exhibit a disruption in microbiota rhythmicity and metabolic alterations, such as glucose intolerance and obesity (Thaiss et al., 2014). Fecal transplantation of microbiota from jet-lagged mice and humans into germ-free mice recapitulated these metabolic deficits, demonstrating a role for the gut microbiota in the metabolic disturbances observed after circadian disruption (Thaiss et al., 2014). Microbiome alterations in response to host circadian disruption may be influenced by diet, as mice undergoing weekly light-dark reversals showed alterations in the gut microbiome composition that were dependent on being fed a high-fat or high-sugar diet (Voigt et al., 2014). Furthermore, a high-fat diet disrupts diurnal oscillations in microbiota composition and metabolite production, which may alter hepatic circadian rhythms in the host (Leone et al., 2015). Similarly, diet-induced obesity disrupts diurnal variations in microbial composition, which is partially restored by time-restricted feeding (Zarrinpar, Chaix, Yooseph, & Panda, 2014). Together, these studies demonstrate an important role for diet in the relationship between circadian rhythms and the microbiome. The role of the host central clock in regulating microbial rhythms has also been demonstrated in genetic mouse models with mutations in circadian clock genes (Voigt, Forsyth, Green, Engen, & Keshavarzian, 2016). For example, microbiota rhythmicity is disrupted in mice with mutations in Per1/2 (Thaiss et al., 2014) and Bmal1 (Liang, Bushman, & FitzGerald, 2015). Studies using the ClockΔ19 mice have found a reduction in microbial diversity compared to wild-type mice (Voigt, Summa, et al., 2016) and intestinal barrier dysfunction (i.e., increased hyperpermeability) that is worsened by alcohol intake (Summa et al., 2013). Alterations in intestinal barrier integrity can lead to gut leakiness and increase systemic inflammation, which may increase the risk for psychiatric diseases (Bauer & Teixeira, 2018).
There are a variety of proposed mechanisms by which the microbiome may influence brain function, including the vagus nerve, enteric nervous system, microbiota metabolite production, the neuroendocrine system, immune signaling, and inflammation (Cussotto et al., 2018). Increased levels of lipopolysaccharide from gram-negative enterobacteria have been observed in the serum of patients with MDD (Maes, Kubera, & Leunis, 2008). This translocation may result in systemic inflammation, which has been observed in patients with mood disorders (Bauer & Teixeira, 2018). Metabolites produced by the microbiome also influence metabolism, behavior, and perhaps mood. Short-chain fatty acids, such as butyrate, acetate, and propionate, are common metabolic byproducts released by gut microbiota and have been shown to alter host immune responses, metabolism, and neurotransmitter synthesis (Cussotto et al., 2018; Rogers et al., 2016). Gut bacteria also produce a wide variety of neurotransmitters, including GABA, dopamine, norepinephrine, serotonin, and acetylcholine (Cussotto et al., 2018). However, it is currently unknown what effect these microbial-derived neurotransmitters might have (if any) on brain function, as many of these neurotransmitters are not able to cross the blood-brain barrier. Nevertheless, microbial-derived neurotransmitter precursors could play a role in mood regulation. For example, tryptophan is produced by gut microbiota and may influence mood through altering serotonin production. Interestingly, germfree mice have increased plasma tryptophan and hippocampal 5-HT levels (Clarke et al., 2013). Lastly, a recent study found that the ketogenic diet, a known treatment for intractable epilepsy in children, alters the intestinal microbiota and these changes are necessary and sufficient for seizure protection in mice (Olson et al., 2018). Ketogenic diet- and microbiota-dependent seizure protection was correlated with reductions in peripheral gamma-glutamyl amino acids and increases in hippocampal GABA/glutamate ratios, suggesting that the gut microbiome may modulate seizure susceptibility in response to diet through alterations in host metabolism (Olson et al., 2018). Together, the studies described above suggest a role for the microbiome in both metabolism and mood regulation and highlight a need for future studies to investigate how the circadian system may be important for this connection.
7 |. NEUROINFLAMMATION
In recent years, there has been a great deal of interest in the potential role of inflammation and the production of pro-inflammatory cytokines in the pathophysiology of depression. Major depression is common in patients suffering from chronic inflammatory conditions (Menard, Pfau, Hodes, & Russo, 2017). Subsets of MDD patients also have higher levels of both pro-inflammatory cytokines and circulating leukocytes. Chronic stress can increase pro-inflammatory cytokines, and nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) signaling in the periphery and CNS, as well as microglial activation in the brain (Frank, Baratta, Sprunger, Watkins, & Maier, 2007; Pace et al., 2006). Furthermore, when cytokines produced from macrophages are given to healthy subjects, they induce depressive-like symptoms (Smith, 1991). Cytokines, like interleukin-1β, can alter monoamine levels by increasing metabolism of norepinephrine and 5-HT, and increase the production of CRF in the hypothalamus, activating the HPA stress axis (Takahashi, Flanigan, McEwen, & Russo, 2018). These mechanisms may contribute to the regulation of mood in people with increased cytokine production. Moreover, anti-inflammatory treatments can be beneficial in certain people with increased levels of inflammatory cytokines and depression-related symptoms (Kopschina Feltes et al., 2017).
Even modest circadian rhythm and sleep disruption can result in elevated levels of pro-inflammatory cytokines in humans and exaggerated responses to a lipopolysaccharide (LPS) challenge in mice (Castanon-Cervantes et al., 2010; Fonken, Weil, & Nelson, 2013; Vgontzas et al., 2004). In turn, LPS can induce phase delays in free-running hamsters and alter the response to light in the SCN of mice (Marpegan, Bekinschtein, Costas, & Golombek, 2005; Palomba & Bentivoglio, 2008). LPS treatment can also transiently alter expression of clock genes, like Per1 and Per2, in the hypothalamus and liver (Okada et al., 2008; Takahashi et al., 2001). Inflammatory cytokines can decrease the frequency of excitatory and inhibitory currents in the SCN and ablate the circadian rhythm of spontaneous activity in the SCN, reducing frequency to nadir levels across the 24-hr cycle (Lundkvist, Hill, & Kristensson, 2002). This is similar to what is reported with aging, suggesting that the effects of age on SCN function could be mediated by increased levels of inflammatory cytokines in the brain (Nygard, Hill, Wikstrom, & Kristensson, 2005; Watanabe, Shibata, & Watanabe, 1995). Circadian genes are expressed rhythmically in peripheral immune cells, including macrophages and natural killer (NK) cells (Arjona & Sarkar, 2005, 2006; Hayashi, Shimba, & Tezuka, 2007; Kusanagi et al., 2004). Mice with a mutation in Per2 lose the well-defined circadian pattern of resistance to an LPS challenge that is normally seen in mice, and they are more resistant to LPS-induced mortality (Liu et al., 2006). These mice are deficient in the production of IFN-γ and IL-10 by NK cells, which suggests that PER2 is involved in the production of certain cytokines following an immune challenge (Liu et al., 2006).
Nuclear factor kappa-light-chain-enhancer of activated B cells signaling plays a central role in the cellular response to stress, inflammation, and immunity. Importantly, CLOCK is found in protein complexes with the transcription factor, p65, a member of the NFκB family (Spengler et al., 2012). In the absence of BMAL1, CLOCK can upregulate NFκB-mediated transcription and the immune response is reduced in cells derived from Clock-deficient mice (Spengler et al., 2012). Moreover, hCLOCK is induced by insults like hypoxia and activates inflammatory responses through its interactions with the NFκB pathway (Tang et al., 2017). Thus, there is a bidirectional relationship between immune stimulation and the clock such that inflammation can alter circadian rhythms, and the circadian clock helps to regulate immune responses. Future studies are needed to determine how these immune factors impact mood and to what extent genetic or environmental changes to circadian rhythms influence inflammatory responses.
8 |. MITOCHONDRIAL FUNCTION
Mitochondria are the primary energy-producing organelles in cells. Neurons in the brain require high levels of energy to function. In fact, the brain, with its high aerobic activity, requires approximately 20 times more energy than the rest of the body by weight and a cortical neuron consumes 4.7 billion ATP molecules per second in a resting human brain (Kety, 1950; Zhu et al., 2012). In addition, a by-product of energy production by mitochondria is the creation of reactive oxygen species (ROS), which can have toxic effects if not properly cleared by antioxidants. This makes the brain highly vulnerable to changes in mitochondrial function or antioxidant production. Indeed, several studies have linked mitochondrial dysfunction to psychiatric disorders including bipolar disorder, schizophrenia, and major depression (Allen, Romay-Tallon, Brymer, Caruncho, & Kalynchuk, 2018; Clay, Sillivan, & Konradi, 2011; Manji et al., 2012; Marazziti et al., 2011). In turn, patients with mitochondrial diseases often display symptoms which are characteristic of psychiatric disorders.
Mitochondrial morphology including fusion and fission, as well as the formation of new mitochondria, is dependent on a functional molecular clock (de Goede, Wefers, Brombacher, Schrauwen, & Kalsbeek, 2018). For example, both Bmal1 KO and ClockΔ19 mutant mice have reduced muscle mitochondrial volume and respiratory function and altered levels of pgc1a and pgc1b, key genes involved in mitochondrial biogenesis (Andrews et al., 2010). While mitochondrial mass and content do not seem to change over the light/dark cycle, mitochondrial respiration and fusion and fission processes do display diurnal rhythms (de Goede et al., 2018). Some of these diurnal differences are due to changes in activity and food consumption at different times of day, but the molecular clock also seems to play a role. Indeed, the molecular clock regulates a large number of genes that are involved in mitochondrial function and the antioxidant response (Panda et al., 2002). One important link between circadian regulation of transcription in the nucleus and the mitochondrial redox system involves the NAD+-dependent deacetylase, SIRT1 (Figure 2). SIRT1 becomes active in the presence of NAD+, thus it is responsive to the NAD+/NADH ratio indicative of the cellular redox state. Activated SIRT1 can bind to CLOCK and deacetylate BMAL1 and PER2, altering circadian transcription (Asher et al., 2008; Nakahata et al., 2008). In turn, CLOCK and BMAL1 regulate the production of NAMPT, part of the NAD+ salvage pathway, resulting in a feedback loop of SIRT1- CLOCK-BMAL1 interaction (Nakahata, Sahar, Astarita, Kaluzova, & Sassone-Corsi, 2009; Ramsey et al., 2009). SIRT3 is also an important, NAD+-dependent, regulator of mitochondrial function and a recent study found diurnal rhythms in acetylation of SIRT3 protein targets in mouse liver (Mauvoisin et al., 2017). In addition to redox regulation, the molecular clock is involved in the antioxidant response. Studies in the mouse lung find that the nuclear factor erythroid-derived 2-like 2 (NRF2)/glutathione-mediated antioxidant pathway is under direct control of the molecular clock (Pekovic-Vaughan et al., 2014). NRF2 is controlled by CLOCK directly through E-box binding, resulting in rhythmic expression of NRF2, as well as its transcriptional target genes which are involved in glutathione redox homeostasis. ClockΔ19 mice have reduced levels of both NRF2 and glutathione and associated oxidative damage (Pekovic-Vaughan et al., 2014). It is likely that CLOCK is also regulating these pathways in the brain, though this has yet to be determined experimentally.
FIGURE 2.
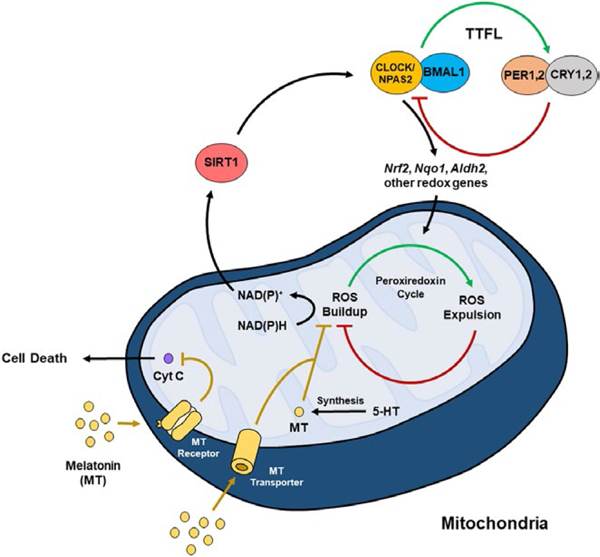
Interactions between the circadian clock and peroxiredoxin cycle. The transcription factors CLOCK, NPAS2, and BMAL1 make up the positive arm of the transcriptional-translational feedback loop (TTFL) and control the expression of period (PER) and cryptochrome (CRY). PER and CRY make up the negative arm of the TTFL and inhibit the activity of CLOCK/BMAL1, forming a negative feedback loop. CLOCK(NPAS2)/BMAL1 controls the expression of various output genes (including Nrf2, Nqo1, and Aldh2), which influences ROS accumulation in the peroxiredoxin cycle. Sirtuin 1 (SIRT1), an NAD+-dependent histone and protein deacetylase, regulates the activity of the CLOCK/BMAL1 complex. Interestingly, melatonin (MT) acts as an antioxidant to inhibit ROS. Melatonin enters the mitochondria through MT transporters and is also synthesized within the mitochondria. Melatonin can also bind to MT receptors on the mitochondria to inhibit cytochrome c release, which may be protective against cell death
Another prominent way in which circadian rhythms might contribute to the antioxidant response is through production of melatonin (Figure 2). Many studies have found that melatonin acts as a potent antioxidant. In fact, this appears to be its original function, as it was discovered to be involved in the antioxidant response in bacteria, which evolved several billion years ago (Manchester, Poeggeler, Alvares, Ogden, & Reiter, 1995; Tan et al., 2010). More recent studies have discovered that melatonin is present at high concentrations in mitochondria due to both internal transport by PEPT1/2 and direct synthesis within the mitochondria (Reiter et al., 2018; Suofu et al., 2017). Moreover, melatonin type 1 receptors (MT1), the associated G protein, and β-arrestins are all expressed on and within neuronal mitochondria (Suofu et al., 2017). Melatonin synthesized in the mitochondria is released by the organelle where it then binds and activates MT1 receptor-associated signal transduction cascades, which inhibit stress-mediated cytochrome c release and caspase activation, protecting the cell from programed cell death (Suofu et al., 2017). While melatonin produced by the pineal gland is strongly controlled by diurnal SCN activity and light exposure, it is unclear whether melatonin production or signaling within the mitochondria is also under circadian control. Suofu et al. (2017) examined levels of the rate limiting enzyme in melatonin synthesis, (AANAT), in brain non-synaptosomal mitochondria at 2:00 a.m. and 2:00 p.m. and found that in contrast to pineal tissue, there was no fluctuation in mitochondrial AANAT at these two timepoints. Thus, it is possible that only melatonin, which is transported into mitochondria, displays such diurnal variations and would be altered by disrupted environmental rhythms. Future studies are needed to determine the exact role of melatonin signaling within mitochondria and how disruptions to this system may contribute to psychiatric disorders.
9 |. CONCLUSIONS
Our knowledge of the circadian clock and its potential role in mood regulation has expanded over the recent years. The circadian clock interacts reciprocally with multiple systems and processes in the central nervous system and periphery, including monoaminergic and glutamatergic transmission, HPA axis function, metabolism, and immune function (Figure 3). Given the diversity of processes regulated by the circadian clock, it is likely that circadian rhythm disruption may alter mood through multiple systems. However, despite the progress that has been made over the years, some questions remain regarding the exact mechanisms by which circadian rhythms influence mood. Future studies using animal models will be necessary to clarify the causative roles that circadian rhythms play in mood regulation in each of the systems described above. Additional genetic studies will also further elucidate the roles that individual circadian clock genes in central and peripheral tissues play in mood regulation. Ultimately, a better understanding of the molecular mechanisms underlying the interactions between circadian rhythms and mood may lead to the development of novel therapeutic targets for the treatment of mood disorders and the circadian rhythm disturbances associated with these conditions.
FIGURE 3.
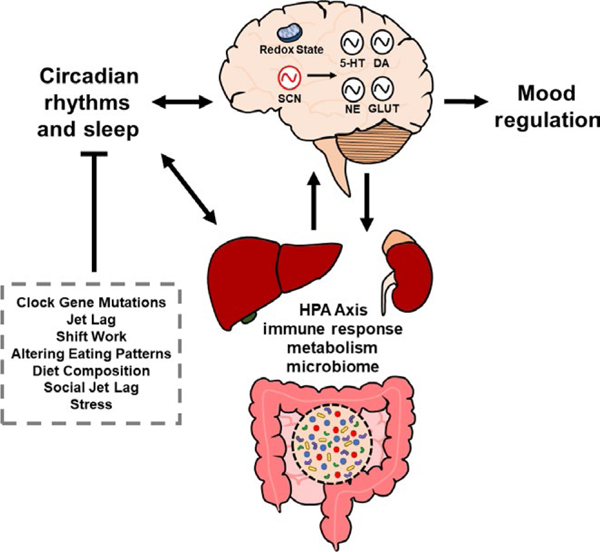
The circadian system interacts reciprocally with a variety of physiological processes and systems that are involved in mood regulation. These processes include monoamine and glutamatergic signaling, HPA axis function, immune response, metabolism, and microbiome. Both environmental and genetic disruptions to circadian rhythms may produce alterations in these systems that ultimately affect mood. SCN, suprachiasmatic nucleus; 5-HT, serotonin; DA, dopamine; NE, norepinephrine; GLUT, glutamate
ACKNOWLEDGEMENTS
Work from our group was funded by NARSAD, IMHRO, MH106460, DA039865, and T32MH016804 (KDK).
Funding information
National Alliance for Research on Schizophrenia and Depression; National Institute on Drug Abuse, Grant/Award Number: DA039865; National Institute of Mental Health, Grant/Award Number: MH106460 and T32MH016804; International Mental Health Research Organization
Abbreviations:
- 5-HT
5-hydroxytryptamine
- AANAT
aralkylamine N-acetyltransferase
- ACTH
adrenocorticotropic hormone
- ATP
adenosine triphosphate
- BDNF
brain-derived neurotrophic factor
- BMAL1
Brain and Muscle Arnt-like Protein 1
- CCK
cholecystokinin
- CHRONO
chlP-derived repressor of network oscillator
- CLOCK
Circadian Locomotor Output Cycles Kaput
- CNS
central nervous system
- CREB
cAMP response element-binding protein
- CRF
corticotropin-releasing factor
- CRY
cryptochrome
- DA
dopamine
- DAT
dopamine transporter
- E-box
enhancer box
- EEG
electroencephalography
- GABA
γ-aminobutyric acid
- GLUT
glutamate
- GR
glucocorticoid receptor
- GSK
glycogen synthase kinase
- GWAS
genome-wide association studies
- HPA
hypothalamic-pituitary-adrenal
- IFN
interferon
- IL
interleukin
- ipRGC
intrinsically photosensitive retinal ganglion cells
- LPS
lipopolysaccharide
- MAOA
monoamine oxidase A
- MDD
major depressive disorder
- MT1
melatonin type 1 receptor
- MT
melatonin
- NAc
nucleus accumbens
- NAD
nicotinamide adenine dinucleotide
- NAMPT
nicotinamide phosphoribosyltransferase
- NE
norepinephrine
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NK
natural killer
- NMDA
N-methyl-D-aspartate
- NPAS2
Neuronal PAS Domain Protein 2
- NREM
non-rapid eye movement
- NRF2
nuclear factor erythroid-derived 2-like 2
- PER
period
- PFC
prefrontal cortex
- PVN
paraventricular nucleus of the hypothalamus
- ROS
reactive oxygen species
- SCN
suprachiasmatic nucleus
- SD
sleep deprivation
- SIRT
Sirtuin
- SST
somatostatin
- SWA
slow-wave activity
- TH
tyrosine hydroxylase
- TTFL
transciptional-translational feedback loop
- VTA
ventral tegmental area
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest.
REFERENCES
- Adamantidis A, & de Lecea L (2008). Sleep and metabolism: Shared circuits, new connections. Trends in Endocrinology and Metabolism, 19, 362–370. 10.1016/j.tem.2008.08.007 [DOI] [PubMed] [Google Scholar]
- Albrecht U, & Ripperger JA (2018). Circadian clocks and sleep: Impact of rhythmic metabolism and waste clearance on the brain. Trends in Neurosciences, 41, 677–688. 10.1016/j.tins.2018.07.007 [DOI] [PubMed] [Google Scholar]
- Allen J, Romay-Tallon R, Brymer KJ, Caruncho HJ, & Kalynchuk LE (2018). Mitochondria and mood: Mitochondrial dysfunction as a key player in the manifestation of depression. Frontiers in Neuroscience, 12, 386 10.3389/fnins.2018.00386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Safadi S, Al-Safadi A, Branchaud M, Rutherford S, Dayanandan A, Robinson B, & Amir S (2014). Stress-induced changes in the expression of the clock protein PERIOD1 in the rat limbic forebrain and hypothalamus: Role of stress type, time of day, and predictability. PLoS ONE, 9, e111166 10.1371/journal.pone.0111166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Safadi S, Branchaud M, Rutherford S, & Amir S (2015). Glucocorticoids and stress-induced changes in the expression of PERIOD1 in the rat forebrain. PLoS ONE, 10, e0130085 10.1371/journal.pone.0130085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amare AT, Schubert KO, Klingler-Hoffmann M, Cohen-Woods S, & Baune BT (2017). The genetic overlap between mood disorders and cardiometabolic diseases: A systematic review of genome wide and candidate gene studies. Translational Psychiatry, 7, e1007 10.1038/tp.2016.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anafi RC, Lee Y, Sato TK, Venkataraman A, Ramanathan C, Kavakli IH, ... Hogenesch JB (2014). Machine learning helps identify CHRONO as a circadian clock component. PLoS Biology, 12, e1001840 10.1371/journal.pbio.1001840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews JL, Zhang X, McCarthy JJ, McDearmon EL, Hornberger TA, Russell B, ... Esser KA (2010). CLOCK and BMAL1 regulate MyoD and are necessary for maintenance of skeletal muscle phenotype and function. Proceedings of the National Academy of Sciences of the United States of America, 107, 19090–19095. 10.1073/pnas.1014523107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arey RN, Enwright JF 3rd, Spencer SM, Falcon E, Ozburn AR, Ghose S, ... McClung CA (2014). An important role for cho-lecystokinin, a CLOCK target gene, in the development and treatment of manic-like behaviors. Molecular Psychiatry, 19, 342–350. 10.1038/mp.2013.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arjona A, & Sarkar DK (2005). Circadian oscillations of clock genes, cytolytic factors, and cytokines in rat NK cells. Journal of Immunology, 174, 7618–7624. 10.4049/jimmunol.174.12.7618 [DOI] [PubMed] [Google Scholar]
- Arjona A, & Sarkar DK (2006). Evidence supporting a circadian control of natural killer cell function. Brain, Behavior, and Immunity, 20, 469–476. 10.1016/j.bbi.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Asaoka S, Aritake S, Komada Y, Ozaki A, Odagiri Y, Inoue S, ... Inoue Y (2013). Factors associated with shift work disorder in nurses working with rapid-rotation schedules in Japan: The nurses’ sleep health project. Chronobiology International, 30, 628–636. 10.3109/07420528.2012.762010 [DOI] [PubMed] [Google Scholar]
- Asher G, Gatfield D, Stratmann M, Reinke H, Dibner C, Kreppel F, ... Schibler U (2008). SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell, 134, 317–328. 10.1016/j.cell.2008.06.050 [DOI] [PubMed] [Google Scholar]
- Ashkenazy T, Einat H, & Kronfeld-Schor N (2009a). Effects of bright light treatment on depression- and anxiety-like behaviors of diurnal rodents maintained on a short daylight schedule. Behavioral Brain Research, 201, 343–346. https ://doi.org/10.1016/j.bbr.2009.03.005 [DOI] [PubMed] [Google Scholar]
- Ashkenazy T, Einat H, & Kronfeld-Schor N (2009b). We are in the dark here: Induction of depression- and anxiety-like behaviours in the diurnal fat sand rat, by short daylight or melatonin injections. International Journal of Neuropsychopharmacology, 12, 83–93. 10.1017/S1461145708009115 [DOI] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, ... Monteggia LM (2011). NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature, 475, 91–95. 10.1038/nature10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsalobre A, Brown SA, Marcacci L, Tronche F, Kellendonk C, Reichardt HM, ... Schibler U (2000). Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science, 289, 2344–2347. 10.1126/science.289.5488.2344 [DOI] [PubMed] [Google Scholar]
- Barandas R, Landgraf D, McCarthy MJ, & Welsh DK (2015). Circadian clocks as modulators of metabolic comorbidity in psychiatric disorders. Current Psychiatry Reports, 17, 98 10.1007/s11920-015-0637-2 [DOI] [PubMed] [Google Scholar]
- Barik J, Parnaudeau S, Saint Amaux AL, Guiard BP, Golib Dzib JF, Bocquet O, ... Tronche F (2010). Glucocorticoid receptors in dopaminoceptive neurons, key for cocaine, are dispensable for molecular and behavioral morphine responses. Biological Psychiatry, 68, 231–239. 10.1016/j.biopsych.2010.03.037 [DOI] [PubMed] [Google Scholar]
- Bauer ME, & Teixeira AL (2018). Inflammation in psychiatric disorders: What comes first? Annals of the New York Academy of Sciences, 10.1111/nyas.13712. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Becker-Krail D, & McClung C (2016). Implications of circadian rhythm and stress in addiction vulnerability. F1000Research, 5, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellet MM, Vawter MP, Bunney BG, Bunney WE, & SassoneCorsi P (2011). Ketamine influences CLOCK:BMAL1 function leading to altered circadian gene expression. PLoS ONE, 6, e23982 10.1371/journal.pone.0023982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Hamo M, Larson TA, Duge LS, Sikkema C, Wilkinson CW, de la Iglesia HO, & Gonzalez MM (2016). Circadian forced desynchrony of the master clock leads to phenotypic manifestation of depression in rats. eNeuro, 3, 10.1523/ENEURO.0237-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bering T, Carstensen MB, Wortwein G, Weikop P, & Rath MF (2018). The circadian oscillator of the cerebral cortex: Molecular, biochemical and behavioral effects of deleting the arntl clock gene in cortical neurons. Cerebral Cortex, 28, 644–657. [DOI] [PubMed] [Google Scholar]
- Blasiak A, Gundlach AL, Hess G, & Lewandowski MH (2017). Interactions of circadian rhythmicity, stress and orexigenic neuropeptide systems: Implications for food intake control. Frontiers in Neurosciences, 11, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnavion P, Jackson AC, Carter ME, & de Lecea L (2015). Antagonistic interplay between hypocretin and leptin in the lateral hypothalamus regulates stress responses. Nature Communications, 6, 6266 10.1038/ncomms7266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borbely AA, & Wirz-Justice A (1982). Sleep, sleep deprivation and depression. A hypothesis derived from a model of sleep regulation. Human Neurobiology, 1, 205–210. [PubMed] [Google Scholar]
- Brancaccio M, Patton AP, Chesham JE, Maywood ES, & Hastings MH (2017). Astrocytes control circadian timekeeping in the suprachiasmatic nucleus via glutamatergic signaling. Neuron, 93, 1420–1435 e1425. 10.1016/j.neuron.2017.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon-Cervantes O, Wu M, Ehlen JC, Paul K, Gamble KL, Johnson RL, ... Davidson AJ (2010). Dysregulation of inflammatory responses by chronic circadian disruption. Journal of Immunology, 185, 5796–5805. 10.4049/jimmunol.1001026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, Chrousos GP, Lambrou GI, Pavlaki A, Koide H, Ng SS, & Kino T (2011). Peripheral CLOCK regulates target-tissue glucocorticoid receptor transcriptional activity in a circadian fashion in man. PLoS ONE, 6, e25612 10.1371/journal.pone.0025612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chenu F, El Mansari M, & Blier P (2013). Electrophysiological effects of repeated administration of agomelatine on the dopamine, norepinephrine, and serotonin systems in the rat brain. Neuropsychopharmacology, 38, 275–284. 10.1038/npp.2012.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung S, Lee EJ, Yun S, Choe HK, Park SB, Son HJ, ... Kim K (2014). Impact of circadian nuclear receptor REV-ERBalpha on midbrain dopamine production and mood regulation. Cell, 157, 858–868. 10.1016/jcell.2014.03.039 [DOI] [PubMed] [Google Scholar]
- Ciarleglio CM, Resuehr HE, & McMahon DG (2011). Interactions of the serotonin and circadian systems: Nature and nurture in rhythms and blues. Neuroscience, 197, 8–16. 10.1016/j.neuroscience.2011.09.036 [DOI] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, ... Cryan JF (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry, 18, 666–673. 10.1038/mp.2012.77 [DOI] [PubMed] [Google Scholar]
- Clay HB, Sillivan S, & Konradi C (2011). Mitochondrial dys-function and pathology in bipolar disorder and schizophrenia. International Journal of Developmental Neuroscience, 29, 311–324. 10.1016/jijdevneu.2010.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comai S, Ochoa-Sanchez R, Dominguez-Lopez S, Bambico FR, & Gobbi G (2015). Melancholic-Like behaviors and circadian neurobiological abnormalities in melatonin MT1 receptor knock-out mice. International Journal of Neuropsychopharmacology, 18, 10.1093/ijnp/pyu075 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coque L, Mukherjee S, Cao JL, Spencer S, Marvin M, Falcon E, ... McClung CA (2011). Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology, 36, 1478–1488. 10.1038/npp.2011.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuesta M, Clesse D, Pevet P, & Challet E (2009). New light on the serotonergic paradox in the rat circadian system. Journal of Neurochemistry, 110, 231–243. 10.1111/j.1471-4159.2009.06128.x [DOI] [PubMed] [Google Scholar]
- Cuesta M, Mendoza J, Clesse D, Pevet P, & Challet E (2008). Serotonergic activation potentiates light resetting of the main circadian clock and alters clock gene expression in a diurnal rodent. Experimental Neurology, 210, 501–513. 10.1016/j.expneurol.2007.11.026 [DOI] [PubMed] [Google Scholar]
- Cui H, Lopez M, & Rahmouni K (2017). The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nature Reviews Endocrinology, 13, 338–351. 10.1038/nrendo.2016.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cussotto S, Sandhu KV, Dinan TG, & Cryan JF (2018). The neuroendocrinology of the microbiota-gut-brain axis: A behavioural perspective. Frontiers in Neuroendocrinology, 51, 80–101. [DOI] [PubMed] [Google Scholar]
- Der-Avakian A, Bland ST, Schmid MJ, Watkins LR, Spencer RL, & Maier SF (2006). The role of glucocorticoids in the uncontrollable stress-induced potentiation of nucleus accumbens shell dopamine and conditioned place preference responses to morphine. Psychoneuroendocrinology, 31, 653–663. 10.1016/j.psyneuen.2006.02.004 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Loddo P, & Tanda G (1999). Reciprocal changes in prefrontal and limbic dopamine responsiveness to aversive and rewarding stimuli after chronic mild stress: Implications for the psychobiology of depression. Biological Psychiatry, 46, 1624–1633. 10.1016/S0006-3223(99)00236-X [DOI] [PubMed] [Google Scholar]
- Dinan TG, & Cryan JF (2017). Gut-brain axis in 2016: Brain-gut-microbiota axis – mood, metabolism and behaviour. Nature Reviews Gastroenterology & Hepatology, 14, 69–70. 10.1038/nrgastro.2016.200 [DOI] [PubMed] [Google Scholar]
- Du F, Zhu XH, Zhang Y, Friedman M, Zhang N, Ugurbil K, & Chen W (2008). Tightly coupled brain activity and cerebral ATP metabolic rate. Proceedings of the National Academy of Sciences of the United States of America, 105, 6409–6414. 10.1073/pnas.0710766105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulcis D, Jamshidi P, Leutgeb S, & Spitzer NC (2013). Neurotransmitter switching in the adult brain regulates behavior. Science, 340, 449–453. 10.1126/science.1234152 [DOI] [PubMed] [Google Scholar]
- Duman RS (2018). Ketamine and rapid-acting antidepressants: A new era in the battle against depression and suicide. F1000Research, 7, 10.12688/f1000research.14344.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC Jr, Ballard ED, & Zarate CA (2017). Ketamine-induced glutamatergic mechanisms of sleep and wakefulness: Insights for developing novel treatments for disturbed sleep and mood. Handbook of Experimental Pharmacology, 10.1007/164_2017_51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, ... Zarate CA (2013). Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. The International Journal of Neuropsychopharmacology/official scientific journal of the Collegium Internationale Neuropsychopharmacologicum, 16, 301–311. 10.1017/S1461145712000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC Jr,Selter J, Brutsche N, Sarasso S, & Zarate CA Jr (2013). Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. Journal of Affective Disorders, 145, 115–119. 10.1016/j.jad.2012.05.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan WC Jr, Slonena E, Hejazi NS, Brutsche N, Yu KC, Park L, ... Zarate CA Jr (2017). Motor-activity markers of circadian timekeeping are related to Ketamine’s rapid antidepressant properties. Biological Psychiatry, 82, 361–369. 10.1016/j.biopsych.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers CL, Frank E, & Kupfer DJ (1988). Social zeitgebers and biological rhythms. A unified approach to understanding the etiology of depression. Archives of General Psychiatry, 45, 948–952. 10.1001/archpsyc.1988.01800340076012 [DOI] [PubMed] [Google Scholar]
- Einat H, Kronfeld-Schor N, & Eilam D (2006). Sand rats see the light: Short photoperiod induces a depression-like response in a diurnal rodent. Behavioral Brain Research, 173, 153–157. 10.1016/j.bbr.2006.06.006 [DOI] [PubMed] [Google Scholar]
- Etain B, Milhiet V, Bellivier F, & Leboyer M (2011). Genetics of circadian rhythms and mood spectrum disorders. European Neuropsychopharmacology, 21(Suppl. 4), S676–S682. 10.1016/j.euroneuro.2011.07.007 [DOI] [PubMed] [Google Scholar]
- Feillet CA (2010). Food for thoughts: Feeding time and hormonal secretion. Journal of Neuroendocrinology, 22, 620–628. 10.1111/j.1365-2826.2010.01998.x [DOI] [PubMed] [Google Scholar]
- Fernandez DC, Fogerson PM, Lazzerini Ospri L, Thomsen MB, Layne RM, Severin D, ... Hattar S (2018). Light affects mood and learning through distinct retina-brain pathways. Cell, 175, 71–84 e18. 10.1016/j.cell.2018.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay JM, Zigmond MJ, & Abercrombie ED (1995). Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: Effects of diazepam. Neuroscience, 64, 619–628. 10.1016/0306-4522(94)00331-X [DOI] [PubMed] [Google Scholar]
- Fonken LK, Weil ZM, & Nelson RJ (2013). Mice exposed to dim light at night exaggerate inflammatory responses to lipopoly-saccharide. Brain, Behavior, and Immunity, 34, 159–163. 10.1016/j.bbi.2013.08.011 [DOI] [PubMed] [Google Scholar]
- Frank MG, Baratta MV, Sprunger DB, Watkins LR, & Maier SF (2007). Microglia serve as a neuroimmune substrate for stress-induced potentiation of CNS pro-inflammatory cytokine responses. Brain, Behavior, and Immunity, 21, 47–59. 10.1016/j.bbi.2006.03.005 [DOI] [PubMed] [Google Scholar]
- Garza JC, Guo M, Zhang W, & Lu XY (2012). Leptin restores adult hippocampal neurogenesis in a chronic unpredictable stress model of depression and reverses glucocorticoid-induced inhibition of GSK-3beta/beta-catenin signaling. Molecular Psychiatry, 17, 790–808. 10.1038/mp.2011.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain A, & Kupfer DJ (2008). Circadian rhythm disturbances in depression. Human Psychopharmacology, 23, 571–585. 10.1002/hup.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Goede P, Wefers J, Brombacher EC, Schrauwen P, & Kalsbeek A (2018). Circadian rhythms in mitochondrial respiration. Journal of Molecular Endocrinology, 60, R115–R130. 10.1530/JME-17-0196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold PW, & Chrousos GP (2002). Organization of the stress system and its dysregulation in melancholic and atypical depression: High vs low CRH/NE states. Molecular Psychiatry, 7, 254–275. 10.1038/sj.mp.4001032 [DOI] [PubMed] [Google Scholar]
- Gonzalez MM, & Aston-Jones G (2008). Light deprivation damages monoamine neurons and produces a depressive behavioral phenotype in rats. Proceedings of the National Academy of Sciences of the United States of America, 105, 4898–4903. 10.1073/pnas.0703615105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goriki A, Hatanaka F, Myung J, Kim JK, Yoritaka T, Tanoue S, ... Takumi T (2014). A novel protein, CHRONO, functions as a core component of the mammalian circadian clock. PLoS Biology, 12, e1001839 10.1371/journal.pbio.1001839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafe LA, & Bhatnagar S (2018). Orexins and stress. Frontiers in Neuroendocrinology, 51, 132–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardiola-Lemaitre B, De Bodinat C, Delagrange P, Millan MJ, Munoz C, & Mocaer E (2014). Agomelatine: Mechanism of action and pharmacological profile in relation to antidepressant properties. British Journal of Pharmacology, 171, 3604–3619. 10.1111/bph.12720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Huang TY, Garza JC, Chua SC, & Lu XY (2013). Selective deletion of leptin receptors in adult hippocampus induces depression-related behaviours. International Journal of Neuropsychopharmacology, 16, 857–867. 10.1017/S1461145712000703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M, Lu Y, Garza JC, Li Y, Chua SC, Zhang W, ... Lu XY (2012). Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Translational Psychiatry, 2, e83 10.1038/tp.2012.9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Shimba S, & Tezuka M (2007). Characterization of the molecular clock in mouse peritoneal macrophages. Biological and Pharmaceutical Bulletin, 30, 621–626. 10.1248/bpb.30.621 [DOI] [PubMed] [Google Scholar]
- Hinkelmann K, Moritz S, Botzenhardt J, Muhtz C, Wiedemann K, Kellner M, & Otte C (2012). Changes in cortisol secretion during antidepressive treatment and cognitive improvement in patients with major depression: A longitudinal study. Psychoneuroendocrinology, 37, 685–692. 10.1016/j.psyneuen.2011.08.012 [DOI] [PubMed] [Google Scholar]
- Inder ML, Crowe MT, & Porter R (2016). Effect of transmeridian travel and jetlag on mood disorders: Evidence and implications. Australian and New Zealand Journal of Psychiatry, 50, 220–227. 10.1177/0004867415598844 [DOI] [PubMed] [Google Scholar]
- Ishida A, Mutoh T, Ueyama T, Bando H, Masubuchi S, Nakahara D, ... Okamura H (2005). Light activates the adrenal gland: Timing of gene expression and glucocorticoid release. Cell Metabolism, 2, 297–307. 10.1016/j.cmet.2005.09.009 [DOI] [PubMed] [Google Scholar]
- James MH, Campbell EJ, & Dayas CV (2017). Role of the orexin/hypocretin system in stress-related psychiatric disorders. Current Topics in Behavioral Neurosciences, 33, 197–219. 10.1007/978-3-319-57535-3 [DOI] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, ... Ruan B (2015). Altered fecal microbiota composition in patients with major depressive disorder. Brain, Behavior, and Immunity, 48, 186–194. 10.1016/j.bbi.2015.03.016 [DOI] [PubMed] [Google Scholar]
- Johansson AS, Brask J, Owe-Larsson B, Hetta J, & Lundkvist GB (2011). Valproic acid phase shifts the rhythmic expression of Period2:Luciferase. Journal of Biological Rhythms, 26, 541–551. 10.1177/0748730411419775 [DOI] [PubMed] [Google Scholar]
- Jokela M, Hamer M, Singh-Manoux A, Batty GD, & Kivimaki M (2014). Association of metabolically healthy obesity with depressive symptoms: Pooled analysis of eight studies. Molecular Psychiatry, 19, 910–914. 10.1038/mp.2013.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmbach DA, Pillai V, Cheng P, Arnedt JT, & Drake CL (2015). Shift work disorder, depression, and anxiety in the transition to rotating shifts: The role of sleep reactivity. Sleep Medicine, 16, 1532–1538. https://doi.org/10.1016Zj.sleep.2015.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, O’Brien C, Patterson E, El Aidy S, Deane J, ... Dinan TG (2016). Transferring the blues: Depression-associated gut microbiota induces neurobehavioural changes in the rat. Journal of Psychiatric Research, 82, 109–118. 10.1016/j.jpsychires.2016.07.019 [DOI] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, & Hyland NP (2015). Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Frontiers in Cellular Neuroscience, 9, 392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy PJ, Cryan JF, Dinan TG, & Clarke G (2014). Irritable bowel syndrome: A microbiome-gut-brain axis disorder? World Journal of Gastroenterology, 20, 14105–14125. 10.3748/wjg.v20.i39.14105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS (1950). Blood flow and metabolism of the human brain in health and disease. Transactions and Studies of the College of Physicians of Philadelphia, 18, 103–108. [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, ... Takahashi JS (1997). Positional cloning of the mouse circadian clock gene. Cell, 89, 641–653. 10.1016/S0092-8674(00)80245-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kino T, & Chrousos GP (2011). Acetylation-mediated epigenetic regulation of glucocorticoid receptor activity: Circadian rhythm-associated alterations of glucocorticoid actions in target tissues. Molecular and Cellular Endocrinology, 336, 23–30. 10.1016/j.mce.2010.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch CE, Leinweber B, Drengberg BC, Blaum C, & Oster H (2017). Interaction between circadian rhythms and stress. Neurobiology of Stress, 6, 57–67. 10.1016/).ynstr.2016.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopschina Feltes P, Doorduin J, Klein HC, Juarez-Orozco LE, Dierckx RA, Moriguchi-Jeckel CM, & de Vries EF (2017). Anti-inflammatory treatment for major depressive disorder: Implications for patients with an elevated immune profile and non-responders to standard antidepressant therapy. Journal of Psychopharmacology, 31, 1149–1165. 10.1177/0269881117711708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen M, Nierenberg AA, & Ostergaard SD (2018). Face and predictive validity of the ClockDelta19 mouse as an animal model for bipolar disorder: A systematic review. Molecular Psychiatry, 23, 70–80. 10.1038/mp.2017.192 [DOI] [PubMed] [Google Scholar]
- Kusanagi H, Mishima K, Satoh K, Echizenya M, Katoh T, & Shimizu T (2004). Similar profiles in human period1 gene expression in peripheral mononuclear and polymorphonuclear cells. Neuroscience Letters, 365, 124–127. 10.1016/j.neulet.2004.04.065 [DOI] [PubMed] [Google Scholar]
- Lamia KA, Papp SJ, Yu RT, Barish GD, Uhlenhaut NH, Jonker JW, ... Evans RM (2011). Cryptochromes mediate rhythmic repression of the glucocorticoid receptor. Nature, 480, 552–556. 10.1038/nature10700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, Long JE, Proulx CD, Barandas R, Malinow R, & Welsh DK (2016). Genetic disruption of circadian rhythms in the suprachiasmatic nucleus causes helplessness, behavioral despair, and anxiety-like behavior in mice. Biological Psychiatry, 80, 827–835. 10.1016/j.biopsych.2016.03.1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf D, McCarthy MJ, & Welsh DK (2014). Circadian clock and stress interactions in the molecular biology of psychiatric disorders. Current Psychiatry Reports, 16, 483 10.1007/s11920-014-0483-7 [DOI] [PubMed] [Google Scholar]
- Lazaroff M, Patankar S, Yoon SO, & Chikaraishi DM (1995). The cyclic AMP response element directs tyrosine hydroxylase expression in catecholaminergic central and peripheral nervous system cell lines from transgenic mice. The Journal of Biological Chemistry, 270, 21579–21589. 10.1074/jbc.270.37.21579 [DOI] [PubMed] [Google Scholar]
- LeGates TA, Altimus CM, Wang H, Lee HK, Yang S, Zhao H, ... Hattar S (2012). Aberrant light directly impairs mood and learning through melanopsin-expressing neurons. Nature, 491, 594–598. 10.1038/nature11673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leliavski A, Shostak A, Husse J, & Oster H (2014). Impaired glucocorticoid production and response to stress in Arntl-deficient male mice. Endocrinology, 155, 133–142. 10.1210/en.2013-1531 [DOI] [PubMed] [Google Scholar]
- Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, ... Chang EB (2015). Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host & Microbe, 17, 681–689. 10.1016/j.chom.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, ... Bunney WE (2013). Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proceedings of the National Academy of Sciences of the United States of America, 110, 9950–9955. 10.1073/pnas.1305814110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, ... Duman RS (2010). mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science, 329, 959–964. 10.1126/science.1190287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lu WQ, Beesley S, Loudon AS, & Meng QJ (2012). Lithium impacts on the amplitude and period of the molecular circadian clockwork. PLoS ONE, 7, e33292 10.1371/journal.pone.0033292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Bushman FD, & FitzGerald GA (2015). Rhythmicity of the intestinal microbiota is regulated by gender and the host circadian clock. Proceedings of the National Academy of Sciences of the United States of America, 112, 10479–10484. 10.1073/pnas.1501305112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, ... Li Q (2017). Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. Journal of Affective Disorders, 207, 300–304. 10.1016/j.jad.2016.09.051 [DOI] [PubMed] [Google Scholar]
- Linkowski P, Mendlewicz J, Kerkhofs M, Leclercq R, Golstein J, Brasseur M, ... Van Cauter E (1987). 24-hour profiles of adrenocorticotropin, cortisol, and growth hormone in major depressive illness: Effect of antidepressant treatment. Journal of Clinical Endocrinology and Metabolism, 65, 141–152. 10.1210/jcem-65-1-141 [DOI] [PubMed] [Google Scholar]
- Liu W, Liu J, Xia J, Xue X, Wang H, Qi Z, & Ji L (2017). Leptin receptor knockout-induced depression-like behaviors and attenuated antidepressant effects of exercise are associated with STAT3/SOCS3 signaling. Brain, Behavior, and Immunity, 61, 297–305. 10.1016/j.bbi.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Liu J, Malkani G, Shi X, Meyer M, Cunningham-Runddles S, Ma X, & Sun ZS (2006). The circadian clock Period 2 gene regulates gamma interferon production of NK cells in host response to lipopolysaccharide-induced endotoxic shock. Infection and Immunity, 74, 4750–4756. 10.1128/IAI.00287-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Edgar N, Gillman AG, Hoffman D, Zhu X, & McClung CA (2015). Chronic stress induces brain region-specific alterations of molecular rhythms that correlate with depression-like behavior in mice. Biological Psychiatry, 78, 249–258. 10.1016/j.biopsych.2015.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, & McClung CA (2016). Animal models of bipolar mania: The past, present and future. Neuroscience, 321, 163–188. 10.1016/j.neuroscience.2015.08.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Parekh PK, Kaplan GN, Becker-Krail DD, Williams WP 3rd, Yamaguchi S, ... McClung CA (2018). NAD+ cellular redox and SIRT1 regulate the diurnal rhythms of tyrosine hydroxylase and conditioned cocaine reward. Molecular Psychiatry, 10.1038/s41380-018-0061-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY (2007). The leptin hypothesis of depression: A potential link between mood disorders and obesity? Current Opinion in Pharmacology, 7, 648–652. 10.1016/j.coph.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Kim CS, Frazer A, & Zhang W (2006). Leptin: A potential novel antidepressant. Proceedings of the National Academy of Sciences of the United States of America, 103, 1593–1598. 10.1073/pnas.0508901103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundkvist GB, Hill RH, & Kristensson K (2002). Disruption of circadian rhythms in synaptic activity of the suprachiasmatic nuclei by African trypanosomes and cytokines. Neurobiology of Disease, 11, 20–27. 10.1006/nbdi.2002.0536 [DOI] [PubMed] [Google Scholar]
- Maes M, Kubera M, & Leunis JC (2008). The gut-brain barrier in major depression: Intestinal mucosal dysfunction with an increased translocation of LPS from gram negative enterobacteria (leaky gut) plays a role in the inflammatory pathophysiology of depression. Neuro Endocrinology Letters, 29, 117–124. [PubMed] [Google Scholar]
- Manchester LC, Poeggeler B, Alvares FL, Ogden GB, & Reiter RJ (1995). Melatonin immunoreactivity in the photosynthetic prokaryote Rhodospirillum rubrum: Implications for an ancient antioxidant system. Cellular and Molecular Biology Research, 41, 391–395. [PubMed] [Google Scholar]
- Manji H, Kato T, Di Prospero NA, Ness S, Beal MF, Krams M, & Chen G (2012). Impaired mitochondrial function in psychiatric disorders. Nature Reviews. Neuroscience, 13, 293–307. 10.1038/nrn3229 [DOI] [PubMed] [Google Scholar]
- Mansur RB, Brietzke E, & McIntyre RS (2015). Is there a “metabolic-mood syndrome”? A review of the relationship between obesity and mood disorders. Neuroscience and Biobehavioral Reviews, 52, 89–104. 10.1016/j.neubiorev.2014.12.017 [DOI] [PubMed] [Google Scholar]
- Marazziti D, Baroni S, Picchetti M, Landi P, Silvestri S, Vatteroni E, & Catena Dell’Osso M (2011). Mitochondrial alterations and neuropsychiatric disorders. Current Medicinal Chemistry, 18, 4715–4721. 10.2174/092986711797379221 [DOI] [PubMed] [Google Scholar]
- Marpegan L, Bekinschtein TA, Costas MA, & Golombek DA (2005). Circadian responses to endotoxin treatment in mice. Journal of Neuroimmunology, 160, 102–109. 10.1016/j.jneuroim.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Mauvoisin D, Atger F, Dayon L, Nunez Galindo A, Wang J, Martin E, ... Gachon F (2017). Circadian and feeding rhythms orchestrate the diurnal liver acetylome. Cell Reports, 20, 1729–1743. 10.1016/j.celrep.2017.07.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MJ, & Welsh DK (2012). Cellular circadian clocks in mood disorders. Journal of Biological Rhythms, 27, 339–352. 10.1177/0748730412456367 [DOI] [PubMed] [Google Scholar]
- McClung CA (2007). Circadian genes, rhythms and the biology of mood disorders. Pharmacology & Therapeutics, 114, 222–232. 10.1016/j.pharmthera.2007.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA (2011). Circadian rhythms and mood regulation: Insights from pre-clinical models. European Neuropsychopharmacology, 21 (Suppl. 4), S683–S693. 10.1016/j.euroneuro.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA (2013). How might circadian rhythms control mood? Let me count the ways. Biological Psychiatry, 74, 242–249. 10.1016/j.biopsych.2013.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClung CA, Sidiropoulou K, Vitaterna M, Takahashi JS, White FJ, Cooper DC, & Nestler EJ (2005). Regulation of dopaminergic transmission and cocaine reward by the Clock gene. Proceedings of the National Academy of Sciences of the United States of America, 102, 9377–9381. 10.1073/pnas.0503584102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menard C, Pfau ML, Hodes GE, & Russo SJ (2017). Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology, 42, 62–80. 10.1038/npp.2016.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JH (2008). Applying neuroimaging ligands to study major depressive disorder. Seminars in Nuclear Medicine, 38, 287–304. 10.1053/j.semnuclmed.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Micale V, Arezzi A, Rampello L, & Drago F (2006). Melatonin affects the immobility time ofrats in the forced swim test: The role of serotonin neurotransmission. European Neuropsychopharmacology, 16, 538–545. 10.1016/j.euroneuro.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Lamers F, Bot M, Drent ML, & Penninx BW (2017). Leptin dysregulation is specifically associated with major depression with atypical features: Evidence for a mechanism connecting obesity and depression. Biological Psychiatry, 81, 807–814. 10.1016/j.biopsych.2015.10.023 [DOI] [PubMed] [Google Scholar]
- Milaneschi Y, Simmons WK, van Rossum EFC, & Penninx BW (2018). Depression and obesity: Evidence of shared biological mechanisms. Molecular Psychiatry, 10.1038/s41380-018-0017-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Moore RY, & Eichler VB (1972). Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research, 42, 201–206. 10.1016/0006-8993(72)90054-6 [DOI] [PubMed] [Google Scholar]
- Morris LS, Voon V, & Leggio L (2018). Stress, motivation, and the gut-brain axis: A focus on the ghrelin system and alcohol use disorder. Alcoholism, Clinical and Experimental Research, 42, 1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, ... McClung CA (2010). Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biological Psychiatry, 68, 503–511. 10.1016/j.biopsych.2010.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, & Holtzman DM (2016). Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science, 354, 1004–1008. 10.1126/science.aah4968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Lim MM, Yang G, Bauer AQ, Qi L, Lee Y, ... Fitzgerald GA (2013). Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. The Journal of Clinical Investigation, 123, 5389–5400. 10.1172/JCI70317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, & Kino T (2009). Circadian rhythm transcription factor CLOCK regulates the transcriptional activity of the glucocorticoid receptor by acetylating its hinge region lysine cluster: Potential physiological implications. The FASEB Journal, 23, 1572–1583. 10.1096/fj.08-117697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader N, Chrousos GP, & Kino T (2010). Interactions of the circadian CLOCK system and the HPA axis. Trends in Endocrinology and Metabolism, 21, 277–286. 10.1016/j.tem.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Kaluzova M, Grimaldi B, Sahar S, Hirayama J, Chen D, ... Sassone-Corsi P (2008). The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell, 134, 329–340. 10.1016/j.cell.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakahata Y, Sahar S, Astarita G, Kaluzova M, & Sassone-Corsi P (2009). Circadian control of the NAD+ salvage pathway by CLOCK-SIRT1. Science, 324, 654–657. 10.1126/science.1170803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nollet M, & Leman S (2013). Role of orexin in the pathophysiology of depression: Potential for pharmacological intervention. CNS Drugs, 27, 411–422. 10.1007/s40263-013-0064-z [DOI] [PubMed] [Google Scholar]
- Nygard M, Hill RH, Wikstrom MA, & Kristensson K (2005). Age-related changes in electrophysiological properties of the mouse suprachiasmatic nucleus in vitro. Brain Research Bulletin, 65, 149–154. 10.1016/j.brainresbull.2004.12.006 [DOI] [PubMed] [Google Scholar]
- Okabe T, Chavan R, Fonseca Costa SS, Brenna A, Ripperger JA, & Albrecht U (2016). REV-ERBalpha influences the stability and nuclear localization of the glucocorticoid receptor. Journal of Cell Science, 129, 4143–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Yano M, Doki Y, Azama T, Iwanaga H, Miki H, ... Monden M (2008). Injection of LPS causes transient suppression of biological clock genes in rats. The Journal of Surgical Research, 145, 5–12. https://doi.org/10.1016Zj.jss.2007.01.010 [DOI] [PubMed] [Google Scholar]
- de Oliveira C, Scarabelot VL, de Souza A, de Oliveira CM, Medeiros LF, de Macedo IC, ... Torres IL (2014). Obesity and chronic stress are able to desynchronize the temporal pattern of serum levels of leptin and triglycerides. Peptides, 51, 46–53. 10.1016/j.peptides.2013.10.024 [DOI] [PubMed] [Google Scholar]
- Olson CA, Vuong HE, Yano JM, Liang QY, Nusbaum DJ, & Hsiao EY (2018). The gut microbiota mediates the anti-seizure effects of the ketogenic diet. Cell, 174, 497 10.1016/j.cell.2018.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco-Solis R, Montellier E, Aguilar-Arnal L, Sato S, Vawter MP, Bunney BG, ... Sassone-Corsi P (2017). A circadian genomic signature common to ketamine and sleep deprivation in the anterior cingulate cortex. Biological Psychiatry, 82, 351–360. 10.1016/j.biopsych.2017.02.1176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Challet E, Ott V, Arvat E, de Kloet ER, Dijk DJ, ... Van Cauter E (2017). The functional and clinical significance of the 24-hour rhythm of circulating glucocorticoids. Endocrine Reviews, 38, 3–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oster H, Damerow S, Kiessling S, Jakubcakova V, Abraham D, Tian J, ... Eichele G (2006). The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metabolism, 4, 163–173. 10.1016/j.cmet.2006.07.002 [DOI] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, & Heim CM (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. The American Journal of Psychiatry, 163, 1630–1633. 10.1176/ajp.2006.163.9.1630 [DOI] [PubMed] [Google Scholar]
- Palomba M, & Bentivoglio M (2008). Chronic inflammation affects the photic response of the suprachiasmatic nucleus. Journal of Neuroimmunology, 193, 24–27. 10.1016/j.jneuroim.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, ... Hogenesch JB (2002). Coordinated transcription of key pathways in the mouse by the circadian clock. Cell, 109, 307–320. 10.1016/S0092-8674(02)00722-5 [DOI] [PubMed] [Google Scholar]
- Parekh PK, & McClung CA (2015). Circadian mechanisms underlying reward-related neurophysiology and synaptic plasticity. Frontiers in Psychiatry, 6, 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariante CM, & Lightman SL (2008). The HPA axis in major depression: Classical theories and new developments. Trends in Neurosciences, 31, 464–468. 10.1016/j.tins.2008.06.006 [DOI] [PubMed] [Google Scholar]
- Pekovic-Vaughan V, Gibbs J, Yoshitane H, Yang N, Pathiranage D, Guo B, ... Meng QJ (2014). The circadian clock regulates rhythmic activation of the NRF2/glutathione-mediated antioxidant defense pathway to modulate pulmonary fibrosis. Genes & Development, 28, 548–560. 10.1101/gad.237081.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piech-Dumas KM, & Tank AW (1999). CREB mediates the cAMP-responsiveness of the tyrosine hydroxylase gene: Use of an antisense RNA strategy to produce CREB-deficient PC12 cell lines. Brain Research. Molecular Brain Research, 70, 219–230. 10.1016/S0169-328X(99)00149-7 [DOI] [PubMed] [Google Scholar]
- Prosser RA, Lee HM, & Wehner A (2006). Serotonergic pretreatments block in vitro serotonergic phase shifts of the mouse suprachiasmatic nucleus circadian clock. Neuroscience, 142, 547–555. 10.1016/j.neuroscience.2006.06.014 [DOI] [PubMed] [Google Scholar]
- Ramsey KM, Yoshino J, Brace CS, Abrassart D, Kobayashi Y, Marcheva B, ... Bass J (2009). Circadian clock feedback cycle through NAMPT-mediated NAD+ biosynthesis. Science, 324, 651–654. 10.1126/science.1171641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Tan DX, Rosales-Corral S, Galano A, Zhou XJ, & Xu B (2018). Mitochondria: Central organelles for melatonin’s antioxidant and anti-aging actions. Molecules, 23, 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong ML, Licinio J, & Wesselingh S (2016). From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Molecular Psychiatry, 21, 738–748. 10.1038/mp.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roubos EW, Dahmen M, Kozicz T, & Xu L (2012). Leptin and the hypothalamo-pituitary-adrenal stress axis. General and Comparative Endocrinology, 177, 28–36. 10.1016/j.ygcen.2012.01.009 [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, ... McClung CA (2007). Mania-like behavior induced by disruption of CLOCK. Proceedings of the National Academy of Sciences of the United States of America, 104, 6406–6411. 10.1073/pnas.0609625104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RT, Poland RE, Lesser IM, Winston RA, & Blodgett AL (1987). Neuroendocrine aspects of primary endogenous depression. I. Cortisol secretory dynamics in patients and matched controls. Archives of General Psychiatry, 44, 328–336. 10.1001/archpsyc.1987.01800160032006 [DOI] [PubMed] [Google Scholar]
- Salomon RM, Ripley B, Kennedy JS, Johnson B, Schmidt D, Zeitzer JM, ... Mignot E (2003). Diurnal variation of cerebrospinal fluid hypocretin-1 (Orexin-A) levels in control and depressed subjects. Biological Psychiatry, 54, 96–104. 10.1016/S0006-3223(02)01740-7 [DOI] [PubMed] [Google Scholar]
- Schade R, Vick K, Sohr R, Ott T, Pfister C, Bellach J, ... Lemmer B (1993). Correlative circadian rhythms of cholecystokinin and dopamine content in nucleus accumbens and striatum of rat brain. Behavioral Brain Research, 59, 211–214. 10.1016/0166-4328(93)90168-P [DOI] [PubMed] [Google Scholar]
- Segall LA, Milet A, Tronche F, & Amir S (2009). Brain glucocorticoid receptors are necessary for the rhythmic expression of the clock protein, PERIOD2, in the central extended amygdala in mice. Neuroscience Letters, 457, 58–60. 10.1016/j.neulet.2009.03.083 [DOI] [PubMed] [Google Scholar]
- Sharma AN, Elased KM, Garrett TL, & Lucot JB (2010). Neurobehavioral deficits in db/db diabetic mice. Physiology & Behavior, 101, 381–388. 10.1016/j.physbeh.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri-Kojori E, Wang GJ, Wiers CE, Demiral SB, Guo M, Kim SW, ... Volkow ND (2018). beta-Amyloid accumulation in the human brain after one night of sleep deprivation. Proceedings of the National Academy of Sciences of the United States of America, 115, 4483–4488. 10.1073/pnas.1721694115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidor MM, Spencer SM, Dzirasa K, Parekh PK, Tye KM, Warden MR, ... McClung CA (2015). Daytime spikes in dopaminergic activity drive rapid mood-cycling in mice. Molecular Psychiatry, 20, 1406–1419. 10.1038/mp.2014.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RS (1991). The macrophage theory of depression. Medical Hypotheses, 35, 298–306. 10.1016/0306-9877(91)90272-Z [DOI] [PubMed] [Google Scholar]
- So AY, Bernal TU, Pillsbury ML, Yamamoto KR, & Feldman BJ (2009). Glucocorticoid regulation of the circadian clock modulates glucose homeostasis. Proceedings of the National Academy of Sciences of the United States of America, 106, 17582–17587. 10.1073/pnas.0909733106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrino Crespo C, Perianes Cachero A, Puebla Jimenez L, Barrios V, & Arilla Ferreiro E (2014). Peptides and food intake. Frontiers in Endocrinology (Lausanne), 5, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son GH, Chung S, & Kim K (2011). The adrenal peripheral clock: Glucocorticoid and the circadian timing system. Frontiers in Neuroendocrinology, 32, 451–465. 10.1016/j.yfrne.2011.07.003 [DOI] [PubMed] [Google Scholar]
- Souetre E, Salvati E, Belugou JL, Pringuey D, Candito M, Krebs B, ... Darcourt G (1989). Circadian rhythms in depression and recovery: Evidence for blunted amplitude as the main chronobiological abnormality. Psychiatry Research, 28, 263–278. 10.1016/0165-1781(89)90207-2 [DOI] [PubMed] [Google Scholar]
- Spencer RL, Chun LE, Hartsock MJ, & Woodruff ER (2018). Glucocorticoid hormones are both a major circadian signal and major stress signal: How this shared signal contributes to a dynamic relationship between the circadian and stress systems. Frontiers in Neuroendocrinology, 49, 52–71. 10.1016/j.yfrne.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Spencer SJ, Emmerzaal TL, Kozicz T, & Andrews ZB (2015). Ghrelin’s role in the hypothalamic-pituitary-adrenal axis stress response: Implications for mood disorders. Biological Psychiatry, 78, 19–27. 10.1016/j.biopsych.2014.10.021 [DOI] [PubMed] [Google Scholar]
- Spengler ML, Kuropatwinski KK, Comas M, Gasparian AV, Fedtsova N, Gleiberman AS, ... Antoch MP (2012). Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proceedings of the National Academy of Sciences of the United States of America, 109, E2457–E2465. 10.1073/pnas.1206274109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse J, Braselton J, & Reynolds L (2006). Fluoxetine modulates the circadian biological clock via phase advances of suprachiasmatic nucleus neuronal firing. Biological Psychiatry, 60, 896–899. 10.1016/j.biopsych.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Summa KC, Voigt RM, Forsyth CB, Shaikh M, Cavanaugh K, Tang Y, ... Keshavarzian A (2013). Disruption of the circadian clock in mice increases intestinal permeability and promotes alcohol-induced hepatic pathology and inflammation. PLoS ONE, 8, e67102 10.1371/journal.pone.0067102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suofu Y, Li W, Jean-Alphonse FG, Jia J, Khattar NK, Li J, ... Friedlander RM (2017). Dual role of mitochondria in producing melatonin and driving GPCR signaling to block cytochrome c release. Proceedings of the National Academy of Sciences of the United States of America, 114, E7997–E8006. 10.1073/pnas.1705768114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi A, Flanigan ME, McEwen BS, & Russo SJ (2018). Aggression, social stress, and the immune system in humans and animal models. Frontiers in Behavioral Neuroscience, 12, 56 10.3389/fnbeh.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Yamada T, Tsukita S, Kaneko K, Shirai Y, Munakata Y, ... Katagiri H (2013). Chronic mild stress alters circadian expressions of molecular clock genes in the liver. American Journal of Physiology. Endocrinology and Metabolism, 304, E301–E309. 10.1152/ajpendo.00388.2012 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Yokota S, Hara R, Kobayashi T, Akiyama M, Moriya T, & Shibata S (2001). Physical and inflammatory stressors elevate circadian clock gene mPer1 mRNA levels in the paraventricular nucleus of the mouse. Endocrinology, 142, 4910–4917. 10.1210/endo.142.11.8487 [DOI] [PubMed] [Google Scholar]
- Tan DX, Hardeland R, Manchester LC, Paredes SD, Korkmaz A, Sainz RM, ... Reiter RJ (2010). The changing biological roles of melatonin during evolution: From an antioxidant to signals of darkness, sexual selection and fitness. Biological Reviews of the Cambridge Philosophical Society, 85, 607–623. [DOI] [PubMed] [Google Scholar]
- Tang X, Guo D, Lin C, Shi Z, Qian R, Fu W, ... Fan L (2017). hCLOCK induction by hypoxia promotes inflammatory responses by activating the NFkappaB pathway. Molecular Medicine Reports, 15, 1401–1406. 10.3892/mmr.2017.6127 [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Levy M, Korem T, Dohnalova L, Shapiro H, Jaitin DA, ... Elinav E (2016). Microbiota diurnal rhythmicity programs host transcriptome oscillations. Cell, 167, 1495–1510 e1412. 10.1016/j.cell.2016.11.003 [DOI] [PubMed] [Google Scholar]
- Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, ... Elinav E (2014). Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell, 159, 514–529. 10.1016/j.cell.2014.09.048 [DOI] [PubMed] [Google Scholar]
- Tuma J, Strubbe JH, Mocaer E, & Koolhaas JM (2005). Anxiolytic-like action of the antidepressant agomelatine (S 20098) after a social defeat requires the integrity of the SCN. European Neuropsychopharmacology, 15, 545–555. 10.1016/j.euroneuro.2005.02.004 [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, ... Bass J (2005). Obesity and metabolic syndrome in circadian Clock mutant mice. Science, 308, 1043–1045. 10.1126/science.1108750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vadnie CA, & McClung CA (2017). Circadian rhythm disturbances in mood disorders: Insights into the role of the suprachiasmatic nucleus. Neural Plasticity, 2017, 1504507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, & Chrousos GP (2004). Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. The Journal of Clinical Endocrinology and Metabolism, 89, 2119–2126. 10.1210/jc.2003-031562 [DOI] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Green SJ, Engen PA, & Keshavarzian A (2016). Circadian rhythm and the gut microbiome. International Review of Neurobiology, 131, 193–205. 10.1016/bs.irn.2016.07.002 [DOI] [PubMed] [Google Scholar]
- Voigt RM, Forsyth CB, Green SJ, Mutlu E, Engen P, Vitaterna MH, ... Keshavarzian A (2014). Circadian disorganization alters intestinal microbiota. PLoS ONE, 9, e97500 10.1371/journal.pone.0097500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt RM, Summa KC, Forsyth CB, Green SJ, Engen P, Naqib A, ... Keshavarzian A (2016). The circadian clock mutation promotes intestinal dysbiosis. Alcoholism, Clinical and Experimental Research, 40, 335–347. 10.1111/acer.12943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, & Chen D (2013). Evidence for seasonal mania: A review. The Journal of Psychiatric Practice, 19, 301–308. 10.1097/01.pra.0000432600.32384.c5 [DOI] [PubMed] [Google Scholar]
- Watanabe A, Shibata S, & Watanabe S (1995). Circadian rhythm of spontaneous neuronal activity in the suprachiasmatic nucleus of old hamster in vitro. Brain Research, 695, 237–239. 10.1016/0006-8993(95)00713-Z [DOI] [PubMed] [Google Scholar]
- Weber M, Lauterburg T, Tobler I, & Burgunder JM (2004). Circadian patterns of neurotransmitter related gene expression in motor regions of the rat brain. Neuroscience Letters, 358, 17–20. 10.1016/j.neulet.2003.12.053 [DOI] [PubMed] [Google Scholar]
- Wittekind DA, & Kluge M (2015). Ghrelin in psychiatric disorders - A review. Psychoneuroendocrinology, 52, 176–194. 10.1016/j.psyneuen.2014.11.013 [DOI] [PubMed] [Google Scholar]
- Wu JC, & Bunney WE (1990). The biological basis of an anti-depressant response to sleep deprivation and relapse: Review and hypothesis. The American Journal of Psychiatry, 147, 14–21. [DOI] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, ... Nedergaard M (2013). Sleep drives metabolite clearance from the adult brain. Science, 342, 373–377. 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada N, Katsuura G, Ochi Y, Ebihara K, Kusakabe T, Hosoda K, & Nakao K (2011). Impaired CNS leptin action is implicated in depression associated with obesity. Endocrinology, 152, 2634–2643. 10.1210/en.2011-0004 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakahata Y, Tanaka M, Yoshida M, Soma H, Shinohara K, ... Takumi T (2005). Acute physical stress elevates mouse period1 mRNA expression in mouse peripheral tissues via a glucocorticoid-responsive element. The Journal of Biological Chemistry, 280, 42036–42043. 10.1074/jbc.M509600200 [DOI] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, & Hu H (2018). Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature, 554, 317–322. 10.1038/nature25509 [DOI] [PubMed] [Google Scholar]
- Yang S, Van Dongen HP, Wang K, Berrettini W, & Bucan M (2009). Assessment of circadian function in fibroblasts of patients with bipolar disorder. Molecular Psychiatry, 14, 143–155. 10.1038/mp.2008.10 [DOI] [PubMed] [Google Scholar]
- Young JW, Cope ZA, Romoli B, Schrurs E, Aniek J, van Enkhuizen J, . Dulcis D (2018). Mice with reduced DAT levels recreate seasonal-induced switching between states in bipolar disorder. Neuropsychopharmacology, 43, 1721–1731. 10.1038/s41386-018-0031-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JW, & Dulcis D (2015). Investigating the mechanism(s) underlying switching between states in bipolar disorder. European Journal of Pharmacology, 759, 151–162. 10.1016/j.ejphar.2015.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA Jr , Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, … Manji HK (2006). A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Archives of General Psychiatry, 63, 856–864. 10.1001/archpsyc.63.8.856 [DOI] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Yooseph S, & Panda S (2014). Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metabolism, 20, 1006–1017. 10.1016/j.cmet.2014.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, ... Xie P (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Molecular Psychiatry, 21, 786–796. 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
- Zhu XH, Qiao H, Du F, Xiong Q, Liu X, Zhang X, ... Chen W (2012). Quantitative imaging of energy expenditure in human brain. NeuroImage, 60, 2107–2117. 10.1016/j.neuroimage.2012.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]


