Highlights
-
•
CT-guided fine needle aspirate is accurate in the diagnoses of subsolid nodules.
-
•
CT-guided fine needle aspirate of subsolid nodules is safe.
-
•
A core needle biopsy is not always necessary.
Keywords: CT guided lung biopsy, Subsolid nodule, Fine needle aspirate, Core biopsy, Lung cancer, Ground glass opacity
Abstract
Purpose
To assess the success of determining malignancy in subsolid lung nodules by fine needle aspirate of CT-guided transthoracic needle biopsy.
Material and method
This IRB approved retrospective study analyzed CTguided transthoracic needle biopsy of 86 consecutive subsolid nodules (size 25 + 14 mm; Age 71 + 10 years: M: F, 27:59), with ground glass opacity of = 50% in 64 (74%) and size < 2 cm in 38 (44%). Fine needle aspirate was performed in all and additional core biopsy in 21 (24%). The biopsy results were correlated with resected surgical pathology in 59 (69%) and by long term clinical and imaging follow-up in 27 (31%). The statistical analysis was performed by Fischer exact test to determine the success rate in < 2cm and =2cm nodules and those with <50% and =50% ground glass opacity.
Results
The technical success of performing the biopsy was 94.7%. The sensitivity for making a diagnosis of malignancy in small and large subsolid nodules was 88.6 and 95.6% (p=>0.05), with a specificity 100% in both groups. Core biopsy altered the diagnosis only in 1/21 (4.8%). The nondiagnostic biopsy rate was 18 and 11% for lesions with =50% and <50% ground glass opacity (p=>0.05). The incidence of pneumothorax was 21%, none requiring chest tube, and mild hemoptysis in 8%.
Conclusion
CT-guided transthoracic needle biopsy of both small and large subsolid nodules is highly sensitive and very specific for making the diagnosis of malignancy with a low rate of complications. Additional core biopsy offered no significant advantage over fine needle aspirate biopsy alone.
1. Introduction
Subsolid lung nodules are defined as nodules containing a ground glass component. A ground glass opacity (GGO) is defined as hazy increased attenuation of the lung with visible bronchial and vascular structures [1]. A subsolid nodule (SSN) can be either purely ground glass in attenuation or have a part solid component along with ground glass opacity. Pulmonary nodules, including pure GGOs, are increasingly encountered in clinical practice due to increased utilization and the improved resolution of CT imaging. The reported incidence of a subsolid nodule is 4.2% in baseline screening for lung cancer and 0.7% in annual repeat screening [2].
Approximately 75% of persistent GGO are attributable to adenocarcinoma in situ (adenocarcinoma consisting entirely of lepidic/bronchioloalveolar pattern) or minimally invasive adenocarcinoma [3]. However, the differential diagnosis of GGO also includes premalignant lesions such as atypical adenomatous hyperplasia (AAH) and benign conditions such as focal fibrosis and inflammation [4]. Some reports suggest that focal GGOs with a solid component (part solid) are more likely to be associated with malignancy [3,5]. Henschke et al report that 63% of part solid nodules and 18% of pure GG nodules detected on CT screening are malignant [6].
Hasegawa et al note nearly half of screening-detected cancer on low-dose CT are either part solid or pure GG nodules [7].
In clinical practice, there is controversy regarding management of persistent SSN. Positron emission tomography is generally not useful for assessment of GGOs, and published guidelines differ regarding when to consider tissue sampling or
surgical resection. Criteria to guide management of SSN include size greater than 1 cm, presence of interval growth, development of a solid component, and a solid component that is > 4–8 mm [[8], [9], [10]]. Some studies advocate a more conservative approach with imaging follow-up of pulmonary nodules < 3 cm with a GGO component > 50% [11]. To further complicate the matter, studies report moderate inter-reader variability with regard to the size and the characterization of pulmonary nodules [12,13].
The reported accuracy of CT guided fine needle aspirate has been low for subsolid and predominantly ground glass nodules, particularly those that measure less 2 cm [14,15]. Subsolid nodules are more challenging to characterize with the small sample of tissue obtained through image guided biopsy, particularly fine needle aspirates. The purpose of this study is to assess the technical success and diagnostic yield of CT guided transthoracic fine needle aspiration and core needle biopsy of subsolid nodules.
2. Material and methods
Approval for this retrospective study was obtained from the hospital ethics committee on human studies. The study was HIPAA compliant, and the requirements for patient’s informed consent was waived.
2.1. Medical record review
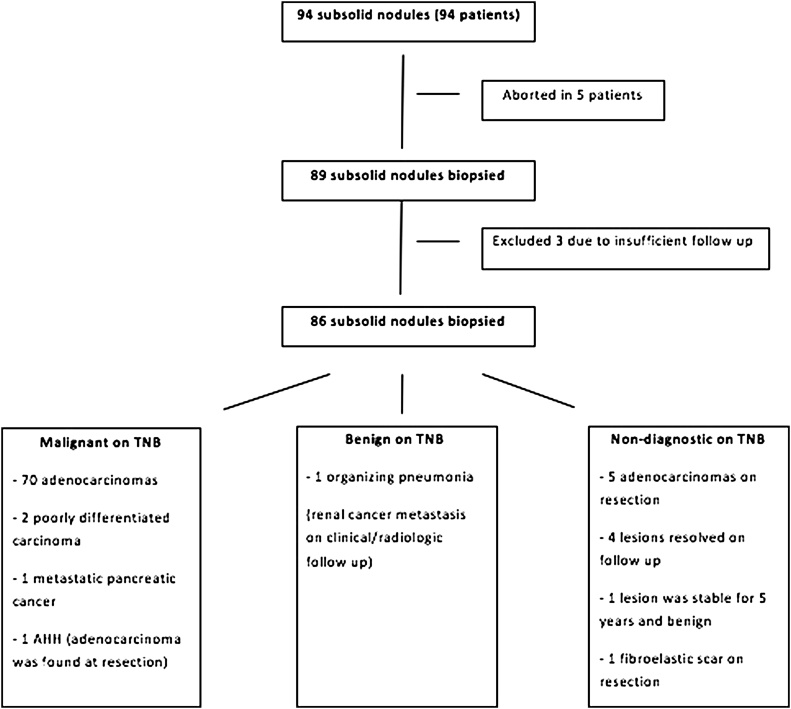
A search of the radiology database was performed to identify patients who had undergone CT-guided transthoracic needle biopsy of a subsolid nodule (SSN) for the period from July 2009 through June 2013. Patients who underwent biopsy of either a pure ground glass opacity (GGO) or a part solid nodule were enrolled in this study. During this period 94 biopsies were attempted on subsolid nodules among a total of 1090 percutaneous CT-guided lung biopsies. Of the excluded patients, in 5 tissue samples were not obtained and in 3 there was insufficient follow-up to determine a final diagnosis (Fig. 1). The characteristics of the patients and the nodules are summarized in Table 1. Fine needle aspirate was performed in all and core biopsy was done in 21 patients (24%). Surgical resection was performed in 59 (69%) subsolid nodules and allowed correlation with surgical pathology of completely resected specimen.
Fig. 1.
A flowchart of the patients included in the biopsy, TNB result and final diagnoses.
Table 1.
Demographics, Lesion and Procedural Characteristics (n = 86).
| Characteristic | No (%) |
|---|---|
| Age (mean + SD) | 71 + 10 |
| Sex | |
| Male | 27 |
| Female | 59 |
| Smoking history | |
| Never smoker | 23 (27) |
| Smoker | 11 (13) |
| Ex-smoker | 52 (60) |
| Underlying lung cancer | |
| Yes | 21 (24) |
| No | 65 (76) |
| Lesion size, mm (mean + SD) | 25 + 14 (8.8-95.3) |
| Lesion size | |
| < 2 cm | 38 |
| ≥ 2 cm | 48 |
| Compared with previous CT | |
| No comparison CT | 14 (16) |
| Stable | 26 (30) |
| Growth | 46 (54) |
|
Emphysema None |
56 (65) |
| Background Background & surrounding lesion |
11 (13) 19 (22) |
| Location | |
| RUL | 29 (34) |
| RML | 2 (2) |
| RLL | 16 (19) |
| LUL | 25 (29) |
| LLL | 14 (16) |
| % GGO | |
| < 50 % | 22 (26) |
| ≥ 50 % | 64 (74) |
| Needle path, mm (Mean + SD) | 34 + 18 |
| Samples obtained | |
| FNA | 86 (100) |
| FNA & CNB | 21 (24) |
One investigator (NK) retrospectively reviewed and abstracted data from the electronic medical records, to document if patient needed admission after the procedure if performed on an outpatient, discharge notes; diagnostic and procedure-related imaging; and surgical, pathologic, and other laboratory reports. Abstracted data included patient demographics, smoking history, indication for biopsy, coagulation profile (international normalized ratio, prothrombin time, partial thromboplastin time, platelet count), and current anticoagulation. Two investigators (NK and SM) retrospectively reviewed the imaging studies in the radiology PACS (Impax 4.1, Agfa HealthCare). The size, location and estimated percentage of ground glass component of the nodule (either < or ≥ 50% ground glass), and the presence or absence of emphysema was recorded.
With regard to the lung biopsy, whether fine-needle aspiration or core biopsy was performed, length of the needle path in the lung, histopathologic results of the biopsy specimen, and complications were recorded. Follow-up imaging, post-biopsy surgery and pathologic findings, and final diagnoses were also recorded.
2.2. Biopsy procedure
All biopsies were performed by experienced interventional thoracic radiologists and according to a standard protocol as previously described [16]. All procedures were performed under CT guidance using a multidetector scanner (Advantage, GE healthcare). Patients were placed in a prone (preferably), supine, or lateral decubitus position, depending on the location of the lesion, to obtain the most direct route, to avoid crossing bullae or emphysematous lung, and to minimize the number of pleural surfaces crossed by the needle path. Patients were strapped securely in place to minimize movement. Prior to the start of the biopsy, unless contraindicated, patients were given medication for conscious sedation intravenously, using a two-drug combination of fentanyl citrate (Sublimaze, Taylor) and midazolam hydrochloride (Versed, Roche). The medications were administered by interventional radiology nurse under the supervision of interventional thoracic radiologist. A localizing CT scan was performed using initially 5 mm and then 2.5 mm slices to decide on the point of entry. If needed, the CT gantry was angled to avoid vessels, fissures and ribs.
Local anesthesia was achieved with 1% xylocaine without epinephrine subcutaneously. All biopsies were performed with a 19-guage thin-walled coaxial needle (Chiba-Ultrathin, Cook). Prior to crossing the pleura, multiple fine adjustments of the introducer needle were made in the chest wall to ensure the
needle trajectory was correct. The pleura was punctured only once during the procedure to minimize the risk of a pneumothorax. Once in the lung the needle was advanced in small increments of 1–2 cm. A CT was performed after every manipulation to confirm needle position. After the introducer needle reached the periphery of the lesion (Fig. 2), the inner stylet was exchanged for a 22-gauge Chiba needle attached to an empty 10-ml syringe. When feasible the needle was directed towards the solid component, if present. Tissue samples were obtained in quick, short moments while suction was applied with an attached 10-mL syringe. Saline solution was placed in the well of the introducer needle during needle exchanges to provide a water seal and to reduce the risk of potential air embolism. Throughout the procedure, small doses of the sedation medications were given to keep the patient comfortable and to minimize variation in the depth of respiration.
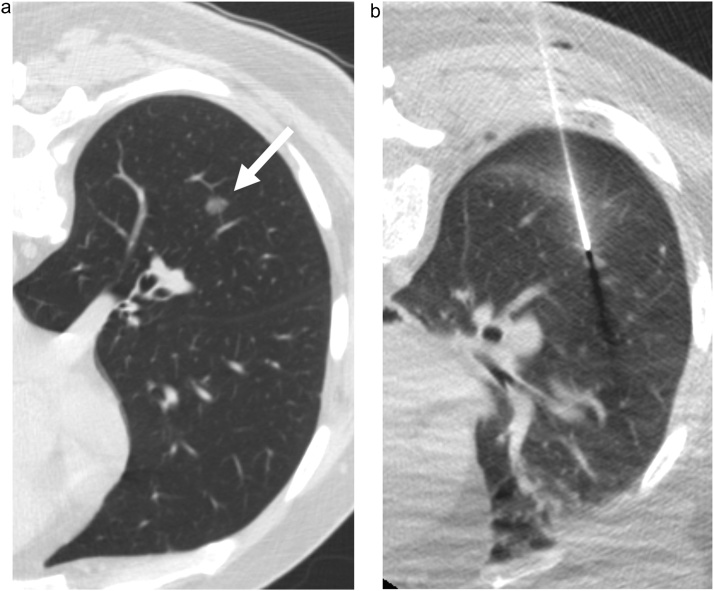
Fig. 2.
58-year-old male. a) Axial CT shows a 12 mm subsolid nodule in the right lower lobe (arrow). b) Axial image at the time of biopsy shows the introducer needle at the edge of the nodule.
Aspirated specimens were placed on sterile glass slides and were handed to an onsite cytotechnologist who prepared the slides immediately. The attending cytopathologist provided an opinion regarding the adequacy of each cytologic specimen for diagnosis. When needed additional aspirates were obtained from different direction and depth until a satisfactory specimen was procured. Core biopsy samples were obtained if a definite diagnosis of malignancy was not made on rapid pathology and where the nodule location didn’t preclude using a 20-gauge spring-loaded core biopsy needle with a 1- or 2-cm needle throw, such as nodule close to hilum and overlying a segmental or larger pulmonary artery and vein. If infection was being considered further aspirates were obtained and sent to microbiology laboratory for staining and culture.
Prior to removal of the biopsy needle, a CT was performed to assess for
pneumothorax and hemorrhage. If pneumothorax was present, air was aspirated at the time of removal of the biopsy needle. After removal of the biopsy needle, the patient was immediately rolled to a puncture site down position to minimize the risk of pneumothorax [17]. Chest radiographs were obtained at 1 and 3 h after the procedure to monitor for complications.
Complications were categorized in accordance with the Society of Interventional Radiology clinical practice guidelines [18]. The presence of a pneumothorax at the time of the biopsy as detected on CT scans, or after the biopsy as detected on chest radiographs, and the need for intervention, either aspiration, chest tube placement, or both, was recorded. The presence and degree of hemorrhage on last CT images prior to the removal of the biopsy needle was also recorded.
2.3. Statistical analysis
Technical success for all attempts was calculated. Technical success was defined as completion of the biopsy procedure and technical failure as inability to obtain a tissue sample. The causes of technical failure included lack of safe window for needle placement, excessive and unpredictable displacement of the lesion during respiration and the termination of biopsy due to complications before sample could be obtained.
The sensitivity, specificity and diagnostic accuracy were calculated for patients who had surgical correlation or sufficient follow-up data on the basis of clinical and imaging follow-up for at least 2 years. Three patients not undergoing surgery were excluded due to insufficient follow-up to determine the final diagnosis.
The biopsy results were divided into three categories: positive for malignancy, negative for malignancy, and a non-diagnostic. The positive category included both definite malignant or premalignant diagnoses such as atypical adenomatous hyperplasia (AAH). A positive biopsy result was deemed true-positive when there was surgical or clinical confirmation based on nodule evolution. A positive CT biopsy result was deemed false positive, if there was no malignancy in surgically resected specimen or if the lesion resolved without treatment at follow up. A negative or non-diagnostic biopsy result was considered true-negative when a benign neoplasm such as hamartoma or specific infection was diagnosed, or when the lesion resolved or decreased in size at follow-up CT. Finally, if a pathological diagnosis of malignancy was made after a negative or non-diagnostic biopsy, the CT biopsy result was considered false-negative.
Patients were classified into two groups according to lesion size (< 2 cm vs ≥ 2 cm) and GGO component (< 50% vs ≥ 50%). Comparison of sensitivity, specificity, diagnostic accuracy and non-diagnostic results was performed between the two groups by using Fisher’s exact test. A p value < 0.05 was considered statistically significant.
3. Results
The technical success rate was 94.7% (89/94). In 5 instances of technical failure, a tissue sample was not obtained. The procedure was abandoned prior to crossing the pleura because of no safe window or excessive motion of the nodule in 3 patients, and the procedure was aborted after crossing the pleura because of the development of small pneumothoraces, which made redirecting the needle difficult in two patients.
Of the 89 biopsies in which tissue was obtained, 3 patients were excluded as there was insufficient follow-up period to determine a final diagnosis. Out of a total 86 patients, histological examination of the needle biopsies diagnosed 74 malignancies, including 70 adenocarcinomas, 1 AAH, 2 poorly differentiated carcinomas and 1 metastatic pancreatic cancer; 59 cases of those were confirmed by surgery. 12 nodules returned with a benign or non-diagnostic biopsy result. One lesion was diagnosed as organizing pneumonia, but later determined to be metastatic renal cell carcinoma by clinical and imaging follow-up., 11 biopsies were non-diagnostic. Subsequently, 5 nodules were confirmed to be adenocarcinoma by surgery, 4 nodules resolved or decreased in size on follow-up CT, 1 nodule was stable on CT at 5-year follow-up period, and 1 nodule was diagnosed to be fibrous scar after surgical resection. The association between the TNB result and final diagnosis is outlined in Table 2. The overall accuracy of TNB for determining malignancy was 93% (74/80). The sensitivity and specificity for malignant lesions was 92 and 100%. The concordance with surgery was 90% (53/59). The positive predictive value for malignancy is 100% (74/74) and the negative predictive value is 50% (6/12) when results for all the patients including those with non-diagnostic tests (86) were included.
Table 2.
Association between diagnosis obtained from CT-guided Transthoracic Needle Biopsy and Final Diagnosis (N = 86).
| Lesion | CT-guided TNB |
Final Diagnosis |
|---|---|---|
| Malignant Lesion | ||
| Lung adenocarcinoma | 70 | 76 |
| AAH | 1 | 0 |
| Poorly differentiated adenocarcinoma | 2 | 2 |
| Metastatic pancreatic cancer | 1 | 1 |
| Metastatic renal cell carcinoma | 0 | 1 |
| Benign Lesions | ||
| Organizing pneumonia* | 1 | 0 |
| Scar | 0 | 1 |
| Non-specific benign (inflammation/fibrosis) | 0 | 5 |
| Non-diagnostic | 11 | 0 |
This was later diagnosed to be metastatic renal cell carcinoma.
Fine needle aspiration was done for all of the biopsies (86 nodules) and additional core needle biopsy (CNB) was done in 21 patients. Core biopsy was diagnostic, whereas the FNA was not, in only one patient (4.7%). In this instance, FNA showed scant fibrous tissue, but core biopsy suggested at least AAH. Surgical resection ultimately found the nodule to be adenocarcinoma. A final diagnosis of malignancy was made in 20 of the 21 nodules that had samples obtained by both fine needle aspiration and core needle biopsy. For 7 of these 20 nodules (35%) CNB specimens failed to diagnose malignancy. Interestingly, FNA correctly diagnosed malignancy in 4 of the 7 nodules not diagnosed by CNB. FNA failed to correctly identify malignancy in 3 (15%) of these nodules and the diagnosis was established only after surgical resection.
The sensitivity, specificity, and diagnostic accuracy for nodules ≥ 2 cm were 95.6%, 100%; and 95.8% (n = 48), which were not statistically different from nodules <2 cm (n = 38; 88.6%, 100%, 89.5%, respectively) (p > 0.05) (Table 3). Six nondiagnostic samples occurred in nodules < 2 cm and five in nodules ≥ 2 cm and was not statistically significant (P = 0.526). The sensitivity, specificity, and diagnosis accuracy for nodules with ≥ 50% GGO component were 95%, 100% and 95.3%, respectively (n = 64), which were not statistically different from those for nodules with < 50% GGO component (n = 22; 85%, 100%, 86.4%, respectively) (p > 0.05) (Table 4). Seven nondiagnostic samples occurred in nodules with GGO ≥ 50% and four in nodules with GGO < 50% (p = 0.461).
Table 3.
Comparison of diagnostic yield and accuracy based on lesion size.
| Variable | Lesion size |
P value | |
|---|---|---|---|
| ≥ 2 cm (n = 48) | < 2 cm (n = 38) | ||
| Sensitivity (%) | 95.56% (43/45) | 88.57% (31/35) | 0.396 |
| Specificity (%) | 100% (3/3) | 100% (3/3) | 1 |
| Accuracy (%) | 95.83% (46/48) | 89.47% (34/38) | 0.399 |
| Nondiagnostic samples | 13.16% (5/48) | 15.79% (6/38) | 0.526 |
Table 4.
Comparison of diagnostic yield and accuracy based on ground glass opacity.
| Variable | Ground glass opacity |
P value | |
|---|---|---|---|
| < 50% (n = 22) | ≥ 50% (n = 64) | ||
| Sensitivity (%) | 85% (17/20) | 95% (57/60) | 0. |
| Specificity (%) | 100% (4/4) | 100% (2/2) | 1 |
| Accuracy (%) | 86.36% (19/22) | 95.31% (61/64) | 0.172 |
| Nondiagnostic samples | 18.18% (4/22) | 10.94% (7/64) | 0.461 |
Of the 94 patients with subsolid nodules, 3 patients for whom the procedure was aborted prior to crossing the pleura because of no safe window or excess motion were excluded from the calculation of complication. Out of a total 91 procedures, focal alveolar hemorrhage surrounding the lesion occurred in 48 (53%) patients on post-procedural CT. However, only 7 (8%) patients had mild hemoptysis and were managed conservatively by observation. Pneumothorax was seen in 19 (21%) patients on post-procedural CT; of these, 15 (79%) were aspirated at the time of removal of the biopsy needle and no patient required a chest tube insertion.
4. Discussion
Our study demonstrates that CT guided FNA of subsolid nodules can be performed with a high rate of technical success and accuracy, even for nodules < 2 cm in size or those with < 50% GGO. The performance of an additional core biopsy has no significant benefit for most patients.
Ground glass nodules are a common finding on imaging, particularly in lung cancer screening CT [2]. Kim et al found that 75% of persistent GGO are attributable to adenocarcinoma in situ (adenocarcinoma consisting solely of lepidic pattern; bronchioalveolar carcinoma, BAC) or adenocarcinoma with predominant lepidic component [3]. Kobayashi et al. found that only approximately 20% of pure GGOs and 40% of part solid nodules gradually grew or increased their solid component, whereas others remained unchanged for years [11]. The size and histologic classification influences treatment and may determine whether sublobar resection or lobectomy is performed [19].
Not all persistent subsolid nodules are malignant. In our study 7% of nodules were attributable to benign causes on subsequent clinical follow up and surgery and there were no false positives for diagnosis of malignancy. Our incidence of benign lesions is less than the 9–39% rate reported in literature based on CT-guided biopsy of ground glass nodules [4]. This low incidence could be partly explained by our process of careful case selection based on image morphology and in many cases persistence or growth of nodules over multiple imaging time points. This selection process could have eliminated biopsy of many transient inflammatory ground glass nodules or stable and extremely indolent neoplastic nodules. Despite our careful selection process, 7% of the nodules were attributable to benign processes and indicates that routine surgical resection may not be ideal, in terms of cost and potential complications. It is also worth noting though in our study we had 100% positive predictive value to determine malignancy, the negative predictive value was only 50% when non-diagnostic samples were included and should be taken in to consideration when determining the management.
CT-guided transthoracic needle biopsy (TNB) has been shown to be a safe and accurate method for establishing the diagnosis of pulmonary lesions. Prior studies have calculated the accuracy of CT-guided biopsy of GGO lesions to range from 51%–97% [14,15,[20], [21], [22], [23], [24], [25], [26]]. The accuracy of fine needle aspirates was much lower than core biopsy biopsies. In our study, the FNA results had similar accuracy to core biopsy studies, with an accuracy was 93%. It is well documented that core biopsy results in higher complications compared to fine needle aspirate. Heerink et al in their meta-analysis showed significant increase in complication rates for core biopsy compared to fine needle aspirate [27]. In our study core biopsies were obtained in less than a quarter of the cases and improved accuracy in only one case. Our findings support that core biopsy is not always required and carefully performed FNA with onsite pathology can yield accurate results. We also found improved diagnostic yield in FNA group compared to core biopsy. In our study FNA correctly diagnosed malignancy in 4 of the 7 cases where CNB was non-diagnostic. Our improved results can perhaps be partly explained by the availability of rapid on-site cytopathology evaluation by pathologists who can rapidly interpret the
specimens. Our findings differ from Aviram et al, who compared sequential FNA and core needle biopsy to either technique alone, and found that sequential procedures significantly improved the diagnostic accuracy of malignancy compared to either technique alone [22]. In our study there was a single case where the fine needle aspirate showed scant fibrous tissue, but the core biopsy suggested at least AHH, and adenocarcinoma was found at surgical resection. It is not uncommon for AAH and adenocarcinoma to co-exist, and was also reported by Inoue et al who reported on 8 patients with a TNB diagnosis of AAH before adenocarcinomas was found on pathological examination of the resected surgical specimen [26].
For patients undergoing surgical resection, the preoperative diagnosis of malignancy by TNB facilitates anatomic resection, such as segmentectomy and lobectomy, without the need for intraoperative frozen section analysis. Frozen sections of lung nodules can be difficult to interpret, as reactive proliferative epithelial changes in inflamed lung tissue can closely simulate a malignancy. A study assessing the accuracy of frozen section diagnoses of small pulmonary nodules found that the sensitivities for the diagnosis of neoplasia were 86.9% and 94.1% for nodules smaller than 1.1 cm in diameter and measuring 1.1–1.5 cm, respectively [28]. The preoperative identification of favorable histology also allows the surgeon to plan and perform sublobar resection, wedge or segmentectomy, with the associated advantages of preservation of pulmonary function, improved perioperative morbidity and mortality, and increased potential for a second resection with a subsequent primary tumor [29].
Treatment options for early lung cancer also include non-surgical options such as radiotherapy, radiofrequency ablation, microwave ablation and cryoablation therapy. In patients where a treatment option other than surgery is chosen, it is important that the diagnosis of lung cancer is confirmed, when feasible, prior to determine treatment. A recent study found that resected GG nodules which were negative for EGFR, KRAS, ALK, or HER2 mutations were associated with a lack of growth [30]. Early genetic diagnosis of GG nodules, therefore, could predict growth potential and play a role in clinical decision making, including prolonged interval between CT scans for mutant-negative tumors and more stringent follow-up or treatment of mutation-positive GG nodules. It has also been suggested that mutations in KRAS may enhance cellular resistance to radiation [31], which if known may affect the treatment offered. Either CT-guided or transbronchial biopsy could be used to assess mutation status. Though in our study we have not tested for mutations, studies have shown such testing is possible on both fine needle aspirate and core biopsy specimens [32].
Our low-rate of biopsy-related complications, including mild hemoptysis in 7 patients and no patient requiring a chest tube for pneumothorax, confirms that CT- guided TNB of subsolid lesions is a safe, minimally invasive procedure with an acceptable complication rate, that is similar to those published for TNB of solid pulmonary nodules and less than in high risk groups [33,34].
This study has several limitations. The retrospective nature of the study may have introduced a selection bias. Secondly, our results are based on the experience at a single academic medical center and may not be widely applicable to centers which are not as experienced in CT-guided transthoracic needle biopsy, or where on-site cytopathology is not available. Lastly, although we followed up patients for two years after the biopsy, malignant pure ground glass nodules are very slow growing with mean doubling time of adenocarcinomas demonstrating pure ground glass reported to be greater than 800 days [3,7,35]. Therefore, 2 years follow-up period of this study may not be sufficient to determine whether lesions which were diagnosed as benign on biopsy and were stable in size on follow-up imaging were malignant or benign. Due to negative predictive value of 50%, imaging follow up should be considered when determining the management.
In conclusion, the results of this study suggest that CT-guided fine needle aspirate biopsy with or without CNB of pulmonary subsolid nodules can yield high sensitivity and specificity with acceptable complication rates.
Author agreement
Each author has made substantial contributions to all of the following: 1) the conception and design of the study, or acquisition of data, or analysis and interpretation of data, 2) drafting the article or revising it critically for important intellectual content, 3) final approval of the version to be submitted. This manuscript is not under consideration for publication elsewhere
Conflict of interest
None of the authors or their institutions have a conflict of interest.
References
- 1.Austin J.H., Muller N.L., Friedman P.J., Hansell D.M., Naidich D.P., Remy-Jardin M. Glossary of terms for CT of the lungs: recommendations of the nomenclature committee of the fleischner society. Radiology. 1996;200(2):327–331. doi: 10.1148/radiology.200.2.8685321. [DOI] [PubMed] [Google Scholar]
- 2.Yankelevitz D.F., Yip R., Smith J.P., Liang M., Liu Y., Xu D.M. CT screening for lung Cancer: nonsolid nodules in baseline and annual repeat rounds. Radiology. 2015 doi: 10.1148/radiol.2015142554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H.Y., Shim Y.M., Lee K.S., Han J., Yi C.A., Kim Y.K. Persistent pulmonary nodular ground-glass opacity at thin-section CT: histopathologic comparisons. Radiology. 2007;245(1):267–275. doi: 10.1148/radiol.2451061682. [DOI] [PubMed] [Google Scholar]
- 4.Collins J., Stern E.J. Ground-glass opacity at CT: the ABCs. AJR Am. J. Roentgenol. 1997;169(2):355–367. doi: 10.2214/ajr.169.2.9242736. [DOI] [PubMed] [Google Scholar]
- 5.Nakata M., Saeki H., Takata I., Segawa Y., Mogami H., Mandai K. Focal ground-glass opacity detected by low-dose helical CT. Chest. 2002;121(5):1464–1467. doi: 10.1378/chest.121.5.1464. [DOI] [PubMed] [Google Scholar]
- 6.Henschke C.I., Yankelevitz D.F., Mirtcheva R., McGuinness G., McCauley D., Miettinen O.S. CT screening for lung cancer: frequency and significance of part-solid and nonsolid nodules. AJR Am. J. Roentgenol. 2002;178(5):1053–1057. doi: 10.2214/ajr.178.5.1781053. [DOI] [PubMed] [Google Scholar]
- 7.Hasegawa M., Sone S., Takashima S., Li F., Yang Z.G., Maruyama Y. Growth rate of small lung cancers detected on mass CT screening. Br. J. Radiol. 2000;73(876):1252–1259. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 8.Naidich D.P., Bankier A.A., MacMahon H., Schaefer-Prokop C.M., Pistolesi M., Goo J.M. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304–317. doi: 10.1148/radiol.12120628. [DOI] [PubMed] [Google Scholar]
- 9.American College of Radiology Lung CT Screening Reporting and Data System (Lung-RADS) https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/Lung-Rads (Accessed 9/1/2018.
- 10.Gould M.K., Donington J., Lynch W.R., Mazzone P.J., Midthun D.E., Naidich D.P. Evaluation of individuals with pulmonary nodules: when is it lung cancer? Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e93S–120S. doi: 10.1378/chest.12-2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kobayashi Y., Mitsudomi T. Management of ground-glass opacities: should all pulmonary lesions with ground-glass opacity be surgically resected? Transl. Lung Cancer Res. 2013;2(5):354–363. doi: 10.3978/j.issn.2218-6751.2013.09.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gierada D.S., Pilgram T.K., Ford M., Fagerstrom R.M., Church T.R., Nath H. Lung cancer: interobserver agreement on interpretation of pulmonary findings at low-dose CT screening. Radiology. 2008;246(1):265–272. doi: 10.1148/radiol.2461062097. [DOI] [PubMed] [Google Scholar]
- 13.Penn A., Ma M., Chou B.B., Tseng J.R., Phan P. Inter-reader variability when applying the 2013 Fleischner guidelines for potential solitary subsolid lung nodules. Acta radiol. 2014 doi: 10.1177/0284185114551975. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu K., Ikeda N., Tsuboi M., Hirano T., Kato H. et al. Percutaneous CT-guided fine needle aspiration for lung cancer smaller than 2 cm and revealed by ground- glass opacity at CT. Lung Cancer. 2006;51(February (2)):173–179. doi: 10.1016/j.lungcan.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 15.De Filippo M., Saba L., Concari G., Nizzoli R., Ferrari L., Tiseo M. Predictive factors of diagnostic accuracy of CT-guided transthoracic fine-needle aspiration for solid noncalcified, subsolid and mixed pulmonary nodules. Radiol. Med. 2013;118(October (7)):1071–1081. doi: 10.1007/s11547-013-0965-4. [DOI] [PubMed] [Google Scholar]
- 16.Wu C.C., Maher M.M., Shepard J.A. CT-guided percutaneous needle biopsy of the chest: preprocedural evaluation and technique. AJR Am. J. Roentgenol. 2011;196(5):W511–4. doi: 10.2214/AJR.10.4657. [DOI] [PubMed] [Google Scholar]
- 17.O’Neill A.C., McCarthy C., Ridge C.A., Mitchell P., Hanrahan E., Butler M. Rapid needle-out patient-rollover time after percutaneous CT-guided transthoracic biopsy of lung nodules: effect on pneumothorax rate. Radiology. 2012;262(1):314–319. doi: 10.1148/radiol.11103506. [DOI] [PubMed] [Google Scholar]
- 18.Gupta S., Wallace M.J., Cardella J.F., Kundu S., Miller D.L., Rose S.C. Quality improvement guidelines for percutaneous needle biopsy. J. Vasc. Interv. Radiol. 2010;21(7):969–975. doi: 10.1016/j.jvir.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 19.Chen D., Dai C., Kadeer X., Mao R., Chen Y., Chen C. New horizons in surgical treatment of ground-glass nodules of the lung: experience and controversies. Ther. Clin. Risk Manag. 2018;14:203–211. doi: 10.2147/TCRM.S152127. Published 2018 Jan 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim T.J., Lee J.H., Lee C.T., Jheon S.H., Sung S.W., Chung J.H. Diagnostic accuracy of CT-guided core biopsy of ground-glass opacity pulmonary lesions. AJR Am. J. Roentgenol. 2008;190(1):234–239. doi: 10.2214/AJR.07.2441. [DOI] [PubMed] [Google Scholar]
- 21.Hur J., Lee H.J., Nam J.E., Kim Y.J., Kim T.H., Choe K.O. Diagnostic accuracy of CT fluoroscopy-guided needle aspiration biopsy of ground-glass opacity pulmonary lesions. AJR Am. J. Roentgenol. 2009;192(3):629–634. doi: 10.2214/AJR.08.1366. [DOI] [PubMed] [Google Scholar]
- 22.Aviram G., Greif J., Man A., Schwarz Y., Marmor S., Graif M. Diagnosis of intrathoracic lesions: are sequential fine-needle aspiration (FNA) and core needle biopsy (CNB) combined better than either investigation alone? Clin. Radiol. 2007;62(3):221–226. doi: 10.1016/j.crad.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 23.Lu C.H., Hsiao C.H., Chang Y.C., Lee J.M., Shih J.Y., Wu L.A. Percutaneous computed tomography-guided coaxial core biopsy for small pulmonary lesions with ground-glass attenuation. J. Thorac. Oncol. 2012;7(1):143–150. doi: 10.1097/JTO.0b013e318233d7dd. [DOI] [PubMed] [Google Scholar]
- 24.Yamauchi Y., Izumi Y., Nakatsuka S., Inoue M., Hayashi Y., Mukai M. Diagnostic performance of percutaneous core needle lung biopsy under multi-CT fluoroscopic guidance for ground-glass opacity pulmonary lesions. Eur. J. Radiol. 2011;79(2):e85–9. doi: 10.1016/j.ejrad.2011.03.088. [DOI] [PubMed] [Google Scholar]
- 25.Maxwell A.W., Klein J.S., Dantey K., Mount S.L., Butnor K.J., Leiman G. CT-guided transthoracic needle aspiration biopsy of subsolid lung lesions. J. Vasc. Interv. Radiol. 2014;25(3):340-6–6 e1. doi: 10.1016/j.jvir.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 26.Inoue D., Gobara H., Hiraki T., Mimura H., Kato K., Shibamoto K. CT fluoroscopy-guided cutting needle biopsy of focal pure ground-glass opacity lung lesions: diagnostic yield in 83 lesions. Eur. J. Radiol. 2012;81(2):354–359. doi: 10.1016/j.ejrad.2010.11.025. [DOI] [PubMed] [Google Scholar]
- 27.Heerink W.J., de Bock G.H., de Jonge G.J., Groen H.J., Vliegenthart R. Oudkerk M.COmplication rates of CT-guided transthoracic lung biopsy: meta-analysis. Eur. Radiol. 2017;27(January (1)):138–148. doi: 10.1007/s00330-016-4357-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marchevsky A.M., Changsri C., Gupta I., Fuller C., Houck W., McKenna R.J., Jr. Frozen section diagnoses of small pulmonary nodules: accuracy and clinical implications. Ann. Thorac. Surg. 2004;78(5):1755–1759. doi: 10.1016/j.athoracsur.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Blasberg J.D., Pass H.I., Donington J.S. Sublobar resection: a movement from the lung Cancer study group. J. Thorac. Oncol. 2010;5(10):1583–1593. doi: 10.1097/jto.0b013e3181e77604. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi Y., Mitsudomi T., Sakao Y., Yatabe Y. Genetic features of pulmonary adenocarcinoma presenting with ground-glass nodules: the differences between nodules with and without growth. Ann. Oncol. 2015;26(1):156–161. doi: 10.1093/annonc/mdu505. [DOI] [PubMed] [Google Scholar]
- 31.Wang M., Han J., Marcar L., Black J., Liu Q., Li X. Radiation resistance in KRAS-Mutated lung Cancer Is enabled by stem-like properties mediated by an Osteopontin-EGFR pathway. Cancer Res. 2017;77(8):2018–2028. doi: 10.1158/0008-5472.CAN-16-0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lian W., Ouyang Y. CT-guided aspiration lung biopsy for EGFR and ALK gene mutation analysis of lung cancer. Oncol. Lett. 2017;13(May (5)):3415–3422. doi: 10.3892/ol.2017.5921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y., Du Y., Yang H.F., Yu J.H., Xu X.X. CT-guided percutaneous core needle biopsy for small (≤20 mm) pulmonary lesions. Clin. Radiol. 2013;68(January (1)):e43–8. doi: 10.1016/j.crad.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 34.Digumarthy S.R., Kovacina B., Otrakji A., Lanuti M., Shepard J.A., Sharma A. Percutaneous CT guided lung biopsy in patients with pulmonary hypertension:assessment of complications. Eur. J. Radiol. 2016;85(February (2)):466–471. doi: 10.1016/j.ejrad.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 35.Aoki T., Nakata H., Watanabe H., Nakamura K., Kasai T., Hashimoto H. Evolution of peripheral lung adenocarcinomas: CT findings correlated with histology and tumor doubling time. AJR Am. J. Roentgenol. 2000;174(3):763–768. doi: 10.2214/ajr.174.3.1740763. [DOI] [PubMed] [Google Scholar]