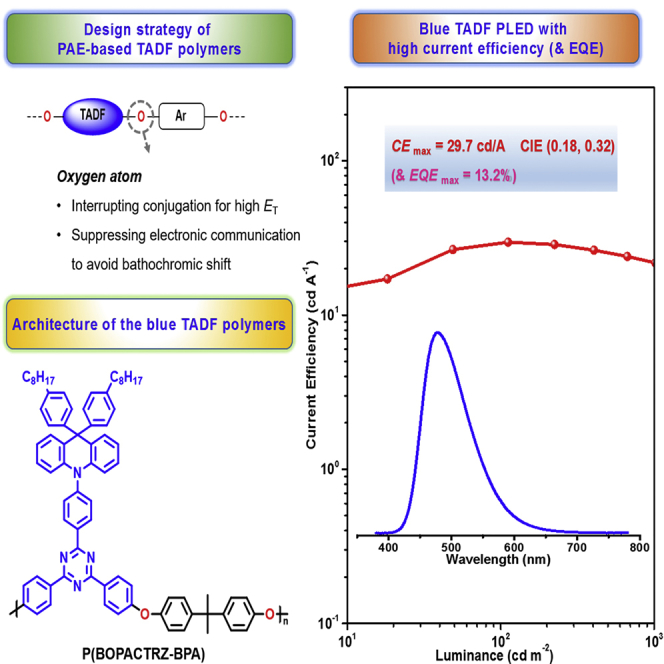
Summary
Polymer light-emitting diodes are attractive for optoelectronic applications owing to their brightness and ease of processing. However, often metals have to be inserted to increase the luminescence efficiency, and producing blue emitters is a challenge. Here we present a strategy to make blue thermally activated delayed fluorescence (TADF) polymers by directly embedding a small molecular blue TADF emitter into a poly(aryl ether) (PAE) backbone. Thanks to the oxygen-induced negligible electronic communication between neighboring TADF fragments, its corresponding blue delayed fluorescence can be inherited by the developed polymers. These polymers are free from metal catalyst contamination and show improved thermal stability. Through device optimization, a current efficiency of 29.7 cd/A (21.2 lm/W, 13.2%) is realized together with Commission Internationale de L'Eclairage coordinates of (0.18, 0.32). The value is competitive with blue phosphorescent polymers, highlighting the importance of the PAE backbone in achieving high-performance blue delayed fluorescence at a macromolecular level.
Subject Areas: Chemistry, Polymer Chemistry, Physics, Optoelectronics, Materials Science
Graphical Abstract
Highlights
-
•
Directly embedding a small molecular blue TADF emitter in a poly(aryl ether) backbone
-
•
Oxygen-induced negligible electron communication leads to blue delayed fluorescence
-
•
The achieved device efficiency is competitive with blue phosphorescent polymers
Chemistry; Polymer Chemistry; Physics; Optoelectronics; Materials Science
Introduction
Polymer light-emitting diodes (PLEDs) fabricated via cost-effective wet methods have attracted much attention because of their potential applications in large-area and flexible flat panel displays and solid-state lightings (Kraft et al., 1998, Grimsdale et al., 2009, Shao et al., 2018). Dating back to the 1990s, the early developed electroluminescent polymers for PLEDs were based on fluorescence (Burroughes et al., 1990), in which only singlet excitons could be utilized to give limited internal quantum efficiency (IQE) below 25% (Segal et al., 2003, Baldo et al., 1999). To harvest both singlet and triplet excitons, phosphorescent polymers containing noble metal complexes were subsequently demonstrated and achieved a near-unity IQE (Xu et al., 2015, Liang et al., 2014). Until recently, thermally activated delayed fluorescent (TADF) polymers have caught the public's eye (Liu et al., 2018, Zou et al., 2018). Without any noble metals, they can realize comparable efficiency to phosphorescent polymers through a rapid reverse intersystem crossing (RISC) from the lowest triplet state (T1) to the lowest singlet state (S1) with the help of the environmental thermal energy (Endo et al., 2009, Uoyama et al., 2012, Zhang et al., 2014). Although great progress has been made in developing green to red TADF polymers nowadays (Nikolaenko et al., 2015, Lee et al., 2016, Ren et al., 2016, Zhu et al., 2016, Freeman et al., 2017, Li et al., 2017, Xie et al., 2017, Yang et al., 2018a, Yang et al., 2018b), there are a few examples of efficient TADF polymers with blue emission (Shao et al., 2017, Zeng et al., 2018).
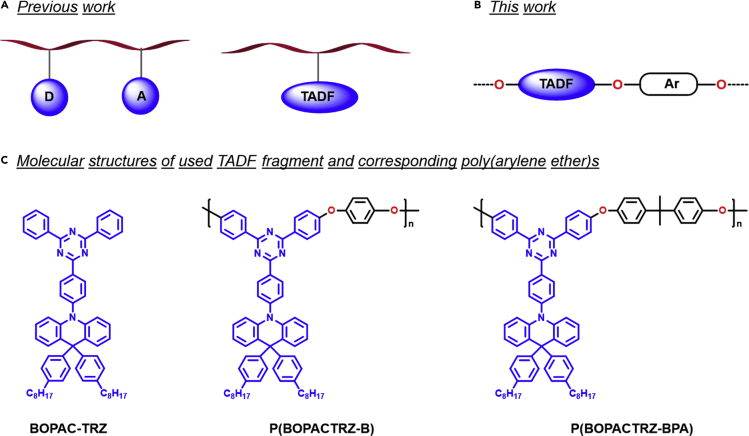
The challenge lies in the dearth of a polymer backbone with high triplet energy (ET) (van Dijken et al., 2004) because most of the conjugated polymers have too low ET to prevent the triplet energy back transfer from the TADF unit to the backbone (Sudhakar et al., 2003, Youn Lee et al., 2012). As an alternative, a nonconjugated backbone is adopted for the design of blue TADF polymers. For example, Shao et al. reported a polyethylene pended with suitable electron donor (D) and acceptor (A) units (Figure 1A) (Shao et al., 2017). Benefiting from the through-space charge transfer (TSCT) effect, a bright blue emission was obtained accompanied by a maximum current efficiency of 24.8 cd/A and an external quantum efficiency (EQE) of 12.1%. However, it is not an easy task to delicately control the distance between D and A, for the bulky solubilizing substituents may weaken TSCT and result in poor device performance. Then Zeng et al. developed blue TADF polymers by simply introducing a blue TADF emitter into the side chain of polynorbornene (Figure 1A) (Zeng et al., 2018). Owing to the insulating nature, the corresponding PLEDs showed extremely low current density, leading to an inferior current efficiency of 13.5 cd/A (7.3%). Therefore both a simple and an effective molecular design are highly desirable for blue TADF polymers.
Figure 1.
Schematic Representation of Blue TADF Polymers
(A) Routes of TADF polymers' molecular design in the literature.
(B) Routes of TADF polymers' molecular design in this work.
(C) Schematic representation of used TADF fragment and corresponding blue TADF polymers in this work.
Also see Scheme S1 and Figures S12–S21.
Herein, we propose a novel strategy to efficient blue TADF from an old poly(arylene ether) (PAE), where a small molecular blue TADF emitter is directly embedded in its backbone. As depicted in Figure 1B, the involved oxygen atom can not only interrupt the conjugation to keep high ET for the polymer backbone but also suppress the electronic communication between neighboring TADF fragments to avoid the unwanted bathochromic shift. Combined with the freedom of metal catalyst contamination and intrinsic thermal stability, PAE is believed to be a promising platform to realize blue TADF. As a proof of concept, BOPAC-TRZ is selected as the embedded TADF unit (Figure 1C), because its analog DPAC-TRZ without octyl groups possesses efficient blue delayed fluorescence (Lin et al., 2016) and the triazine component has enough nucleophilic aromatic substitution polymerization reactivity (Strukelj and Hedrick, 1994, Yu et al., 2009). With the commercially available comonomer 1,4-dihydroxybenzene or bisphenol A in hand, two blue TADF polymers P(BOPACTRZ-B) and P(BOPACTRZ-BPA) have been successfully constructed based on the PAE backbone. The corresponding devices reveal a record-high current efficiency of 29.7 cd/A (13.2%) as well as Commission Internationale de L'Eclairage (CIE) coordinates of (0.18, 0.32). To the best of our knowledge, the obtained efficiency is the highest value reported so far for blue TADF polymers.
Results and Discussion
The monomer and polymer synthesis is presented in Scheme S1. Starting from 2-(phenylamino) benzoic acid, the key intermediate 9,9-bis(4-octylphenyl)-9,10-dihydroacridine (2) was first prepared through esterification and subsequent in situ cyclization, in which the two octyl groups were introduced to ensure PAE solubility. Then a copper-catalyzed selective C-N coupling between 2 and 1-bromo-4-iodobenzene was performed to give 10-(4-bromophenyl)-9,9-bis(4-octylphenyl)-9,10-dihydroacridine (3). After being converted to its corresponding boric acid ester (4), the difluorinated blue TADF monomer M1 was produced via a Suzuki-Miyaura reaction in a moderate yield of 52%. For comparison, the small-molecular blue TADF emitter BOPAC-TRZ without fluorine atoms was also synthesized as the reference. Finally, a nucleophilic aromatic substitution polymerization between M1 and 1,4-dihydroxybenzene or bisphenol A was carried out to afford the polymers P(BOPACTRZ-B) and P(BOPACTRZ-BPA) (Johnson et al., 1967, Hale et al., 1967, Johnson and Farnham, 1967). In this case, K2CO3 instead of palladium was used as the catalyst, thus eliminating the detrimental effect of metal catalyst residue on device performance (Krebs et al., 2004). Moreover, the number-average molecular weight (Mn) and polydispersity index determined by gel permeation chromatography are 10.7 kDa and 1.6 for P(BOPACTRZ-B) and 56.5 kDa and 1.4 for P(BOPACTRZ-BPA). Owing to the introduction of two additional octyl groups in BOPAC-TRZ, they are readily soluble in common organic solvents, such as toluene, chlorobenzene, and tetrahydrofuran (THF). They are also thermally stable with a decomposition temperature (Td) above 410°C and a glass transition temperature (Tg) above 150°C (Figure S1). Compared with BOPAC-TRZ (Td = 360°C, Tg = 50°C), the improved thermal stability clearly suggests the advantage of the PAE backbone.
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energy levels of P(BOPACTRZ-B) and P(BOPACTRZ-BPA) are estimated to be −5.41∼-5.43 eV and −2.55 eV, respectively. The values are nearly identical to those of the small molecule BOPAC-TRZ (Figure S2), well consistent with the theoretical simulation (Figure S3). Similar to BOPAC-TRZ, the HOMO of P(BOPACTRZ-B) and P(BOPACTRZ-BPA) distributes on the acridine donor, whereas their LUMO is mainly localized on the triazine acceptor. We note that the direct electron communication between neighboring TADF units is blocked by the oxygen atom in the main chain. This is a key factor to keep the independent blue TADF from BOPAC-TRZ when it is imbedded in the PAE backbone.
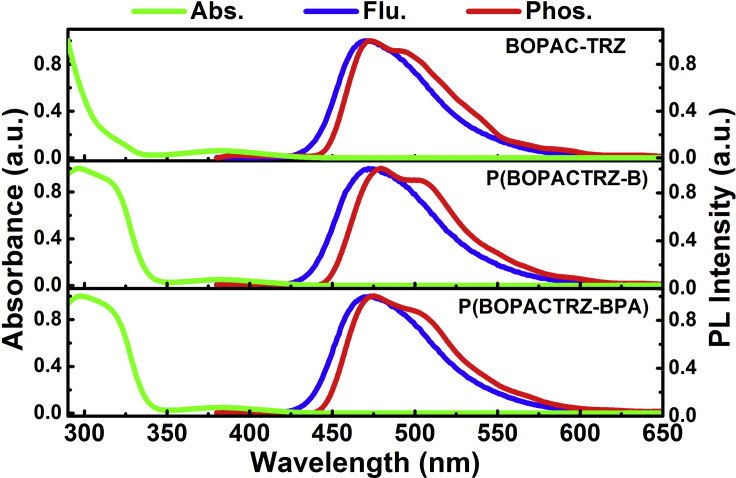
Figure 2 compares the ultraviolet-visible absorption spectra in toluene and fluorescence and phosphorescence spectra in films for BOPAC-TRZ, P(BOPACTRZ-B), and P(BOPACTRZ-BPA). They all display a weak lowest energy absorption band in the range of 350–450 nm, which is related to the charge transfer transition from acridine to triazine (Lin et al., 2016). With regard to BOPAC-TRZ, an additional intense absorption around 295–350 nm newly appears in P(BOPACTRZ-B) and P(BOPACTRZ-BPA), indicative of the formation of a PAE backbone (Strukelj and Hedrick, 1994, Yu et al., 2009). Meanwhile, the fluorescence spectra of P(BOPACTRZ-B) and P(BOPACTRZ-BPA) turn out to be the same as that of BOPAC-TRZ, revealing a blue light peak at about 472 nm. The observation can be reasonably ascribed to the oxygen-induced negligible electron communication between neighboring TADF units. This is further verified by the matched phosphorescence among BOPAC-TRZ, P(BOPACTRZ-B), and P(BOPACTRZ-BPA). From the onsets of fluorescence and phosphorescence spectra, their corresponding singlet-triplet energy splitting (ΔEST) is calculated to be about 0.06 eV (Table 1), small enough to ensure efficient RISC from T1 to S1 followed by delayed fluorescence.
Figure 2.
Ultraviolet-Visible Absorption Spectra in Toluene, Fluorescence and Phosphorescence Spectra in the Neat Films of P(BOPACTRZ-B) and P(BOPACTRZ-BPA) Compared with BOPAC-TRZ
Table 1.
Physical Properties of P(BOPACTRZ-B) and P(BOPACTRZ-BPA) Compared with BOPAC-TRZ
| Compound | λabsa (nm) | λemb (nm) | ΦPLc (%) | τpd (ns) | τdd (ns) | ES/ET/ΔESTe (eV) | Egf (eV) | HOMOg (eV) | LUMOg (eV) | Tgh (oC) | Tdi (oC) | Mn (kDa) | PDI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BOPAC-TRZ | 290, 381 | 472 | 75.5 | 25.2 | 1,654.4 | 2.82/2.76/0.06 | 2.86 | −5.44 | −2.58 | 50 | 360 | – | – |
| P(BOPACTRZ-B) | 296, 382 | 472 | 44.5 | 19.7 | 1,172.4 | 2.83/2.76/0.07 | 2.88 | −5.43 | −2.55 | 150 | 436 | 10.7 | 1.6 |
| P(BOPACTRZ-BPA) | 298, 383 | 473 | 75.3 | 21.8 | 1,623.5 | 2.84/2.78/0.06 | 2.86 | −5.41 | −2.55 | 167 | 410 | 56.5 | 1.4 |
Also see Figures S1–S5 and S9, and Table S1.
PDI, polydispersity index.
Measured in toluene at a concentration of 1 × 10−4 M.
Measured in neat films.
Measured by integrating sphere in neat films under N2.
The prompt and delayed fluorescence lifetimes detected in neat films under N2 at 298 K.
ΔEST = ES – ET, where ES and ET are estimated from the onsets of the fluorescence and phosphorescence spectra, respectively.
Optical band gap estimated from the absorption onset.
HOMO = -e (Eonset, ox + 4.8 V), LUMO = HOMO + Eg, where Eonset, ox is the onset of the first oxidation wave.
Glass transition temperatures determined by differential scanning calorimetry.
Decomposition temperatures corresponding to a 5% weight loss determined by thermo gravimetric analysis (TGA).
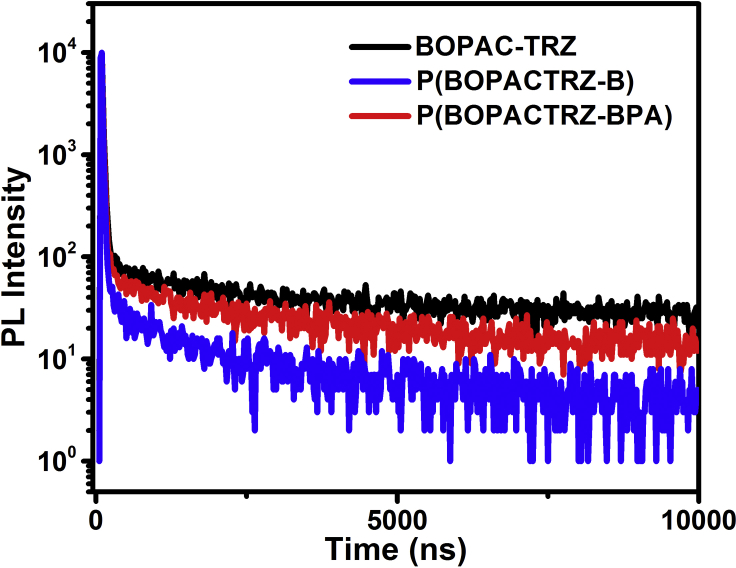
To ascertain their TADF features, subsequently, the transient photoluminescence (PL) spectra of P(BOPACTRZ-B) and P(BOPACTRZ-BPA) were detected. As expected, they possess obvious delayed fluorescence originating from BOPAC-TRZ, which is found to disappear or reduce in the presence of O2 (Figure S4). This is a typical characteristic of TADF emitters as a result of the triplet quenching effect from the ground-state O2 molecules. Going from BOPAC-TRZ to P(BOPACTRZ-B), noticeably, the delayed component is significantly decreased (Figure 3). Also, the related lifetime is down from 1,654.4 ns to 1,172.4 ns (Table 1 and Figure S5). In contrast, P(BOPACTRZ-BPA) shows a close delayed fluorescence to BOPAC-TRZ, giving a lifetime of 1,623.5 ns. Correspondingly, the film PL quantum yield of P(BOPACTRZ-BPA) (ΦPL = 75.3%) is competent with that of BOPAC-TRZ (ΦPL = 75.5%), but much higher than that of P(BOPACTRZ-B) (ΦPL = 44.5%). Supposing the similar influence from the interchain triplet-triplet annihilation (TTA) (Baldo et al., 2000), the intrachain TTA is tentatively responsible for the different TADF behavior for P(BOPACTRZ-B) and P(BOPACTRZ-BPA). As one can see, they only differ from the backbone structure, where BOPAC-TRZ as the blue TADF moiety is separated by 1,4-dihydroxybenzene and bisphenol A, respectively. The distance between neighboring BOPAC-TRZ (denoted as the distance between two triazine rings) is found to increase from 17.5 Å of P(BOPACTRZ-B) to 21.5 Å of P(BOPACTRZ-BPA) (Figure S3). So a much stronger intrachain TTA could be anticipated in P(BOPACTRZ-B), which is well in agreement with its slower RISC rate constant of T1-to-S1 upconversion (kRISC) and faster non-radiative rate constant of T1 (knrT) relative to P(BOPACTRZ-BPA) (Table S1). Further experiments should be carried out to prove this hypothesis, but is beyond the aim of this work.
Figure 3.
PL Decay Measured at 298 K under N2 for the Neat Films of P(BOPACTRZ-B) and P(BOPACTRZ-BPA) Compared with BOPAC-TRZ
Also see Figures S4, S5, and S9, and Table S1.
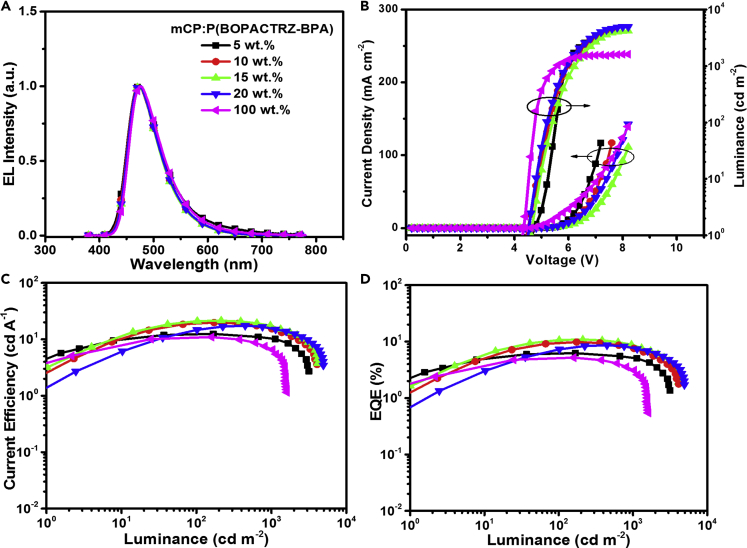
As discussed above, P(BOPACTRZ-BPA) other than P(BOPACTRZ-B) can well inherit the blue TADF of the small molecule BOPAC-TRZ. Therefore P(BOPACTRZ-BPA) has great potential in electroluminescence (EL). To evaluate this point, PLEDs were assembled with a configuration of ITO/PEDOT: PSS (40 nm)/mCP: P(BOPACTRZ-BPA) (50 nm)/TSPO1 (8 nm)/TmPyPB (42 nm)/LiF (1 nm)/Al (100 nm) (Figure S6). Here PEDOT:PSS (poly(3,4-ethylenedioxythiophene): poly(styrene sulfonate)), mCP (1,3-bis(9H-carbazol-9-yl)benzene), TSPO1 (diphenyl-4-triphenylsilylphenyl-phosphine oxide), and TmPyPB (1,3,5-tri[(3-pyridyl)-phen-3-yl]benzene) act as the hole-injection, host, exciton-blocking, and electron-transporting materials, respectively. P(BOPACTRZ-BPA) is doped into mCP to constitute the emitting layer. The EL spectra, current density-voltage-luminance characteristics, current efficiency, and EQE as a function of luminance are depicted in Figure 4, and the related data are summarized in Table 2. With varied doping concentration, the EL spectra remain nearly unchanged (Figure 4A). All devices emit a bright blue light merely from P(BOPACTRZ-BPA), showing similar Commission Internationale de L'Eclairage (CIE) coordinates of (0.17–0.19, 0.27–0.30).
Figure 4.
Device Performance for P(BOPACTRZ-BPA) with PEDOT:PSS as the Hole-Injection Layer
(A) EL spectra at a driving voltage of 6 V.
(B) Current density-voltage-luminance characteristics.
(C) Current efficiency as a function of luminance.
(D) EQE as a function of luminance.
Also see Figures S6–S10.
Table 2.
Device Performance for P(BOPACTRZ-BPA)
| Doping Concentration | Vona (V) | Lmax (cd/m2) | ηcb (cd/A) | ηpb (lm/W) | EQEb (%) | λem (nm) | CIE (x, y) |
|---|---|---|---|---|---|---|---|
| 5 wt %c | 4.8 | 3,159 | 12.5/11.0/10.1 | 7.0/6.0/5.3 | 6.2/5.5/5.1 | 472 | (0.19, 0.28) |
| 10 wt %c | 4.6 | 4,133 | 19.6/18.9/15.2 | 11.4/10.6/7.9 | 9.8/9.4/7.6 | 472 | (0.17, 0.27) |
| 15 wt %c | 4.6 | 4,147 | 21.4/21.4/17.4 | 12.2/12.0/7.4 | 10.8/10.8/8.7 | 472 | (0.17, 0.27) |
| 20 wt %c | 4.6 | 4,926 | 17.4/16.8/15.0 | 9.8/9.8/7.9 | 8.6/8.3/7.4 | 472 | (0.17, 0.27) |
| 100 wt %c | 4.4 | 1,607 | 11.0/9.9/6.5 | 7.2/6.2/3.7 | 5.2/4.7/3.1 | 476 | (0.18, 0.30) |
| 15 wt %d | 3.6 | 7,270 | 29.7/28.7/21.8 | 21.2/19.6/13.2 | 13.2/12.8/9.7 | 477 | (0.18, 0.32) |
Also see Figures S6–S8, S10, and S11, and Tables S2 and S3.
Turn-on voltage at 1 cd/m2.
Data at maximum, 200 cd/m2, and 1,000 cd/m2 for current efficiency (ηc), power efficiency (ηp), and EQE, respectively.
Devices with PEDOT:PSS as the hole-injection layer.
Devices with PEDOT:PSS + perfluorinated ionomer (v/v 3:2) as the hole-injection layer.
Despite this, it is found that the current density is distinctly dependent on the doping concentration (Figure 4B). At a low doping concentration, charge trap (Li et al., 2014) plays an important role because the HOMO and LUMO levels of P(BOPACTRZ-BPA) lie between those of mCP (Figure S7). Thereby the current density at the same driving voltage is gradually decreased from 5 to 15 wt %. When the content is further up to 100 wt %, carriers can be injected and transported directly on P(BOPACTRZ-BPA), resulting in reversely enhanced current density. Moreover, the turn-on voltage at 1 cd/m2 is down from 4.8 V to 4.4 V in the absence of the mCP host. The observations imply the good charge-transporting capability of P(BOPACTRZ-BPA), even though the non-conjugated bisphenol A is involved in the backbone. Among these devices, the best performance is achieved at a 15 wt % content (Figures 4C and 4D), revealing a maximum luminance of 4,147 cd/m2, a peak current efficiency of 21.4 cd/A, a peak power efficiency of 12.2 lm/W, and a peak EQE of 10.8%. As the transient PL behaviors of P(BOPACTRZ-BPA) doped into mCP are not obviously influenced by the doping concentration (Figure S9), here the mCP host is used not to alleviate the negative concentration quenching as usual, but to provide a charge trap center (Figure S7) to improve hole-electron recombination possibility and thus device performance.
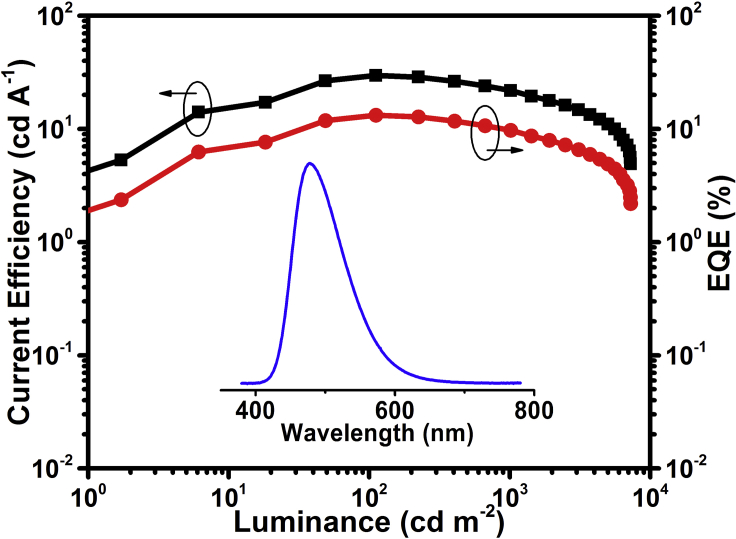
It should be noted that there is a large hole injection barrier of 0.7 eV between PEDOT:PSS and mCP. To solve this problem, PEDOT:PSS is then modified by a perfluorinated ionomer (see Figure S6). According to the literature (Lee et al., 2007), a work function gradient could be formed via self-organization to favor efficient hole injection. Consequently, the turn-on voltage is greatly reduced from 4.6 to 3.6 V without obviously changing the emission color (CIE: (0.18, 0.32)). The luminance, current efficiency, power efficiency, and EQE are further optimized to be 7,270 cd/m2, 29.7 cd/A, 21.2 lm/W, and 13.2%, respectively (Figure 5). Even at a high luminance of 200 and 1,000 cd/m2, the current efficiency still remains at 28.7 and 21.8 cd/A, respectively, indicative of the small efficiency roll-off. The values are the highest ever reported for blue TADF polymers, and even superior to blue phosphorescent polymers (Table S2), which suggests that the PAE backbone is a promising platform to design efficient blue TADF polymers.
Figure 5.
Current Efficiency and EQE as a Function of Luminance for the Optimized Device of P(BOPACTRZ-BPA) at a Doping Concentration of 15 wt % with PEDOT:PSS + Perfluorinated Ionomer (v/v 3:2) as the Hole-Injection Layer
Inset: EL spectra at a driving voltage of 6 V. Also see Figures S6, S10, and S11, and Tables S2 and S3.
Limitations of Study
With respect to P(BOPACTRZ-BPA), the control device based on the reference BOPAC-TRZ exhibits nearly the same blue EL with CIE coordinates of (0.18, 0.32) but a lower current efficiency of 20.2 cd/A (15.1 lm/W, 8.9%) (Figure S10 and Table S3). As mentioned above, P(BOPACTRZ-BPA) has a better thermal stability than BOPAC-TRZ, thus leading to the improved device efficiency. Accordingly, because of the unstable acridine moiety and the limited device configuration (Song and Lee, 2017), a comparable short device lifetime is also observed for P(BOPACTRZ-BPA) relative to BOPAC-TRZ (Figure S11 and Table S3). These results mean that the device efficiency, color purity, and lifetime of P(BOPACTRZ-BPA) are mainly determined by BOPAC-TRZ. If a suitable small molecular blue TADF emitter is delicately selected to be embedded into the PAE backbone, high-performance blue TADF polymers, which show comparable efficiency to solution-processible small molecules and dendrimers (Huang et al., 2018), good color purity with a CIE y-coordinate value <0.20 (Sun et al., 2015), as well as longer lifetime toward practical applications, can be further constructed through such a design strategy. And the related work is under way.
Conclusion
In summary, highly efficient blue TADF polymers have been demonstrated by simply embedding a small molecular TADF emitter into the PAE backbone. They are free of metal catalyst contamination and exhibit improved thermal stability. Also, they are able to inherit the blue delayed fluorescence from the TADF fragment thanks to the oxygen-induced negligible electronic communication. As a result, the corresponding device realizes a state-of-art current efficiency as high as 29.7 cd/A (21.2 lm/W, 13.2%) together with CIE coordinates of (0.18, 0.32). The obtained performance can well compete with that of blue phosphorescent polymers, representing the first example of PAE-based blue TADF polymers. This work, we believe, will open up a new era in PAE used for PLEDs (Shao et al., 2016).
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors acknowledge the National Natural Science Foundation of China (Nos. 51873205, 51573183 and 51703223), the National Key Research and Development Program (2016YFB0401301), and the 973 Project (2015CB655001) for the financial support.
Author Contributions
J.-Q.D. and L.-X.W. coordinated and directed the study. S.-M.W. and J.-Q.D. conceived the idea and designed the experiments. X.-R.L. and J.-C.R. synthesized and characterized the polymers. X.-F.L. fabricated and characterized the devices. X.-R.L., S.-M.W., and J.-Q.D. wrote and revised the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: May 31, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.04.020.
Contributor Information
Shumeng Wang, Email: wangshumeng@ciac.ac.cn.
Junqiao Ding, Email: junqiaod@ciac.ac.cn.
Supplemental Information
References
- Baldo M.A., O’brien D.F., Thompson M.E., Forrest S.R. Excitonic singlet-triplet ratio in a semiconducting organic thin film. Phys. Rev. B. 1999;60:14422–14428. [Google Scholar]
- Baldo M.A., Adachi C., Forrest S.R. Transient analysis of organic electrophosphorescence. II. Transient analysis of triplet-triplet annihilation. Phys. Rev. B. 2000;62:10967. [Google Scholar]
- Burroughes J.H., Bradley D.D.C., Brown A.R., Marks R.N., Mackay K., Friend R.H., Burns P.L., Holmes A.B. Light-emitting diodes based on conjugated polymers. Nature. 1990;347:539–541. [Google Scholar]
- van Dijken A., Bastiaansen J.J., Kiggen N.M., Langeveld B.M., Rothe C., Monkman A., Bach I., Stossel P., Brunner K. Carbazole compounds as host materials for triplet emitters in organic light-emitting diodes: polymer hosts for high-efficiency light-emitting diodes. J. Am. Chem. Soc. 2004;126:7718–7727. doi: 10.1021/ja049771j. [DOI] [PubMed] [Google Scholar]
- Endo A., Ogasawara M., Takahashi A., Yokoyama D., Kato Y., Adachi C. Thermally activated delayed fluorescence from Sn4+-porphyrin complexes and their application to organic light-emitting diodes - a novel mechanism for electroluminescence. Adv. Mater. 2009;21:4802–4806. doi: 10.1002/adma.200900983. [DOI] [PubMed] [Google Scholar]
- Freeman D.M.E., Musser A.J., Frost J.M., Stern H.L., Forster A.K., Fallon K.J., Rapidis A.G., Cacialli F., McCulloch I., Clarke T.M. Synthesis and exciton dynamics of donor-orthogonal acceptor conjugated polymers: reducing the singlet−triplet energy gap. J. Am. Chem. Soc. 2017;139:11073–11080. doi: 10.1021/jacs.7b03327. [DOI] [PubMed] [Google Scholar]
- Grimsdale A.C., Leok Chan K., Martin R.E., Jokisz P.G., Holmes A.B. Synthesis of light-emitting conjugated polymers for applications in electroluminescent devices. Chem. Rev. 2009;109:897–1091. doi: 10.1021/cr000013v. [DOI] [PubMed] [Google Scholar]
- Hale W.F., Farnham A.G., Johnson R.N., Clendinning R.A. Poly (aryl ethers) by nucleophilic aromatic substitution. II. Thermal stability. J. Polym. Sci. A Polym. Chem. 1967;5:2399–2414. [Google Scholar]
- Huang T., Jiang W., Duan L. Recent progress in solution processable TADF materials for organic light-emitting diodes. J. Mater. Chem. C. 2018;6:5577–5596. [Google Scholar]
- Johnson R.N., Farnham A.G. Poly (aryl ethers) by nucleophilic aromatic substitution. III. Hydrolytic side reactions. J. Polym. Sci. A Polym. Chem. 1967;5:2415–2427. [Google Scholar]
- Johnson R.N., Farnham A.G., Clendinning R.A., Hale W.F., Merriam C.N. Poly (aryl ethers) by nucleophilic aromatic substitution. I. Synthesis and properties. J. Polym. Sci. A Polym. Chem. 1967;5:2375–2398. [Google Scholar]
- Kraft A., Grimsdale A.C., Holmes A.B. Electroluminescent conjugated polymers-seeing polymers in a new light. Angew. Chem. Int. Ed. 1998;37:402–428. doi: 10.1002/(SICI)1521-3773(19980302)37:4<402::AID-ANIE402>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Krebs F.C., Nyberg R.B., Jørgensen M. Influence of residual catalyst on the properties of conjugated polyphenylenevinylene materials: palladium nanoparticles and poor electrical performance. Chem. Mater. 2004;16:1313–1318. [Google Scholar]
- Lee T.W., Chung Y., Kwon O., Park J.J. Self-organized gradient hole injection to improve the performance of polymer electroluminescent devices. Adv. Funct. Mater. 2007;17:390–396. [Google Scholar]
- Lee S.Y., Yasuda T., Komiyama H., Lee J., Adachi C. Thermally activated delayed fluorescence polymers for efficient solution-processed organic light-emitting diodes. Adv. Mater. 2016;28:4019–4024. doi: 10.1002/adma.201505026. [DOI] [PubMed] [Google Scholar]
- Li H., Li C., Duan L., Qiu Y. Charge transport in amorphous organic semiconductors: effects of disorder, carrier density, traps, and scatters. Isr. J. Chem. 2014;54:918–926. [Google Scholar]
- Li C., Nobuyasu R.S., Wang Y., Dias F.B., Ren Z., Bryce M.R., Yan S. Solution-processable thermally activated delayed fluorescence white OLEDs based on dual-emission polymers with tunable emission colors and aggregation-enhanced emission properties. Adv. Opt. Mater. 2017;5:1700435. [Google Scholar]
- Liang A., Ying L., Huang F. Recent progresses of iridium complex-containing macromolecules for solution-processed organic light-emitting diodes. J. Inorg. Organomet. Polym. 2014;24:905–926. [Google Scholar]
- Lin T.-A., Chatterjee T., Tsai W.-L., Lee W.-K., Wu M.-J., Jiao M., Pan K.-C., Yi C.-L., Chung C.-L., Wong K.T. Sky-blue organic light emitting diode with 37% external quantum efficiency using thermally activated delayed fluorescence from spiroacridine-triazine hybrid. Adv. Mater. 2016;28:6976–6983. doi: 10.1002/adma.201601675. [DOI] [PubMed] [Google Scholar]
- Liu Y., Li C., Ren Z., Yan S., Bryce M.R. All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes. Nat. Rev. Mater. 2018;3:18020. [Google Scholar]
- Nikolaenko A.E., Cass M., Bourcet F., Mohamad D., Roberts M. Thermally activated delayed fluorescence in polymers: a new route toward highly efficient solution processable OLEDs. Adv. Mater. 2015;27:7236–7240. doi: 10.1002/adma.201501090. [DOI] [PubMed] [Google Scholar]
- Ren Z., Nobuyasu R.S., Dias F.B., Monkman A.P., Yan S., Bryce M.R. Pendant homopolymer and copolymers as solution-processable thermally activated delayed fluorescence materials for organic light-emitting diodes. Macromolecules. 2016;49:5452–5460. [Google Scholar]
- Segal M., Baldo M.A., Holmes R.J., Forrest S.R., Soos Z.G. Excitonic singlet-triplet ratios in molecular and polymeric organic materials. Phys. Rev. B. 2003;68:075211. [Google Scholar]
- Shao S., Ding J., Wang L. New applications of poly(arylene ether)s in organic light-emitting diodes. Chin. Chem. Lett. 2016;27:1201–1208. [Google Scholar]
- Shao S., Hu J., Wang X., Wang L., Jing X., Wang F. Blue thermally activated delayed fluorescence polymers with nonconjugated backbone and through-space charge transfer effect. J. Am. Chem. Soc. 2017;139:17739–17742. doi: 10.1021/jacs.7b10257. [DOI] [PubMed] [Google Scholar]
- Shao S., Ding J., Wang L. Research progress on electroluminescent polymers. Acta Polym. Sin. 2018:198–216. [Google Scholar]
- Song W., Lee J.Y. Degradation mechanism and lifetime improvement strategy for blue phosphorescent organic light-emitting diodes. Adv. Opt. Mater. 2017;5:1600901. [Google Scholar]
- Strukelj M., Hedrick J.C. Synthesis and characterization of novel poly (aryl ether pyridyltriazine) s. Macromolecules. 1994;27:7511–7521. [Google Scholar]
- Sudhakar M., Djurovich P.I., Hogen-Esch T.E., Thompson M.E. Phosphorescence quenching by conjugated polymers. J. Am. Chem. Soc. 2003;125:7796–7797. doi: 10.1021/ja0343297. [DOI] [PubMed] [Google Scholar]
- Sun J.W., Baek J.Y., Kim K.-H., Moon C.-K., Lee J.-H., Kwon S.K., Kim Y.-H., Kim J.-J. Thermally activated delayed fluorescence from azasiline based intramolecular charge-transfer emitter (DTPDDA) and a highly efficient blue light emitting diode. Chem. Mater. 2015;27:6675–6681. [Google Scholar]
- Uoyama H., Goushi K., Shizu K., Nomura H., Adachi C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature. 2012;492:234–238. doi: 10.1038/nature11687. [DOI] [PubMed] [Google Scholar]
- Xie G., Luo J., Huang M., Chen T., Wu K., Gong S., Yang C. Inheriting the characteristics of TADF small molecule by side-chain engineering strategy to enable bluish-green polymers with high PLQYs up to 74% and external quantum efficiency over 16% in light-emitting diodes. Adv. Mater. 2017;29:1604223. doi: 10.1002/adma.201604223. [DOI] [PubMed] [Google Scholar]
- Xu F., Kim H.U., Kim J.H., Jung B.J., Grimsdale A.C., Hwang D.H. Progress and perspective of iridium-containing phosphorescent polymers for light-emitting diodes. Prog. Polym. Sci. 2015;47:92–121. [Google Scholar]
- Yang Y., Wang S., Zhu Y., Wang Y., Zhan H., Cheng Y. Thermally activated delayed fluorescence conjugated polymers with backbone-donor/pendant-acceptor architecture for nondoped OLEDs with high external quantum efficiency and low roll-off. Adv. Funct. Mater. 2018;28:1706916. [Google Scholar]
- Yang Y., Zhao L., Wang S., Ding J., Wang L. Red-emitting thermally activated delayed fluorescence polymers with poly(fluorene-co-3,3up to 74yl diphenyl ether) as the backbone. Macromolecules. 2018;51:9933–9942. [Google Scholar]
- Youn Lee S., Yasuda T., Nomura H., Adachi C. High-efficiency organic light-emitting diodes utilizing thermally activated delayed fluorescence from triazine-based donor–acceptor hybrid molecules. Appl. Phys. Lett. 2012;101:093306. [Google Scholar]
- Yu G., Liu C., Wang J., Chu C., Jian X. Synthesis of phenyl-s-triazine-based copoly(aryl ether)s derived from hydroquinone and resorcinol. Polym. Degrad. Stab. 2009;94:2065–2071. [Google Scholar]
- Zeng X., Luo J., Zhou T., Chen T., Zhou X., Wu K., Zou Y., Xie G., Gong S., Yang C. Using ring-opening metathesis polymerization of norbornene to construct thermally activated delayed fluorescence polymers: high-efficiency blue polymer light-emitting diodes. Macromolecules. 2018;51:1598–1604. [Google Scholar]
- Zhang Q., Li B., Huang S., Nomura H., Tanaka H., Adachi C. Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence. Nat. Photon. 2014;8:326–332. [Google Scholar]
- Zhu Y., Zhang Y., Yao B., Wang Y., Zhang Z., Zhan H., Zhang B., Xie Z., Wang Y., Cheng Y. Synthesis and electroluminescence of a conjugated polymer with thermally activated delayed fluorescence. Macromolecules. 2016;49:4373–4377. [Google Scholar]
- Zou Y., Gong S., Xie G., Yang C. Design strategy for solution-processable thermally activated delayed fluorescence emitters and their applications in organic light-emitting diodes. Adv. Opt. Mater. 2018;6:1800568. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.