Abstract
We present a case of Langerhans’ cell histiocytosis in a 40-year-old woman presenting with central diabetes insipidus and right ear pain. As this disease process is often clinically challenging, the presence of certain imaging findings should raise the possibility of this diagnosis. We review the pertinent imaging and correlate with histology and immunohistochemistry leading to the diagnosis.
Keywords: Langerhans cell histiocytosis, Adult, Histology, CT temporal bone, MRI
Case report
A 40-year-old woman was admitted to our institution for increased thirst, polydipsia, and polyuria with associated dry mouth and dry eyes. Clinical symptoms were concerning for Sjogren's syndrome; although, antinuclear antibodies, anti-Ro/SSA, and anti-La/SSB antibodies were negative. Subsequent outpatient laboratory tests had found an elevated serum sodium level of 158 mEq/L (normal 135-145) with low urine osmolality. Further work-up for diabetes insipidus demonstrated a positive response after Vasopressin challenge. These findings conformed central diabetes insipidus.
A pituitary magnetic resonance image demonstrated a markedly thickened hypothalamic infundibulum (Fig. 1). The infundibulum was deemed too difficult to biopsy and the patient was managed with desmopressin.
Fig. 1.
Sagittal T1-weighted contrast-enhanced image of the pituitary MRI demonstrating marked thickening of the pituitary infundibulum.
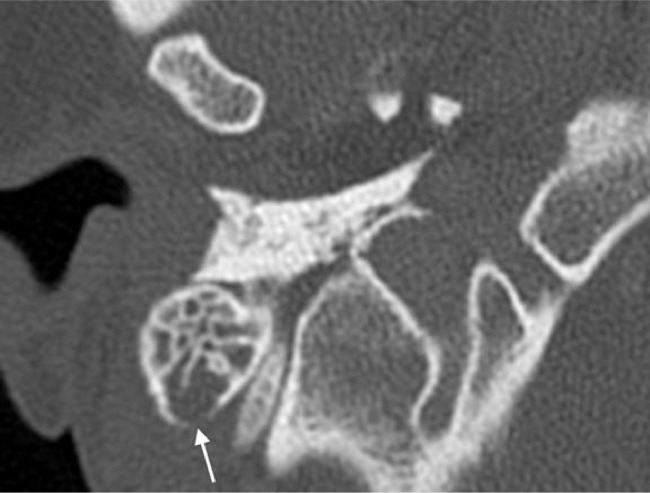
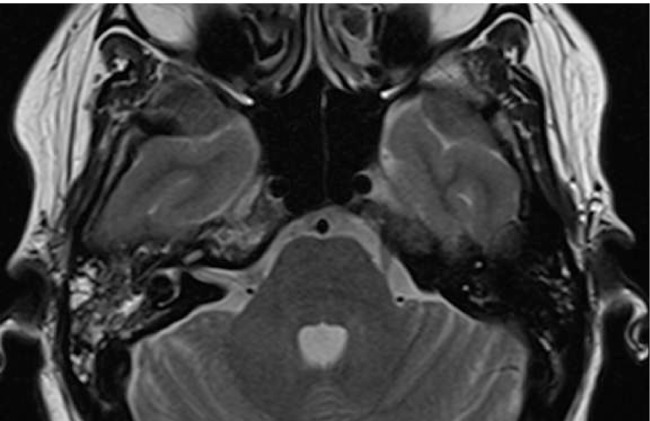
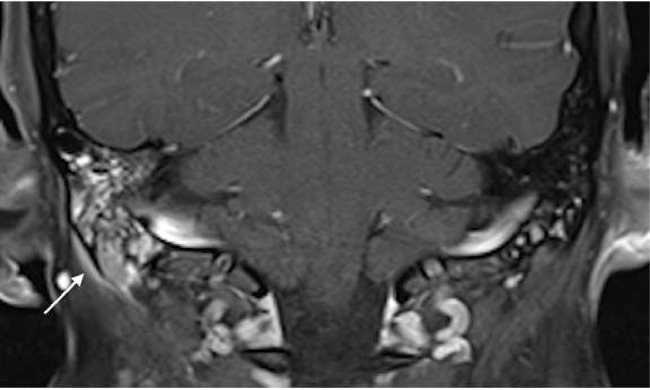
The patient was readmitted 4 months later with right ear pain, which had been a consistent complaint for an year. A temporal bone CT was obtained demonstrating opacification of the right mastoid air cells and a focal area of resorption at the mastoid tip (Fig. 2). Subsequent skull base magnetic resonance image showed fluid signal intensity in the right mastoid air cells with abnormal enhancement of the mastoid process and accompanying subperiosteal soft tissue enhancement (Figs. 2 and 3). The pituitary infundibulum again showed marked thickening (Fig. 4). The constellation of these imaging findings was concerning for an inflammatory or granulomatous process. A right mastoidectomy was performed which revealed avascular tissue filling the air cells and eroding the mastoid tip to the sigmoid sinus. An excisional biopsy of the right mastoid was obtained.
Fig. 2.
Axial section from high resolution CT of the temporal bone demonstrating opacification of the right mastoid air cells with focal bony resorption at the posterior cortex (arrow).
Fig. 3.
Axial T2-weighted MRI of the temporal bone reveals mixed intensity signal changes within the right mastoid air cells. Note that the right petrous apex has also increased T2-weighted signal.
Fig. 4.
Coronal T1-weighted contrast-enhanced fat saturated image of the right temporal bone demonstrating heterogeneous enhancement within the right mastoid air cells with accompanying subperiosteal soft tissue thickening and enhancement beneath the mastoid tip (arrow).
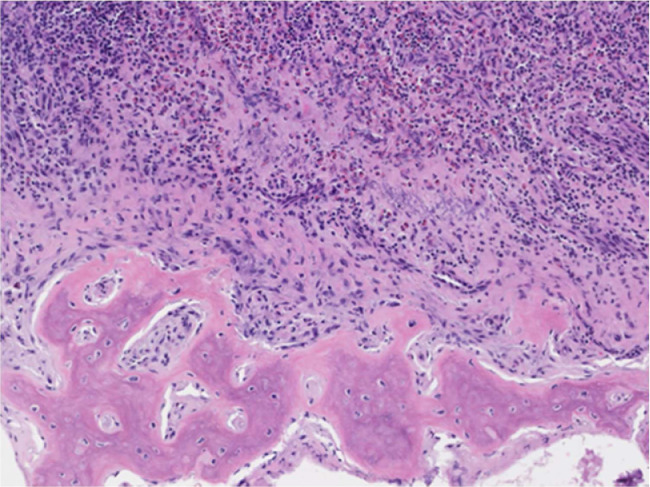
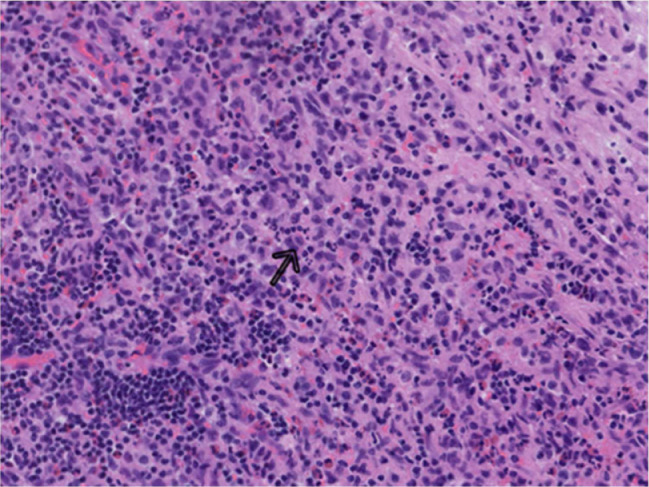
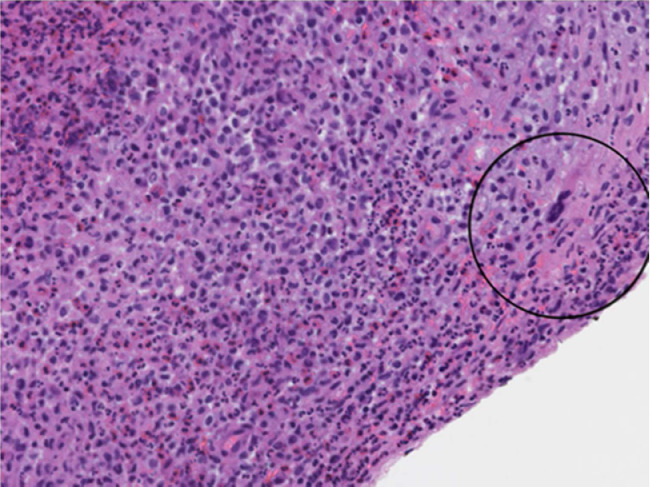
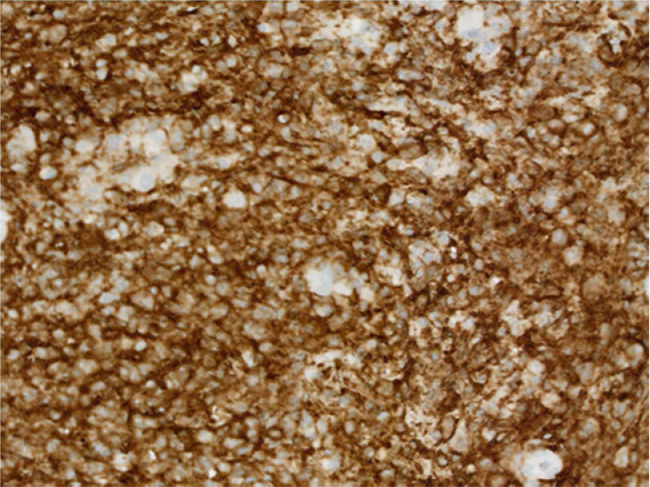
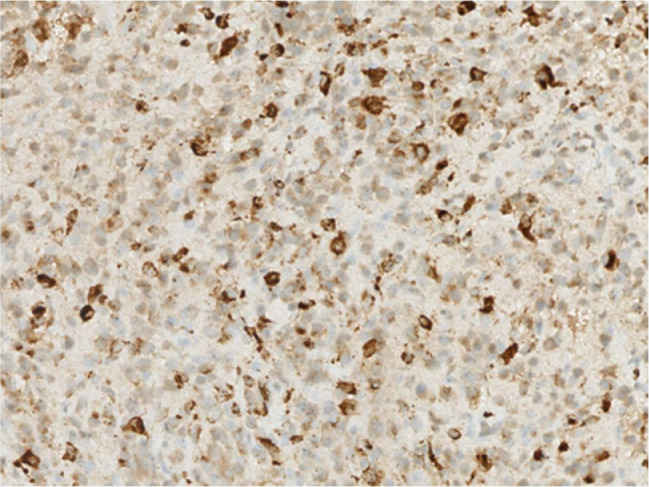
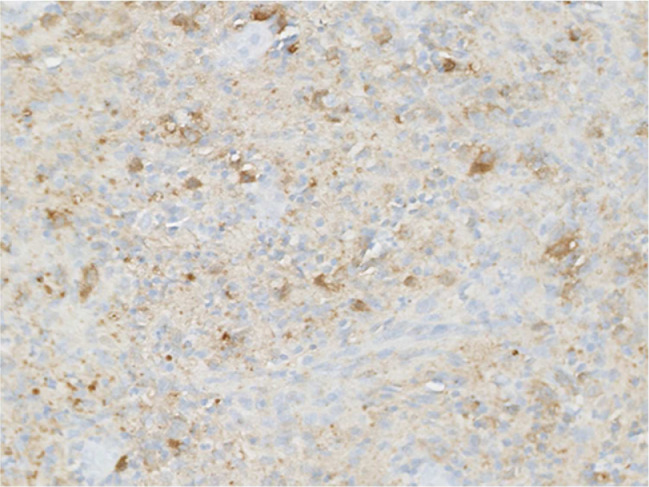
The gross tissue examination revealed multiple fragments of bone admixed with tan-red pieces of rubbery tissue. Microscopic examination showed fragments of bone and a mixture of histiocytes, eosinophils, neutrophils, and small lymphocytes (Fig. 5). Few eosinophilic microabscesses were also seen. Numerous Langerhans’ cells were recognized based on their characteristic grooved, folded, and indented nuclei, with fine chromatin, inconspicuous nucleoli, and thin nuclear membrane (Fig. 6). Occasional multinucleated forms were also appreciated (Fig. 7). The sheets of Langerhans cells were strongly positive for CD1a (Fig. 8), CD68 (Fig. 9), and S-100 (Fig. 10). Special Acid-fast bacilli and Periodic acid–Schiff stains were negative for Acid-fast bacilli and fungal elements. These histologic findings confirmed the diagnosis of Langerhans’ cell histiocytosis.
Fig. 5.
Bony lesion shows infiltration of polygonal cells with eosinophilic cytoplasm and oval nuclei (Langerhans cells); admixed with numerous eosinophils and lymphocytes; ×100 (H&E).
Fig. 6.
Cells with nuclei containing linear grooves (arrow), typical cytologic feature of Langerhans cells; ×200 (H&E).
Fig. 7.
Multinucleated form (circled); ×200 (H&E).
Fig. 8.
Immunohistochemistry showing presence of Langerhans cell CD1a antigen. CD1a; ×200.
Fig. 9.
Immunohistochemistry showing presence of Langerhans cell CD68 protein; ×200.
Fig. 10.
Immunohistochemistry showing presence of Langerhans cell S100 protein; ×200.
Discussion
Langerhans’ cell histiocytosis (LCH) is a rare neoplastic proliferation of Langerhans’ cells, which are derived from myeloid progenitor cells in the bone marrow induced by somatic mutations of several genes in the cell signaling mitogen-activated protein kinase pathway in myeloid precursors [1]. LCH can occur at any age, with most cases presenting in the first 4 years of life. LCH reportedly affects 4-8 children per million [2], [3], [4], [5], [6] and 1 to 2 adults per million [7] each year. There is a slight predominance in males with the incidence reportedly higher in whites of northern European descent [8]. LCH has a highly variable clinical presentation, ranging from a single lesion to potentially fatal disseminated disease. The disease process is therefore commonly classified as 3 clinical variants based on the organ involvement. These categories are designated as eosinophilic granuloma, the most common form which is characterized by a solitary osseous lesion; Hand-Schuller-Christian disease, the chronic recurrent form, which classically shows a triad of a skull lesion, exophthalmos, and diabetes insipidus; and the fulminant form termed Letterer-Siwe disease characterized by multiple organ involvement [9], [10], [11].
Typical radiologic findings in LCH are also based on the organ involved. This disease process favors the skull, spine, and long bones with characteristic punched out lytic lesions and vertebra plana. Central nervous system involvement usually demonstrates infundibular thickening, absence of the normal posterior pituitary bright spot, and neurodegenerative lesions [12], [14]. Lastly, LCH can also involve the lungs with upper lobe predominant centrilobular nodules and cysts of varying sizes and morphology. This case report emphasizes the neuroradiologic and central nervous system findings which predominantly involve the mastoid process, temporal bones, orbit, and sphenoid [12]. Given the rarity of this disease and unpredictable clinical course, one must have a high index of suspicion when interpreting the imaging findings. Temporal bone disease is often nonspecific and most commonly presents as a temporal bone mass, otitis media, or externa. However, the presence of a lytic lesion in the temporal bone that spares the osseous labyrinth should raise the possibility of LCH [12]. When the disease is present in the temporal bone, orbit or sphenoid, they are classified as “CNS risk” due to increased incidence of pituitary and brain involvement [13]. Central diabetes insipidus is often the initial symptom but not commonly recognized as a manifestation of LCH [14]. As a result, patients are often delayed in diagnosis until additional symptoms and radiologic findings develop [4], [5].
It is recommended that biopsy confirmation of suspected LCH be performed in all cases, especially for patients requiring therapy. Biopsies of involved tissue usually demonstrate heterogeneous collections of Langerhans cells with eosinophils, neutrophils, small lymphocytes, and histiocytes (with some multinucleated giant cells formation). Langerhans cell histiocytosis lesions are characterized by CD1a+/CD207+ histiocytes. Detection of these Langerhans cell immunohistochemical markers is mandatory to confirm the diagnosis.
Conclusion
Langerhans’ cell histiocytosis is a rare disease that is difficult to diagnose due to the variable clinical and radiologic findings. We hope to bring awareness of this disease process in the central nervous system in patients presenting with central diabetes insipidus who are found to have a thickened pituitary infundibulum. Consider this disease entity in the differential diagnosis of resorptive lesions in the mastoid process and temporal bone which spares the osseous labyrinth.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
References
- 1.Swerdlow S.H., Campo E., Harris N.L., Jaffe E.S., Pileri S.A., Stein H., Thiele J., Vardiman J.W. Fourth Edition. World Health Organization; 2017. WHO Classification of Tumors of Hematopoietic and Lymphoid Tissues; pp. 400–439. Revised edition. [Google Scholar]
- 2.Stalemark H., Laurencikas E., Karis J. Incidence of Langerhans cell histiocytosis in children: a population-based study. Pediatr Blood Cancer. 2008;51:76–81. doi: 10.1002/pbc.21504. [DOI] [PubMed] [Google Scholar]
- 3.Salotti J.A., Nanduri V., Pearce M.S. Incidence and clinical features of Langerhans cell histiocytosis in the UK and Ireland. Arch Dis Child. 2009;94:376–380. doi: 10.1136/adc.2008.144527. [DOI] [PubMed] [Google Scholar]
- 4.Broadbent V., Egeler R.M., Nesbit M.E., Jr. Langerhans cell histiocytosis—clinical and epidemiological aspects. Br J Cancer. 1994;23(Suppl):S11–S16. [PMC free article] [PubMed] [Google Scholar]
- 5.Guyot-Goubin A., Donadieu J., Barkaoui M. Descriptive epidemiology of childhood Langerhans cell histiocytosis in France, 2000-2004. Pediatr Blood Cancer. 2008;51:71–75. doi: 10.1002/pbc.21498. [DOI] [PubMed] [Google Scholar]
- 6.Nicholson S.H., Egeler M.R., Nesbit M.E. Langerhans cell histiocytosis: the epidemiology of Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12:379–384. doi: 10.1016/s0889-8588(05)70517-7. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner I., von Hochstetter A., Baumert B. Langerhans’ cell histiocytosis in adults. Med Pediatr Oncol. 1997;28:9–14. doi: 10.1002/(sici)1096-911x(199701)28:1<9::aid-mpo3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 8.Ribeiro K.B., Degar B., Antoneli C.B., Rollins B., Rodriguez-Galindo C. Ethnicity, race, and socioeconomic status influence incidence of Langerhans cell histiocytosis. Pediatr Blood Cancer. 2015;62:982–987. doi: 10.1002/pbc.25404. [DOI] [PubMed] [Google Scholar]
- 9.Kim S., Hong S., Shin H., Hwang J., Jou S., Choi S. Adult Langerhans’ cell histiocytosis with multisystem involvement. Medicine. Nov 2018;97(48):1–6. doi: 10.1097/MD.0000000000013366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stull M., Kransdorf M., Devaney K. Langerhans’ cell histiocytosis of bone. RadioGraphic. 1992;12(4) doi: 10.1148/radiographics.12.4.1636041. [DOI] [PubMed] [Google Scholar]
- 11.Zinn D.J., Chakraborty R., Allen C.E. Langerhans cell histiocytosis: emerging insights and clinical implications. Oncology (Williston Park) Feb 2016;30(2):122–132. 139. [PubMed] [Google Scholar]
- 12.Coleman M., Matsumoto J., Carr C., Eckel L., Rao A. Bilateral temporal bone langerhans cell histiocytosis: radiologic pearls. Open Neuroimag J. 2013;7:53–57. doi: 10.2174/1874440001307010053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grois N., Pötschger U., Prosch H., Minkov M., Arico M., Braier J. Risk factors for diabetes insipidus in langerhans cell histiocytosis. DALHX- and LCH I and II Study Committee. Pediatr Blood Cancer. 2006;46(2):228. doi: 10.1002/pbc.20425. [DOI] [PubMed] [Google Scholar]
- 14.Zaveri J., La Q., Yarmish G., Neuman J. More than just Langerhans cell histiocytosis: a radiologic review of histiocyic disorders. Radiographics. 2014;43(7):1–18. doi: 10.1148/rg.347130132. [DOI] [PubMed] [Google Scholar]