Abstract
The aim of this study was to evaluate the risk of asthma in rheumatoid arthritis patients using matched control group for socioeconomic factors and past medical history. Adults >20 years old were collected from the Korean Health Insurance Review and Assessment Service - National Sample Cohort (HIRA-NSC) from 2002 through 2013. A total of 6,695 individuals with rheumatoid arthritis were matched for age, sex, income, region of residence, hypertension, diabetes, and dyslipidemia with 26,780 individuals included in a control group. In both the rheumatoid arthritis and control groups, subjects’ history of asthma was evaluated. Asthma (J45 and J46) and rheumatoid arthritis (M05 and M06) were included based on the International Classification of Disease-10 (ICD-10) codes and medication history. The crude and adjusted (depression and Charlson Comorbidity Index) hazard ratios (HRs) and 95% confidence intervals (CI) of asthma for rheumatoid arthritis patients were analyzed using a stratified Cox proportional hazard model. Subgroup analyses were conducted according to age and sex, number of treatment histories, and medication histories. Approximately 16.4% (1,095/6,695) of rheumatoid arthritis group and 13.0% (3,469/26,780) of the control group had asthma (P < 0.001). The rheumatoid arthritis group demonstrated a higher adjusted HR for asthma than the control group (adjusted HR = 1.23, 95% CI = 1.15–1.32, P < 0.001). This result was consistent in all subgroups. Rheumatoid arthritis was related to an increase risk of asthma.
Subject terms: Asthma, Rheumatoid arthritis
Introduction
Rheumatoid arthritis is an autoimmune disease that manifests as a synovial inflammation, hyperplasia and joint, cartilage and bone destruction1. The prevalence of rheumatoid arthritis is varies according to ethnicity and residential region from approximately 0.48–1% in adult population2. In Korea, approximately 0.27% of the general population has rheumatoid arthritis3. Rheumatoid arthritis has been reported to increase the risk of several morbidities including acute myocardial infarction, chronic obstructive pulmonary disease and asthma4–6. Thus, considerable attention has been paid to minimize the burden of rheumatoid arthritis by elucidating associated factors. The etiology of rheumatoid arthritis is yet to be completely elucidated, and multiple factors including genetic and environmental triggers and immunologic factors are known to be involved in the pathophysiology of rheumatoid arthritis1. Because of these multiple causative factors, several systemic diseases, such as cardiovascular disease and diabetes, have been described to be related to rheumatoid arthritis7,8.
Asthma is a chronic inflammatory disease of the airway with a heterogeneous pathophysiology9,10. The prevalence of asthma is approximately 4.3–8.6% in the adult population globally11,12. In Korea, approximately 5.7% of adults have asthma13. In addition to causing well-known pathologies in the airway, several studies have proposed systemic inflammatory dysfunction in asthma patients14,15. In addition to generating T helper cell type 2 (Th2) immune responses, Th1-predominant inflammatory factors such as tumor necrosis factor (TNF)-α are elevated in asthma patients16. In line with this, other inflammatory diseases, such as rheumatoid arthritis, have been suggested to be associated with asthma5,17–19. A cross-sectional study reported a positive association between rheumatoid arthritis and asthma (adjusted odds ratio [OR] = 3.12, 95% confidence interval [CI] = 2.77–3.51)19. However, a causal relationship between asthma and rheumatoid arthritis could not be determined due to the cross-sectional study design. A few prior longitudinal follow-up studies reported an increased risk of rheumatoid arthritis in asthma patients20,21. On the other hand, only one recent population cohort study described a high risk of asthma in rheumatoid arthritis patients5.
The running hypothesis of the present study was that rheumatoid arthritis could increase the risk of asthma in an adult population independent of demographic factors and patients’ past medical history. To prove this hypothesis, we used a large, population cohort database of medical claim codes. To minimize possible bias from medical accessibility, participants’ level of income and region of residence were additionally matched for in the control group. Moreover, participants’ past medical history of hypertension, diabetes, and dyslipidemia were matched between the study and control groups.
Results
The time duration from index date to asthma was 45.6 months (Standard deviation, SD = 34.5) in asthma group and 46.2 months (SD = 35.1) in control group. The rate of asthma was higher in the rheumatoid arthritis group (16.4% [1,095/6,695]) than in the control group (13.0% [3,469/26,780], P < 0.001, Table 1). The general characteristics (age, sex, income, region of residence, and hypertension, diabetes, and dyslipidemia) of the participants were the same due to the matching procedure (P = 1.000). The rates of depression, and Charlson Comorbidity Index (CCI) score ≥2 were higher in the rheumatoid arthritis group than in the control group (each P < 0.05).
Table 1.
General Characteristics of Participants.
| Characteristics | Total participants | ||
|---|---|---|---|
| Rheumatoid arthritis (n, %) | Control (n, %) | P-value | |
| Age (years old) | 1.000 | ||
| 20–24 | 176 (2.6) | 704 (2.6) | |
| 25–29 | 281 (4.2) | 1,124 (4.2) | |
| 30–34 | 382 (5.7) | 1,528 (5.7) | |
| 35–39 | 509 (7.6) | 2,036 (7.6) | |
| 40–44 | 667 (10.0) | 2,668 (10.0) | |
| 45–49 | 914 (13.7) | 3,656 (13.7) | |
| 50–54 | 1,059 (15.8) | 4,236 (15.8) | |
| 55–59 | 828 (12.4) | 3,312 (12.4) | |
| 60–64 | 767 (11.5) | 3,068 (11.5) | |
| 65–69 | 548 (8.2) | 2,192 (8.2) | |
| 70–74 | 339 (5.1) | 1,356 (5.1) | |
| 75–79 | 168 (2.5) | 672 (2.5) | |
| 80–84 | 49 (0.7) | 196 (0.7) | |
| 85+ | 8 (0.1) | 32 (0.1) | |
| Sex | 1.000 | ||
| Male | 1,569 (23.4) | 6,276 (23.4) | |
| Female | 5,126 (76.6) | 20,504 (76.6) | |
| Income | 1.000 | ||
| 1 (lowest) | 1,077 (16.1) | 4,308 (16.1) | |
| 2 | 979 (14.6) | 3,916 (14.6) | |
| 3 | 1,195 (17.8) | 4,780 (17.8) | |
| 4 | 1,516 (22.6) | 6,064 (22.6) | |
| 5 (highest) | 1,928 (28.8) | 7,712 (28.8) | |
| Region of residence | 1.000 | ||
| Urban | 2,952 (44.1) | 11,808 (44.1) | |
| Rural | 3,743 (55.9) | 14,972 (55.9) | |
| Hypertension | 2,829 (42.3) | 11,316 (42.3) | 1.000 |
| Diabetes | 1,387 (20.7) | 5,548 (20.7) | 1.000 |
| Dyslipidemia | 2,272 (33.9) | 9,088 (33.9) | 1.000 |
| Depression | 859 (12.8) | 2,740 (10.2) | <0.001* |
| CCI (score) | <0.001* | ||
| 0 | 2,061 (30.8) | 11,988 (44.8) | |
| 1 | 1,036 (15.5) | 3,602 (13.5) | |
| ≥2 | 3,598 (53.7) | 11,190 (41.8) | |
| Asthma ≥2 times | 1,095 (16.4) | 3,469 (13.0) | <0.001* |
| Asthma ≥3 times | 786 (11.7) | 2,435 (9.1) | <0.001* |
| Asthma ≥4 times | 626 (9.4) | 1,862 (7.0) | <0.001* |
| Asthma ≥5 times | 500 (7.5) | 1,477 (5.5) | <0.001* |
*Chi-square test or Fisher’s exact test. Significance at P < 0.05.
CCI: Charlson Comorbidity Index.
The adjusted HR of asthma was 1.23 in the rheumatoid arthritis group compared to the control group (95% CI = 1.15–1.32, P < 0.001, Table 2 and Fig. 1).
Table 2.
Crude and adjusted hazard ratios (95% confidence interval) of rheumatoid arthritis for asthma.
| Characteristics | Asthma | |||
|---|---|---|---|---|
| Crude† | P-value | Adjusted†,‡ | P-value | |
| Rheumatoid arthritis | 1.31 (1.22–1.40) | <0.001* | 1.23 (1.15–1.32) | <0.001* |
| Control | 1.00 | 1.00 | ||
*Cox-proportional hazard regression model, Significance at P < 0.05.
†Stratified model for age, income, and region of residence, hypertension, diabetes mellitus, and dyslipidemia histories.
‡Adjusted model for depression histories, and Charlson Comorbidity Index.
Figure 1.
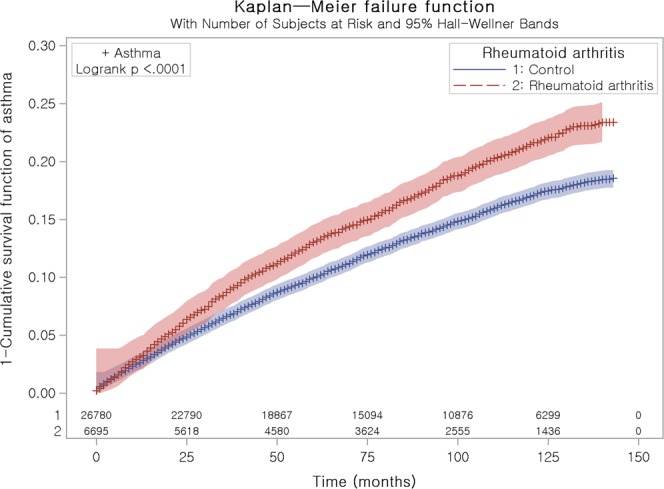
Kaplan Meier curve of rheumatoid arthritis for asthma. It was explained as 1 – survival function curve.
In subgroup analyses performed according to age and sex, all the crude and adjusted HRs of asthma were higher in the rheumatoid arthritis group than in the control group (each of P < 0.05, Table 3). The adjusted HRs were 1.61 (95% CI = 1.06–2.45) in men <40 years old; 1.40 (95% CI = 1.15–1.71) in women <40 years old; 2.20 (95% CI = 1.71–2.82) in men 40–59 years old; 1.16 (95% CI = 1.05–1.29) in women 40–59 years old; 1.47 (95% CI = 1.13–1.93) in men ≥60 years old; and 1.19 (95% CI = 1.05–1.35) in women ≥60 years old. According to the number of treatment histories of asthma, the rate of ≥3, ≥4, and ≥5 times of treatment histories of asthma were elevated in rheumatoid arthritis patients (all P < 0.001, Table 4). According to the types of medications, rheumatoid arthritis patients who treated with methotrexate, lefunomide, and other medications demonstrated 1.27 (95% CI = 1.14–1.43), 1.23 (95% CI = 1.02–1.48), and 1.20 (95% CI = 1.09–1.32) higher adjusted HRs for asthma (Table 5).
Table 3.
Subgroup analysis of crude and adjusted hazard ratios (95% confidence interval) of rheumatoid arthritis for asthma according to age and sex.
| Characteristics | Asthma | |||
|---|---|---|---|---|
| Crude† | P-value | Adjusted†,‡ | P-value | |
| Age <40 years old, men (n = 2,080) | ||||
| Rheumatoid arthritis | 1.64 (1.08–2.49) | 0.021* | 1.50 (0.98–2.30) | 0.063 |
| Control | 1.00 | 1.00 | ||
| Age <40 years old, women (n = 4,660) | ||||
| Rheumatoid arthritis | 1.40 (1.14–1.70) | 0.001* | 1.28 (1.05–1.57) | 0.017* |
| Control | 1.00 | 1.00 | ||
| Age 40–59 years old, men (n = 3,370) | ||||
| Rheumatoid arthritis | 2.32 (1.80–2.99) | <0.001* | 2.21 (1.71–2.85) | <0.001* |
| Control | 1.00 | 1.00 | ||
| Age 40–59 years old, women (n = 13,970) | ||||
| Rheumatoid arthritis | 1.17 (1.05–1.30) | 0.003* | 1.10 (0.99–1.22) | 0.074 |
| Control | 1.00 | 1.00 | ||
| Age ≥60 years old, men (n = 2,395) | ||||
| Rheumatoid arthritis | 1.55 (1.18–2.04) | 0.002* | 1.46 (1.11–1.93) | 0.007* |
| Control | 1.00 | 1.00 | ||
| Age ≥60 years old, women (n = 7,000) | ||||
*Cox-proportional hazard regression model, Significance at P < 0.05.
†Stratified model for age, income, and region of residence, hypertension, diabetes mellitus, and dyslipidemia histories.
‡Adjusted model for depression histories, and Charlson Comorbidity Index.
Table 4.
Analysis of crude and adjusted hazard ratios (95% confidence interval) of rheumatoid arthritis for asthma according the number of clinic visits of asthma.
| Characteristics | Asthma | |||
|---|---|---|---|---|
| Crude | P-value | Adjusted† | P-value | |
| Asthma ≥3 times of clinics visit with medication histories | ||||
| Rheumatoid arthritis | 1.32 (1.23–1.44) | <0.001* | 1.25 (1.16–1.36) | <0.001* |
| Control | 1.00 | 1.00 | ||
| Asthma ≥4 times of clinics visit with medication histories | ||||
| Rheumatoid arthritis | 1.38 (1.26–1.51) | <0.001* | 1.30 (1.19–1.43) | <0.001* |
| Control | 1.00 | 1.00 | ||
| Asthma ≥5 times of clinics visit with medication histories | ||||
| Rheumatoid arthritis | 1.38 (1.25–1.53) | <0.001* | 1.30 (1.17–1.44) | <0.001* |
| Control | 1.00 | 1.00 | ||
*Cox-proportional hazard regression model, Significance at P < 0.05.
†Stratified model for age, income, and region of residence, hypertension, diabetes mellitus, and dyslipidemia histories.
‡Adjusted model for depression histories, and Charlson Comorbidity Index.
Table 5.
Crude and adjusted hazard ratios (95% confidence interval) of rheumatoid arthritis for asthma according to their treatments.
| Characteristics | Asthma | |||
|---|---|---|---|---|
| Crude† | P-value | Adjusted†‡ | P-value | |
| Methotrexate (n = 12,335) | ||||
| Rheumatoid arthritis | 1.32 (1.18–1.48) | <0.001* | 1.27 (1.14–1.43) | <0.001* |
| Control | 1.00 | 1.00 | ||
| Leflunomide (n = 3,955) | ||||
| Rheumatoid arthritis | 1.31 (1.09–1.58) | 0.004* | 1.23 (1.02–1.48) | 0.033* |
| Control | 1.00 | 1.00 | ||
| Other treatments (n = 17,185) | ||||
| Rheumatoid arthritis | 1.28 (1.16–1.41) | <0.001* | 1.20 (1.09–1.32) | <0.001* |
| Control | 1.00 | 1.00 | ||
*Cox-proportional hazard regression model, Significance at P < 0.05.
†Stratified model for age, income, and region of residence, hypertension, diabetes mellitus, and dyslipidemia histories.
‡Adjusted model for depression histories, and Charlson Comorbidity Index.
Discussion
The present study demonstrated a higher risk of asthma in rheumatoid arthritis patients than in participants in control group matched for age, sex, income, region of residence, and past medical history. The rheumatoid arthritis patients demonstrated a 1.23-times higher risk of asthma than those in the control group (95% CI = 1.15–1.32, P < 0.001). In addition, the subgroup analyses demonstrated that the increased risk of asthma was consistent according to age and sex and the medication histories of rheumatoid arthritis. This study has added to previous findings by using a control group matched for socioeconomic factors and past medical history. Moreover, various comorbidities were adjusted using CCI.
Similar to the present results, several studies have reported a relationship between asthma and rheumatoid arthritis5,17,19. However, the majority of previous studies were based on cross-sectional analyses, which could not demonstrate causality between asthma and rheumatoid arthritis17,18. A recent population-based cohort study demonstrated a 2.07-times increased risk of asthma in rheumatoid arthritis patients (95% CI = 1.99–2.15)5. However, they used control group matched only for age and sex. Thus, the possible influence of socioeconomic factors could not be excluded in that study. In particular, because they used medical claim code data, medical accessibility should have been unbiased between the study and control groups. However, medical accessibility might have been different according to socioeconomic status. Thus, socioeconomic factors should be considered when analyzing medical claim code data. From the present results, it can be concluded that the risk of asthma was increased in rheumatoid arthritis patients independent of socioeconomic factors, demographic factors and past medical history. The verified definitions of both asthma and rheumatoid arthritis potentiated the feasibility of the present study3,13,22. To improve the fidelity of the diagnosis of asthma, subgroup analyses were performed according to the number of treatment histories of asthma in this study. As results, all the asthma subgroups with ≥3, ≥4, and ≥5 times of treatment histories were elevated in rheumatoid arthritis patients.
The plausible pathophysiologic mechanisms for the increased risk of asthma in rheumatoid arthritis could be explained by immune imbalance, heterogeneous asthma characteristics, and bystander effects.
The abnormal Th1 response that occurs in rheumatoid arthritis patients could influence an uncontrolled Th2 response, which induces asthma. The counterregulation between Th1-type immunity and Th2-type immunity has been found, with some exceptions, in several studies18,23. Th1 and Th2 lymphocytes are classified based on their distinct cytokine secretion profiles24. However, Th2-type cytokines have been identified to contribute to the development of some autoimmune diseases25,26. In addition, an animal study demonstrated a switch from Th1 lymphocyte phenotypes from proinflammatory IFN-gamma-secreting Th1 cells to IL-4-secreting Th2 cells upon stimulation with cytokines27. Abnormalities in the cytokines affecting the Th1-Th2 balance may contribute to the development of asthma in rheumatoid arthritis patients. Moreover, Th17 cells have been reported to be increased in both asthma and rheumatoid arthritis patients18. The overlapping effects of some subsets of Th1 or Th2 cells could link rheumatoid arthritis and a high risk of asthma.
The systemic inflammatory conditions of rheumatoid arthritis could increase the risk of asthma with variety of immunologic mechanisms in addition to skewed Th2 responses. Nonallergic asthma might be caused by the inflammatory responses associated with rheumatoid arthritis. A recent microarray study demonstrated numerous novel pathways, such as those associated with neuronal function, WNT pathways, and actin cytoskeleton pathways, in asthma patients in addition to the classical type 2 inflammation-related genes16. Compared to childhood-onset asthma, adult asthma has several subtypes that have pathological heterogeneity28. Several asthma endotypes have been proposed based on distinct pathophysiologic mechanisms including nonallergic origins of noneosinophilic (neutrophilic) asthma and airflow obstruction caused by obesity29. Therefore, systemic inflammatory or immune dysfunctions in rheumatoid arthritis patients might lead to the several types of asthma endotypes. For instance, neutrophilic asthma could be induced due to deprivation of anti-inflammatory responses in rheumatoid arthritis patients30.
The medication of rheumatoid arthritis could increase the risk of asthma. The disease-modifying drugs and immunosuppressant used in rheumatoid arthritis patients were suggested to have toxic effects to lung function31. They reported that hypersensitivity reaction and susceptibility to infection following the use of rheumatoid arthritis medication could be predisposed the rheumatoid arthritis patients to lung diseases31. However, the effects of these disease-modifying drugs and immunosuppressant on airway hyperresponsiveness and asthma were controversial. A few experimental studies demonstrated the attenuation of allergen-induced airway inflammation and sensitization after long-term use of leflunomide32,33. In addition, immunosuppressant was suggested to relieving asthma by inhibition of T cell activation34. To evaluate the effects of rheumatoid arthritis medications on the risk of asthma, the present study analyzed the risk of asthma according to the types of rheumatoid arthritis medications. As results, all the types of rheumatoid arthritis medication groups showed the increased risk of asthma. Therefore, the association between rheumatoid arthritis and asthma is beyond the effects of rheumatoid arthritis medications.
The common pathophysiological causes of asthma and rheumatoid arthritis could mediate the risk of asthma in rheumatoid arthritis patients. Unidentified common comorbidities could result in both rheumatoid arthritis and asthma. Cardiovascular diseases have been reported to be related to both asthma and rheumatoid arthritis7,35. Likewise, diabetes also increases the risk of both rheumatoid arthritis and asthma8,36. Although we matched and adjusted for these comorbidities, a possible influence of these or other unconsidered confounders could not be excluded. In addition, other environmental factors including smoking, could link rheumatoid arthritis with asthma. Because the HIRA-NSC data does not include lifestyle factors, such as smoking, alcohol consumption, and body mass index, these factors could not be considered in the present study.
In the subgroup analyses performed in this study, all of the age and sex subgroups showed an increased risk of asthma in rheumatoid arthritis patients. On the other hand, a previous study demonstrated a differential association between asthma and rheumatoid arthritis according age and sex5. A population cohort study reported the risk of asthma in rheumatoid arthritis patients was higher in subgroups of women and younger subjects (<40 years old)5. Differences in medical care could result in highly detectable rates in women and the younger population included in that study.
This study based on the large, population cohort encompassing a whole nation. The current data minimized missing participants by basing the sample on the national medical registry database. In Korea, all citizens are registered and covered by the HIRA. This HIRA data was sampled by a statistician to represent the whole national population, which was verified in a previous study13,37. Both rheumatoid arthritis and asthma were defined by ICD-10 codes and medication history. The unbiased control group was another advantage of the present study. The control group was sampled with a random number order and matched for age, sex, income, region of residence, and past medical history of hypertension, diabetes, and dyslipidemia. Because rheumatoid arthritis is influenced by socioeconomic status, the consideration of this factor might be crucial38.
However, the limited information on the severity and treatment of both rheumatoid arthritis and asthma limited the interpretations of the present study. Detailed laboratory data including serum levels of autoantibodies and lung function testing could not be accessed in this study. Thus, the heterogeneous nature of rheumatoid arthritis or asthma could not be classified in this study. Lastly, the confounding effects of unavailable variables, such as smoking, alcohol consumption, obesity, diet, and nutrition, are still possible.
In conclusion, rheumatoid arthritis increased the risk of asthma in an adult Korean population. The high risk of asthma was consistent according to age and sex subgroups.
Materials and Methods
Study population and data collection
The ethics committee of Hallym University (2017-I102) approved the use of these data. Written informed consent was exempted by the Institutional Review Board.
This national cohort study relied on data from the Korean Health Insurance Review and Assessment Service-National Sample Cohort (HIRA-NSC). The detailed description of this data was described in our previous studies39,40.
Participant selection
Of 1,125,691 cases with 114,369,638 medical claim codes, the 7,783 of rheumatoid arthritis patients were selected following the previous studies that reported the prevalence and incidence of rheumatoid arthritis of Korea (Supplement File S1)3,22. The 230,764 of asthma patients were selected using the modified method of a previous study (Supplement File S1)13.
The rheumatoid arthritis group was matched 1:4 with participants (control group) who were not diagnosed with rheumatoid arthritis from 2002 through 2013. The control group was selected from the total population (n = 1,117,908). Matching was performed for age, group, sex, income group, region of residence, and past medical history (hypertension, diabetes, and dyslipidemia). To prevent selection bias when selecting the matched participants, the control participants were sorted using a random number order and were then selected from top to bottom. We set the index date as the date of the diagnosis of rheumatoid arthritis. It was assumed that the matched control participants were involved at the same time as the rheumatoid arthritis participants (index date). Therefore, the control patients who died before the index date were excluded. The participants with a history of asthma before the index date were excluded from both the rheumatoid arthritis and control groups. In the rheumatoid arthritis group, 965 participants were excluded. The rheumatoid arthritis patients for whom we could not identify sufficient matching participants were excluded (n = 8). We also excluded the participants younger than 20 years of age (n = 115). The mean follow up time from index date to last date (Dec. 31, 2013) or death date was almost similar in both rheumatoid arthritis (89.5 months, SD = 43.0) and control group (90.0 months, SD = 43.2). Finally, 1:4 matching resulted in the inclusion of 6,695 rheumatoid arthritis patients and 26,780 control participants (Fig. 2).
Figure 2.
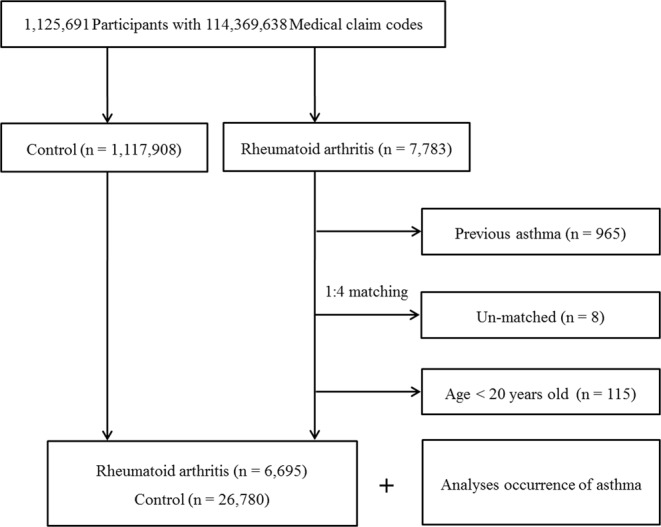
A schematic illustration of the participant selection process used in the present study. Of a total of 1,125,691 participants, 6,695 rheumatoid arthritis patients were matched with 26,780 control participants for age, group, sex, income group, region of residence, and past medical history.
Variables
The age, sex, income, region of residence, hypertension, diabetes, dyslipidemia, and depression were defined as previous studies39. Depression was adjusted due to the previously reported association between asthma and depression41. CCI was used for 16 comorbidities as the continuous variable (0 [no comorbidity] through 27 [multiple comorbidities]) except for pulmonary disease and rheumatoid42.
Statistical analyses
Chi-square tests were used to compare the general characteristics between the rheumatoid arthritis and control groups.
Stratified Cox proportional hazard models were used to assess hazard ratios (HRs) for rheumatoid arthritis with respect to asthma. In this analysis, crude (simple) and adjusted (for depression and CCI) models were used, and 95% CIs were calculated. In these analyses, age, sex, income, region of residence, hypertension, diabetes, and dyslipidemia were stratified. Kaplan Meier analysis and Log rank test was used.
For the subgroup analyses, we divided the participants by age (20–39, 40–59, and 60+ years) and sex (men and women) to confirm that these relations were reliable according to age and sex. For the sensitivity analyses, we analyzed the limited number of participants who were visited clinics ≥3 times, 4 times, and 5 times for asthma were included. In addition, the rheumatoid arthritis patients were classified according to the most frequently used rheumatoid arthritis medications including methotrexate, leflunomide, and other medication and analyzed the relation with the rate of asthma.
Two-tailed analyses were conducted, and P values less than 0.05 were considered to indicate significance. The results were statistically analyzed using SPSS v. 21.0 (IBM, Armonk, NY, USA).
Supplementary information
Acknowledgements
The manuscript was edited for proper English language, grammar, punctuation, spelling, and overall style by the highly qualified native English-speaking editors at American Journal Experts (E288-00CA-4EB3-15B0-EA2P). This work was supported in part by a research grant (NRF-2018-R1D1A1A02085328 and NRF-2017R1C1B1007696) from the National Research Foundation (NRF) of Korea.
Author Contributions
H.G.C. designed the study, participated in data collection and interpretation of data, and revised the manuscript. S.Y.K. and D.J.O. participated in interpretation of data and drafted and revised the manuscript. H.G.C., S.Y.K., C.M. and D.J.O. participated in data analysis, interpretation of data, and revised the manuscript. All authors approved the final version of the manuscript.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43481-3.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England journal of medicine. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Costenbader KH, Chang SC, Laden F, Puett R, Karlson EW. Geographic variation in rheumatoid arthritis incidence among women in the United States. Archives of internal medicine. 2008;168:1664–1670. doi: 10.1001/archinte.168.15.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sung YK, Cho SK, Choi CB, Bae SC. Prevalence and incidence of rheumatoid arthritis in South Korea. Rheumatol Int. 2013;33:1525–1532. doi: 10.1007/s00296-012-2590-2. [DOI] [PubMed] [Google Scholar]
- 4.Shen TC, et al. Increased risk of chronic obstructive pulmonary disease in patients with rheumatoid arthritis: a population-based cohort study. QJM: monthly journal of the Association of Physicians. 2014;107:537–543. doi: 10.1093/qjmed/hcu027. [DOI] [PubMed] [Google Scholar]
- 5.Shen TC, Lin CL, Wei CC, Tu CY, Li YF. The risk of asthma in rheumatoid arthritis: a population-based cohort study. QJM: monthly journal of the Association of Physicians. 2014;107:435–442. doi: 10.1093/qjmed/hcu008. [DOI] [PubMed] [Google Scholar]
- 6.Chung WS, et al. Rheumatoid arthritis and risk of acute myocardial infarction–a nationwide retrospective cohort study. International journal of cardiology. 2013;168:4750–4754. doi: 10.1016/j.ijcard.2013.07.233. [DOI] [PubMed] [Google Scholar]
- 7.Cioffi, G. et al. High prevalence of occult heart disease in normotensive patients with rheumatoid arthritis. Clinical cardiology, 10.1002/clc.22926 (2018). [DOI] [PMC free article] [PubMed]
- 8.Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clinical and experimental rheumatology. 2015;33:115–121. [PubMed] [Google Scholar]
- 9.Wesolowska-Andersen A, Seibold MA. Airway molecular endotypes of asthma: dissecting the heterogeneity. Current opinion in allergy and clinical immunology. 2015;15:163–168. doi: 10.1097/ACI.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papi A, Brightling C, Pedersen SE, Reddel HK. Asthma. Lancet. 2018;391:783–800. doi: 10.1016/S0140-6736(17)33311-1. [DOI] [PubMed] [Google Scholar]
- 11.To T, et al. Global asthma prevalence in adults: findings from the cross-sectional world health survey. BMC public health. 2012;12:204. doi: 10.1186/1471-2458-12-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asher MI, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 13.Kim S, et al. Healthcare use and prescription patterns associated with adult asthma in Korea: analysis of the NHI claims database. Allergy. 2013;68:1435–1442. doi: 10.1111/all.12256. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, et al. Systemic inflammation mediates the detrimental effects of obesity on asthma control. Allergy and asthma proceedings. 2018;39:43–50. doi: 10.2500/aap.2018.39.4096. [DOI] [PubMed] [Google Scholar]
- 15.Fu JJ, McDonald VM, Baines KJ, Gibson PG. Airway IL-1beta and Systemic Inflammation as Predictors of Future Exacerbation Risk in Asthma and COPD. Chest. 2015;148:618–629. doi: 10.1378/chest.14-2337. [DOI] [PubMed] [Google Scholar]
- 16.Modena BD, et al. Gene expression in relation to exhaled nitric oxide identifies novel asthma phenotypes with unique biomolecular pathways. American journal of respiratory and critical care medicine. 2014;190:1363–1372. doi: 10.1164/rccm.201406-1099OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luo, Y. et al. Rheumatoid arthritis is associated with increased in-hospital mortality in asthma exacerbations: a nationwide study. Clinical rheumatology, 10.1007/s10067-018-4114-2 (2018). [DOI] [PubMed]
- 18.Rolfes MC, Juhn YJ, Wi CI, Sheen YH. Asthma and the Risk of Rheumatoid Arthritis: An Insight into the Heterogeneity and Phenotypes of Asthma. Tuberculosis and respiratory diseases. 2017;80:113–135. doi: 10.4046/trd.2017.80.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeong HE, Jung SM, Cho SI. Association between Rheumatoid Arthritis and Respiratory Allergic Diseases in Korean Adults: A Propensity Score Matched Case-Control Study. International journal of rheumatology. 2018;2018:3798124. doi: 10.1155/2018/3798124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemminki K, Li X, Sundquist J, Sundquist K. Subsequent autoimmune or related disease in asthma patients: clustering of diseases or medical care? Annals of epidemiology. 2010;20:217–222. doi: 10.1016/j.annepidem.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 21.Tirosh A, et al. Autoimmune diseases in asthma. Annals of internal medicine. 2006;144:877–883. doi: 10.7326/0003-4819-144-12-200606200-00004. [DOI] [PubMed] [Google Scholar]
- 22.Cho SK, et al. Development of an algorithm for identifying rheumatoid arthritis in the Korean National Health Insurance claims database. Rheumatol Int. 2013;33:2985–2992. doi: 10.1007/s00296-013-2833-x. [DOI] [PubMed] [Google Scholar]
- 23.Kero J, Gissler M, Hemminki E, Isolauri E. Could TH1 and TH2 diseases coexist? Evaluation of asthma incidence in children with coeliac disease, type 1 diabetes, or rheumatoid arthritis: a register study. The Journal of allergy and clinical immunology. 2001;108:781–783. doi: 10.1067/mai.2001.119557. [DOI] [PubMed] [Google Scholar]
- 24.Romagnani S, et al. An update on human Th1 and Th2 cells. International archives of allergy and immunology. 1997;113:153–156. doi: 10.1159/000237532. [DOI] [PubMed] [Google Scholar]
- 25.Yoshikawa H, et al. Elevation of IL-12 p40 and its antibody in myasthenia gravis with thymoma. Journal of neuroimmunology. 2006;175:169–175. doi: 10.1016/j.jneuroim.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Afanasyeva M, et al. Experimental autoimmune myocarditis in A/J mice is an interleukin-4-dependent disease with a Th2 phenotype. The American journal of pathology. 2001;159:193–203. doi: 10.1016/S0002-9440(10)61685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Assenmacher M, et al. Commitment of individual Th1-like lymphocytes to expression of IFN-gamma versus IL-4 and IL-10: selective induction of IL-10 by sequential stimulation of naive Th cells with IL-12 and IL-4. Journal of immunology. 1998;161:2825–2832. [PubMed] [Google Scholar]
- 28.Kim MA, Shin YS, Pham le D, Park HS. Adult asthma biomarkers. Current opinion in allergy and clinical immunology. 2014;14:49–54. doi: 10.1097/ACI.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 29.Lotvall J, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. The Journal of allergy and clinical immunology. 2011;127:355–360. doi: 10.1016/j.jaci.2010.11.037. [DOI] [PubMed] [Google Scholar]
- 30.Gao P, et al. Anti-inflammatory deficiencies in neutrophilic asthma: reduced galectin-3 and IL-1RA/IL-1beta. Respiratory research. 2015;16:5. doi: 10.1186/s12931-014-0163-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shaw M, Collins BF, Ho LA, Raghu G. Rheumatoid arthritis-associated lung disease. European respiratory review: an official journal of the European Respiratory Society. 2015;24:1–16. doi: 10.1183/09059180.00008014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uhlig T, et al. Effects of long-term oral treatment with leflunomide on allergic sensitization, lymphocyte activation, and airway inflammation in a rat model of asthma. Clinical and experimental allergy: journal of the British Society for Allergy and Clinical Immunology. 1998;28:758–764. doi: 10.1046/j.1365-2222.1998.00309.x. [DOI] [PubMed] [Google Scholar]
- 33.Malaviya R, Uckun FM. Leflunomide metabolite analogue alpha-cyano-beta-hydroxy-beta-methyl-N-[3-(trifluoromethyl)phenyl]propenamide inhibits IgE/FcepsilonRI receptor-mediated mast cell leukotriene release and allergic asthma in mice. American journal of therapeutics. 2001;8:309–316. doi: 10.1097/00045391-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Corrigan CJ. Asthma refractory to glucocorticoids: the role of newer immunosuppressants. American journal of respiratory medicine: drugs, devices, and other interventions. 2002;1:47–54. doi: 10.1007/BF03257162. [DOI] [PubMed] [Google Scholar]
- 35.Cazzola M, et al. Cardiovascular disease in asthma and COPD: a population-based retrospective cross-sectional study. Respiratory medicine. 2012;106:249–256. doi: 10.1016/j.rmed.2011.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Perez MK, Piedimonte G. Metabolic asthma: is there a link between obesity, diabetes, and asthma? Immunology and allergy clinics of North America. 2014;34:777–784. doi: 10.1016/j.iac.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee, J., Lee, J. S., Park, S. H., Shin, S. A. & Kim, K. Cohort Profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. International journal of epidemiology, 10.1093/ije/dyv319 (2016). [DOI] [PubMed]
- 38.Mackie SL, et al. Relationship between area-level socio-economic deprivation and autoantibody status in patients with rheumatoid arthritis: multicentre cross-sectional study. Annals of the rheumatic diseases. 2012;71:1640–1645. doi: 10.1136/annrheumdis-2011-201003. [DOI] [PubMed] [Google Scholar]
- 39.Kim SY, et al. Bidirectional association between gastroesophageal reflux disease and depression: Two different nested case-control studies using a national sample cohort. Scientific reports. 2018;8:11748. doi: 10.1038/s41598-018-29629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim SY, Lim JS, Kong IG, Choi HG. Hearing impairment and the risk of neurodegenerative dementia: A longitudinal follow-up study using a national sample cohort. Scientific reports. 2018;8:15266. doi: 10.1038/s41598-018-33325-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choi, H. G. et al. Association Between Asthma and Depression: A National Cohort Study. The journal of allergy and clinical immunology. In practice, 10.1016/j.jaip.2018.10.046 (2018). [DOI] [PubMed]
- 42.Quan H, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. American journal of epidemiology. 2011;173:676–682. doi: 10.1093/aje/kwq433. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


