Abstract
Thalamocortical (TC) relay cells exhibit different temporal patterns of activity, including tonic mode and burst mode, to transmit sensory information to the cortex. Our aim was to quantify the metabolic cost of different temporal patterns of neural activity across a range of average firing rates. We used a biophysically-realistic model of a TC relay neuron to simulate tonic and burst patterns of firing. We calculated the metabolic cost by converting the calculated ion fluxes into the demand for ATP to maintain homeostasis of intracellular ion concentrations. Most energy was expended on reversing Na+ entry during action potentials and pumping Ca2+ out of the cell. Average firing rate determined the ATP cost across firing patterns by controlling the overall number of spikes. Varying intraburst frequency or spike number in each burst influenced the metabolic cost by altering the interactions of inward and outward currents on multiple timescales, but temporal pattern contributed substantially less to the metabolic demand of neural activity as compared to average firing rate. These predictions should be considered when interpreting findings of functional imaging studies that rely of estimates of neuronal metabolic demand, e.g., functional magnetic resonance imaging.
Subject terms: Computational models, Biophysical models
Introduction
Thalamocortical (TC) relay neurons exhibit two distinct firing patterns - tonic and burst1–3 - that determine their manner of information processing and transmission of signals to the cortex1,4. Differences in metabolic demand between these firing modes are critical to interpreting functional brain imaging methods that infer neural activity from related metabolic mechanisms, for example functional magnetic resonance imaging (fMRI). Specifically, in vivo recordings suggest a tight correspondence between blood-oxygen-level dependent (BOLD) fMRI signals and neural activity5–9. However, it is not clear how the temporal pattern of neural activity, as opposed to the average firing rate of neurons, contributes to metabolic demand, and therefore might influence measures in functional brain imaging.
Processes consuming metabolic energy include maintaining rest potential, restoring ionic concentration gradients after action potentials (APs), reversing transmitter-evoked ionic fluxes through postsynaptic receptors, neurotransmitter recycling, reversing presynaptic AP-triggered Ca2+ entry, and vesicle cycling10,11. An energy budget of neural computation showed that most signaling-related energy was expended to pump Na+ and Ca2+ ions out of cells against their electrochemical gradients10. By converting ion fluxes into the number of ATP molecules, the metabolic cost of APs was determined in different cell types and species, and the effects of AP shape12, temperature13, channel densities and kinetics14, cell size15, and dendritic nonlinearity16 were considered. The metabolic cost of a single AP was dependent on the firing frequency11,13–16, and computational models predicted that metabolic cost per spike varied inversely with the firing rate during spike frequency adaptation16,17. Indeed, experimental recordings made in lateral superior olive, hippocampal CA1 region, and cerebral cortex indicated that the kinetics of ATP consumption and regeneration of energy equivalents, including nicotinamide adenine dinucleotide, flavin adenine dinucleotide, and O2 concentration, were correlated with the frequency of neural activity18,19.
The objective of this study was to estimate the metabolic cost of different temporal patterns of neural activity across a range of average firing rates. We used a computational model to simulate tonic and burst patterns of firing in TC relay neurons. Our results revealed that the metabolic demand to restore ionic concentration gradients during neural activity was determined by average firing rate, and the pattern of activity contributed little to the energy cost.
Methods
TC relay model
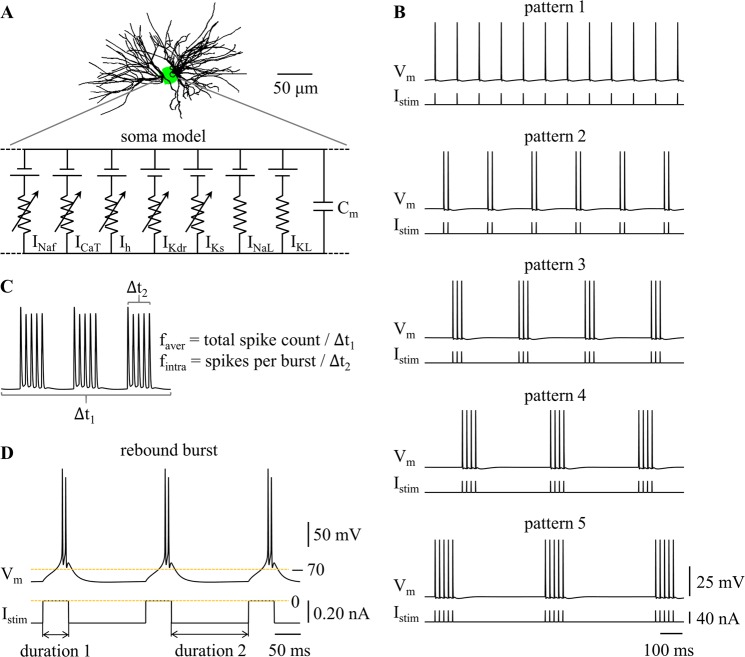
We used a computational model to simulate different temporal patterns of neural activity in TC relay neurons. The model included a dendritic tree, a cell body, and a myelinated axon, and its morphology was reconstructed from a filled TC cell from rat ventrobasal nucleus20. We examined the metabolic cost of neural activity in the cell body, as due to accessibility, this was the common site of recording in most experiments. There were three compartments in the soma (Fig. 1A), which were modeled with non-linear membrane dynamics including the parallel combination of fast Na+ (INaf), delayed rectifier K+ (IKdr), T-type Ca2+ (ICaT), hyperpolarization-activated cation (Ih), slow K+ (IKs), Na+ linear leakage (INaL), and K+ linear leakage (IKL) currents, and membrane capacitance (Cm). The details of the model followed McIntyre et al.21, and the model replicated a wide range of electrophysiological properties of TC neurons.
Figure 1.
Firing patterns in a computational model of a TC relay neuron. (A) Schematic of the model. (B) Temporal patterns of neural activity. Transmembrane voltage Vm was measured in the cell body. Depolarizing pulse train Istim (pulse width: 0.1 ms, pulse amplitude: 40 nA) was applied to cell body. (C) Graphical depiction of faver and fintra. faver = total spike count/Δt1, and fintra = spikes per burst/Δt2. (D) Rebound burst at faver = 10 Hz. Hyperpolarizing current (amplitude: −0.2 nA) was injected in the cell body. Duration 1 was 50 ms, and duration 2 was 150 ms.
Calculation of metabolic cost
The metabolic cost of different patterns of neural activity was determined by the number of ATP molecules expended to pump Na+ and Ca2+ ions out of the cell. Since the Na+/K+ ATPase pump did not differentiate between Na+ channel subtypes, we determined Na+ influx by summing INaL and INaf10. Ca2+ entry during APs or membrane hyperpolarization occurred through T-type Ca2+ channels, and to restore and maintain intracellular Ca2+ levels, the Ca2+-ATPase or Na+-Ca2+ exchanger pumped the Ca2+ entry out of the cell11. Therefore, the calculation of ATP consumption included the Na+ influx during APs, the Na+ influx at rest, and Ca2+ entry.
The total Na+ entry was computed by integrating the area under INaf(t) and INaL(t) curves. Since one Ca2+ ion has two positive charges, the Ca2+ entry was computed by integrating ICaT(t) curve, divided by 2. The Na+/K+ pump hydrolyzes one ATP molecule for every three Na+ ions extruded, and the Ca2+-ATPase or Na+-Ca2+ exchanger consumes one ATP for every Ca2+ ion extruded10,11. Thus, the number of ATP molecules consumed in each compartment was calculated by: , where Scom was the membrane area of the compartment (ionic currents I were current densities), NA was Avagadro’s number, and F was Faraday’s constant. The total metabolic cost was the sum of the ATP consumed in each of the three somatic compartments. This value was then divided by the time duration of the simulation to get a rate of metabolic energy consumed (in mol/s) during different rates and patterns of neural firing.
The resting potential was −70.40 mV in TC model neuron, which was an emergent property dependent upon all conductances and reversal potentials that were represented in the model. Although the net membrane current was 0 mA/cm2 at rest, there were still ion fluxes through relevant channels. Specifically, the Na+ influx mainly occurred through Na+ leakage channels (INaL = 1.10 × 10−3 mA/cm2), and the fast Na+ current (INaf = 3.17 × 10−6 mA/cm2) made little contribution. The Ca2+ influx occurred through T-type Ca2+ channels (ICaT = 1.65 × 10−4 mA/cm2). To maintain the resting potential, the Na+/K+ and Ca2+ pumps reversed these ion fluxes to maintain the concentration gradient for each ion, which consumed the ATP at a rate of 8.7770 × 107 mol/s. 81.51% of the ATP was consumed to reverse the Na+ leak, 18.31% was to pump the Ca2+ ions, and less than 1% was consumed to reverse leakage through the fast Na+ channels.
Simulation
We implemented the TC model in NEURON (v7.5)22, and the simulations were run with a time step of 0.025 ms. We quantified the metabolic cost of five temporal patterns of neural activity, which are illustrated in Fig. 1B. Pattern 1 was tonic firing, and patterns 2–5 were burst firing. Each pattern was recorded for 10 periods after a 1000 ms period of initialization. The average firing rate faver and intraburst frequency fintra were used to quantify the burst patterns, and faver was used to quantify the tonic patterns. faver referred to the average firing rate during the whole stimulation train, and fintra was the firing rate within each burst (Fig. 1C). We used 0 mV as a threshold to detect APs in the cell body.
We applied depolarizing pulse trains Istim to the cell body to generate the five firing patterns. We recorded transmembrane potential and transmembrane currents in the soma. We examined the effects of faver and fintra on the metabolic cost of each pattern of firing. Pulse amplitude was 40 nA and pulse width was 0.1 ms, which were suprathreshold for activation of APs in the cell body as faver or fintra varied. The current pulses controlled the firing patterns in the cell body while themselves producing little effect on the metabolic cost of neural activity. Thus, this approach allowed us to quantify how the average firing rate and temporal pattern influenced the metabolic cost of neural activity.
Tonic and burst firing in TC relay neurons is typically generated by a large number of synaptic inputs arriving at the dendrites. We conducted additional simulations to quantify the metabolic cost of the five firing patterns generated by synaptic inputs, rather than by current injection into the soma. Both approaches – synaptic inputs distributed across the dendritic tree and somatic current injection – generated identical patterns of neural activity (Supplementary Fig. S1). Further, these patterns generated very similar metabolic costs across changes in rate and pattern (Supplementary Figs S2, S3). Thus, our approach of using somatic current injection to generate and control firing activity did not influence our predictions of the effects of firing pattern on metabolic demand.
De-inactivation of ICaT generates low-threshold Ca2+ spikes (i.e., rebound bursts) in TC neurons1,3, and also results in burst patterns of firing. During Ca2+-mediated spikes, the intraburst frequency fintra was dominated by the rebound depolarization, and we only examined the effects of faver on the metabolic cost of neural activity. We applied constant hyperpolarizing current pulses to the cell body3 and 50 ms pauses in the hyperpolarizing current (duration 1, i.e., current switched off) resulted in low-threshold rebound spikes (Fig. 1D). The interval between pauses in the hyperpolarizing current (duration 2) changed the average firing rate faver, and increasing the amplitude of the hyperpolarizing phase increased the size of rebound depolarization, thereby increasing the number of spikes in each burst. Since the number of spikes in each burst also varied with duration 2, we used the smallest-amplitude of the hyperpolarizing current required to generate firing patterns at each average firing rate.
Results
Effects of average firing rate
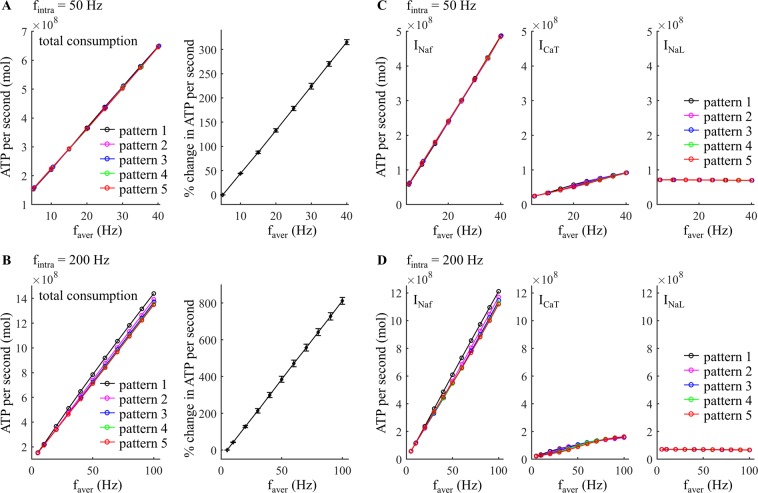
We first quantified the effects of average firing rate faver on the metabolic cost of each firing pattern generated by trains of depolarizing pulses injected into the cell body. ATP demand increased linearly as a function of faver and exhibited very similar trends across the five temporal patterns (Fig. 2A,B). The depolarization phase of the APs generated abundant Na+ entry through fast Na+ channels (Fig. 3A), which dominated the metabolic cost of neural activity. Increasing faver increased the number of spikes in each pattern of firing, and thus increased the ATP consumption to pump out the Na+ that entered through the fast Na+ channel (Fig. 2C,D, left). Although depolarizing pulses inactivated low-threshold ICaT, a small number of Ca2+ ions entered through T-type Ca2+ channels during each AP (Fig. 3A), and the ATP expended on Ca2+ extrusion also increased with faver (Fig. 2C,D, center). In contrast, Na+ entry through INaL was constant during the interspike intervals and was much smaller than INaf (Fig. 3A). Since increasing numbers of APs reduced INaL, the ATP expended to reverse Na+ leak decreased with faver (Fig. 2C,D, right).
Figure 2.
Effect of average firing rate, faver, on estimated metabolic cost of different temporal patterns of neural activity. (A,B) Left: total ATP consumption at fintra = 50 Hz and 200 Hz. Right: percent change in total ATP consumption (mean ± SD across five patterns). (C,D) ATP consumption required to reverse the ionic fluxes through each of INaf, ICaT, and INaL at fintra = 50 Hz and 200 Hz.
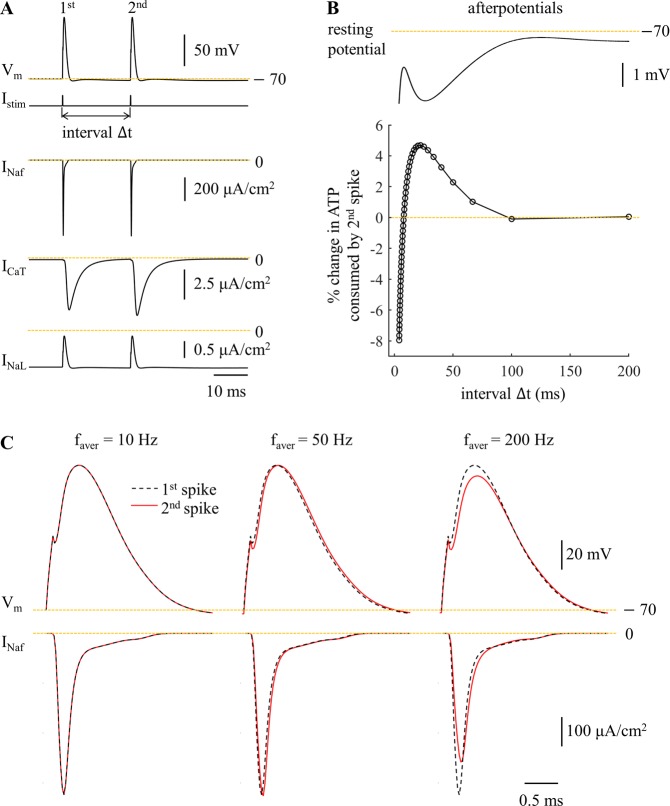
Figure 3.
Metabolic cost of pairs of APs as a function of the inter-pulse interval reflect the contributions of afterpotentials. (A) Sample responses and underlying currents (including INaf, ICaT, and INaL). Negative currents were inward. Two depolarizing pulses (amplitude: 40 nA, width: 0.1 ms) were applied to the cell body, and the inter-pulse interval was Δt = 20 ms. (B) Top: afterpotentials on the same time scale as the inter-stimulus interval, Δt. Bottom: percent change in ATP cost for the 2nd AP relative to the 1st AP as a function of Δt. The interval Δt was calculated by 1/faver, and faver was from 5 Hz to 250 Hz at a step size of 5 Hz. (C) Fast Na+ currents underlying two APs at faver = 10 Hz, 50 Hz, and 200 Hz.
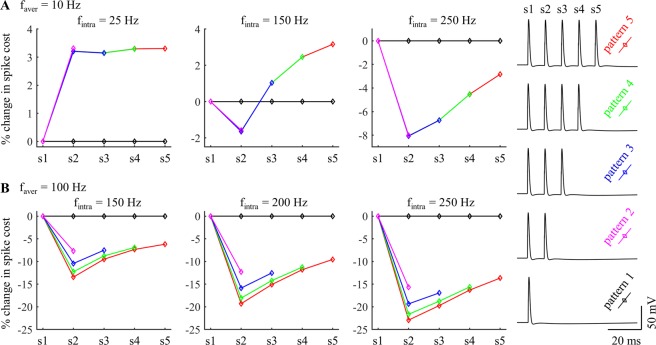
At fintra = 50 Hz, there was little difference in total ATP consumption between firing patterns (Fig. 2A, left). At fintra = 200 Hz, burst patterns consumed slightly less ATP than tonic patterns, and increasing numbers of spikes in each burst slightly reduced the metabolic cost of neural activity in comparison to patterns with fewer spikes per burst (Fig. 2B, left). To understand further the role of pattern on metabolic demand, we applied two suprathreshold pulses (amplitude: 40 nA, width: 0.1 ms) to the cell body to evoke pairs of APs, and the metabolic cost of the 2nd spike was determined as a function of the inter-pulse interval (Fig. 3B). The Na+ influx by the 2nd spike was dominated by the voltage-dependent INaf, which exhibited different degrees of activation dependent upon the hyperpolarizing afterpotentials from the 1st spike. At low frequencies (≤10 Hz), the inter-pulse interval was long enough that there was little change in the INaf underlying the two spikes (Fig. 3C), and thus the metabolic costs were similar. At moderate frequencies (from 15 Hz to 125 Hz), INaf activated more slowly during the 2nd AP, which resulted in a small delay of the fast Na+ current. This increased Na+ influx during the 2nd spike, and its ATP demand was higher than the 1st spike. However, the ATP consumed to extrude Ca2+ entry was reduced from pattern 1 to pattern 5 (Fig. 2C, center), and the total consumption of neural activity exhibited little difference between firing patterns at fintra = 50 Hz. At high frequencies (≥130 Hz), the afterpotentials attenuated the INaf underlying the 2nd spike, thereby reducing total Na+ influx and the ATP cost of burst patterns.
Effects of intraburst frequency
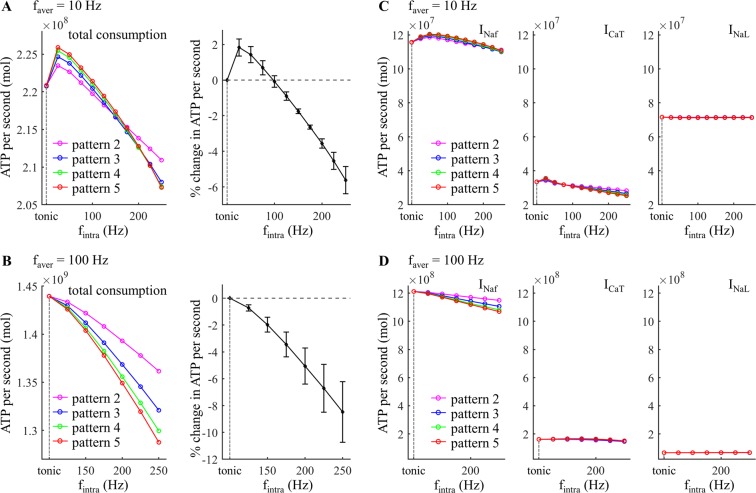
We quantified the effects of fintra on the metabolic cost of five temporal patterns of activity generated by applying depolarizing pulses to the cell body. Compared to varying faver, increasing fintra produced much smaller effects on the ATP demand. A 100 Hz increment in fintra resulted in less than 10% change of the mean ATP cost across firing patterns (Fig. 4A,B, right), but an only 5 Hz increment in faver increased the mean ATP cost by more than 43% (Fig. 2A,B, right). At faver = 10 Hz, the metabolic cost of burst patterns declined slightly as fintra increased (Fig. 4A). The increased number of spikes in each burst from pattern 2 to pattern 5 increased the ATP cost of neural activity at low fintra (<150 Hz) but reduced the energy consumption at high fintra. With fintra ≤75 Hz, the tonic patterns consumed less ATP than burst patterns. Note that the higher ATP cost required to reverse Na+ leakage at faver = 10 Hz arose from the long inter-burst intervals. At faver = 100 Hz, the ATP cost of burst patterns decreased as fintra increased, and increasing number of spikes in each burst reduced the metabolic cost of neural activity (Fig. 4B). In this case, tonic patterns consumed more ATP than burst patterns.
Figure 4.
Effect of fintra on estimated metabolic cost of different temporal patterns of neural activity. (A,B) Left: total ATP consumption at faver = 10 Hz and 100 Hz. Right: percent change in total ATP consumption (mean ± SD across five patterns). (C,D) ATP consumption required to reverse the ionic fluxes through each of INaf, ICaT, and INaL at faver = 10 Hz and 100 Hz.
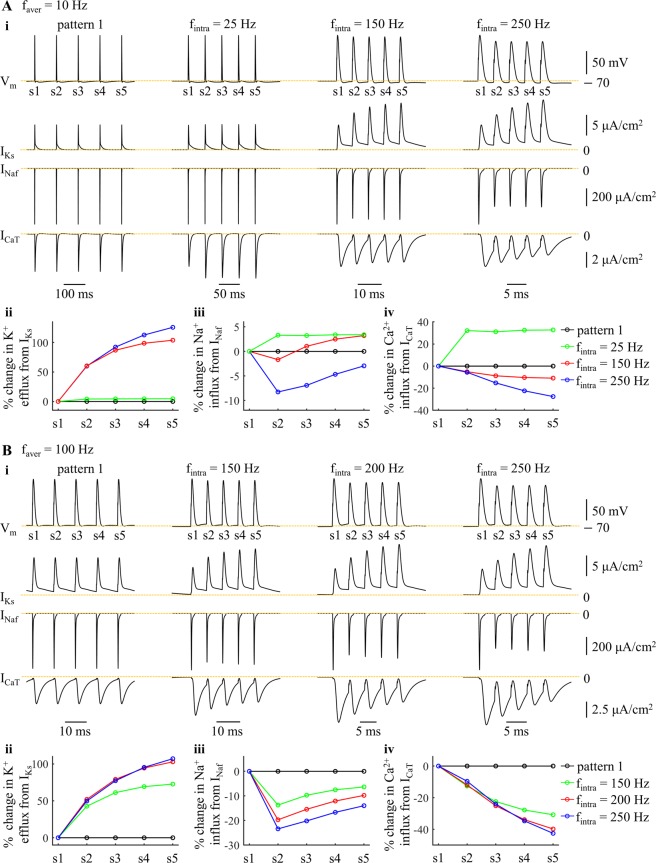
The IKs, INaf, and ICaT in each burst showed distinct changes with fintra (Fig. 5A,B, i). Increasing fintra reduced the interspike intervals, and the activation of the kinetically-slow IKs built up from one spike to the next, especially at frequencies >100 Hz. This effectively increased the K+ efflux from IKs during each spike (Fig. 5A,B, ii). The changes in Na+ influx during each spike were the net effects of the afterpotentials and the augmented activation of IKs (Fig. 5A,B, iii). Due to the afterpotentials (Fig. 3B), the inward INaf in each burst was substantially reduced at high fintra, which resulted in a pronounced reduction in the Na+ load from APs. In contrast, the hyperpolarizing IKs overlapped with Na+ influx, and more Na+ influx was required to achieve AP depolarization after the 2nd spike in each burst. Note that the changes in the Na+ influx of the 2nd spike were dominated by the afterpotentials of the 1st spike (Fig. 3B), since IKs was not sufficiently activated at this time. Unlike IKs, the Ca2+ influx increased after the 2nd spike in each burst at fintra = 25 Hz, while it was reduced from one spike to the next at high fintra (Fig. 5A,B, iv). Thus, the ATP expended on Ca2+ extrusion was higher at fintra = 25 Hz than for tonic patterns, and then decreased with fintra (Fig. 4C,D, center).
Figure 5.
Effects of fintra on ionic currents underlying patterns 1 and 5 at (A) faver = 10 Hz and (B) 100 Hz. (i) slow IKs, fast INaf, and low-threshold ICaT underlying five APs. The mark numbers (i.e., s1–s5) of the spikes from each pattern were indicated in the top panels. For pattern 1 (left panels), IKs, ICaT, and INaf were measured from 5 APs after 1000 ms. For pattern 5, currents were measured from a burst after 1000 ms. (ii) percent change in K+ efflux from IKs during each spike. (iii) percent change in Na+ influx from INaf during each spike. (iv) percent change in Ca2+ influx from ICaT during each spike.
With faver = 10 Hz, the inter-burst interval was long enough that the afterpotentials of the last spike of the prior burst had little effect on the metabolic demand of the subsequent burst (Fig. 6A). At low fintra, the afterpotentials of the cell body and the augmented activation of IKs both increased the Na+ influx during the APs (Fig. 6A, fintra = 25 Hz), and, as well, the ATP consumed on extruding Ca2+ entry was increased from pattern 1 to pattern 5 (Fig. 5A, iv), resulting in higher metabolic costs of burst patterns than tonic patterns. Similarly, increasing the number of spikes in each burst increased the ATP cost of neural activity. At moderate fintra, the afterpotentials reduced the Na+ influx during each AP, but the augmented IKs of patterns 3–5 dominated the effects of afterpotentials after the 3rd AP in each burst, resulting in a higher metabolic cost per spike than in tonic patterns (Fig. 6A, fintra = 150 Hz). At high fintra, the effects of afterpotentials overpowered the effects of slow IKs, and the ATP cost per spike in each burst were all lower than that of tonic patterns (Fig. 6A, fintra = 250 Hz). Since the ATP cost from ICaT was reduced from pattern 1 to pattern 5 at fintra ≥100 Hz (Fig. 4C, center), tonic patterns required more ATP than burst patterns and increasing spike number in each burst reduced the metabolic cost of neural activity at high fintra. With faver = 100 Hz, the prior burst produced effects on the metabolic demand of subsequent bursts (Fig. 6B). Due to short interspike intervals in each burst, the reduction in INaf from the afterpotentials substantially reduced the AP metabolic cost, which dominated the effects of augmented IKs, and burst patterns required less ATP than tonic patterns. Thus, the effects of varying fintra on ATP consumption of neural activity arose from the interactions of these two currents and the different timescales of their kinetics.
Figure 6.
Effects of fintra on percent change in spike cost of five patterns at (A) faver = 10 Hz and (B) 100 Hz. The APs were recorded from one period of each pattern after 1000 ms, and the mark numbers (i.e., s1–s5) of the APs are indicated in the right panel.
Metabolic cost with rebound activation
Burst and tonic modes differ in more than just firing pattern, and include differences in resting potential and spike threshold. By explicitly representing ICaT in the model, we accounted for the effects of these other changes on the metabolic costs of neural activity. Hyperpolarization de-inactivates inward ICaT in TC neurons and subsequent depolarization generates a low-threshold Ca2+ spike, which depends on the resting potential. The low-threshold spikes bring the membrane potential to the threshold for generating fast Na+ spikes, and result in a rebound burst of APs. We examined the effect of faver on the metabolic cost of firing patterns with rebound excitation generated by applying hyperpolarizing pulses to the cell body.
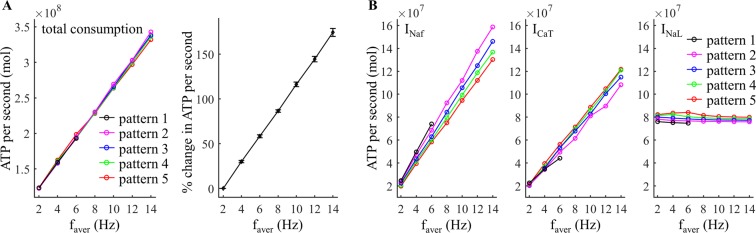
Increasing faver increased the number of APs, and the ATP cost of each pattern increased as a function of faver (Fig. 7A). A 2 Hz increment in faver increased the mean ATP cost across five patterns by ~29% (Fig. 7A, right), which was higher than ATP demand without rebound excitation (Fig. 2A,B, right). The increased number of spikes increased the ATP cost from fast Na+ and T-type Ca2+ currents, but did not alter appreciably the energy demand resulting from the Na+ leakage channel (Fig. 7B). When ICaT was de-inactivated by hyperpolarization, Ca2+ extrusion consumed much more ATP than without rebound excitation (Fig. 8) and made an appreciable contribution to overall ATP demand.
Figure 7.
Effect of faver on estimated metabolic cost of different temporal patterns of neural activity resulting from rebound activation. (A) Left: total ATP consumption by neural activity. Right: percent change in total ATP consumption (mean ± SD across five patterns). (B) ATP consumption required to reverse the ionic fluxes through each of INaf, ICaT, and INaL. With faver ≥8 Hz, the interspike interval was so short that ICaT was unable to steadily generate 1 spike during each rebound activation. Thus, the faver of pattern 1 was only from 2 Hz to 6 Hz.
Figure 8.
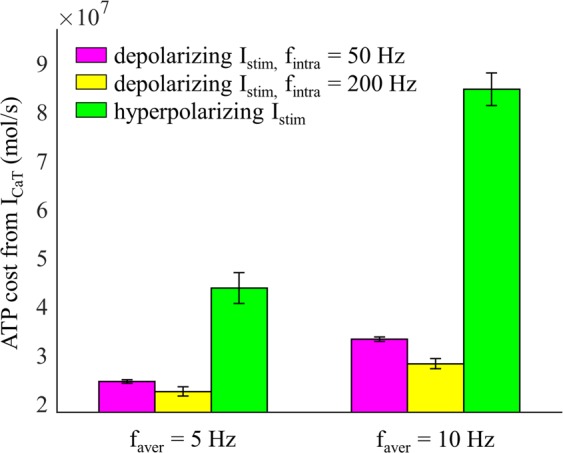
ATP cost per second of Ca2+ extrusion (mean ± SD). At faver = 5 Hz, the ATP cost was averaged over five firing patterns. At faver = 10 Hz, the ATP cost was averaged over patterns 2–5. With depolarizing Istim, inward ICaT was inactivated and no rebound spikes occurred.
The fintra was high (>150 Hz) during rebound bursts following de-inactivation of ICaT, and with the same faver, increasing numbers of spikes in each burst resulted in more APs falling within afterpotentials (Fig. 9). This reduced INaf, and thus the ATP cost from the fast Na+ current. In contrast, stronger hyperpolarization to increase the number of spikes in each burst increased ICaT, which required more ATP for reversal. Stronger hyperpolarization also slightly increased INaL, which resulted in a higher ATP cost. As a result, there was little difference in the total energy consumption between firing patterns, similar to when activity was driven by depolarizing current pulses.
Figure 9.
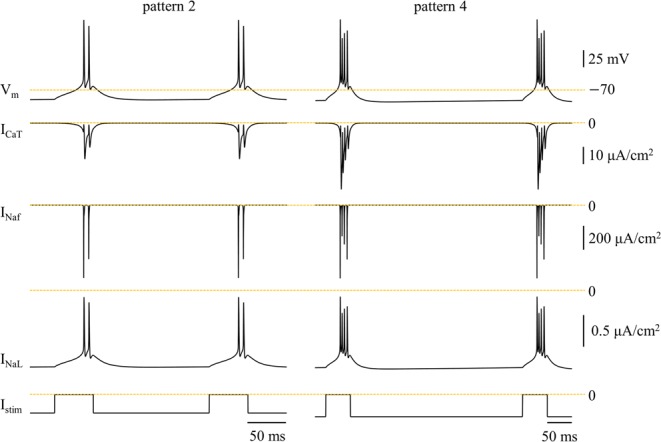
Ionic currents during rebound bursts at faver = 10 Hz. Vm, ICaT, INaf, and INaL were measured from firing patterns 2 (left) and 4 (right). Hyperpolarizing pulse train Istim was applied to the cell body. The amplitude of the pulses was respectively −0.2 nA (left) and −0.24 nA (right).
Discussion
We used a biophysically-based computational model of a TC relay neuron to estimate the metabolic cost of different temporal patterns of neural activity. We applied current pulses to the cell body to generate the tonic and burst modes of firing and quantified the effects of average firing rate, intraburst frequency, and spike number in each burst on ATP demand. The average firing rate was the dominant factor in determining the metabolic cost of neural activity, and the temporal pattern of activity contributed substantially less to determining the ATP required to maintain homeostasis of ion concentrations within the cell.
Estimates of energy requirements of neural computation indicated that APs made a significant contribution to the overall energy demand10,11. To restore ion concentrations, the Na+/K+ pump extrudes Na+ ions and imports K+ ions against their electrochemical gradients, thus consuming ATP. The energy demand from APs in different cell types and species was determined in earlier studies12–15,23, and it was predicted that ATP consumption was dependent on the firing rate13–17,24. The stimulus applied in these studies was a constant current, which not only altered the firing rates but also itself affected the AP cost. Unlike these prior studies, we focused on the metabolic costs of different temporal patterns of neural activity. The applied stimuli were brief current pulses, which themselves produced little effect on the energy cost of an evoked spike while controlling the rate and pattern of neural activity. Using a computational model, we showed that the average firing rate dominated the ATP consumption in both tonic and burst patterns of firing. Our simulations were consistent with experimental recordings18,19, which suggested that the metabolic demand of neural activity in the lateral superior olive, hippocampal CA1 region, and cerebral cortex exhibited a strong dependence on firing rate.
Earlier studies predicted that temperature13,24, cell size15, spike shape12, AP threshold25, channel densities and kinetics12,14, spike-frequency adaptation17, and dendritic properties16 influenced the energy cost of an AP by altering the underlying ionic currents. We extended these results to show that the afterpotentials in the cell body affected the activation of voltage-dependent Na+ currents and thus Na+ influx and spike metabolic cost. Further, biophysical models16,17 predicted that slow currents interact with the fast Na+ current to modulate the AP cost in a spike train, including the Ca2+-activated K+ (IAHP) current, voltage-activated K+ (IM) current, and dendritic high-voltage activated Ca2+ current. Our simulations indicated that varying the intraburst frequency altered the slow K+ and T-type Ca2+ currents underlying the APs in each burst, and these currents interacted with fast Na+ currents to contribute to the small differences in metabolic cost between firing patterns. These findings highlight the importance of slow ionic currents in determining the spike pattern-related energy demand in the brain.
The Ca2+ influx via T-type Ca2+ channels is a key regulator of cellular excitability26–28 and controls the switch between tonic and burst firing modes in TC relay neurons1. Further, intracellular Ca2+ signaling powerfully regulates the metabolism in multiple subcellular process27,29. The Ca2+ ATPase and the Na+-Ca2+ exchanger both participate in the control of neuronal Ca2+ signaling11. The former consumes one ATP for extruding one Ca2+ ion and predominates at low Ca2+ load. The Na+-Ca2+ exchanger uses the energy stored in the Na+ gradient to drive Ca2+ extrusion, which predominates at higher Ca2+ loads. It exports one Ca2+ ion in exchange for the import of three Na+ ions, which requires additional ATP for the extrusion of extra Na+ by the Na+/K+ pump. Our simulations showed that Ca2+ extrusion made a significant contribution to the metabolic cost of neural activity when the T-type Ca2+ current was de-inactivated. Intracellular Ca2+ signaling also participates in ATP production in mitochondria27, and we did not consider this in our simulations.
Quantifying the metabolic demand of neural activity influences the interpretation of functional brain imaging data through related metabolic mechanisms. Deep brain stimulation (DBS) is a neurosurgical method successful in treating movement disorders and alters the temporal patterns of neural activity30–32. fMRI is based on the changes in local circulation and metabolism33, and is used to measure and interpret changes in brain activity during DBS. fMRI studies of thalamic DBS reported that the evoked BOLD responses were sensitive to the stimulus frequency34–36. Our studies showed that the average firing rate dominated the metabolic cost of neural activity in TC relay neurons, suggesting that metabolism-dependent functional imaging methods may be sensitive to stimulation frequency-dependent changes in neural activity. In vivo experiments also indicate that the temporal pattern of DBS is a factor determining its effectiveness37–39, but we did not observe the significant effects of firing pattern on ATP demand. However, this does not necessarily mean the temporal patterns may not differentially affect the BOLD signal. It is possible that the burst and tonic modes of firing evoke different neurovascular coupling mechanisms and lead to distinct blood flow responses, which would be apparent in fMRI.
There were several limitations of our modeling approach. First, due to the limited data available on the magnitude and location of all the voltage-gated channels underlying the neural activity in TC cells, there may be over- or under-estimates of absolute metabolic costs, but this should not impact our comparisons of relative metabolic demands across firing rates and patterns. Second, we only examined the metabolic cost of firing patterns in the cell body. There are substantial differences in the energy efficiency of APs in the dendrites, soma, axon initial segment, and nodes of Ranvier40, and these differences may influence the overall metabolic cost of different patterns of firing. Third, we only considered the metabolic cost of tonic and burst patterns in a TC relay neuron model. These firing patterns may also be generated in other cell types, and their ionic currents and morphologies are substantially different than TC relay neurons, thus influencing the metabolic cost of firing activity. Fourth, the synaptic conductances were set such that when we used synaptic inputs to generate the desired firing patterns each presynaptic event evoked a spike in the soma. In our simulations there were only 165 excitatory synaptic inputs on the dendrites, and this represented only a small fraction of the total synapses on TC relay neurons, estimated to be 5584–8797, with ~65% being non-GABAergic41,42. Thus, the individual synaptic conductance for each modeled synapse was larger than that measured for single synapses on thalamic neurons, estimated to be 9–15 pS43,44. Finally, we did not include synaptic transmission in metabolic cost calculation, and this could influence overall ATP consumption. Pre- and post-synaptic mechanisms mediating synaptic transmission made a significant contribution to the overall usage of signaling-related energy in neurons and glia10,45.
In summary, we quantified the metabolic cost of different rates and patterns of activity in a model TC relay cell. The average firing rate determined the ATP demand of neural activity by directly determining the number of APs. The temporal pattern contributed to the metabolic energy by altering the interactions of ionic currents on multiple timescales, but made a much smaller contribution than average firing rate to the overall energy demand. These predictions are important for understanding information processing in TC relay neurons and critical for interpreting the signals from metabolism-dependent modalities of functional brain imaging (e.g., fMRI).
Supplementary information
Acknowledgements
This work was funded by grants from the National Institutes of Health (R37 NS040894) and the National Natural Science Foundation of China (61601320).
Author Contributions
G.Y. and W.M.G. conceived and designed the work. G.Y. performed the simulations. G.Y. and W.M.G. analyzed and interpreted the data. G.Y. and W.M.G. wrote the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43460-8.
References
- 1.Sherman SM. Tonic and burst firing: dual modes of thalamocortical relay. Trends Neurosci. 2001;24:122–6. doi: 10.1016/S0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- 2.Zeldenrust F, Chameau P, Wadman WJ. Spike and burst coding in thalamocortical relay cells. PLoS Comput. Biol. 2018;14:e1005960. doi: 10.1371/journal.pcbi.1005960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jahnsen H, Llinás R. Electrophysiological properties of guinea-pig thalamic neurones: an in vitro study. J. Physiol. 1984;349:205–26. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guillery RW, Sherman SM. Thalamic relay functions and their role in corticocortical communication: generalizations from the visual system. Neuron. 2002;33:163–75. doi: 10.1016/S0896-6273(01)00582-7. [DOI] [PubMed] [Google Scholar]
- 5.Mukamel R, et al. Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science. 2005;309:951–4. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- 6.He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. Electrophysiological correlates of the brain’s intrinsic large-scale functional architecture. Proc. Natl. Acad. Sci. USA. 2008;105:16039–44. doi: 10.1073/pnas.0807010105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shmuel A, Augath M, Oeltermann A, Logothetis NK. Negative functional MRI response correlates with decreases in neuronal activity in monkey visual area V1. Nat. Neurosci. 2006;9:569–77. doi: 10.1038/nn1675. [DOI] [PubMed] [Google Scholar]
- 8.Butler R, Bernier PM, Lefebvre J, Gilbert G, Whittingstall K. Decorrelated input dissociates narrow band γ power and BOLD in human visual cortex. J. Neurosci. 2017;37:5408–5418. doi: 10.1523/JNEUROSCI.3938-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Herron P, et al. Neural correlates of single-vessel haemodynamic responses in vivo. Nature. 2016;534:378–82. doi: 10.1038/nature17965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Howarth C, Gleeson P, Attwell D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow Metab. 2012;32:1222–1232. doi: 10.1038/jcbfm.2012.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Attwell D, Laughlin SB. An energy budget for signaling in the grey matter of the brain. J. Cereb. Blood Flow Metab. 2001;21:1133–1145. doi: 10.1097/00004647-200110000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Sengupta B, Stemmler M, Laughlin SB, Niven JE. Action potential energy efficiency varies among neuron types in vertebrates and invertebrates. PLoS Comput. Biol. 2010;6:e1000840. doi: 10.1371/journal.pcbi.1000840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu Y, Hill AP, McCormick DA. Warm body temperature facilitates energy efficient cortical action potentials. PLoS Comput. Biol. 2012;8:e1002456. doi: 10.1371/journal.pcbi.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hasenstaub A, Otte S, Callaway E, Sejnowski TJ. Metabolic cost as a unifying principle governing neuronal biophysics. Proc. Natl. Acad. Sci. USA. 2010;107:12329–12334. doi: 10.1073/pnas.0914886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sengupta B, Faisal AA, Laughlin SB, Niven JE. The effect of cell size and channel density on neuronal information encoding and energy efficiency. J. Cereb. Blood Flow Metab. 2013;33:1465–1473. doi: 10.1038/jcbfm.2013.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yi GS, Wang J, Wei XL, Deng B. Dendritic properties control energy efficiency of action potentials in cortical pyramidal cells. Front. Cell. Neurosci. 2017;11:265. doi: 10.3389/fncel.2017.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi GS, Wang J, Li HY, Wei XL, Deng B. Metabolic energy of action potentials modulated by spike frequency adaptation. Front. Neurosci. 2016;10:534. doi: 10.3389/fnins.2016.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brosel S, Grothe B, Kunz L. An auditory brainstem nucleus as a model system for neuronal metabolic demands. Eur. J. Neurosci. 2018;47:222–235. doi: 10.1111/ejn.13789. [DOI] [PubMed] [Google Scholar]
- 19.Hall CN, Klein-Flugge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J. Neurosci. 2012;32:8940–51. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Destexhe A, Neubig M, Ulrich D, Huguenard J. Dendritic low-threshold calcium currents in thalamic relay cells. J. Neurosci. 1998;18:3574–88. doi: 10.1523/JNEUROSCI.18-10-03574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIntyre CC, Grill WM, Sherman DL, Thakor NV. Cellular effects of deep brain stimulation: model-based analysis of activation and inhibition. J. Neurophysiol. 2004;91:1457–69. doi: 10.1152/jn.00989.2003. [DOI] [PubMed] [Google Scholar]
- 22.Hines ML, Carnevale NT. Neuron: a tool for neuroscientists. Neuroscientist. 2001;7:123–35. doi: 10.1177/107385840100700207. [DOI] [PubMed] [Google Scholar]
- 23.Nawroth JC, Greer CA, Chen WR, Laughlin SB, Shepherd GM. An energy budget for the olfactory glomerulus. J. Neurosci. 2007;27:9790–800. doi: 10.1523/JNEUROSCI.1415-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moujahid A, d’Anjou A. Metabolic efficiency with fast spiking in the squid axon. Front. Comput. Neurosci. 2012;6:95. doi: 10.3389/fncom.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yi GS, Wang J, Tsang KM, Wei XL, Deng B. Input-output relation and energy efficiency in the neuron with different spike threshold dynamics. Front. Comput. Neurosci. 2015;9:62. doi: 10.3389/fncom.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cueni L, Canepari M, Adelman JP, Luthi A. Ca2+ signaling by T-type Ca2+ channels in neurons. Pflugers Arch. 2009;457:1161–1172. doi: 10.1007/s00424-008-0582-6. [DOI] [PubMed] [Google Scholar]
- 27.Brini M, Calì T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell. Mol. Life Sci. 2014;71:2787–814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sotelo, J. R. & Benech, J. C. Calcium and cellular metabolism: transport and regulation (Plenum Press, New York, 1997).
- 29.Jayakumar S, Hasan G. Neuronal calcium signaling in metabolic regulation and adaptation to nutrient stress. Front. Neural Circuits. 2018;12:25. doi: 10.3389/fncir.2018.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grafton ST, et al. Normalizing motor-related brain activity: subthalamic nucleus stimulation in Parkinson disease. Neurology. 2006;66:1192–9. doi: 10.1212/01.wnl.0000214237.58321.c3. [DOI] [PubMed] [Google Scholar]
- 31.Grill WM, Snyder AN, Miocinovic S. Deep brain stimulation creates an informational lesion of the stimulated nucleus. Neuroreport. 2004;15:1137–40. doi: 10.1097/00001756-200405190-00011. [DOI] [PubMed] [Google Scholar]
- 32.Kuncel AM, Cooper SE, Wolgamuth BR, Grill WM. Amplitude- and frequency-dependent changes in neuronal regularity parallel changes in tremor with thalamic deep brain stimulation. IEEE Trans. Neural Syst. Rehabil. Eng. 2007;15:190–7. doi: 10.1109/TNSRE.2007.897004. [DOI] [PubMed] [Google Scholar]
- 33.Raichle ME, Mintun MA. Brain work and brain imaging. Annu. Rev. Neurosci. 2006;29:449–76. doi: 10.1146/annurev.neuro.29.051605.112819. [DOI] [PubMed] [Google Scholar]
- 34.Chao TH, Chen JH, Yen CT. Repeated BOLD-fMRI imaging of deep brain stimulation responses in rats. PLoS One. 2014;9:e97305. doi: 10.1371/journal.pone.0097305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shih YY, Yash TV, Rogers B, Duong TQ. FMRI of deep brain stimulation at the rat ventral posteromedial thalamus. Brain Stimul. 2014;7:190–3. doi: 10.1016/j.brs.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paek SB, et al. Frequency-dependent functional neuromodulatory effects on the motor network by ventral lateral thalamic deep brain stimulation in swine. Neuroimage. 2015;105:181–188. doi: 10.1016/j.neuroimage.2014.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birdno MJ, Kuncel AM, Dorval AD, Turner DA, Grill WM. Tremor varies as a function of the temporal regularity of deep brain stimulation. Neuroreport. 2008;19:599–602. doi: 10.1097/WNR.0b013e3282f9e45e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brocker DT, et al. Improved efficacy of temporally non-regular deep brain stimulation in Parkinson’s disease. Exp. Neurol. 2013;239:60–7. doi: 10.1016/j.expneurol.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swan BD, et al. Short pauses in thalamic deep brain stimulation promote tremor and neuronal bursting. Clin Neurophysiol. 2016;127:1551–9. doi: 10.1016/j.clinph.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallermann S, de Kock CP, Stuart GJ, Kole MH. State and location dependence of action potential metabolic cost in cortical pyramidal neurons. Nat. Neurosci. 2012;15:1007–14. doi: 10.1038/nn.3132. [DOI] [PubMed] [Google Scholar]
- 41.Liu XB, Honda CN, Jones EG. Distribution of four types of synapse on physiologically identified relay neurons in the ventral posterior thalamic nucleus of the cat. J. Comp. Neurol. 1995;352:69–91. doi: 10.1002/cne.903520106. [DOI] [PubMed] [Google Scholar]
- 42.Sato F, Nakamura Y, Shinoda Y. Serial electron microscopic reconstruction of axon terminals on physiologically identified thalamocortical neurons in the cat ventral lateral nucleus. J. Comp. Neurol. 1997;388:613–31. doi: 10.1002/(SICI)1096-9861(19971201)388:4<613::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 43.Lessmann V, Gottmann K. Fast desensitization of glutamate activated AMPA/kainate receptors in rat thalamic neurones. Neuroreport. 1994;5:2253–6. doi: 10.1097/00001756-199411000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Kielland A, Heggelund P. AMPA and NMDA currents show different short-term depression in the dorsal lateral geniculate nucleus of the rat. J. Physiol. 2002;542:99–106. doi: 10.1113/jphysiol.2002.019240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.