Abstract
Thalamic neurons have two firing modes: tonic and bursting. It was originally suggested that bursting occurs only during states such as slow-wave sleep, when little or no information is relayed by the thalamus. However, bursting occurs during wakefulness in the visual and somatosensory thalamus, and could theoretically influence sensory processing. Here we used chronically implanted electrodes to record from the ventroposterior medial thalamic nucleus (VPM) and primary somatosensory cortex (SI) of awake, freely moving rats during different behaviors. These behaviors included quiet immobility, exploratory whisking (large-amplitude whisker movements), and whisker twitching (small-amplitude, 7- to 12-Hz whisker movements). We demonstrated that thalamic bursting appeared during the oscillatory activity occurring before whisker twitching movements, and continued throughout the whisker twitching. Further, thalamic bursting occurred during whisker twitching substantially more often than during the other behaviors, and a neuron was most likely to respond to a stimulus if a burst occurred ≈120 ms before the stimulation. In addition, the amount of cortical area activated was similar to that during whisking. However, when SI was inactivated by muscimol infusion, whisker twitching was never observed. Finally, we used a statistical technique called partial directed coherence to identify the direction of influence of neural activity between VPM and SI, and observed that there was more directional coherence from SI to VPM during whisker twitching than during the other behaviors. Based on these findings, we propose that during whisker twitching, a descending signal from SI triggers thalamic bursting that primes the thalamocortical loop for enhanced signal detection during the whisker twitching behavior.
It has been demonstrated that thalamic relay neurons have two distinct modes of firing. These are the tonic firing mode, in which neurons fire single action potentials, and the bursting mode, in which cells fire bursts of two to seven action potentials (1, 2). During the tonic firing mode, cells respond to stimuli with individual spikes that can closely follow incoming activity (3–5). In contrast, during the burst mode, cells respond to incoming stimuli with bursts that do not directly resemble the afferent activity because the bursts are all-or-nothing events, and there is a long refractory period between bursts (4, 5).
It was originally thought that the tonic mode was associated with behavioral states, such as wakefulness and sleep, during which afferent stimuli are readily relayed by the thalamus (3, 6–8). In contrast, the bursting mode was thought to occur only during times when no afferent information was, in theory, transmitted by the thalamus (refs. 1, 9, and 10; see ref. 11 for review), such as during slow-wave sleep, barbiturate anesthesia, and pathological conditions such as seizure activity. However, it has recently been demonstrated that thalamic bursting can occur during awake states as well, in multiple species (12–18), and that sensory information can be conveyed to the cortex during the burst activity (16).
Because the bursting mode can occur during wakefulness, it is possible that instead of corresponding to little or no thalamic relay of afferent activity, this mode of firing could involve the transmission of incoming activity and could actually play a role in sensory processing (12, 13, 19). In previous studies of the rat vibrissal system, our laboratory has demonstrated that responses to afferent stimuli differ in multiple ways depending on the behavioral state of an animal (20). We conducted the present study to determine whether thalamic bursting occurs during any of these behavioral states and, if so, what its role may be. In particular, we focused on a behavioral state called “whisker twitching,” during which rats twitch their whiskers in small-amplitude, rhythmic movements at 7–12 Hz (21–23). During the whisker twitching, there are very robust, coherent oscillations of neural activity in the vibrissal areas of the brainstem, thalamus, and cortex (21, 23). These oscillations begin ≈550 ms before the onset of the whisker twitching movements, and are similar to the μ-rhythm described in humans (24), and the somatosensory-motor rhythm in cats (25). Because this activity is a prime candidate to lead to burst firing, we investigated whether the whisker twitching behavior could involve thalamic burst firing. Further, we propose that the whisker twitching behavior may be designed for very specific types of enhanced stimulus detection and sensory processing.
Methods
Implantation of Recording Electrodes.
Neural activity was recorded by using stainless-steel microwire electrodes implanted into the vibrissal regions of the ventroposterior medial thalamic nucleus (VPM) and the primary somatosensory cortex (SI) of three female Long–Evans hooded rats. The electrodes and implantation procedures have been described elsewhere (26). Briefly, with the animals under pentobarbital anesthesia, craniotomies were made above VPM and SI (27). Electrode bundles (for VPM) or arrays (for SI) each containing 16 microwires were slowly lowered to the appropriate depth (VPM, ≈5.5 mm; SI, ≈1.1 mm) and their locations were verified by evoking responses to manual stimulation of the vibrissae. When the electrodes were in the correct location, they were cemented to the skull with dental acrylic. Rats were allowed to recover for 1 week before recording sessions began.
Inactivation of SI Activity by Muscimol Infusion.
A cannula was attached to the array of electrodes implanted in SI that allowed controlled delivery of muscimol to that area. The cannula tip was positioned such that the muscimol was infused directly next to the microwires. For each experiment, 500 nl of muscimol in sterile saline (1 ng/nl) was slowly infused into SI (28, 29). Inactivation of SI was verified in two ways: (i) action potentials recorded by the electrodes implanted in SI were completely eliminated by 30 min after infusion and remained inactivated for ≈8–9 h; and (ii) there was a consistent decrement in the overall level of activity in VPM after the infusion, caused by the inactivation of corticothalamic inputs, which lasted throughout the time action potentials in SI were blocked.
Construction and Implantation of Nerve Cuff Electrode.
Chronically implanted nerve cuff electrodes were used to stimulate the infraorbital nerve. The construction and implantation of these electrodes have been described previously (20). Briefly, nerve cuff electrodes consisted of two bands of platinum (Goodfellow, Berwyn, PA) 0.5 mm wide, embedded in Sylgard (Factor II, Lakeside, AZ). The platinum bands were constructed to run around the circumference of the nerve and current was passed between the bands to activate axons in the nerve. Lead wires were run beneath the skin to a connector that was attached to the skull with dental acrylic.
Responses in VPM and SI to nerve cuff stimulation were similar to those evoked by manual whisker stimulation (20). Each stimulus was 100 μs in duration and the current level was set individually for each rat (range: 5–9 mA, see ref. 20 for details).
Recording Neural Activity.
Neural activity was recorded from the implanted microwires and processed by using a Multineuron Acquisition Processor (Plexon, Dallas). Voltage-time threshold windows were used to identify waveforms of single units (26). From these, spike times were recorded, and these data were processed as described below. Recordings were made both of single-unit activity and of the activity of multiunit clusters. A total of 63 single-unit recordings in VPM and 58 single-unit recordings in SI were made during these experiments. Recording sessions were each ≈2 h long.
Behavioral Analyses.
During recording sessions, rats were put into a recording chamber (60 cm × 30 cm) and allowed to move freely. Signals from the electrodes were transmitted to the recording device via headstage wires, which did not impair the rat's normal activity. High-resolution video recordings were made during the recording sessions, which were analyzed in order to identify the behavioral states throughout each recording session. Four behavioral states were defined for these experiments: (i) quiet immobility: rat awake, standing or sitting, displaying no voluntary movement; (ii) active: involves any form of motor activity other than that of the whiskers; (iii) whisking: large-amplitude whisker movements associated with exploratory behavior; and (iv) whisker twitching: small-amplitude movements of the whiskers at a frequency of 7–12 Hz, accompanied by robust oscillatory neural activity in the vibrissal representations in the brainstem nuclei, VPM, and SI (22, 23).
Data Analysis.
The time periods during which each behavior occurred were determined by video analysis. In addition, periods of whisker twitching were verified by finding the first principal component (PC1) of all of the neural responses within a given area (23). The oscillatory activity during the whisker twitching state is clearly evident in PC1 as high-amplitude modulations of activity at ≈8 Hz (e.g., Figs. 1 a and c, and 5c).
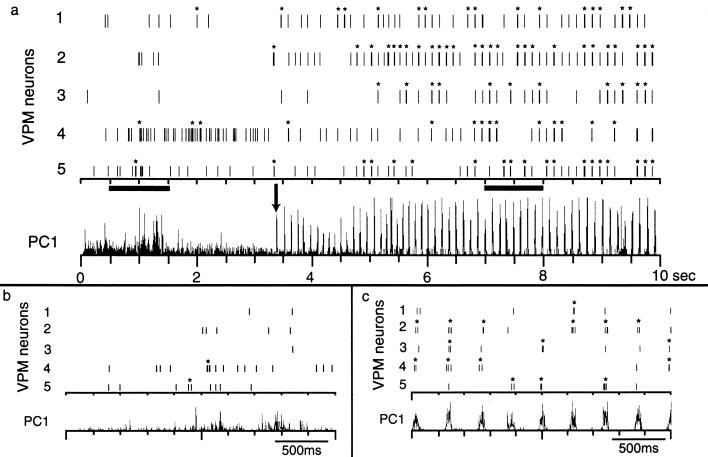
Figure 1.
Neural activity in VPM during quiet immobility and whisker twitching behavior. (a) During quiet immobility (before arrow in PC1), neuronal firing was not periodic and did not frequently involve bursting. However, after the oscillations began, the firing was very phasic and tended to occur in bursts. Note that not every neuron fired on every cycle. (b and c) Enlargements of activity in the left and right dark bars in a, respectively. Asterisks indicate activity that qualified as bursts according to the criteria described in Methods; note that individual spikes cannot be resolved at this time scale.
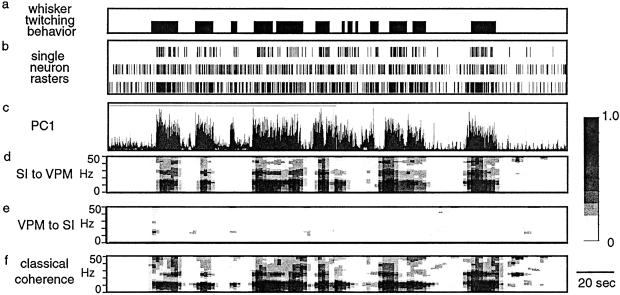
Figure 5.
PDC. The traces in this figure are from 250 s of a recording that includes periods of whisker twitching behavior. (a) Bars indicate times when the rat was engaged in the whisker twitching behavior. (b) Raster plots showing spike times for three single-neuron recordings. (c) PC1 of activity in VPM. (d) PDC from SI to VPM. (e) PDC from VPM to SI. (f) Classical coherence between VPM and SI. Gray scale bar indicates fraction of total power at each frequency for a given source (VPM or SI).
We assessed the amount of bursting activity of single units during different periods of our recordings by using software developed for such analyses (NEX software, Plexon). A burst was defined as having a minimum of two spikes and a maximum interspike interval of 10 ms, and had to be separated from other bursts by more than 100 ms. Burst activity is reported in bursts per second.
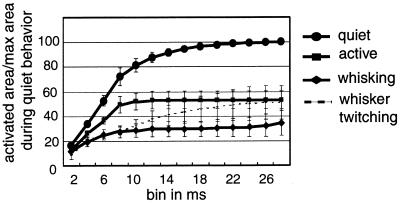
The amount of cortical area activated in response to a stimulus was assessed during each behavioral state by measuring the amount of activity on each electrode in the SI electrode array, in 2-ms time bins from 2 to 28 ms after stimulus. The data are presented as a cumulative summation of the number of electrodes active in each successive time bin. The data were normalized to the total cumulative sum in the quiet state. This allowed us to determine how much cortical territory was activated across the electrode array, which sampled a 2 mm × 0.5 mm area.
To identify the direction of activity between VPM and SI during different behavioral states, we used the recently introduced method of partial directed coherence (PDC) (30–32). This technique has been described in detail elsewhere (30–32). Briefly, PDC is a frequency domain representation of the key concept of Granger causality, which states that an observed time-series x(n) Granger-causes another series y(n), if knowledge of x(n)'s past significantly improves prediction of y(n). This relation between time-series is not reciprocal, i.e., x(n) may Granger-cause y(n) without y(n) necessarily Granger-causing x(n). Nullity of PDC between two structures at a given frequency suggests the lack of a direct link between those structures. Because existing direct feedback relationships between each pair of channels are explicitly exposed, PDC allows the uncovering of coactivations among multichannel neuronal recordings by highlighting neuronal groups that possibly drive other neuronal groups.
To compute PDC multivariate autoregressive modeling of the data,
in the context of signals derived from neuronal spiking, one must first
convolve the spike impulse trains,
si(t) =
∑kδ(t − tk), with a
suitable kernel such as h(t) =
sin(πt/Tw)/(πt/Tw),
and sample the resulting signal at the appropriate rate (30, 33). One
also needs to subtract the dc component of each signal. Formally, this
leads to discrete time signals
xi(n), 1 ≤
i ≤ N, one for each structure under
scrutiny whose mutual relations can be described by a set of
p (model order) matrices Ar of
the autoregressive model. For Ā(f) =
I−A(f) with
A(f) =
|∑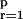 Arz−r|z=e−j2πf
and āij(f) being
Ā 's i, jth element, it follows
aij(f) = 0
for all f, whenever the past of
xj(n) implies no prediction
improvement on xj(n)'s present
(i.e., equivalent to lack of Granger causality because the condition
aij(r) = 0 is required.).
In this paper we used the following normalized definition for partial
directed coherence:
Arz−r|z=e−j2πf
and āij(f) being
Ā 's i, jth element, it follows
aij(f) = 0
for all f, whenever the past of
xj(n) implies no prediction
improvement on xj(n)'s present
(i.e., equivalent to lack of Granger causality because the condition
aij(r) = 0 is required.).
In this paper we used the following normalized definition for partial
directed coherence:
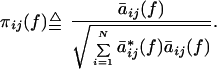 |
Note that the name “partial directed coherence” follows from its relationship to partial coherence (32). The classical coherence functions, which measure the degree of simultaneous activation between two areas (34), were calculated in the framework of a multivariate autoregressive model as explained elsewhere (32).
For the PDC analysis, we used the PC1 of the activity collected from all of the electrodes in a given brain area (VPM or SI). We found the mean value of coherence across 1–50 Hz for each 2-s analysis time window throughout the recording session. These values were calculated for classical coherence, directed coherence from VPM to SI, and directed coherence from SI to VPM. They were then correlated with the behavioral states described above, as determined by videotape analysis. Results for each coherence type and behavior were normalized to the classical coherence value for the quiet state for a given experiment.
ANOVAs were performed where appropriate to compare results. When post hoc tests were indicated, “Tukey's honestly significant difference” test was used.
Results
Bursting During Different Behavioral States.
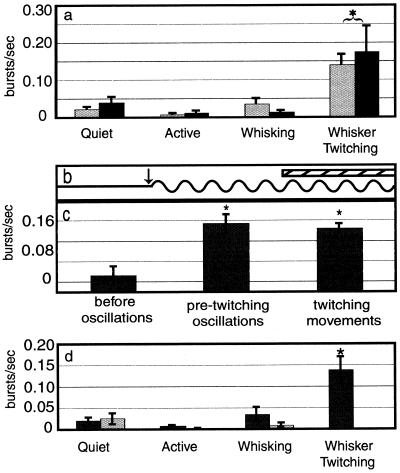
During the whisker twitching behavior, robust oscillations of neuronal activity were observed in VPM and SI. During these oscillations, neurons in VPM and SI frequently fired bursts of action potentials that were synchronized with the oscillatory activity as well as with the whisker twitching movements (Fig. 1a). Note that not every neuron fired with every oscillatory cycle (Fig. 1c). In contrast, rhythmic burst activity was not observed during periods when no whisker twitching and oscillatory activity was present (Fig. 1b). We quantified the burst rate during the four behavioral states we analyzed: quiet immobility, active, exploratory whisking, and whisker twitching. The results show that in both VPM and SI, there was substantially more bursting activity during the whisker twitching behavior (VPM, 0.139 ± 0.032 burst per second ± SEM; SI, 0.174 ± 0.071; Fig. 2a) than during any of the other three behaviors (VPM: quiet, 0.022 ± 0.007; active, 0.008 ± 0.03; whisking, 0.035 ± 0.018; SI: quiet, 0.040 ± 0.018; active, 0.011 ± 0.006; whisking, 0.013 ± 0.006; Fig. 2a, P < 0.0001).
Figure 2.
Burst firing in VPM and SI. (a) During the whisker twitching behavior there was significantly more bursting activity in both VPM and SI than during the other three behavioral states. Gray bars are for VPM and black bars are for SI. Asterisk and bracket indicate that the VPM and SI values are both different from values during the other behaviors. (b and c) The amount of burst activity increased relative to baseline during both the pre-twitching oscillations (after arrow in b) and after the whisker twitching movements began (hatched bar in b). The burst rate during the pre-twitching oscillations was not significantly different from that after the whisker twitching movements began. Asterisks in c indicate values significantly different from the “before oscillations” condition. (d) After inactivation of SI cortex, bursting activity in VPM was still higher during whisker twitching than during any of the other behaviors. Black bars show premuscimol and gray bars show postmuscimol values. Asterisk indicates value is significantly different from all others.
Bursting During the Pre-Twitching Oscillation Period.
Oscillatory neural activity in VPM preceded the onset of the small-amplitude whisker twitching movements by an average of 576 ± 28 ms. We quantified the amount of burst activity during this period, termed the pre-twitching oscillation period (arrow in Fig. 2b), and found that there was no statistical difference between the rate of bursting during this period and during the whisker twitching movements themselves (hatched bar in Fig. 2b), during which the oscillatory activity continued (0.134 ± 0.018 and 0.124 ± 0.010 burst per second, respectively; Fig. 2c). However, the amount of bursting activity in both of these cases was significantly different from the level of bursting during baseline periods when there was no oscillatory activity or whisker twitching movements (0.032 ± 0.019; P < 0.01).
Burst Activity During Inactivation of SI.
After SI was inactivated by muscimol infusion, animals never exhibited the whisker twitching behavior, and neither the thalamic oscillatory activity nor the accompanying burst activity was observed in VPM. However, during SI inactivation, the amount of bursting activity was not altered in VPM during the quiet, active, and whisking behaviors (Fig. 2d). The whisker twitching behavior did fully return after the muscimol dose wore off (≈8–9 h).
Resetting of Oscillation Phase by a Stimulus.
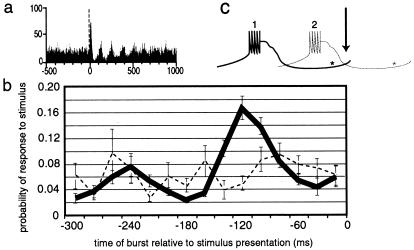
The presentation of a stimulus can reset the phase of the oscillations. This can be observed in Fig. 3a, which is a histogram of ≈200 trials of stimulus presentation during the whisker twitching behavior. Before the presentation of the stimulus (before vertical broken line in Fig. 3a), the activity of the neuron is not synchronous across trials, despite the fact that there is ongoing oscillatory activity throughout the trials. This is because the phase at which a stimulus is presented is different from trial to trial, so there is no coherence from one trial to the next before the stimulus is presented. However, after the stimulus, there is clear synchronous oscillatory activity, the magnitude of which damps out over ≈500 ms. The fact that this poststimulus activity is synchronized across trials, and is thus evident in the histogram, suggests that the stimulus resets the phase of the oscillations.
Figure 3.
Resetting of oscillation phase and probability of a response to infraorbital nerve stimulation after a burst. (a) Histogram showing that the phase of oscillations during whisker twitching is reset with the presentation of a stimulus (vertical broken line). (b) The probability of a response to the stimulus depended on when the stimulus was presented relative to the timing of the previous burst. (c) This schematic diagram illustrates this point by showing a “burst” of action potentials and the period after the burst when the cell is unable to fire, relative to when the stimulus occurs (arrow/0 ms on graph in b). Asterisks show approximately where the IT current becomes de-inactivated and the cell is ready to fire. If the burst labeled 1 occurs, the neuron is likely to respond when the stimulus occurs, whereas if burst 2 occurs instead, the neuron is much less likely to fire in response to the stimulus.
Probability of a Response to a Stimulus After a Burst.
The probability of a cell responding to infraorbital nerve stimulation differs according to whether there was a burst preceding the stimulus, and the time between the burst and the stimulus presentation. This effect is shown in Fig. 3 b and c. This graph shows the probability of a neuron responding to a stimulus is highest if a burst occurred at ≈120 ms preceding the stimulus. The level of probability at this point is higher than at any other point during whisker twitching or at any point during the quiet state.
Cumulative Cortical Area Activated.
The total amount of cortical area activated by a single stimulus was much larger during the quiet state than during any of the other three states (all values normalized to total cumulative area activated for quiet state for each experiment, i.e., quiet = 100% (active, 53.21% ± 6.94%; whisking, 34.23% ± 10.27%; whisker twitching, 50.79% ± 13.79% ; Fig. 4). This activity developed over the course of ≈28 ms after the stimulus was presented. In addition, the rate at which the cortical area was activated was fastest during the quiet state (18.60% ± 0.90% of quiet state maximum area per second), intermediate during the active state (12.36% ± 1.12%), and slowest during the whisking and whisker twitching states (5.22%± 1.81% and 6.88 ± 4.24, respectively).
Figure 4.
Cumulative sum of activated cortical area. This graph shows the total amount of cortical area activated in the first 28 ms after stimulation of the infraorbital nerve. Values are presented as a percent of cumulative area activated during the quiet behavior.
Direction of Information Flow.
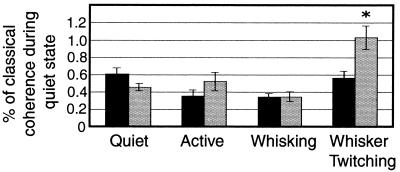
We used PDC analysis to determine the direction of information flow from one region (VPM or SI) to another during the behavioral states we studied (Fig. 5). During the quiet, active, and whisking states, there was no statistically significant difference in the amount of directed coherence from VPM to SI compared with that from SI to VPM (quiet, 61.18 ± 6.71% of quiet classical coherence value, 45.92 ± 4.20; active, 52.41 ± 10.44, 35.51 ± 7.53; whisking, 35.06 ± 5.52, 34.95 ± 4.12, respectively, Fig. 6). In addition, there was no statistically significant difference in the amount of directed coherence between these three behaviors in either direction. In contrast, during the whisker twitching behavior, there was a significantly larger amount of directed coherence from SI to VPM than from VPM to SI (103.25 ± 13.45, 56.36 ± 8.3 respectively; P < 0.03). The amount of directed coherence from VPM to SI during whisker twitching was indistinguishable from that during the other behaviors.
Figure 6.
PDC during different behavioral states. During the whisker twitching state there was a significantly larger amount of directed coherence from SI to VPM than from VPM to SI (∗). In addition, the amount of directed coherence between SI and VPM during whisker twitching was larger than during any other behavioral state. Black bars show directed coherence from VPM to SI, and gray bars show directed coherence from SI to VPM. Values were normalized to the amount of classical coherence for each measure.
Discussion
The results of this study demonstrate that thalamocortical neurons can fire in the bursting mode during the whisker twitching behavior of awake rats. Contrary to what has typically been believed, we showed that the SI cortex does respond to stimuli during this behavior, demonstrating that the bursting mode is not limited to states in which there is no relay of sensory information by the thalamus to the cortex. In addition, the whisker twitching behavior depends on, and is likely initiated by, the cortex. The finding that there is more descending influence from SI to VPM, as measured by the PDC technique, during whisker twitching compared with the other behavioral states, as well as the advantages that burst activity can have for particular types of sensory transmission (4, 5, 14, 35, 36), suggest to us that thalamic bursting activity during the whisker twitching behavior in awake rats may serve to optimize the detection of afferent stimuli, as suggested by Sherman and colleagues (4, 5, 37) based on experiments in the visual system.
The Burst Firing Mode Is Not Limited to Sleep and Pathological States.
We demonstrated here that thalamocortical cells are capable of firing high-frequency bursts of action potentials during whisker twitching, a normal waking behavioral state in the rat. During this state, which is accompanied by μ-oscillations throughout the brainstem, thalamic, and cortical levels of the vibrissal system (23), bursts occur substantially more frequently than during any other behavioral state analyzed. Previously, Fanselow and Nicolelis (20) showed that there are responses to tactile stimuli during the whisker twitching behavior in both VPM and SI. Therefore, our results provide a further challenge to the notion that thalamic bursting activity is associated only with states during which there is no relay of sensory information by the thalamus to the cortex.
The bursts of action potentials described here were similar to those observed both in vitro (refs. 11 and 38; for review see ref., 39) and in vivo (1, 40). These results corroborate other studies that have shown bursting activity during wakefulness in cats (13, 14), guinea pigs (15), rabbits (16), and monkeys (17, 18). Thus, it is clear that bursting can occur during wakefulness and, indeed, can be observed during periods of sensory processing (12–14).
Cortical Activation During Whisker Twitching.
When a stimulus was presented to the infraorbital nerve, the total amount of cortical area activated varied according to behavioral state. The total cortical area activated by a stimulus during the whisker twitching state was similar to other states involving vigilance and sensory processing (the active and exploratory whisking states), but was not similar to that during quiet immobility when there is little afferent sensory input. This observation provides further support for the notion that whisker twitching is a normal state, during which tactile information can be processed and relayed to the cortex.
Descending Control of Oscillatory Activity.
This study also showed that the whisker twitching behavior, during which a substantial amount of bursting activity occurs, depends on the integrity of the cortex. When activity in SI was blocked by infusion of muscimol, the animals ceased to display the whisker twitching movements, as well as the accompanying oscillatory neural activity and the thalamic bursting. These results confirm those of Semba and Komisaruk (22), who showed that ablation of neocortex eliminates the whisker twitching behavior. Moreover, Nicolelis et al. (23) demonstrated that during whisker twitching, the cortical oscillations phase-led those of the thalamus by an average of 9.19 ms, and began before oscillations in the thalamus in ≈80% of whisker twitching episodes.
In addition, by using the statistical technique of PDC, we demonstrated that during the whisker twitching there was ≈2 times the amount of directed coherence from SI to VPM than in any other behavioral state analyzed. The implication of the PDC analysis technique is that when there is partial directed coherence from one structure to another, activity in the first structure is likely to be influencing activity in the second structure. Thus, our results indicate that during the whisker twitching state, there is a large degree of descending influence from SI to VPM, compared with the other behavioral states. Taken together, these findings suggest that the cortex plays a much bigger role in driving activity in the thalamus during whisker twitching than it does during other states.
Function of the Bursting Mode and Whisker Twitching Behavior.
Based on the results presented in this paper and other studies discussed here, we postulate that the thalamic bursting mode is not associated only with situations during which there is little relay of sensory information through the thalamus. Instead, we propose that during the whisker twitching mode, the vibrissal system is actually primed to detect incoming stimuli and that the thalamus is quite capable of transmitting sensory information during this time (e.g., see ref. 20 for responses to stimulation during whisker twitching). Our results corroborate those by several authors (4, 5, 12, 14, 18, 37) who demonstrated in cat and monkey lateral geniculate nucleus (LGN) that bursting activity can occur during wakefulness and that cells respond reliably to visual stimuli during the bursting mode.
There are several reasons why bursting activity could be advantageous for signal detection. First, it has been demonstrated that the probability of firing of VP neurons differs depending on the phase of the 10- to 15-Hz wave activity recorded in the thalamus of the cat (35), and our results demonstrate this effect as well. During the whisker twitching state, the probability of a cell responding to a stimulus was highest when a burst occurred ≈120 ms before the stimulus presentation. This time period, 120 ms, corresponds well with the duration of time a thalamocortical neuron must remain hyperpolarized after a burst occurs before it is ready to fire again. This effect is caused by the involvement of a low-threshold Ca2+ conductance that, once activated, rapidly inactivates and requires ≈100 ms to de-inactivate so the cell can fire again (38). Thus, there is a period after a burst when the cell in unresponsive (≈100 ms after the burst), but when this period is over, the cell can respond to incoming stimuli. If a stimulus occurs during this sensitive period, after the Ca2+ current is de-inactivated, the cell can respond to the stimulus (see ref. 4 for a review). It can be seen in Fig. 3b that during whisker twitching the probability of a response during this sensitive period is substantially higher than that during any other period during whisker twitching and any period in the quiet state. Moreover, Nicolelis et al. (23) showed that in VPM this hypersensitive phase of the oscillations begins around the onset of whisker protraction in the whisker twitching movements. Thus, the presence of a “hypersensitive” period, combined with the fact that it coincides with the onset of whisker protraction, suggests that the oscillations serve to generate a period during which neurons are highly sensitive to incoming stimuli, and that this period is timed to occur when the whiskers are beginning to move forward, a time when they are likely to encounter a stimulus if one becomes present. Thus, one function of the rhythmic bursts could be to provide cyclic periods of heightened sensitivity to incoming stimulation (21). Note also that because the oscillations occur at ≈8 Hz, this period of high sensitivity is reset by the subsequent burst if no stimulus is encountered in the meantime, and thus the oscillatory cycle ensures that the sensitive period recurs at the onset of each whisker protraction. The idea that the burst mode provides an animal with a highly sensitive stimulus detection period has also been put forth by Sherman and his colleagues (4, 5, 37) as well as Guido and Weyand (14), who observed responses to visual stimuli in LGN and showed that the bursting mode can actually augment the detection of stimuli, and, in particular, novel or faint stimuli.
The whisker twitching behavior occurs when animals are standing or sitting still, but are alert. We propose that this behavior represents a time during which animals are anticipating potential incoming stimuli, likely ones that are difficult to detect, and that because of the period of high sensitivity at the beginning of whisker protractions, this behavior may allow them to be in a hyperalert state. This is contrasted with the quiet immobility state in which animals are awake, but less alert and not anticipating any imminent stimulus (e.g., sitting in a safe environment), and there is less sensitivity to stimulation than during the hypersensitive period of the whisker twitching state. This hyperalert state would be particularly appropriate if an animal was in a potentially dangerous situation and needed to detect any type of stimulus, such as one that might signal the approach of a predator, but did not want to divulge its presence by making gross movements such as the large-amplitude exploratory whisker movements.
The utility of the oscillatory activity is supported by Ahissar and colleagues' work (41) showing that cortical neurons can entrain their oscillatory firing to the frequency of peripheral whisker stimulation, and thus set up a thalamic comparator that is ideal for detecting changes in incoming stimuli. The whisker twitching-related oscillations may serve to set up this comparator, which is highly sensitive to a novel stimulus. This comparator can then be continued when an animal switches to exploratory whisker movements after initial stimulus detection during the whisker twitching mode.
Another function of the oscillatory activity is that, in the rat thalamocortical loop, 10-Hz stimulation has been shown to elicit long-term potentiation in corticothalamic synapses (36). Thus, oscillatory activity originating in the SI cortex and whose frequency range is centered near 10 Hz, such as that during the whisker twitching behavior, could enhance the ability of VPM neurons to respond to trains of tactile stimuli conveyed by the ascending trigeminal–lemniscal pathways.
Finally, a burst of action potentials is very effective at activating neurons, and therefore provides a very robust signal to its downstream target (14). In this way, though trains of single action potentials may be capable of closely following the details of a stimulus in a linear fashion, the neuronal bursts may be an effective way to alert the thalamocortical loop to the presence of a previously undetected or unattended stimulus (5, 14). For example, Guido and Weyand (14) showed in the LGN of awake, behaving cats that bursting activity increased during the first cycle of stimulation with a drifting grating, but then shifted into a tonic firing mode as presentation of the stimulus continued. This observation suggests that initial detection of the stimulus involved burst activity, whereas subsequent processing used the more detail-sensitive tonic firing. Thus, bursting activity may serve to optimize the initial detection of a stimulus. This effect may be even stronger in the somatosensory thalamus, where the bursting activity has been shown to be higher than that which occurs in the LGN (18).
For the reasons outlined above, we believe that thalamic burst firing may endow the rat vibrissal system with a very effective signal-detection mode that allows it to detect weak, new, and potentially important tactile information. It has been suggested that both bursting activity in the visual system (4, 5, 14) and the μ- oscillations observed in humans (42) and rats (21) serve as preparatory states, priming a given sensory system for signal detection. The results presented in the current paper corroborate this idea. Taken together, these findings support the postulate that the nervous system is not a passive detector of afferent stimuli, but that it plays an active role in optimizing the detection of potential incoming sensory information. Thus, by using thalamic bursting, the rat vibrissal system can prepare itself, before the activation of any peripheral receptors, to rapidly detect the onset of tactile input, which it can subsequently process in more detail by using the tonic firing mode and shifting into an active exploratory behavior.
Acknowledgments
This work was supported by National Institute of Dental Research Grants DE11121-01 and DE13810-01 (to M.A.L.N.), predoctoral National Research Service Award Grant MH-12316-01A1 (to E.E.F.), and Research Support Foundation of the State of São Paulo Grants FAPESP 96/12118-9 (to K.S.) and FAPESP 99/07641-2 (to L.A.B.).
Abbreviations
- VPM
ventroposterior medial nucleus of the thalamus
- SI
primary somatosensory cortex
- PDC
partial directed coherence
- PC1
first principal component
- LGN
lateral geniculate nucleus
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.McCarley R W, Benoit O, Barrionuevo G. J Neurophysiol. 1983;50:798–818. doi: 10.1152/jn.1983.50.4.798. [DOI] [PubMed] [Google Scholar]
- 2.McCormick D A. J Neurosci. 1992;12:278–289. doi: 10.1523/JNEUROSCI.12-01-00278.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maffei L, Moruzzi G, Rizzolatti G. Arch Ital Biol. 1965;103:596–608. [PubMed] [Google Scholar]
- 4.Sherman S M, Guillery R W. J Neurophysiol. 1996;76:1367–1395. doi: 10.1152/jn.1996.76.3.1367. [DOI] [PubMed] [Google Scholar]
- 5.Sherman S M. Trends Neurosci. 2001;24:122–126. doi: 10.1016/s0166-2236(00)01714-8. [DOI] [PubMed] [Google Scholar]
- 6.Coenen A M, Vendrik A J. Exp Brain Res. 1972;14:227–242. doi: 10.1007/BF00816160. [DOI] [PubMed] [Google Scholar]
- 7.Maffei L, Rizzolatti G. Arch Ital Biol. 1965;103:609–622. [PubMed] [Google Scholar]
- 8.Livingstone M, Hubel D. Nature (London) 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- 9.Fourment A, Hirsch J C, Marc M E. Neuroscience. 1985;14:1061–1075. doi: 10.1016/0306-4522(85)90277-5. [DOI] [PubMed] [Google Scholar]
- 10.Steriade M. Curr Opin Neurobiol. 1993;3:619–625. doi: 10.1016/0959-4388(93)90064-6. [DOI] [PubMed] [Google Scholar]
- 11.McCormick D A. Prog Neurobiol. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- 12.Weyand T G, Boudreaux M, Guido W. J Neurophysiol. 2001;85:1107–1118. doi: 10.1152/jn.2001.85.3.1107. [DOI] [PubMed] [Google Scholar]
- 13.Reinagel P, Godwin D, Sherman S M, Koch C. J Neurophysiol. 1999;81:2558–2569. doi: 10.1152/jn.1999.81.5.2558. [DOI] [PubMed] [Google Scholar]
- 14.Guido W, Weyand T. J Neurophysiol. 1995;74:1782–1786. doi: 10.1152/jn.1995.74.4.1782. [DOI] [PubMed] [Google Scholar]
- 15.Edeline J M, Manunta Y, Hennevin E. J Neurophysiol. 2000;84:934–952. doi: 10.1152/jn.2000.84.2.934. [DOI] [PubMed] [Google Scholar]
- 16.Swadlow H A, Gusev A G. Nat Neurosci. 2001;4:402–408. doi: 10.1038/86054. [DOI] [PubMed] [Google Scholar]
- 17.Ramcharan E J, Cox C L, Zhan X J, Sherman S M, Gnadt J W. J Neurophysiol. 2000;84:1982–1987. doi: 10.1152/jn.2000.84.4.1982. [DOI] [PubMed] [Google Scholar]
- 18.Ramcharan E J, Gnadt J W, Sherman S M. Visual Neurosci. 2000;17:55–62. doi: 10.1017/s0952523800171056. [DOI] [PubMed] [Google Scholar]
- 19.Guido W, Lu S M, Sherman S M. J Neurophysiol. 1992;68:2199–2211. doi: 10.1152/jn.1992.68.6.2199. [DOI] [PubMed] [Google Scholar]
- 20.Fanselow E E, Nicolelis M A. J Neurosci. 1999;19:7603–7616. doi: 10.1523/JNEUROSCI.19-17-07603.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semba K, Szechtman H, Komisaruk B R. Brain Res. 1980;195:281–298. doi: 10.1016/0006-8993(80)90065-7. [DOI] [PubMed] [Google Scholar]
- 22.Semba K, Komisaruk B R. Neuroscience. 1984;12:761–774. doi: 10.1016/0306-4522(84)90168-4. [DOI] [PubMed] [Google Scholar]
- 23.Nicolelis M A, Baccala L A, Lin R C, Chapin J K. Science. 1995;268:1353–1358. doi: 10.1126/science.7761855. [DOI] [PubMed] [Google Scholar]
- 24.Gastout H. Rev Neurol. 1952;87:176–182. [PubMed] [Google Scholar]
- 25.Braizer M. EEG Clin Neurophysiol. 1963;15:287–298. doi: 10.1016/0013-4694(63)90098-1. [DOI] [PubMed] [Google Scholar]
- 26.Nicolelis M A, Ghazanfar A A, Faggin B M, Votaw S, Oliveira L M. Neuron. 1997;18:529–537. doi: 10.1016/s0896-6273(00)80295-0. [DOI] [PubMed] [Google Scholar]
- 27.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic; 1986. [DOI] [PubMed] [Google Scholar]
- 28.Martin J H. Neurosci Lett. 1991;127:160–164. doi: 10.1016/0304-3940(91)90784-q. [DOI] [PubMed] [Google Scholar]
- 29.Krupa D J, Thompson R F. Learn Mem. 1997;3:545–556. doi: 10.1101/lm.3.6.545. [DOI] [PubMed] [Google Scholar]
- 30.Sameshima K, Baccala L A. J Neurosci Methods. 1999;94:93–103. doi: 10.1016/s0165-0270(99)00128-4. [DOI] [PubMed] [Google Scholar]
- 31.Baccala L, Sameshima K. Progr Brain Res. 2001;130:33–47. doi: 10.1016/s0079-6123(01)30004-3. [DOI] [PubMed] [Google Scholar]
- 32.Baccala L, Sameshima K. Biol Cybern. 2001;84:463–474. doi: 10.1007/PL00007990. [DOI] [PubMed] [Google Scholar]
- 33.French A S, Holden A V. Kybernetik. 1971;8:165–171. doi: 10.1007/BF00291117. [DOI] [PubMed] [Google Scholar]
- 34.Bendat J, Piersol A. Random Data: Analysis and Measurement Procedures. New York: Wiley; 1986. [Google Scholar]
- 35.Waller H J, Feldman S M. Brain Res. 1973;57:417–441. doi: 10.1016/0006-8993(73)90147-9. [DOI] [PubMed] [Google Scholar]
- 36.Castro-Alamancos M A, Calcagnotto M E. J Neurosci. 1999;19:9090–9097. doi: 10.1523/JNEUROSCI.19-20-09090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman S M. Visual Neurosci. 1996;13:205–213. doi: 10.1017/s0952523800007446. [DOI] [PubMed] [Google Scholar]
- 38.Jahnsen H, Llinas R. J Physiol. 1984;349:205–226. doi: 10.1113/jphysiol.1984.sp015153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steriade M, Jones E, Llinas R. Thalamic Oscillations and Signaling. New York: Wiley; 1990. [Google Scholar]
- 40.Deschenes M, Paradis M, Roy J P, Steriade M. J Neurophysiol. 1984;51:1196–1219. doi: 10.1152/jn.1984.51.6.1196. [DOI] [PubMed] [Google Scholar]
- 41.Ahissar E, Haidarliu S, Zacksenhouse M. Proc Natl Acad Sci USA. 1997;94:11633–11638. doi: 10.1073/pnas.94.21.11633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salmelin R, Hari R. Neuroscience. 1994;60:537–550. doi: 10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]