Dear Editor,
Plasma cell myeloma (PCM) is the second most common hematologic neoplasm, with a worldwide aged-standardized incidence rate of 1.5/100,000 [1]. The introduction of new therapeutic modalities, including alkylating agents and autologous stem cell transplants (ASCT), has dramatically improved the prognosis of patients with PCM; unfortunately, this has been accompanied by an increase in the incidence of secondary neoplasms [1,2]. Therapy-related ALL (t-ALL) is uncommon and far less understood than therapy-related AML. t-ALL as a secondary neoplasm in PCM patients is extremely rare, and only two cases have been reported so far [3,4,5]. We report two additional cases of t-ALL in PCM patients: one who was treated with alkylating agents and topoisomerase II inhibitors simultaneously and the other one who was treated with only topoisomerase II inhibitors. These two cases are first to be reported in Korea. As this was a case report, the Institutional Review Board of Asan Medical Center, Seoul, Korea, waived the requirement for informed consent.
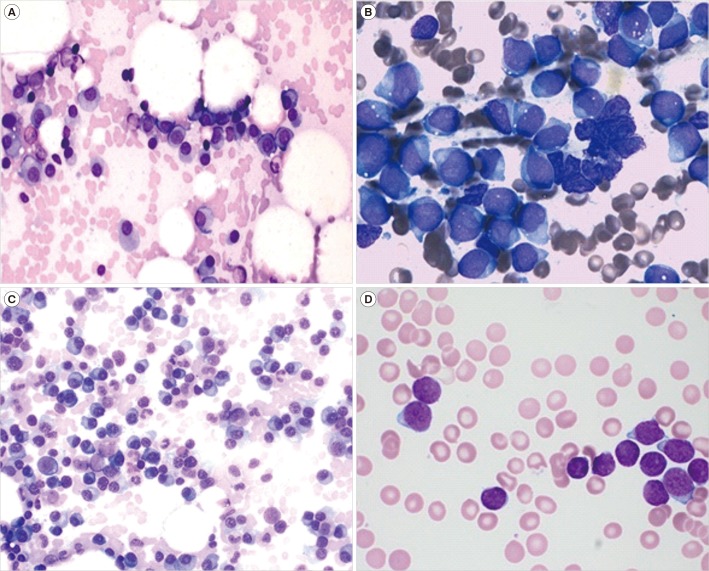
Case 1: A 54-year-old man had been diagnosed as having PCM (IgA κ, plasmablastic type) with a normal karyotype in March 2007 (Fig. 1A). After two cycles of the bortezomib, doxorubicin, and dexamethasone (PAD) regimen, he received ASCT with high-dose melphalan in July 2007. After ASCT, thalidomide monotherapy was given until October 2009. He recovered without complications and was thereafter doing well.
Fig. 1. Bone marrow aspirate findings at initial diagnosis of plasma cell myeloma (PCM) and the diagnosis of ALL in two patients. (A) Neoplastic plasma cells show medium size, round and eccentric nuclei, and blue cytoplasm at initial diagnosis of PCM in Case 1 (Wright stain, ×400). (B) Lymphoblasts show small to large size, fine chromatin pattern, one or two nucleoli, and moderate amount of cytoplasm and occasional cytoplasmic vacuoles at diagnosis of ALL in Case 1 (Wright stain, ×1,000). (C) Neoplastic plasma cells show medium size, round and eccentric nuclei, and blue cytoplasm at initial diagnosis of PCM in Case 2 (Wright stain, ×400). (D) Lymphoblasts show small to medium size, round nuclei and scanty cytoplasm at diagnosis of ALL in Case 2 (Wright stain, ×1,000).
In May 2015, he was admitted with dyspnea. Peripheral blood (PB) analysis indicated the following: white blood cell (WBC) count, 2.6×109/L with 20% blasts; Hb, 84 g/L; and platelet (PLT) count, 14.3×109/L. Protein electrophoresis and immunoelectrophoresis of serum and urine revealed no evidence of PCM. The bone marrow (BM) was massively infiltrated with lymphoblasts (approximately 75.8% of BM nucleated cells) in the hypercellular marrow (cellularity 95%; Fig. 1B) that were immunophenotyped as precursor B-ALL (CD34+, HLA-DR+, CD10+, CD19+, cytoplasmic CD22+, and negative for all myeloid and T-cell antigens). Skull and lumbar-sacrum X-ray did not show any pathologic abnormalities. The karyotypic result of the BM aspirate indicated 45,XY,−7,1dmin[14]/46,XY,−7,+21,1dmin[5]/46,XY[1], which differed from normal karyotype at the initial diagnosis of PCM. The patient was diagnosed as having ALL, and complete remission was achieved after one cycle of induction and two cycles of consolidation chemotherapy.
Case 2: A 52-year-old woman had been diagnosed as having PCM (IgGκ, plasmablastic type) consisting of a malignant hyperdiploid clone with structural abnormalities (52,XX,+add(3)(q12),+5,−6,−8,+9,−10,+11,+15,−17,+18,+19,+20,−22,+3mar [2]/46, XY[7]) in June 2009 (Fig. 1C). She was treated with two cycles of PAD regimen, leading to complete remission, and underwent ASCT. After ASCT, she was given thalidomide monotherapy until August 2016.
In August 2017, she was admitted with fatigue and dizziness. PB analysis showed a WBC count of 2.4×109/L with 6% blasts; Hb, 96 g/L; and PLT, 52.0×109/L. The BM was infiltrated with 92% blasts (Fig. 1D) that were immunophenotyped as precursor B-ALL (CD19+, CD20+, cytoplasmic CD22+, CD10+, and negative for all myeloid and T-cell antigens). Cytogenetics revealed a normal karyotype (46,XX[20]). There was no evidence of relapse with PCM. She was administered six cycles of hyper cyclophosphamide, vincristine, doxorubicin, and dexamethasone chemotherapy, which led to complete remission.
The relationship between t-ALL and prior cytotoxic therapy is controversial, although topoisomerase II inhibitors may also contribute to t-ALL induction [6]. Topoisomerase II inhibitors are associated with several cytogenetic abnormalities, including 11q23, inv(16), and t(9;22), whereas alkylating agents are related to the loss of chromosomes 5 and 7 in secondary ALL [3,7]. The treatment regimens for both of our patients included both alkylating agents and topoisomerase II inhibitors; however, Cases 1 and 2 had monosomy 7 and a normal karyotype, respectively.
Of note, Tang et al. [3] postulated that secondary ALL with a normal karyotype or Ph-positivity may occur regardless of prior chemotherapy, as a causal occurrence or because of inherent genetic instability. They suggested that loss of chromosomes 5, 7, and 17 may be influenced by prior treatment with alkylating agents [3]. We also hypothesized that alkylating agents might cause new lymphoid malignancies with “myeloid-type” chromosomal abnormalities such as monosomy 7 in Case 1. Additionally, various factors, including individual genetic propensity or abnormal cytogenetics, may contribute to t-ALL patient survival; this is in contrast with the poor outcome of therapy-related myeloid neoplasms [2,5,7,8]. Because of the rarity of t-ALL, further studies are required to clarify the association between characteristic cytogenetic alterations and the exposure to various cytotoxic agents in PCM patients. In conclusion, our cases showed t-ALL can occur after PCM treatment including chemotherapy and ASCT. They are first to be reported in Korea.
Footnotes
Authors' Disclosures of Potential Conflicts of Interest: No potential conflicts of interest relevant to this article were reported.
References
- 1.Hong J, Lee JH. Recent advances in multiple myeloma: a Korean perspective. Korean J Intern Med. 2016;31:820–834. doi: 10.3904/kjim.2015.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reddi DM, Lu CM, Fedoriw G, Liu YC, Wang FF, Ely S, et al. Myeloid neoplasms secondary to plasma cell myeloma: an intrinsic predisposition or therapy-related phenomenon? A clinicopathologic study of 41 cases and correlation of cytogenetic features with treatment regimens. Am J Clin Pathol. 2012;138:855–866. doi: 10.1309/AJCPOP7APGDT9JIU. [DOI] [PubMed] [Google Scholar]
- 3.Tang G, Zuo Z, Thomas DA, Lin P, Liu D, Hu Y, et al. Precursor B-acute lymphoblastic leukemia occurring in patients with a history of prior malignancies: is it therapy-related. Haematologica. 2012;97:919–925. doi: 10.3324/haematol.2011.057752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lau LG, Tan LK, Koay ES, Liu TC. Acute lymphoblastic leukemia after tandem autologous stem cell transplantations for multiple myeloma. Leukemia. 2005;19:299–301. doi: 10.1038/sj.leu.2403587. [DOI] [PubMed] [Google Scholar]
- 5.Piszcz J, Bolkun L, Cichocka E, Kloczko J. Secondary acute lymphoblastic leukaemia in a multiple myeloma patient. Contemp Oncol (Pozn) 2012;16:593–595. doi: 10.5114/wo.2012.32497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vardiman JW, Arber AD, et al. Therapy-related myeloid neoplasms. In: Swerdlow SH, Campo E, et al., editors. World Health Organization classification of tumours. Pathology and genetics of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon: IARC Press; 2017. pp. 241–243. [Google Scholar]
- 7.Lee SG, Choi JR, Kim JS, Park TS, Lee KA, Song J. Therapy-related acute lymphoblastic leukemia with t(9;22)(q34;q11.2):a case study and review of the literature. Cancer Genet Cytogenet. 2009;191:51–54. doi: 10.1016/j.cancergencyto.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Wang E, Lu Y, Gaal KK, Huang Q. Therapy-related acute lymphoblastic leukemia without 11q23 abnormality: report of six cases and a literature review. Am J Clin Pathol. 2010;133:75–82. doi: 10.1309/AJCPYWC6AQC7BAVJ. [DOI] [PubMed] [Google Scholar]