Abstract
Myeloid cells are crucial for the host control of a Mycobacterium tuberculosis (M.tb) infection, however the adverse role of specific myeloid subsets has increasingly been appreciated. The relevance of such cells in therapeutic strategies and predictive/prognostic algorithms is to promote interest in regulatory myeloid cells in tuberculosis (TB). Myeloid-derived suppressor cells (MDSC) are a heterogeneous collection of phagocytes comprised of monocytic- and polymorphonuclear cells that exhibit a potent suppression of innate- and adaptive immune responses. Accumulation of MDSC under pathological conditions associated with chronic inflammation, most notably cancer, has been well-described. Evidence supporting the involvement of MDSC in TB is increasing, yet their significance in this infection continues to be viewed with skepticism, primarily due to their complex nature and the lack of genetic evidence unequivocally discriminating these cells from other terminally differentiated myeloid populations. Here we highlight recent advances in MDSC characterization and summarize findings on the TB-induced hematopoietic shift associated with MDSC expansion. Lastly, the mechanisms of MDSC-mediated disease progression and future research avenues in the context of TB therapy and prophylaxis are discussed.
Keywords: myeloid-derived suppressor cells, Mycobacterium tuberculosis, infectious disease, immunosuppression, innate immunity
Introduction
Tuberculosis (TB) remains a leading cause of global mortality (1, 2). Insufficient understanding of TB disease mechanisms represents a major factor impeding its elimination (3). A recent paradigm describes TB as a continuous spectrum of processes, rather than a binary distribution between asymptomatic latent infection and active disease (3–6). This underscores the complex pathophysiology of TB, including multiple cellular effectors, regulators, and checkpoints. Myeloid cells, including neutrophils and monocytes, function both as initial effectors and during the lag phase of T-cell responses to restrict M.tb burden and limit disease progression by activating pro-inflammatory signaling pathways, recruiting additional phagocytes, ingesting bacilli, up-regulating bactericidal mechanisms and inducing antigen-specific adaptive immunity (7–9). Even so, myeloid cells can switch from facilitating protective immunity, to aiding pathological processes, by enhancing TB progression via immunosuppression and dysregulated inflammation (8). Chronic mycobacterial infection triggers the generation of immunosuppressive/tolerogenic myeloid cells, which were initially referred to as “innate natural suppressor cells” (10–12). Subsequent studies have coined these as myeloid-derived suppressor cells (MDSC) (13, 14).
Mycobacteria-Induced Natural Suppressor Cells
Early reports on regulatory myeloid cells in mycobacterial infection came from in vivo and in vitro studies with Mycobacterium bovis Bacillus Calmette-Guerin (BCG) (11, 15–17).In these studies it was indicated that, systemic delivery of mycobacteria induce expansion of hematopoietic progenitor cells in the bone marrow, with the subsequent migration of these cells to the peritoneal cavity and their activation in the spleen (11, 18). It was further reported that BCG could induce the expansion of bone-marrow derived and splenic natural suppressor cells and that these cells could inhibit cell-mediated immunity, notably by suppressing the migratory capacity and proliferation of helper and cytotoxic T-cells (15, 16). T-cell immunosuppression was attributed to the presence of macrophage-like natural suppressor cells, the production of high levels of IL-1 and soluble suppressive factors (16, 19). Natural suppressor cells were later linked to MDSC. Natural suppressor cells from mice exposed to mycobacterial products in Complete Freud's adjuvant (CFA), shared similar phenotypic and functional features with MDSC (10). These cells highly expressed the markers of myeloid origin and differentiation, Gr-1 and CD11b, and inhibition of T-cell proliferation and IFN-γ production was linked to NO production in splenocytes (10). Subsequent studies validated the presence of MDSC during BCG infection (13) and in patients with active TB (14). Thus, initial observations of natural suppressor cells were during mycobacterial insult and established that the generation of these cells was driven by the mycobacterial products.
MDSC Characterization in Mycobacterial Infections
Identification of MDSC requires a combination of assays comprising of immunophenotyping, enzyme measurements, and suppressive tests (20). Markers employed for detection of human MDSC allow, to some extent, their differentiation from monocytes and neutrophils, although this is cumbersome in mice (21). At present, three commonly reported MDSC subsets identified in human TB include early stage MDSC (e-MDSC), polymorphonuclear-MDSC (PMN-MDSC), and monocytic-MDSC (M-MDSC) (14, 22, 23). Immunosuppressive eosinophilic MDSC have recently been described during chronic Staphylococcus aureus infection in vivo but require validation in other diseases (24). MDSC enriched in TB patients, according to recent recommendations using a ficoll density-gradient (22, 23), have been classified as e-MDSC (LIN1−HLA-DR−/loCD11b+CD33+), PMN-MDSC (HLA-DR−/loCD11b+CD14−CD15+CD33+/dim) and M-MDSC (HLA-DR−/lowCD11b+CD14+CD15−CD33+) (20). Instead of a specific subset, M-MDSC population has been described as a heterogenous population of cells, in different maturation stages (20). Since there are no specific markers for MDSC, ambiguity with other myeloid cells that have similar phenotypic characteristics and functional properties exists, especially after pathogen exposure. For instance, infection of monocytes with Candida albicans fungal cells and exposure to fungal components subverts monocyte differentiation to immunosuppressive dendritic cells. The phenotype of the subverted DC is characterized by the expression of CD14 with a lack of CD1a molecule, presence of CD83 and CD86 but a relatively low expression of MHC class II and CD80. These cells produce IL-12 but are associated with the release of IL-10 and IL-6 (25). Similarly our group has demonstrated that CD14+ M-MDSC production of IL-10 and IL-6 is associated with either absent, or relatively low levels of HLA-DR and CD80 (14, 26). Thus, an unequivocal marker that is able to distinguish myeloid cell population and subsets in biological samples such as whole blood culture and tissue is required. Whilst there is no specific marker for M-MDSC yet, utilization of LOX-1 as a unique PMN-MDSC marker has been proposed but (27) requires validation in TB patients.
In murine TB, PMN-MDSC are phenotypically Gr-1+CD11b+Ly6G+Ly6Clo/int and M-MDSC Gr-1+CD11b+Ly6G−/l0Ly6Chi, yet functional assays are essential for their classification (28–30).
Morphological characterization has been used as a confirmatory tool to distinguish MDSC from other myeloid cells in TB samples (22, 28). Immature myeloid cells identified as PMN-MDSC share similar morphological characteristics with neutrophils, as they show ring-shaped or band nuclei. This nuclear shape can, however, be present in neutrophil progenitors and young neutrophils. Utilization of CD10 for human specimens (21) along with suppressive assays may help distinguish PMN-MDSC from non-suppressive immature neutrophils. MDSC likely encompass cells at different maturation stages with a distinct activation status and functional role. For instance, expansion of MDSC with the phenotype Lin−/l0HLA-DR−/loCD11b+CD14+CD33+CD80+, was described in patients with active TB and their frequency correlated with disease progression (14). CD80 up-regulation upon successful TB chemotherapy was associated with MDSC differentiation into macrophages and dendritic cells (14). In mice, accumulation of an immature, heterogeneous population of Gr1dimCD11b+ cells with un-segmented nuclei, which also expresses progenitor markers (CD117+CD135+), was observed during the advanced disease in TB prone animals (28).
Tissue Compartmentalization and Dynamics of MDSC in TB
In murine models MDSC were detected in the blood during BCG vaccination (13). In adults and children suffering from TB, MDSC frequencies in the periphery were comparable to those found in cancer patients (14). All MDSC subsets have been identified in the blood of TB patients, yet relative ratios, within different biological samples/fluids, differ in various studies (14, 22, 23). For instance, PMN-MDSC are enriched in the lung, specifically in bronchoalveolar lavage (BAL) samples of pulmonary TB patients (22) whilst the prevalence of a M-MDSC subset has been described in pleural effusions (14). Compartmentalization of the different MDSC subsets during TB in humans may be site-specific and likely dependent on the disease stage. Such an assumption is supported by findings from experimental TB. In naïve mice, MDSC can be detected at very low frequencies primarily in bone marrow. During acute TB, MDSC mildly accumulates in the lung and upon disease progression their numbers dramatically increase in all aforementioned organs and are also detected in the blood (28). High levels of MDSC in bone-marrow suggests that their genesis occurs primarily via medullary hematopoiesis. A pro-inflammatory environment, abundant in IL-6/G-CSF/PROK-2 may promote myelo- and granulopoiesis, whereas recruitment of MDSC to the lung could be directed by abundant S100-proteins/MMP-9/G-CSF (20, 29). Accumulation of MDSC in the lung parenchyma parallels TB progression in susceptible mice (29, 30). In M.tb-infected-necrosis prone mice, M-MDSC accumulate at the edges of necrotic granulomas (30). A recent study further strengthened the case for MDSC as regulators of granuloma biology. Human ex vivo generated M-MDSC promote mycobacterial replication in in vitro established granulomas, in a process dependent on abundant release of IL-10 (26).
Dynamics of MDSC subsets through-out the course of the TB disease spectrum (31) are relevant for disease pathophysiology. In TB patients, MDSC abundances have not yet been clearly linked with the extent of disease, e.g., by establishing a correlation between their frequencies and lung radiological involvement, smear grading or bacterial burden. Community controls from a high-exposure region and also individuals with remote exposure to M.tb, display very low levels of circulating MDSC, yet frequencies of MDSC increase in recently exposed house hold contacts (HHC) of TB patients (19). MDSC presumably emerge in incipient TB with their increased frequency associated with disease progression. TB-resistant mice that are devoid of necrotic granulomas have minimal levels of MDSC, whilst necrotic prone mouse strains NOS2−/− (knock-out), C3HeB/FeJ, 129S2 (immunocompetent) exhibit higher frequencies with the highest levels observed in immunodeficient (RAG2−/−) animals (29, 30). The accumulation of MDSC in necrotic granulomas has been associated with the inability to control M.tb infection and lung pathology (28, 29). Pulmonary tuberculosis manifests differently than pleural tuberculosis and MDSC biology in pleural cavities still needs further characterization. In TB patients, MDSC are present in pleural effusions and blood and the immunosuppressive potential of MDSC from individuals with a long term infection exceeds the suppression of cells isolated from people with recent M.tb exposure, which also affects CD8 T-cell responsiveness (14). Upon a successful cure, MDSC frequencies decrease to levels observed in healthy controls (14). In children, completion of standard TB treatment was not accompanied by a MDSC decline, likely reflecting the more complex disease presentation of pediatric TB and possibly the polarization of the immune response which may be different to adult immune response (32).
MDSC Directly Interact With Mycobacteria
Lung-residing M-MDSC harbor M.tb and promote bacterial growth through mechanisms involving IL-4/IL4Rα signaling (29). Despite the production of nitric oxide (NO), a potent anti-mycobacterial molecule, MDSC are inefficient at controlling mycobacterial growth (13). Although ex vivo generated human MDSC are not able to provide a niche for fast replication of M.tb when compared to macrophages, they do however exert a potent suppressive activity against T-cells upon infection (26).Recent reports indicate that myeloid cell ontogeny affects their capacity to support mycobacterial growth. Interstitial macrophages, supposedly originating from circulating monocytes, allow lower M.tb. replication rates as compared to fetal germline derived alveolar macrophages (AM). This phenomenon has been linked to the dramatically different metabolic states of AM and interstitial macrophages, with highly up-regulated fatty acid uptake and β-oxidation vs. high glycolytic activity, respectively (33). Pre-existing metabolic bias of myeloid cells controls M.tb growth (33). Of note, tumor-infiltrating MDSC preferentially use fatty acid-β-oxidation (FAO) as a primary energy source, display up-regulation in FAO genes and increases the oxygen consumption rate (34). We, and others have previously shown that MDSC are capable of mycobacterial internalization, however, they display poor microbicidal activity (13, 26). Considering that M.tb uses host fatty acids and cholesterol, the metabolic status of MDSC likely offers a nutritional niche supporting M.tb maintenance (35, 36). Whether FAO affects M.tb survival within MDSC remains to be validated. In the same vein, the metabolic state of M.tb as well as its subcellular localization within MDSC are largely unknown and should be defined.
Mediators of MDSC Expansion and Activation in TB
Expansion and activation of MDSC is mediated by chronic, low-grade inflammation, resulting in the pathological activation of myeloid cells (37). Currently, it is difficult to discriminate signals mediating MDSC expansion from those mediating MDSC activation. Recent findings support a two-step process involving cellular expansion, licensing, and activation (37, 38). First, chronic exposure to GM-CSF, IL-6, prostaglandins, and alarmins such as S100A8/9 (38, 39) promote “emergency myelopoiesis,” impede on terminal maturation of myeloid progenitors. The second phase involves activation of these “licensed” myeloid cells, through the panoply of inflammatory cytokines (e.g., IFN-γ, IL-1β, IL- 6, TNF-α, IL-4), DAMPs (e.g., HMGB1), and likely also PAMPs (e.g., LPS) to obtain suppressive functions (37–39). Such factors are produced during TB and enriched in TB-susceptible mice accumulating MDSC (Figure 1A) (29). Additional molecules detected in TB lesions, including prokineticin 2 (PROK 2) and MMP9, which promote MDSC accumulation in target organs, may also regulate MDSC expansion (29). Recent reports indicate that transmembrane TNF-alpha regulates the activation and expansion of PMN-MDSC and M-MDSC in the pleural cavity of BCG infected mice (40). In mycobacterial infections, M-MDSC are induced regardless of key virulence factors, as M.tb, M.smeg, and BCG have proven to induce MDSC (13). Consequently, due their immunosuppressive activity and high frequency during disease progression, MDSC have been identified as one of the factors that may contribute to a low BCG vaccine efficacy (41). Other factors may include geographical location, helminthic co-infection, route of BCG administration and mycobacterial strain (42). It is important to note that the robust cytokine response often observed following BCG vaccination, contradicts the MDSC functions described above. We suspect that this perceived discrepancy, could be ascribed to the requirement of a 2nd activation signal or the mycobacterial strain-specific differences on MDSC function. Alternatively, the MDSC suppressive function might stretch beyond T-cell immunity and affect other cell subsets which are rarely evaluated following BCG vaccination, with the route of the vaccination and the age of the vaccine, also contributing to the outcome. The role of live bacteria in regions from which MDSC originate, such as immature bone marrow cells, still need to be investigated.
Figure 1.
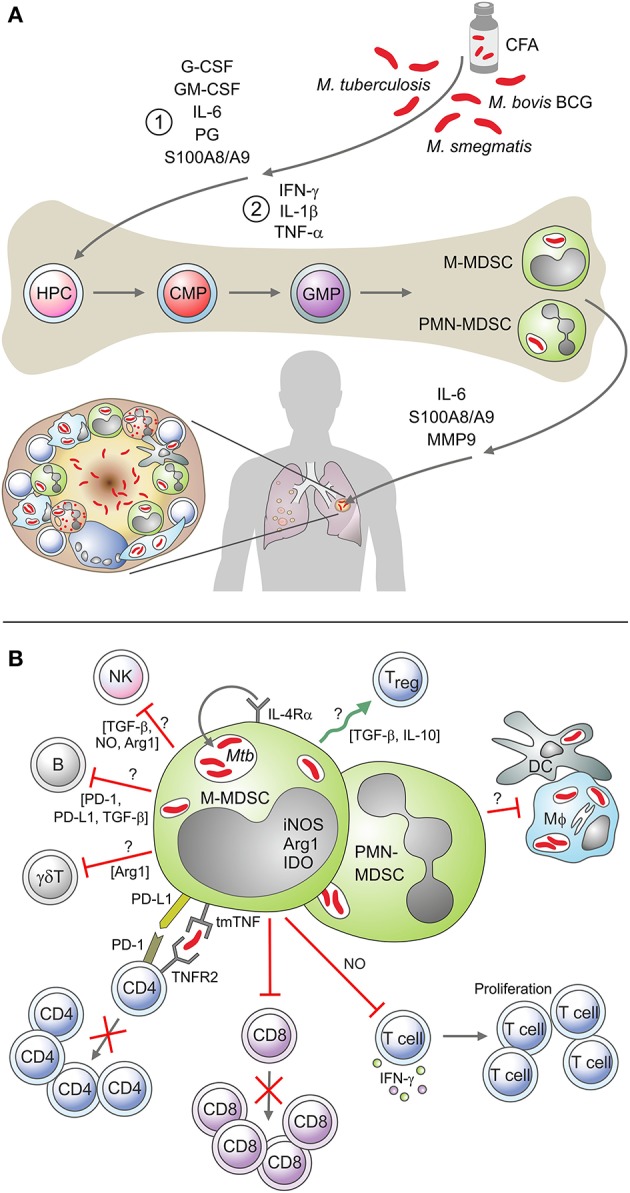
(A) Mediators of MDSC expansion and activation in a mycobacterial environment. A schematic depicting mediators associated with the proposed “two-signal” MDSC expansion and activation process, in a mycobacterial environment. These include cytokines, chemokines, calcium-binding proteins, and matrix metalloproteinases. (B) MDSC cellular interaction and mediators of immunosuppression in a mycobacterial environment. Examples of the known and suggested interactions of MDSC with other immune cells in the mycobacterial setting, including the soluble mediators associated with their immunosuppressive activity.
Mycobacterial glycolipids contained in CFA promote the expansion of the MDSC (10). A comprehensive comparison of “licensed” monocytes, M-MDSC and additional monocytic subsets present in the M.tb infected lung is necessary to distinguish pathways driving MDSC genesis. Advanced techniques such as quantitative shotgun proteomics, RNASeq and chromatin ATAC mapping should provide insights into potentially discriminating markers and differentiation pathways.
MDSC Immunosuppressive Mechanisms During TB Infection
MDSC exert their immunosuppressive activity through mechanisms that involve soluble factors, cell membrane molecules and the modulation of local concentrations off of metabolites and amino acid (20, 43). Most studies focus on T-cell suppression (ref), however MDSC also interact with macrophages and dendritic cells, and induce regulatory B- and T-cells (44–46). Such interactions have not yet been considered in TB (Figure 1B). The interaction of MDSC with T-cells has been established in TB patients, though the effects on antigen-specific responder lymphocytes still await clarification. Suppression of polyclonal stimulated CD4 and CD8 T-cells involves the inhibition of cytokine production, T-cell activation and modulation of T-cell trafficking (14). Whereas, PMN-MDSC expansion correlates with abundant plasma NO (22), phenotypically resembling MDSC present abundant indoleamine 2,3-dioxygenase (IDO) and arginase-1 (ARG-1) (32). In BCG vaccinated mice, iNOS-mediated tendency of MDSC to dampen T-cell priming, suppress polyclonal T-cell proliferation and IFN-γ release (13). iNOS mediates the suppression of lymphocytes also in murine TB, though in situ co-expression of ARG1 and iNOS has been detected in lung lesions (29).Cell surface molecules involved in the regulation of MDSC functions have been identified in experimental TB studies. In mice with mycobacterial pleurisy, tmTNF-α regulates MDSC activity through the cell-to-cell interaction between tmTNF-α expressing MDSC and TNFR2 expressing CD4 T-cells (40). Human MDSC up-regulate PD-L1 upon in vitro mycobacterial infection (26) and employ this check-point molecule to restrict T-cell proliferation (26, 47). IFN-γ counteracts PD-L1 induced suppression (47) and this may explain the profound immunosuppression in end stage TB patients. Relevance of additional enzymes enriched in MDSC purified from cancer patients, such as NADPH and COX2 (20), as well as roles of autophagy molecules (48), remain to be established in TB. Of paramount importance will be the deciphering of interactions between MDSC and macrophages, as those cells harbor and aid restricting bacillary replication. The capacity of MDSC to modulate Treg dynamics, induce Breg and alter NK activity in TB is also unknown. High dimensional analyses, e.g., mass cytometry and histo-cytometry could establish effects on MDSC on various immune cells and facilitate the in-depth functional characterization of these cells. MDSC may further contribute to TB reactivation by exacerbating the immunosuppressive effects of immunotherapy such as anti-TNF agents, absence of TNF-alpha has been associated with an increased bacterial load and T-cell immunosuppression (49, 50).
MDSC and TB Co-Morbidities
Diseases promoting TB development are typically linked to immunosuppression or dysregulation of immunity and encompass HIV (51, 52) and diabetes (53, 54). In addition, undernourishment, alcoholism, and smoking are considered risk factors for TB. Currently, the precise role of MDSC in these conditions and subsequent implications for TB are not clear. MDSC have been reported in HIV infection, but a prevalence of distinct subsets during co-infection has not been unanimously established. Some studies report high frequencies of the PMN-MDSC subset (52, 55–57) whilst others describe increased M-MDSC populations in AIDS patients (58–61). MDSC frequencies correlate with AIDS progression and viral load (51, 59), while anti-retroviral therapy (ART) reduces systemic MDSC frequencies (44, 62, 63). Even HIV exposed uninfected children display abundant circulating MDSC (32). MDSC activity in an HIV environment involves enhanced IL-10 production, induction of CD4+CD25+FoxP3+Tregs and suppression of T-cell responses, notably inhibition of IFN-gamma release by autologous T-cells (52, 60). Such effects may contribute to development of TB in LTBI people infected with HIV, however further studies are required to elucidate the precise role of HIV-induced MDSC in TB reactivation. Very few reports focus on MDSC in diabetes. Recent trials suggest a beneficial effect with MDSC protecting against the development of type-2 diabetes (T2DM) in humans (64). Interestingly, the anti-diabetic drug metformin, showing efficacy as an adjunct therapy in TB (65), causes reduction of MDSC in cancer patients (66). Metformin's effect on MDSC in TB patients has not been evaluated. Smoking is regarded as a predisposing factor that can accelerate TB progression. Although smoking has been associated with MDSC expansion and generation in COPD patients (67, 68), the role of these cells in TB is not clear and should be clarified. Obesity-driven chronic, low-grade inflammation and leptin interaction has also shown to induce MDSC that, although protective against some metabolic dysfunctions, appear to be detrimental to tumor progression (69). At the other end of the spectrum, malnutrition has also been correlated to MDSC induction, suggesting a link with diseases characterized by wasting and malnutrition, such as TB (70). It is tempting to speculate that enhanced MDSC levels in diseases and conditions causing alterations in immune reactivity may contribute to TB reactivation, however this remains to be tested.
Therapeutic Strategies Targeting MDSC in TB
Shortly after identification of MDSC in TB patients and murine models, these cells emerged as promising targets for adjunct host-directed therapy (HDT) approaches (8, 41, 71). The focus of such strategies has been to reverse the impact of MDSC on T-cell immunity in TB by implementing host modulating therapeutic strategies such as those blocking MDSC induction or activation, inhibiting MDSC function or reversing their suppressive function. These strategies have been recently reviewed elsewhere (71). More recently, denileukin diftitox, an anti-neoplastic agent comprised of IL-2 and Diphtheria toxin, potentiates standard TB treatment in a mouse model through the elimination of MDSC and Treg (72). Similarly, combined immunotherapy consisting of ATRA and alpha galactosylceramide as an adjunct immunotherapy improved standard TB treatment (73). Other studies on ATRA have reported the reduction of MDSC and increase in T-cell number with an impact on bacillary loads and lung pathology (13, 29). Tasquinimod (TSQ), a quinoline-3-carboxyamide analog, targets S100A9, a molecule which has been implicated in MDSC accumulation and function. TSQ is in clinical development for the treatment of various cancers and has recently shown to significantly enhance the antitumor effects of immunotherapeutics in cancer mouse models, by inhibiting the suppressive function of MDSC and tumor-associated macrophages (TAM) (74). More recently, TSQ treatment in an acute mouse model of TB, enhanced M.tb clearance, reduced Treg and MDSC frequencies and enhanced the efficacy of the standard treatment regimen (75).
Cytokines indirectly affect MDSC accumulation/function and a recent study has shown that IFN-γ decreases the suppressive function of MDSC by reducing the arginase activity suppressing PD-1/PD-L1 (47). Although not yet tested in TB, a combination treatment of IL-17R and IFN-γ has shown potential in cancer, by reducing the levels of MDSC and increasing T-cells (76). Other MDSC targeting agents tested in cancer, which have shown potential in TB, but with unknown effects on MDSC, include metformin, tyrosine kinase inhibitors (imatinib), PDE-5 inhibitors, and arginase inhibitors (71). The COX-2 inhibitor, etoricoxib, is currently evaluated as HDT for TB and its effect on MDSC levels will be considered in the trial (NCT02503839).
Conclusion
The MDSC arena has experienced several research advances in the context of infectious diseases. Nonetheless, the complex and protracted nature of M.tb infection along with challenges in biology of MDSC research have delayed comprehensive investigations on MDSC in the TB field. Ultimately, MDSC research in TB would be insignificant without an eventual tangible contribution to the clinical benefit of patients. Development of immunotherapies targeting MDSC is undergoing a slow but steady progress, however many TB HDT trials fail to consider the impact of these treatments on MDSC function and frequency. The lack of compounds targeting MDSC specifically, contributes to this problem. The safety, efficacy, dose, and timing of interventions targeting MDSC in TB, will also require careful evaluation, and so too will the effect of novel neonatal vaccines and adult re-vaccination strategies on MDSC genesis. Greater focus on these and other MDSC knowledge gaps is expected to accelerate the discovery of effective TB immunotherapies, thereby contributing to an increased TB cure rate, more durable clinical responses and superior control of drug-resistant M.tb strains.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Diane Schad for assistance with the graphics work.
Footnotes
Funding. The authors declare financial support from the European and Developing Countries Clinical Trials Partnership (EDCTP, CDF1546); the South African Medical Research Council (SA-MRC), the Southern African-Nordic Centre (SANORD), the South-African Department of Science and Technology (SA-DST), and South-African National Research Foundation (SA-NRF) through the South African Research Chair Initiative (SARChi) in TB Biomarkers (#86535). The funders played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication.
References
- 1.Raviglione M, Sulis G. Tuberculosis 2015: burden, challenges and strategy for control and elimination. Infect Dis Rep. (2016) 8:6570. 10.4081/idr.2016.6570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2018. World Health Organization (2018). [Google Scholar]
- 3.Drain PK, Bajema KL, Dowdy D, Dheda K, Naidoo K, Schumacher SG, et al. Incipient and subclinical tuberculosis: a clinical review of early stages and progression of infection. Clin Microbiol Rev. (2018) 31:e00021–18. 10.1128/CMR.00021-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barry CE, Boshoff HI, Dartois V, Dick T, Ehrt S, Flynn J, et al. The spectrum of latent tuberculosis: rethinking the biology and intervention strategies. Nat Rev Microbiol. (2009) 7:845–55. 10.1038/nrmicro2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delogu G, Goletti D. The spectrum of tuberculosis infection: new perspectives in the era of biologics. J Rheumatol Suppl. (2014) 91:11–16. 10.3899/jrheum.140097 [DOI] [PubMed] [Google Scholar]
- 6.Pai M, Behr MA, Dowdy D, Dheda K, Divangahi M, Boehme CC, et al. Tuberculosis. Nat Rev Dis Primers. (2016) 2:16076 10.1038/nrdp.2016.76 [DOI] [PubMed] [Google Scholar]
- 7.Srivastava S, Ernst JD, Desvignes L. Beyond macrophages: the diversity of mononuclear cells in tuberculosis. Immunol Rev. (2014) 262:179–92. 10.1111/imr.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dorhoi A, Kaufmann SHE. Versatile myeloid cell subsets contribute to tuberculosis-associated inflammation: HIGHLIGHTS. Eur J Immunol. (2015) 45:2191–202. 10.1002/eji.201545493 [DOI] [PubMed] [Google Scholar]
- 9.Liu Y, Wang R, Jiang J, Cao Z, Zhai F, Sun W, et al. A subset of CD1c+ dendritic cells is increased in patients with tuberculosis and promotes Th17 cell polarization. Tuberculosis. (2018) 113:189–99. 10.1016/j.tube.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Wang H, Jiang J, Li Z, Zhang J, Qin Z. A Myeloid cell population induced by freund adjuvant suppresses T-cell mediated antitumor immunity. J Immunother. (2010) 33:167–77. 10.1097/CJI.0b013e3181bed2ba [DOI] [PubMed] [Google Scholar]
- 11.Bennett JA, Rao VS, Mitchell MS. Systemic Bacillus Calmette-Guerin (BCG) activates natural suppressor cells. Proc Natl Acad Sci USA. (1978) 75:5142–4. 10.1073/pnas.75.10.5142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kato K, Yamamoto K-I. Suppression of BCG cell wall-induced delayed-type hypersensitivity by BCG pre-treatment. II. Induction of suppressor T cells by heat-killed BCG injection. Immunology. 45:655–61. [PMC free article] [PubMed] [Google Scholar]
- 13.Martino A, Badell E, Abadie V, Balloy V, Chignard M, Mistou M-Y, et al. Mycobacterium bovis bacillus calmette-guérin vaccination mobilizes innate myeloid-derived suppressor cells restraining in vivo T cell priming via IL-1R-dependent nitric oxide production. J Immunol. (2010) 184:2038–47. 10.4049/jimmunol.0903348 [DOI] [PubMed] [Google Scholar]
- 14.du Plessis N, Loebenberg L, Kriel M, von Groote-Bidlingmaier F, Ribechini E, Loxton AG, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent Mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med. (2013) 188:724–32. 10.1164/rccm.201302-0249OC [DOI] [PubMed] [Google Scholar]
- 15.Kato K, Yamamoto K. Suppression of BCG cell wall-induced delayed-type hypersensitivity by BCG pre-treatment. II. Induction of suppressor T cells by heat-killed BCG injection. Immunology. (1982) 45:655–61. [PMC free article] [PubMed] [Google Scholar]
- 16.Klimpel G, Okada M, Henney C. Inhibition of in vitro cytotoxic responses by BCG-induced macrophage-like suppressor cells. II. Suppression occurs at the level of a “helper” T cell. J Immunol. (1979) 123:350–7. [PubMed] [Google Scholar]
- 17.Mellow L, Sabbadini E. Effect of Bacillus Calmette-Guerin on the in vitro generation of cytotoxic T lymphocytes. Immunology. (1985) 56:235–43. [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett J, Marsh J. Relationship of Bacillus Calmette-Guérin-induced suppressor cells to hematopoietic precursor cells. Cancer Res. (1980) 40:80–5. [PubMed] [Google Scholar]
- 19.Kendall L, Sabbadini E. Effect of Bacillus Calmette-Guérin on the in vitro generation of cytotoxic T lymphocytes. I. Effect of BCG on the frequency of cytotoxic T lymphocyte precursors and on the production of helper factors. J Immunol. (1981) 127:234–8. [PubMed] [Google Scholar]
- 20.Bronte V, Brandau S, Chen S-H, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. (2016) 7:12150. 10.1038/ncomms12150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cassetta L, Baekkevold E, Brandau S, Bujiko A, Cassatella M, Dorhoi A, et al. Deciphering myeloid-derived suppressor cells: isolation and markers in humans, mice and non-human primates. Cancer Immunol Immunother. (2019) 68:687–97. 10.1007/s00262-019-02302-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Daker S, Sacchi A, Tempestilli M, Carducci C, Goletti D, Vanini V, et al. Granulocytic myeloid derived suppressor cells expansion during active pulmonary tuberculosis is associated with high nitric oxide plasma level. PLoS ONE. (2015) 10:e0123772 10.1371/journal.pone.0123772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang B, Wang X, Jiang J, Zhai F, Cheng X. Identification of CD244-expressing myeloid-derived suppressor cells in patients with active tuberculosis. Immunol Lett. (2014) 158:66–72. 10.1016/j.imlet.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 24.Goldmann O, Beineke A, Medina E. Identification of a novel subset of myeloid-derived suppressor cells during chronic staphylococcal infection that resembles immature eosinophils. J Infect Dis. (2017) 216:1444–51. 10.1093/infdis/jix494 [DOI] [PubMed] [Google Scholar]
- 25.Nisini R, Torosantucci A, Romagnoli G, Chiani P, Donati S, Gagliardi M, et al. Glucan of Candida albicans cell wall causes the subversion of human monocyte differentiation into dendritic cells. J Leukoc Biol. (2007) 82:1136–42. 10.1189/jlb.0307160 [DOI] [PubMed] [Google Scholar]
- 26.Agrawal N, Streata I, Pei G, Weiner J, Kotze L, Bandermann S, et al. Human monocytic suppressive cells promote replication of Mycobacterium tuberculosis and alter stability of in vitro generated granulomas. Front Immunol. (2018) 9:2417. 10.3389/fimmu.2018.02417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Condamine T, Dominguez GA, Youn J-I, Kossenkov AV, Mony S, Alicea-Torres K, et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci Immunol. (2016) 1:aaf8943. 10.1126/sciimmunol.aaf8943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsiganov EN, Verbina EM, Radaeva TV, Sosunov VV, Kosmiadi GA, Nikitina IY, et al. Gr-1dimCD11b+ immature myeloid-derived suppressor cells but not neutrophils are markers of lethal tuberculosis infection in mice. J Immunol. (2014) 192:4718–27. 10.4049/jimmunol.1301365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Knaul JK, Jörg S, Oberbeck-Mueller D, Heinemann E, Scheuermann L, Brinkmann V, et al. Lung-residing myeloid-derived suppressors display dual functionality in murine pulmonary tuberculosis. Am J Respir Crit Care Med. (2014) 190:1053–66. 10.1164/rccm.201405-0828OC [DOI] [PubMed] [Google Scholar]
- 30.Obregón-Henao A, Henao-Tamayo M, Orme IM, Ordway DJ. Gr1intCD11b+ Myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS ONE. (2013) 8:e80669. 10.1371/journal.pone.0080669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol. (2017) 17:691–702. 10.1038/nri.2017.69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Du Plessis N, Jacobs R, Gutschmidt A, Fang Z, van Helden PD, Lutz MB, et al. Phenotypically resembling myeloid derived suppressor cells are increased in children with HIV and exposed/infected with Mycobacterium tuberculosis: immunity to infection. Eur J Immunol. (2017) 47:107–18. 10.1002/eji.201646658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang L, Nazarova EV, Tan S, Liu Y, Russell DG. Growth of Mycobacterium tuberculosis in vivo segregates with host macrophage metabolism and ontogeny. J Exp Med. (2018) 215:1135–52. 10.1084/jem.20172020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of fatty acid oxidation modulates immunosuppressive functions of myeloid-derived suppressor cells and enhances cancer therapies. Cancer Immunol Res. (2015) 3:1236–47. 10.1158/2326-6066.CIR-15-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Daniel J, Maamar H, Deb C, Sirakova T, Kolattukudy P. Mycobacterium tuberculosis uses host triacylglycerol to accumulate lipid droplets and acquires a dormancy-like phenotype in lipid-loaded macrophages. PLoS Pathog. (2011) 7:e1002093. 10.1371/journal.ppat.1002093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guirado E, Mbawuike U, Keiser TL, Arcos J, Azad AK, Wang S-H, et al. Characterization of host and microbial determinants in individuals with latent tuberculosis infection using a human granuloma model. mBio. (2015) 6:e02537–14. 10.1128/mBio.02537-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ribechini E, Hutchinson JA, Hergovits S, Heuer M, Lucas J, Schleicher U, et al. Novel GM-CSF signals via IFN-γR/IRF-1 and AKT/mTOR license monocytes for suppressor function. Blood Adv. (2017) 1:947–60. 10.1182/bloodadvances.2017006858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gabrilovich DI. Myeloid-derived suppressor cells. Cancer Immunol Res. (2017) 5:3–8. 10.1158/2326-6066.CIR-16-0297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. (2018) 19:108–19. 10.1038/s41590-017-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chavez-Galan L, Vesin D, Uysal H, Blaser G, Benkhoucha M, Ryffel B, et al. Transmembrane tumor necrosis factor controls myeloid-derived suppressor cell activity via TNF receptor 2 and protects from excessive inflammation during BCG-induced pleurisy. Front Immunol. (2017) 8:999. 10.3389/fimmu.2017.00999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jayashankar L, Hafner R. Adjunct strategies for tuberculosis vaccines: modulating key immune cell regulatory mechanisms to potentiate vaccination. Front Immunol. (2016) 7:577. 10.3389/fimmu.2016.00577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moliva J, Turner J, Torrelles J. Immune responses to Bacillus-Calmette-Guerin vaccination: why do they fail to protect against Mycobacterium tuberculosis? Front Immunol. (2017) 8:407. 10.3389/fimmu.2017.00407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dorhoi A, du Plessis N. Monocytic myeloid-derived suppressor cells in chronic infections. Front Immunol. (2018) 8:1895. 10.3389/fimmu.2017.01895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lei G-S, Zhang C, Lee C-H. Myeloid-derived suppressor cells impair alveolar macrophages through PD-1 receptor ligation during Pneumocystis pneumonia. Infect Immun. (2015) 83:572–82. 10.1128/IAI.02686-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park M-J, Lee S-H, Kim E-K, Lee E-J, Park S-H, Kwok S-K, et al. Myeloid-derived suppressor cells induce the expansion of regulatory B cells and ameliorate autoimmunity in the Sanroque mouse model of systemic lupus erythematosus. Arthritis Rheumatol. (2016) 68:2717–27. 10.1002/art.39767 [DOI] [PubMed] [Google Scholar]
- 46.Wang Y, Schafer CC, Hough KP, Tousif S, Duncan SR, Kearney JF, et al. Myeloid-derived suppressor cells impair B cell responses in lung cancer through IL-7 and STAT5. J Immunol. (2018) 201:278–95. 10.4049/jimmunol.1701069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhan X, Fang Y, Hu S, Wu Y, Yang K, Liao C, et al. IFN-γ differentially regulates subsets of Gr-1+CD11b+ myeloid cells in chronic inflammation. Mol Immunol. (2015) 66:451–62. 10.1016/j.molimm.2015.05.011 [DOI] [PubMed] [Google Scholar]
- 48.Alissafi T, Hatzioannou A, Mintzas K, Barouni RM, Banos A, Sormendi S, et al. Autophagy orchestrates the regulatory program of tumor-associated myeloid-derived suppressor cells. J Clin Invest. (2018) 128:3840–52. 10.1172/JCI120888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clay H, Volkman H, Ramakrishnan L. TNF signaling mediates resistance to mycobacteria by inhibiting bacterial growth and macrophage death but not tuberculous granuloma formation. Immunity. (2008) 29:283–94. 10.1016/j.immuni.2008.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller A, Ernst J. Anti-TNF immunotherapy and tuberculosis reactivation: another mechanism revealed. J Clin Invest. (2009) 119:1079–82. 10.1172/JCI39143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vollbrecht T, Stirner R, Tufman A, Roider J, Huber RM, Bogner JR, et al. Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS. (2012) 26:F31–7. 10.1097/QAD.0b013e328354b43f [DOI] [PubMed] [Google Scholar]
- 52.Tumino N, Turchi F, Meschi S, Lalle E, Bordoni V, Casetti R, et al. In HIV-positive patients, myeloid-derived suppressor cells induce T-cell anergy by suppressing CD3ζ expression through ELF-1 inhibition. AIDS. (2015) 29:2397–407. 10.1097/QAD.0000000000000871 [DOI] [PubMed] [Google Scholar]
- 53.Faurholt-Jepsen D, Range N, PrayGod G, Jeremiah K, Faurholt-Jepsen M, Aabye M, et al. Diabetes is a strong predictor of mortality during tuberculosis treatment: a prospective cohort study among tuberculosis patients from Mwanza, Tanzania. Trop Med Int Health. (2013) 18:822–9. 10.1111/tmi.12120 [DOI] [PubMed] [Google Scholar]
- 54.Gil-Santana L, Almeida-Junior J, Oliveira C, Hickson L, Daltro C, Castro S, et al. Diabetes is associated with worse clinical presentation in tuberculosis patients from Brazil: a retrospective cohort study. PLoS ONE. (2016) 11:e0146876. 10.1371/journal.pone.0146876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z-N, Yi N, Zhang T-W, Zhang L-L, Wu X, Liu M, et al. Myeloid-derived suppressor cells associated with disease progression in primary HIV infection: PD-L1 blockade attenuates inhibition. J Acquir Immune Defic Syndr. (2017) 76:200–8. 10.1097/QAI.0000000000001471 [DOI] [PubMed] [Google Scholar]
- 56.Tumino N, Bilotta MT, Pinnetti C, Ammassari A, Antinori A, Turchi F, et al. Granulocytic myeloid-derived suppressor cells increased in early phases of primary HIV infection depending on TRAIL plasma level. J Acquir Immune Defic Syndr. (2017) 74:575–82. 10.1097/QAI.0000000000001283 [DOI] [PubMed] [Google Scholar]
- 57.Bowers NL, Helton ES, Huijbregts RPH, Goepfert PA, Heath SL, Hel Z. Immune Suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog. (2014) 10:e1003993. 10.1371/journal.ppat.1003993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gama L, Shirk E, Russell J, Carvalho K, Li M, Queen S, et al. Expansion of a subset of CD14highCD16negCCR2low/neg monocytes functionally similar to myeloid-derived suppressor cells during SIV and HIV infection. J Leukoc Biol. (2012) 91:803–16. 10.1189/jlb.1111579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin A, Cai W, Pan T, Wu K, Yang Q, Wang N, et al. Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol. (2013) 87:1477–90. 10.1128/JVI.01759-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garg A, Spector SA. HIV type 1 gp120-induced expansion of myeloid derived suppressor cells is dependent on interleukin 6 and suppresses immunity. J Infect Dis. (2014) 209:441–51. 10.1093/infdis/jit469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang L, Zhao J, Ren JP, Wu XY, Morrison ZD, El Gazzar M, et al. Expansion of myeloid-derived suppressor cells promotes differentiation of regulatory T cells in HIV-1+ individuals. AIDS. (2016) 30:1521–31. 10.1097/QAD.0000000000001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Meng Z, Du L, Hu N, Byrd D, Amet T, Desai M, et al. Antiretroviral therapy normalizes autoantibody profile of HIV patients by decreasing CD33+CD11b+HLA-DR+ cells: a cross-sectional study. Medicine. (2016) 95:e3285 10.1097/MD.0000000000003285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grützner EM, Hoffmann T, Wolf E, Gersbacher E, Neizert A, Stirner R, et al. Treatment intensification in HIV-infected patients is associated with reduced frequencies of regulatory T cells. Front Immunol. (2018) 9:811. 10.3389/fimmu.2018.00811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang T, Wen Y, Fan X. Myeloid-derived suppressor cells suppress CD4+ T cell activity and prevent the development of type 2 diabetes. Acta Biochim Biophys Sin. (2018) 50:362–9. 10.1093/abbs/gmy014 [DOI] [PubMed] [Google Scholar]
- 65.Restrepo BI. Metformin: candidate host-directed therapy for tuberculosis in diabetes and non-diabetes patients. Tuberculosis. (2016) 101:S69–72. 10.1016/j.tube.2016.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, et al. Metformin-induced reduction of CD39 and CD73 blocks myeloid-derived suppressor cell activity in patients with ovarian cancer. Cancer Res. (2018) 78:1779–91. 10.1158/0008-5472.CAN-17-2460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scrimini S, Pons J, Agustí A, Clemente A, Sallán M, Bauçà J, et al. Expansion of myeloid-derived suppressor cells in chronic obstructive pulmonary disease and lung cancer: potential link between inflammation and cancer. Cancer Immunol Immunother. (2015) 64:1261–70. 10.1007/s00262-015-1737-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scrimini S, Pons J, Agustí A, Soriano JB, Cosio BG, Torrecilla JA, et al. Differential effects of smoking and COPD upon circulating myeloid derived suppressor cells. Respir Med. (2013) 107:1895–903. 10.1016/j.rmed.2013.08.002 [DOI] [PubMed] [Google Scholar]
- 69.Clements VK, Long T, Long R, Figley C, Smith DMC, Ostrand-Rosenberg S. Frontline science: high fat diet and leptin promote tumor progression by inducing myeloid-derived suppressor cells. J Leukoc Biol. (2018) 103:395–407. 10.1002/JLB.4HI0517-210R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gonda K, Shibata M, Ohtake T, Matsumoto Y, Tachibana K, Abe N, et al. Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncol Lett. (2017) 14:1766–74. 10.3892/ol.2017.6305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.du Plessis N, Kotze LA, Leukes V, Walzl G. Translational potential of therapeutics targeting regulatory myeloid cells in tuberculosis. Front Cell Infect Microbiol. (2018) 8:332 10.3389/fcimb.2018.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gupta S, Cheung L, Pokkali S, Winglee K, Guo H, Murphy J, et al. Suppressor cell-depleting immunotherapy with Denileukin Diftitox is an effective host-directed therapy for tuberculosis. J Infect Dis. (2017) 215:1883–7. 10.1093/infdis/jix208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mourik B, Leenen P, de Knegt G, Huizinga R, van der Eerden B, Wang J, et al. Immunotherapy added to antibiotic treatment reduces relapse of disease in a mouse model of tuberculosis. Am J Respir Cell Mol Biol. (2017) 56:233–41. 10.1165/rcmb.2016-0185OC [DOI] [PubMed] [Google Scholar]
- 74.Shen L, Sundstedt A, Ciesielski M, Miles KM, Celander M, Adelaiye R, et al. Tasquinimod modulates suppressive myeloid cells and enhances cancer immunotherapies in murine models. Cancer Immunol Res. (2015) 3:136–48. 10.1158/2326-6066.CIR-14-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gupta S, Krug S, Pokkali S, Leanderson T, Isaacs JT, Srikrishna G, et al. Pharmacologic exhaustion of suppressor cells with tasquinimod enhances bacterial clearance during tuberculosis. Am J Respir Crit Care Med. (2018) 199:386–89. 10.1164/rccm.201805-0820LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.He D, Li H, Yusuf N, Elmets CA, Li J, Mountz JD, et al. IL-17 promotes tumor development through the induction of tumor promoting microenvironments at tumor sites and myeloid-derived suppressor cells. J Immunol. (2010) 184:2281–8. 10.4049/jimmunol.0902574 [DOI] [PMC free article] [PubMed] [Google Scholar]


