Abstract
Cancer immunotherapy and the emergence of immune checkpoint inhibitors have markedly changed the treatment paradigm for many cancers. They function to disrupt cancer cell evasion of the immune response and activate sustained anti-tumor immunity. Oncolytic viruses have also emerged as an additional therapeutic agent for cancer treatment. These viruses are designed to target and kill tumor cells while leaving the normal cells unharmed. As part of this process, oncolytic virus infection stimulates anti-cancer immune responses that augment the efficacy of checkpoint inhibition. These viruses have the capability of transforming a “cold” tumor microenvironment with few immune effector cells into a “hot” environment with increased immune cell and cytokine infiltration. For this reason, there are multiple ongoing clinical trials that combine oncolytic virotherapy and immune checkpoint inhibitors. This review will detail the key oncolytic viruses in preclinical and clinical studies and highlight the results of their testing with checkpoint inhibitors.
Keywords: oncolytic virus, immunotherapy, immune checkpoint inhibitor
Main Text
As the worldwide cancer incidence continues to rise,1 the need for novel treatment strategies has become increasingly important. Targeting cancers at the molecular level is an attractive option, as demonstrated by the recent successes of systemically delivered immunotherapeutics (Figure 1). The field of immunotherapy seeks to develop treatments that effectively augment the body’s own immune response to cancer in an effort to achieve local and systemic anti-tumor immunity. Unfortunately, certain cancers (e.g., pancreatic cancer) have a unique tumor microenvironment that has a relative paucity of circulating immune effector cells.2 This scenario creates a “cold” tumor microenvironment and results in the tumor’s being less responsive to immunotherapies. Conversely, “hot” tumor microenvironments are known to be immunogenic and have a much higher response rate to immunotherapy.3 Therefore, strategies to transform cold tumor microenvironments to hot ones are especially attractive, as they will help to increase the effectiveness of immune checkpoint inhibitors (ICIs).4 OVs are able to target and kill cancer cells while minimizing toxicities to surrounding normal tissues.5 After infection with these viruses, the local tumor microenvironment is altered with an increase in activated T cells, natural killer (NK) cells, and cytokines.6 This review will explore the combination of ICIs with OVs for cancer therapy and will highlight key preclinical data, along with notable clinical trials.
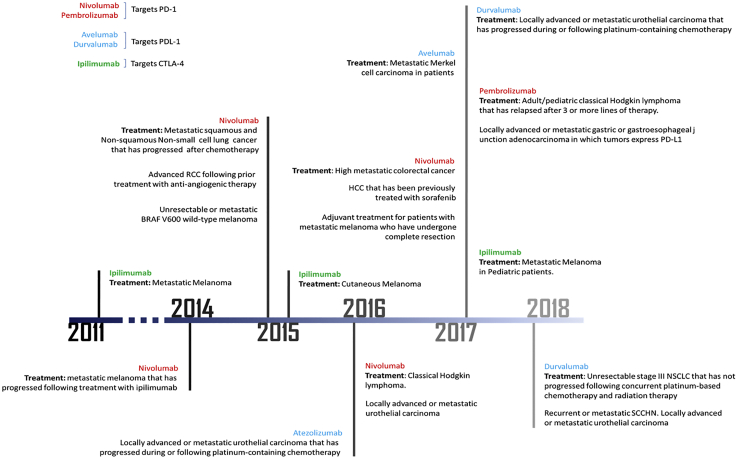
Figure 1.
Timeline of FDA Approval of Immune Checkpoint Inhibitors
ICIs
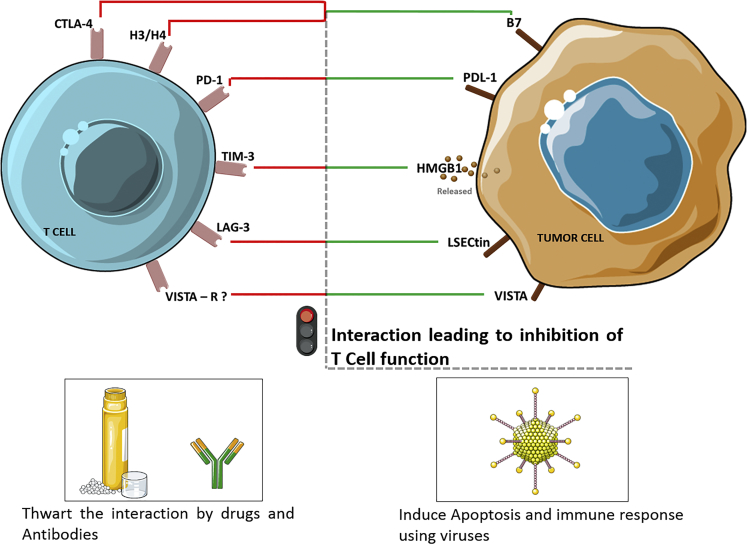
Immune checkpoints are inhibitory pathways in the immune system that modulate the amplitude and duration of immune responses. In some instances, tumors manipulate these immune-checkpoint pathways, resulting in a resistance to the body’s native immune system. ICIs work by disrupting the cancer cells’ signals, thereby exposing the tumors’ T lymphocytes to attack (Figure 2). T lymphocytes have been the major focus of efforts to therapeutically manipulate endogenous antitumor immunity because of their functions in (1) selective peptide recognition, (2) direct cytotoxicity to certain antigen-expressing cells (by CD8+ effector T cells), and (3) their ability to orchestrate diverse immune responses (by CD4+ helper T cells), which involves both adaptive and innate effector mechanisms. T cell-mediated immunity includes multiple sequential steps involving the clonal selection of antigen-specific cells, their activation and proliferation in lymphoid tissues, their translocation (trafficking) to sites where the antigen is presented, the execution of direct effector function, and the provision of help (through cytokines and membrane ligands) for a multitude of effector immune cells. Each of these steps is regulated by counterbalancing stimulatory and inhibitory signals that fine-tune the immune response. Specificity is conferred to the response via antigen-independent second signals that modify the initial signal, which was provided by the interaction of antigenic peptides with T cell receptors.7 The inhibitory signals in the immune response are triggered through membrane receptors, such as those for B7, programmed death receptor ligand-1 (PD-L1), and high-mobility group protein box1 (HMGB-1), and are overexpressed on tumor cells, and hence the interactions of these inhibitory receptor with their ligands (both membrane bound and soluble), such as cytotoxic T lymphocyte-associated antigen-4-B7 (CTLA4-B7), programmed death receptor-1 (PD-1)-PD-L1, and T cell immunoglobulin and mucin domain 3 (TIM3)-HMGB1, limit T cell activation (Figure 3).8, 9, 10, 11 These aforementioned interactions are the targets for currently used ICIs and are explained in more detail in the following sections.
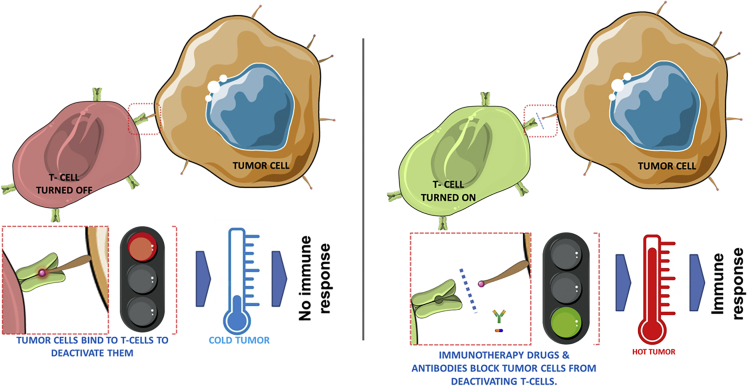
Figure 2.
Immune Checkpoints and the Immune Responsiveness of a Tumor Microenvironment
(Left) Immune checkpoints are triggered by ligand-receptor interactions (red box) wherein T cells are turned OFF making the tumor cold (escape immune response). (Right) Immune checkpoint inhibitors like antibodies, drugs, and recombinant forms of the ligands or the receptors block this ligand–receptor interaction allowing the T cell to turn ON (triggering immune response) and mount an immune response; hence, the cold tumor becomes hot.
Figure 3.
Key Receptor-Ligand Interactions That Turn OFF the T Cell
Oncolytic viruses induce immune responses that, upon infecting tumor cells, induce apoptosis or express the transgenes that, when presented or released by tumor cells, attract immune cells. The transgenes could be (1) key epitopes that attract immune cells, (2) immune-stimulatory blockers of immune checkpoints, or (3) key genes from non-human species that have anti-tumor effects.
CTLA-4
CTLA-4, also known as CD152, is an inhibitory molecule on the surface of activated T cells12 that inhibits the binding of B7 to CD28.13 Its mechanism functions to halt the initial stage of naive T cell activation in the lymph nodes and results in decreased T cell responses.14 Anti-CTLA-4 antibodies are designed to block CTLA-4 binding and prevent the inhibition of T cell function. A notable example is ipilimumab, which was approved by the U.S. Food and Drug Administration (FDA) in 2010 for the treatment of advanced melanoma.8
PD-1
PD-1 has emerged as a promising target that is capable of inducing antitumor immune responses. In contrast to CTLA-4, it is more broadly expressed and functions to limit the activity of T cells in peripheral tissues at the time of an inflammatory response to minimize potential autoimmunity.15, 16, 17 PD-1 is highly expressed on regulatory T (Treg) cells;18 therefore, blockade of the PD-1 pathway may enhance antitumor immune responses by diminishing the number and/or suppressive activity of intratumoral Treg cells.19 PD-L1 and -L2 are the main ligands for PD-1. High expression levels of PD-L1 are reported in most melanoma, ovarian, and lung cancer samples.9 Additionally, PD-L1 is commonly expressed on myeloid cells in the tumor microenvironment,20 suggesting that PD-L1 is adaptively induced as a consequence of immune responses within the tumor microenvironment. Multiple anti-PD-1 and anti-PD-L1 agents have been developed in recent years. For instance, pembrolizumab was the first PD-1 inhibitor approved by the FDA in 2014 for the treatment of melanoma.21 It was later approved in combination with nivolumab for the treatment of metastatic non-small-cell lung cancer and head and neck squamous cell carcinoma.22 Also, atezolizumab is a fully humanized IgG1 antibody against PD-L1 that was FDA approved in 2016 for the treatment of urothelial carcinoma and non-small-cell lung cancer. Furthermore, avelumab and durvalumab are fully humanized IgG1 antibodies that are FDA approved to treat Merkel cell carcinoma, urothelial carcinoma, and non-small-cell lung cancer (H.C. Chung et al., 2016, Am. Soc. Clin. Oncol., abstract).23
Other Immune Checkpoint Targets
Lymphocyte activation gene-3 (Lag-3) is another immune checkpoint receptor, and it is upregulated on activated CD4+/CD8+ T cells and NK cells and has a high affinity for major histocompatibility complex (MHC) class II molecules.24 Clinical trials are ongoing wherein the antibody against LAG-3 is being tested as a monotherapy or in combination with other checkpoint inhibitors.25 For instance, a soluble LAG-3Ig fusion protein was tested in patients with advanced renal cell carcinoma and was well tolerated and led to stabilization of disease in those who received higher doses of the drug in a dose-escalation study.26
TIM-3, is a member of the TIM gene family that plays a critical role in regulating immune response and is expressed on Th1 (T helper), Th17, and CD8+ T cells (Figure 3). Interactions between TIM-3 and its ligands result in suppression of Th1 and Th17 responses and induce immune tolerance, supporting an inhibitory role of TIM-3 in T cell-mediated immune responses.10 Four relevant ligands have been shown to interact with TIM-3 including galectin-9 (Gal-9), HMGB1, carcinoembryonic antigen-related cell adhesion molecule 1 (CEACAM 1), and phosphatidylserine (PS).24 In cancer patients, administration of TIM-3 antibodies increases proliferation and cytokine production by tumor-antigen-specific T cells.27 Preclinical studies with TIM-3 show that it is expressed along with PD-1 on tumor-infiltrating lymphocytes, and combination therapy targeting these two domains may augment anti-tumor responses.28
OVs
OVs are native or recombinant viruses that target cancer cells. The viruses cause the cancerous cells to die at the end of its replication cycles through lysis or by the activation of an antitumor immune response, thus minimizing damage to normal tissues (Figure 4).29, 30
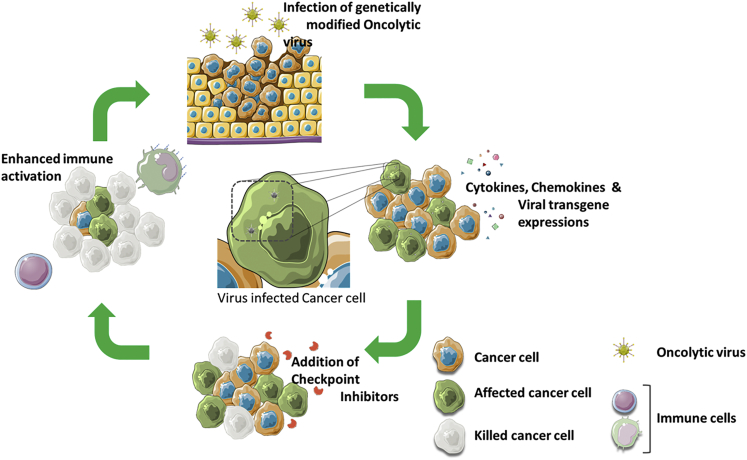
Figure 4.
Oncolytic Viruses Stimulate the Tumor Microenvironment and Synergize with ICI
Schematic representation of different stages of tumor suppression using combination therapy involving oncolytic viruses (OVs) and immune checkpoint inhibitors. Initially, tumor cells are infected with OVs resulting in the release of cytokines, chemokines, and viral transgene which triggers immune responses. A further treatment of those infected cells with immune checkpoint inhibitors mount additional immune responses, resulting in the killing of most cancer cells.
Historically, virologists have been concerned that the immune system may hamper the success of oncolytic virotherapy because of immune-mediated viral clearance, and toxicities have been noted in immune-compromised patients receiving non-engineered viruses.31, 32, 33 Although immune clearance of virus is still a concern, OVs are now recognized as efficient immune-stimulatory agents capable of activating and redirecting innate and adaptive immune responses against the tumor (Figure 5).34, 35 It is these interactions between immune cells and signaling factors (i.e., cytokines and chemokines) that are critical to the induction of antitumor immunity and thus successful immunotherapy. Recent understanding of the mechanism of OV infection and of the resulting changes in the surrounding tumor immune microenvironment further characterizes this unique class of cancer therapeutics and underscores the importance of the field of oncolytic immunotherapy.36
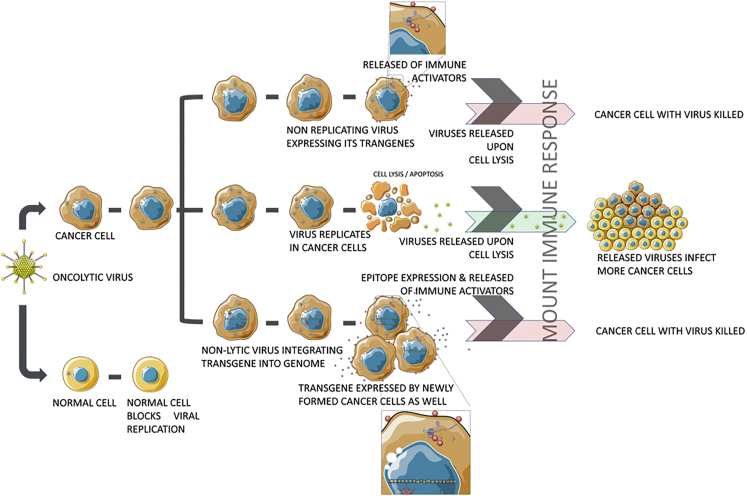
Figure 5.
Oncolytic Viral Means of Immune Stimulation
Oncolytic viruses with different properties have a different mode of action on tumor cells. (1) Non-replicating OVs bearing engineered transgenes allow the infected tumor cells to express those transgenes that trigger an immune response. (2) Replicating OVs lyses the infected tumor cells after infection; the released virus infects the neighboring tumor cells, making the treatment effective. (3) Non-lytic viruses engineered with transgenes integrate the transgene into the tumor cell genome upon infection; the infected cells then express the transgenes and mount an immune response. Because of genome integration, progeny tumor cells are also mitigated, as the integrated transgene is transferred from parental cells to progeny cells.
The activity of an OV is a reflection of the biology of the virus from which it was derived and its host-virus interactions. Typically, OVs fall into two classes. The first class includes naturally replicating viruses that replicate only in cancer cells and are non-pathogenic in humans because of their sensitivity to innate antiviral immunity. These include the parvoviruses, myxoma virus (MYXV), Reovirus, Newcastle disease virus (NDV), and Seneca Valley virus (SVV). The second class includes those that are genetically manipulated for use as vaccines or vectors, such as poliovirus (PV), measles virus (MV), vaccinia virus (VACV), adenovirus, herpes simplex virus (HSV), and vesicular stomatitis virus (VSV).5, 37 These viruses target the tumor cells directly or indirectly, replicate and express proteins that are deemed cytotoxic to the cell’s survival,38 and/or induce anti-tumor response upon expressing key tumor epitopes.39 The specific targeting mechanism is different for different OVs and is based on the viral engineering performed,40, 41 and replication mechanisms can be controlled by using gene promoters that are activated only in tumor cells. 42
OVs in Combination with ICIs
ICIs have helped revolutionize cancer treatment, but oftentimes, even the best response rates to these agents do not exceed 35% to 40%.43, 44, 45, 46 The goal of combining OVs and checkpoint inhibitors is to use the viral infection to prime the tumor by altering the local immune microenvironment to one that is more immunogenic, with the understanding that ICI’s work best in these hot environments.43, 44 Because of the preclinical success of this type of combination therapy, there are multiple ongoing clinical trials that combine OVs and checkpoint inhibitors (Table 1).47, 48, 49
Table 1.
OVs in Combination with Immune Checkpoint Inhibitors That Are in Preclinical and Clinical Testing
| Pre-clinical Works |
Clinical Trials |
|||||||
|---|---|---|---|---|---|---|---|---|
| Virus | DNA/ RNA Virus | Viral Engineering | Immune Checkpoint Inhibitor | Disease Treated | Viral Engineering | Immune Checkpoint Inhibitor | Disease Treated | Reference |
| Measles virus | –ssRNA | for expression of anti-PDL1 and anti-CTLA4 | anti- PDL1 and anti-CTLA4 | malignant melanoma | – | – | – | 118 |
| Adenovirus | dsDNA | for expression of TNFα or IL-2 | anti- PD-1 | melanoma | DNX-2401 (express modified E1A gene) and ONCOS-102 (express modified GM-CSF, E1A gene and fiber knob region) | pembrolizumab (anti-PD1) | glioblastoma and advanced melanoma | 74, 76 ClinicalTrials.gov: NCT02798406,81 and NCT03003676 |
| Herpes simplex virus | dsDNA | for expression of GM-CSF and IL-12 | anti- PD-L1 and anti-CTLA4 | glioblastoma multiforme | T-VEC (express GM-CSF gene) | ipilimumab (anti-CTLA4) and pembrolizumab (anti-PD1) | advanced melanoma | 47, 63, 119, 120 |
| Vaccinia virus | dsDNA | for expression of CXCL-11 | anti-PD-L1 | colon and ovarian cancer | JX-594 phase 3 clinical trial | PDL1 and CTLA-1 | renal carcinoma |
68, 69, 70 H. Chon and C. Kim, 2018, Am. Soc. Cancer Res., abstract |
| WR.B18R-.TK | anti-CTLA4 | renal adenocarcinoma | – | – | – | – | ||
| VVWR/TK−RR−/FCU1 | anti-PD-1 and anti-CTLA-4 | sarcoma | – | – | – | – | ||
| Maraba virus | −ssRNA | MG1 strain (native) | anti-PD-L1 and anti-CTLA4 | breast cancer | MG1-MAGEA3 (express the melanoma-associated antigen A3 [MAGE-A3]) | pembrolizumab (anti-PD1) | non-small cell lung cancer, metastatic melanoma, and cutaneous squamous cell carcinoma | 92 ClinicalTrials.gov: NCT02879760 and NCT03773744 |
| Reovirus | dsRNA | no engineering | anti-PD1 | melanoma | Reolysin | pembrolizumab (anti-PD1) | – | 83, 85 ClinicalTrials.gov: NCT02620423 |
| Coxsackievirus | +ssRNA | Coxsackievirus A21 (native) | anti- PD1 and anti-CTLA4 | melanoma | Coxsackievirus A21 | pembrolizumab(anti-PD1) and ipilimumab(anti-CTLA4) | advanced melanoma and bladder cancer | H. Pandha et al., 2017, Am Soc Cancer Res., abstract; B. Curti et al., 2017, M. Yuan Quah et al., 2016, Am Soc Cancer Res., abstract |
| Vescicular somatitis virus | −ssRNA | for expression of IFNβ and NIS | anti-PD-L1 | glioma | VSV-hIFNβ-NIS | avelumab (anti-PD-L1) and pembrolizumab (anti-PD1) | refractory metastatic solid tumors and refractory non-small cell lung cancer or hepatocellular carcinoma | 108 ClinicalTrials.gov: NCT02923466, NCT03647163 |
| HIF-2α, Sox10, and cMyc | anti-PD-1 and anti-CTLA-4 | myeloid leukemia | – | – | – | 110 | ||
| Myxoma virus | dsDNA | for expression of the soluble form of PD1 | – | melanoma | – | – | – | 113 |
| Newcastle disease virus | −ssRNA | for expression of influenza NS1 | anti-CTLA4 | melanoma, adenocarcinoma, and colon carcinoma | – | – | – | 43 |
| Semliki Forest virus | +ssRNA | for expression of IL-12 | anti-PD1 and anti- PDL1 | melanoma and colon cancer | – | – | – | 117 |
Clinical Testing
HSV
HSV-1 is the member of the Herpesviridae family that has been most extensively tested as a backbone for oncolytic vectors.50 They are large icosahedral viruses that have a double-stranded, linear-DNA genome of approximately 160 kb.6 Cancer gene therapy mediated by HSV-1 vectors has largely focused on the delivery of suicide genes—namely, the thymidine kinase (tk) gene. However, other cancer therapeutic genes have been delivered using replication-defective HSV-1 vectors and include: p53,51 tissue inhibitor of metalloproteinases-2 (TIMP-2),52 interleukin-2 (IL-2),53, 54 IL-12,55 IFN-γ,56 and granulocyte-macrophage colony-stimulating factor (GM-CSF).53, 57
T-VEC
Talimogene laherparepvec (T-VEC) is a genetically modified HSV that was the first FDA-approved oncolytic vector based on the results of the OPTiM (Oncovex [GM-CSF] Pivotal Trial in Melanoma) trial.58 The virus included key deletions in the genome to maximize tumor-specific replication while minimizing neurovirulence.59, 60 In addition, it encoded the transgene for GM-CSF. In the trial, GM-CSF was compared to intralesional T-VEC in patients with advanced, non-resectable melanoma, and T-VEC demonstrated a significant improvement in durable overall response rate (16.2% versus 2.1%; p < 0.001).58 Also, the injection of T-VEC into metastatic melanoma lesions decreased the presence of myeloid-derived suppressor cells, CD4+ Tregs, and other suppressor cell populations.61 These alterations in the immune microenvironment set the stage for the abscopal responses seen with T-VEC administration. In the OPTiM trial, there was a ≥50% reduction in volume of 15% of the non-injected visceral lesions.58 Overall, the vector demonstrated a tolerable safety profile, which consisted of mild constitutional symptoms and local injection site reactions.62
With the initial success of T-VEC as a monotherapy, it was then used in combination with ICIs. Ipilimumab (an anti-CTLA-4 antibody) was used with T-VEC in a phase Ib trial that enrolled 19 patients with previously untreated stages IIIB to IV melanoma. The trial results were significant for an objective response rate of 50%, and there were no dose-limiting toxicities.47 The subsequent phase II portion of the trial randomized 198 patients to receive either T-VEC plus ipilimumab or ipilimumab alone. The objective response rate of the combination therapy arm (39%) was significantly higher than that of the monotherapy arm (18%; p = 0.002). Importantly, 52% of patients demonstrated abscopal responses in distant, noninjected visceral lesions.63
T-VEC has also been combined with an anti-PD-1 antibody with encouraging results. In the phase 1b portion of the Masterkey-265 trial, 21 patients with stage IIB and IV melanoma were treated with T-VEC in combination with intravenous pembrolizumab.64 The side-effect profile was favorable, and there were no dose-limiting toxicities.64 Additionally, the overall objective response rate was 62%, whereas 33% of patients had a complete response.64 The combination therapy had elevated PD-L1 protein expression and increased CD8+ T cells on several tumor cell subsets after T-VEC treatment, suggesting that oncolytic virotherapy may improve the efficacy of anti-PD-1 therapy by altering the tumor microenvironment.
Vaccinia Virus
These vectors are members of the Poxviridae family and are large, enveloped viruses that contain a double-stranded-DNA genome.65 The advantages of using vaccinia virus for cancer therapy include intravenous stability and delivery, enhanced potency, extensive safety history as a live vaccine, demonstrated ability to induce efficient immune responses, and a large transgene-encoding capacity.66
One research group modified a Western Reserve oncolytic vaccinia virus by inserting three different forms of a murine PD-1 binder into the virus and tested it in multiple in vitro and in vivo models. Importantly, intratumoral injection with the vaccinia virus armed with the whole antibody to murine-PD-1 (WR-mAb) demonstrated longer-lasting and higher levels of anti-PD-1 antibody expression when compared to an intratumoral injection of the antibody itself.67 Notably, this increased expression did translate into an improved survival for the mice in the WR-mAb treatment arm when tested in a subcutaneous fibrosarcoma model.
Liu et al.68 demonstrated that, after infection with their oncolytic vaccinia virus that had deletions in the tk and the vgf (virus growth factor) genes (vvDD) in colon and ovarian cancer mouse models, there was a significantly increased level of PD-L1 expression compared with that in animals treated with PBS injection. They went on to show synergistic effects with the combination of a modified oncolytic vaccinia virus (expressing CXCL11) and an anti-PD-L1 antibody in colon and ovarian peritoneal carcinomatosis models, which resulted in statistically significant survival advantages when compared to either monotherapy treatment.
Rojas et al.69 identified the importance of timing of the delivery of immune checkpoint inhibition with respect to the inoculation with an oncolytic vaccinia virus WR.B18R−.TK− (deletion in the tk and the B18R viral genes). When the virus and an anti-CTLA4 antibody were simultaneously delivered in their murine model bearing renal adenocarcinoma, there was no significant antitumor benefit. However, when the checkpoint inhibitor was delivered starting 4 days after the viral injection (to allow for maximum viral replication) there was a survival benefit, as well as a significant reduction in tumor volume, compared with those mice who received virus monotherapy (p < 0.04).69
Other researchers have used an engineered Western Reserve strain of oncolytic vaccinia virus that has deletions of the tk and ribonucleotide reductase genes, with Fcu1 inserted at the tk locus (VVWR/TK−RR−/FCU1) to demonstrate immune-mediated effects on distant lesions. In murine sarcoma models, they demonstrated abscopal responses following a single injection of virus and also showed that these immune-mediated effects were predominantly driven by CD8+ T cells.70 The virus was also tested in combination with both anti-PD-1 and anti-CTLA-4 antibodies. When compared to virus or ICI monotherapy treatments in in vivo mouse models, combination therapy resulted in significant survival advantages.70 CTLA-4 blockade worked best shortly after viral treatment, whereas PD-1 blockade worked better when delivered later (7 days) after viral treatment.70
Pexastimogene Devacirepvec
Pexastimogene devacirepvec (Pexa-Vec; also known as JX-594) is a Wyeth strain oncolytic vaccinia virus with disruption of the viral thymidine kinase gene (tk) and insertion of human GM-CSF and β-galactosidase transgenes to induce both virus replication–dependent oncolysis and tumor-specific immunity.71 Pexa-Vec, in combination with ICIs, is currently undergoing testing in multiple clinical trials. For example, in a phase III clinical trial enrolling patients with renal cell carcinoma, treatment with the virus resulted in a 16-fold increase in intratumoral CD8+ T cells, along with increased expression of PD-1, CTLA-4, and LAG-3 (H. Chong and C. Kim, 2018, Am. Assoc. Cancer Res., abstract).
Adenovirus
Adenoviruses are double-stranded-DNA viruses of the Adenoviridae family that lack a viral envelope and have an icosahedral capsid structure.72 They are approximately 70–90 nm in size and contain a 35 kb genome.6 There are 57 serotypes of adenovirus, but serotype 5 from group C has been the most commonly used backbone for oncolytic vectors.73
Multiple adenovirus constructs are undergoing preclinical testing in combination with ICIs, and some of the promising candidates include those recently reported by the Hemminki group.74 In this study, they described two adenoviruses: one that expresses tumor necrosis factor alpha (TNFα) and another that expresses IL-2. In their in vivo experiments using melanoma tumors established in the flanks of mice, they demonstrated a marked increase in intratumoral CD8+ T cells when viral therapy was combined with the delivery of an anti-PD-1 antibody (compared to virus alone). Furthermore, combination therapy with the anti-PD-1 antibody and viral therapy resulted in statistically significant tumor growth suppression and increased survival when compared to virus monotherapy. A clinical trial employing an oncolytic adenovirus encoding TNF-α and IL-2 (TILT-123) in combination with an anti-PD-1 antibody is forthcoming.
Tasadenoturev
Tasadenoturev (DNX-2401) is a replication-competent oncolytic adenovirus that has been modified with a 24 bp deletion in the E1A region of the genome, which allows for viral replication in cancer cells that lack a functional Rb (retinoblastoma) pathway, but not in normal cells.75 This viral vector was tested in a phase I trial in 37 patients with malignant glioma. A portion of these patients (n = 25) had intratumoral injections at various viral titers to evaluate dosing and response, whereas the others (n = 12) had intratumoral injections via an implanted catheter, followed by surgical resection. In the first group, 72% of patients had a reduction in tumor size and 20% of patients survived more than 3 years after their initial treatment.76 In the second group of patients, immunohistochemical analysis of resected specimens demonstrated decreases in the expression of TIM-3, but not PD-1 or PD-L1.76 DNX-2401 is now undergoing testing in a phase II study combining the virus and pembrolizumab for those glioblastoma patients progressing on initial therapy (CAPTIVE [Combination Adenovirus+Pembrolizumab to Trigger Immune Virus Effects] trial, ClinicalTrials.gov: NCT02798406).
ONCOS-102
Another adenovirus that is currently undergoing testing in clinical trials as part of combination therapy with ICIs is ONCOS-102 (AdV5/3-Δ24-GM-CSF). It is based upon serotype 5, and its key modifications include GM-CSF expression, a 24 bp deletion in the E1A region (conferring replication selectivity to Rb-pathway-deficient cells), and a chimeric fiber knob region.77 In a recent phase I trial in 12 patients with refractory solid tumors, viral administration resulted in large immune cell infiltration into the tumors. When comparing pre- and post-treatment biopsies, ONCOS-102 resulted in a 4-fold increase in CD8+ T cells and an almost 6-fold increase in the expression of CD3.78 Furthermore, there was a subset of mesothelioma patients who demonstrated an increase in tumor PD-L1 expression following injection with the virus.78 These observations have led to interest in combining ONCOS-102 with pembrolizumab, and there is a clinical trial that is ongoing that uses this combination for patients with advanced melanoma (ClinicalTrials.gov: NCT03003676).
Reovirus
They are double-stranded-RNA viruses that belong to the Reoviridae family and are 75–85 nm in size with an icosahedral capsid.79 Reoviruses have direct oncolytic activity against a number of tumor types secondary to viral RNA production that activates the PKR (protein kinase R) pathway, which is inhibited in many Ras-transformed cells.6, 80
Reolysin
Reolysin (pelareorep) is an isolate of the human reovirus type 3 strain and functions as a live, replication-competent vector.81 In addition to performing direct oncolysis, Reolysin can induce dendritic cell maturation as well as NK cell recruitment and activation.82 The combination of an anti-PD-1 antibody with an intratumorally delivered reovirus dramatically improved survival compared to untreated and monotherapy groups in a murine melanoma model.83 Combination therapy enhanced NK killing of virus-infected cells and reduced immune suppression mediated by Foxp3+ Treg cells.83 A series of studies using depletion antibodies clearly demonstrated that NK and CD8+ T cells (but not CD4+ T cells) were responsible for the antitumor efficacy.83, 84 Furthermore, Ilett et al.85 have demonstrated how intratumoral doses of reovirus into subcutaneous melanoma tumors can prime the microenvironment via a Th1 response. Their data also demonstrated that an anti-PD-1 antibody in combination with a systemically delivered reovirus demonstrated significantly improved survival in mouse models compared to control and monotherapy groups and that this enhancement is clearly dependent upon CD8+ T cells.85
In a phase Ib study that enrolled nine patients (primary high-grade glioma and brain metastasis), each individual was given an intravenous infusion of reovirus prior to planned surgical resection. Using immunohistochemistry and transmission electron microscopy, reovirus was detected in the resected specimens of all nine patients.86 Additionally, the resected specimens that were previously inoculated with reovirus demonstrated a marked increase in tumor-infiltrating cytotoxic T cells (CD8+) and elevated levels of PD-L1 expression when compared to untreated controls.86
Reolysin (in combination with the single agent gemcitabine) has been tested in a phase II study of 34 chemotherapy-naive patients with advanced pancreatic cancer and demonstrated a median survival of 10.2 months, a 1-year survival of 45%, and upregulated PD-L1 expression.87 Based upon this data, an additional clinical trial (ClinicalTrials.gov: NCT02620423) is under way investigating the combination of pembrolizumab, chemotherapy, and reovirus administration in patients with metastatic pancreatic adenocarcinoma.
Coxsackievirus
Coxsackieviruses belong to the Picornaviridae family and are non-enveloped viruses with a single-stranded-RNA genome.88 They have an icosahedral capsid and are approximately 30 nm in size.89 Coxsackievirus A21 (CVA21, Cavatak) targets intercellular adhesion molecule-1 (ICAM-1), which is upregulated in a number of cancers, including melanoma, non-small-cell lung, bladder, and prostate cancers.90 Intravenous administration of CVA21 in mouse models of melanoma facilitated immune activation within the tumor as evidenced by gene expression increases of CXCL-10 and PD-L1 (H. Pandha et al., 2015, Am. Soc. Cancer Res., abstract). Furthermore, intravenous delivery of CVA21 in combination with immune checkpoint blockade (anti-PD-1 or anti-CTLA-4) in an immune-competent mouse melanoma model mediated significantly greater antitumor activity and survival benefits when compared to either agent alone (M. Yuan Quah et al., 2016, Am. Soc. Cancer. Res., abstract). In the phase I/II STORM (Selinexor Treatment of Refractory Myeloma) study, patients with advanced cancers received multiple doses of intravenous Cavatak. In particular, the therapy was well tolerated with no grade 3 or 4 adverse treatment-related events (H. Pandha et al., 2015, Am. Soc. Cancer Res., abstract). In an extension study to the CALM (CAVATAK in Late-Stage Melanoma) trial, melanoma patients who received intralesional viral injections demonstrated increased immune cell infiltrates and checkpoint molecule expression (R. Andtbacka et al. 2016, Am Soc. Cancer Res., abstract). Consequently, there have been multiple ongoing trials with Cavatak and ICIs (H. Pandha et al., 2017, Am. Soc. Cancer Res., abstract; B. Curti, et al., 2017, Am. Soc. Cancer Res., abstract).
Maraba Virus
Maraba virus is a member of the Rhabdoviridae family and contains a single-stranded-RNA genome.91 A recent report highlights the utility of an oncolytic Maraba virus in multiple breast cancer models, including a triple-negative-patient-derived xenograft model. The results demonstrated that Maraba virus in combination with immune checkpoint inhibition significantly slowed tumor growth when compared to either monotherapy.92 Additionally, the Lichty research group93 developed an MG1 strain oncolytic Maraba virus modified to express the melanoma-associated antigen A3 (MAGE-A3). In conjunction with a replication-deficient adenovirus expressing the same MAGE-A3 antigen (Ad-MAGEA3), they extensively tested this modified MG1 vector in a prime-boost approach in preclinical models and showed a great expansion in the population of MAGE-A3 antigen-specific CD4+ and CD8+ T cells.93 Currently, the Maraba MG1-MAGEA3 virus (along with Ad-MAGEA3) is being tested in clinical trials with pembrolizumab in patients with non-small-cell lung cancer (ClinicalTrials.gov: NCT02879760) as well as those with metastatic melanoma or cutaneous squamous cell carcinoma (ClinicalTrials.gov: NCT03773744).
VSV
VSV is an enveloped virus of size 70 × 200 nm belonging to the Rhabdoviridae family containing an 11 kb single-stranded-RNA of negative sense.94 VSV in particular is an attractive vector because of (1) its effectiveness against a variety of tumor models,95, 96, 97, 98, 99, 100, 101, 102 including leukemia103 and lymphoma,104 and (2) its sensitivity to type I IFNs, an innate immune mechanism found in normal cells but not in tumor cells, thus making the virus tumor selective and able to boost CD8 T cell responses.105, 106 In clinical trials, recombinant VSV expressing IFNβ has been tested against refractory multiple myeloma, acute myeloid leukemia, T cell lymphoma (ClinicalTrials.gov: NCT03017820) and refractory hepatocellular carcinoma (ClinicalTrials.gov: NCT01628640).107 One vector currently in phase II testing (VSV-hIFNβ-NIS) contains a sodium-iodine symporter engineered in the virus, which facilitates uptake of radioisotopes and non-invasive visualization of viral replication and spread in treated patients (J. Merchan et al., 2018, Eur. Soc. Med. Oncol., abstract). In terms of checkpoint inhibitor combination therapy, many preclinical studies have shown successful combinations with VSV-based vectors. For instance, preclinical studies have shown promise combining anti-PD-L1 antibody with VSV-hIFNβ-NIS which greatly extended survival in a murine acute myeloid leukemia model.108 Moreover, VSV-TAA (engineered VSV expressing tumor-associated antigens [TAAs]—namely, HIF-2α, Sox10, and cMyc) is effective against glioma in mice and showed further extension in survival when combined with anti-PD-1 and anti-CTLA-4.109, 110 Clinical trials are currently ongoing using VSV-hIFNβ-NIS in combination with avelumab in patients with refractory metastatic solid tumors (ClinicalTrials.gov: NCT02923466) or with pembrolizumab for those with refractory non-small-cell lung cancer or hepatocellular carcinoma (ClinicalTrials.gov: NCT03647163).
Select Vector Systems in Preclinical Testing with ICIs
Myxoma Virus
Myxoma viruses belong to the Poxviridae family and are enveloped, double-stranded-DNA viruses with approximately 160 kb.111 The nonpathogenic nature (apart from rabbit host), capacity for genetic modification, ability to produce a long-lived infection in human tumor cells, and the lack of pre-existing antibodies in the human population make myxoma virus an attractive oncolytic agent against human cancer.112 Bartee et al.113 reported a novel recombinant myxoma virus (vPD1) that was designed to secrete a soluble form of PD-1 from host cells following viral infection and replication. The secretory PD-1 inhibition mostly accumulated in tissues at the tumor site, and this high level of expression continued for well after 48 h after viral infection. When tested in a B16/F10 melanoma mouse model, treatment with the vPD1 virus resulted in tumor eradication in 59% of the mice compared to the vGFP+anti-PD1 group that had only a 30% rate of complete response.
Newcastle Disease Virus
This is a large (150–400 nm), single-stranded-RNA virus that is a member of the Paramyxoviridae family.114 Infection with a Newcastle disease virus activates the innate immune system and also causes cancer cell apoptosis, which serves to facilitate the conversion from an immune-suppressive tumor microenvironment into an inflammatory environment with immune effectors capable of anti-tumor effects.6 Zamarin et al.43 used a Newcastle disease virus in multiple melanoma mouse models to demonstrate that although viral replication is restricted to the injected tumor site, there was an increased lymphocytic infiltration innate (e.g., NK cells, myeloid cells) and adaptive (e.g., CD8+ and CD4+FoxP3− T cells) in both local and distant tumor sites. When the virus was combined with an anti-CTLA-4 antibody, the combination resulted in long-term survival of mice, elicited inflammatory recruitment of CD8+ T cells, and demonstrated that virus and ICI combination therapy causes a robust memory response and provides better protection against tumor cell re-challenge.43
Semliki Forest Virus
Semliki Forest virus (Togaviridae family) is a single-stranded-RNA virus with a viral genome that is approximately 11.5 kb.115 There have been multiple preclinical studies with Semliki Forest viruses in combination with checkpoint inhibition that have shown an improvement in antitumor efficacy with such regimens.116 For instance, a Semliki Forest virus expressing murine IL-12 was used to treat syngeneic melanoma and in colon cancer models and demonstrated synergistic effects with both anti-PD-1 and anti-PD-L1 antibodies.117 The results demonstrated significantly prolonged survival, and the vast majority of mice (>80%) that received combination therapy with the virus and checkpoint inhibitor remained tumor free at the end of the experiments.
Conclusions
In recent years, the use of immunotherapies has altered the landscape of cancer treatment. Checkpoint inhibitors have markedly changed the standard of care for many patients with solid tumors, especially those with melanoma. Additionally, oncolytic virotherapy continues to develop, and there is now a much clearer understanding of their mechanisms of action. In the past two decades, genetic engineering has helped the rapid expansion of OVs, enabling even potentially pathogenic viruses to be manipulated for cancer therapies.37 Not only do they lyse cells as part of viral replication, but they can induce changes into the tumor’s local immune microenvironment. These changes serve to increase immune effector cells locally at the tumor site and sensitize those tumors to subsequent treatment with checkpoint inhibitor therapy. Along with oncolytic vectors, the administration of checkpoint inhibitors (either systemically or by viral transgene expression) has demonstrated great success in multiple preclinical models. As a result, there are now multiple ongoing trials that combine checkpoint inhibition and oncolytic virotherapy. The results of these studies are highly anticipated, as they will provide insight into optimal virus structure and transgene payload, timing and method of antibody delivery, and potential side effects of these combination therapies. These novel combination regimens have the potential to have a dramatic impact in the coming years and represent a step forward in the field of immunotherapy.
Conflicts of Interest
The authors declare no competing interests.
Acknowledgments
This work was supported by Susan E. Riley Foundation Grant 2001148, American Cancer Society Mentored Research Scholar Grant MRSG-16-047-01-MPC, and the Hope Portfolio Fund of the City of Hope. The figure materials for this paper were obtained from the website provided by Les Laboratoires Servier, SAS (https://smart.servier.com) with modifications.
References
- 1.Sylla B.S., Wild C.P. A million Africans a year dying from cancer by 2030: what can cancer research and control offer to the continent? Int. J. Cancer. 2012;130:245–250. doi: 10.1002/ijc.26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torphy R.J., Zhu Y., Schulick R.D. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. Ann. Gastroenterol. Surg. 2018;2:274–281. doi: 10.1002/ags3.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haanen J.B.A.G. Converting Cold into Hot Tumors by Combining Immunotherapies. Cell. 2017;170:1055–1056. doi: 10.1016/j.cell.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 4.Brahmer J.R., Tykodi S.S., Chow L.Q., Hwu W.J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russell S.J., Peng K.W., Bell J.C. Oncolytic virotherapy. Nat. Biotechnol. 2012;30:658–670. doi: 10.1038/nbt.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaufman H.L., Kohlhapp F.J., Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 2015;14:642–662. doi: 10.1038/nrd4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pardoll D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolchok J.D., Hodi F.S., Weber J.S., Allison J.P., Urba W.J., Robert C., O’Day S.J., Hoos A., Humphrey R., Berman D.M. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann. N Y Acad. Sci. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong H., Strome S.E., Salomao D.R., Tamura H., Hirano F., Flies D.B., Roche P.C., Lu J., Zhu G., Tamada K. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 2002;8:793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 10.Zhu C., Anderson A.C., Schubart A., Xiong H., Imitola J., Khoury S.J., Zheng X.X., Strom T.B., Kuchroo V.K. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 11.Greenwald R.J., Freeman G.J., Sharpe A.H. The B7 family revisited. Annu. Rev. Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 12.Brunet J.F., Denizot F., Luciani M.F., Roux-Dosseto M., Suzan M., Mattei M.G., Golstein P. A new member of the immunoglobulin superfamily--CTLA-4. Nature. 1987;328:267–270. doi: 10.1038/328267a0. [DOI] [PubMed] [Google Scholar]
- 13.Camacho L.H. CTLA-4 blockade with ipilimumab: biology, safety, efficacy, and future considerations. Cancer Med. 2015;4:661–672. doi: 10.1002/cam4.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder E.I., Desai A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016;39:98–106. doi: 10.1097/COC.0000000000000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okazaki T., Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 2007;19:813–824. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 17.Terme M., Ullrich E., Aymeric L., Meinhardt K., Desbois M., Delahaye N., Viaud S., Ryffel B., Yagita H., Kaplanski G. IL-18 induces PD-1-dependent immunosuppression in cancer. Cancer Res. 2011;71:5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 18.Ishida Y., Agata Y., Shibahara K., Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11:3887–3895. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Francisco L.M., Salinas V.H., Brown K.E., Vanguri V.K., Freeman G.J., Kuchroo V.K., Sharpe A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuang D.M., Zhao Q., Peng C., Xu J., Zhang J.P., Wu C., Zheng L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009;206:1327–1337. doi: 10.1084/jem.20082173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khoja L., Butler M.O., Kang S.P., Ebbinghaus S., Joshua A.M. Pembrolizumab. J. Immunother. Cancer. 2015;3:36. doi: 10.1186/s40425-015-0078-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J., Lee W., Yuan J., Wong P., Ho T.S. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.PACIFIC Investigators. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 24.Anderson A.C., Joller N., Kuchroo V.K. Lag-3, Tim-3, and TIGIT: Co-inhibitory Receptors with Specialized Functions in Immune Regulation. Immunity. 2016;44:989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Andrews L.P., Marciscano A.E., Drake C.G., Vignali D.A. LAG3 (CD223) as a cancer immunotherapy target. Immunol. Rev. 2017;276:80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brignone C., Escudier B., Grygar C., Marcu M., Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin. Cancer Res. 2009;15:6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 27.Fourcade J., Sun Z., Benallaoua M., Guillaume P., Luescher I.F., Sander C., Kirkwood J.M., Kuchroo V., Zarour H.M. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J. Exp. Med. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakuishi K., Apetoh L., Sullivan J.M., Blazar B.R., Kuchroo V.K., Anderson A.C. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warner S.G., O’Leary M.P., Fong Y. Therapeutic oncolytic viruses: clinical advances and future directions. Curr. Opin. Oncol. 2017;29:359–365. doi: 10.1097/CCO.0000000000000388. [DOI] [PubMed] [Google Scholar]
- 30.Liu T.C., Galanis E., Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat. Clin. Pract. Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 31.Garber K. China approves world’s first oncolytic virus therapy for cancer treatment. J. Natl. Cancer Inst. 2006;98:298–300. doi: 10.1093/jnci/djj111. [DOI] [PubMed] [Google Scholar]
- 32.Russell L., Peng K.W. The emerging role of oncolytic virus therapy against cancer. Linchuang Zhongliuxue Zazhi. 2018;7:16. doi: 10.21037/cco.2018.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly E., Russell S.J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 2007;15:651–659. doi: 10.1038/sj.mt.6300108. [DOI] [PubMed] [Google Scholar]
- 34.Chaurasiya S., Warner S. Viroimmunotherapy for Colorectal Cancer: Clinical Studies. Biomedicines. 2017;5:11. doi: 10.3390/biomedicines5010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.LaRocca C.J., Warner S.G. Oncolytic viruses and checkpoint inhibitors: combination therapy in clinical trials. Clin. Transl. Med. 2018;7:35. doi: 10.1186/s40169-018-0214-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Melcher A., Parato K., Rooney C.M., Bell J.C. Thunder and lightning: immunotherapy and oncolytic viruses collide. Mol. Ther. 2011;19:1008–1016. doi: 10.1038/mt.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cattaneo R., Miest T., Shashkova E.V., Barry M.A. Reprogrammed viruses as cancer therapeutics: targeted, armed and shielded. Nat. Rev. Microbiol. 2008;6:529–540. doi: 10.1038/nrmicro1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shtrichman R., Kleinberger T. Adenovirus type 5 E4 open reading frame 4 protein induces apoptosis in transformed cells. J. Virol. 1998;72:2975–2982. doi: 10.1128/jvi.72.4.2975-2982.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marelli G., Howells A., Lemoine N.R., Wang Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Front. Immunol. 2018;9:866. doi: 10.3389/fimmu.2018.00866. article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin C.-Z., Xiang G.L., Zhu X.H., Xiu L.L., Sun J.X., Zhang X.Y. Advances in the mechanisms of action of cancer-targeting oncolytic viruses. Oncol. Lett. 2018;15:4053–4060. doi: 10.3892/ol.2018.7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh P.K., Doley J., Kumar G.R., Sahoo A.P., Tiwari A.K. Oncolytic viruses & their specific targeting to tumour cells. Indian J. Med. Res. 2012;136:571–584. [PMC free article] [PubMed] [Google Scholar]
- 42.Howells A., Marelli G., Lemoine N.R., Wang Y. Oncolytic Viruses-Interaction of Virus and Tumor Cells in the Battle to Eliminate Cancer. Front. Oncol. 2017;7 doi: 10.3389/fonc.2017.00195. article 195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zamarin D., Holmgaard R.B., Subudhi S.K., Park J.S., Mansour M., Palese P., Merghoub T., Wolchok J.D., Allison J.P. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 2014;6:226ra32. doi: 10.1126/scitranslmed.3008095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajani K.R., Vile R.G. Harnessing the Power of Onco-Immunotherapy with Checkpoint Inhibitors. Viruses. 2015;7:5889–5901. doi: 10.3390/v7112914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callahan M.K., Postow M.A., Wolchok J.D. Targeting T Cell Co-receptors for Cancer Therapy. Immunity. 2016;44:1069–1078. doi: 10.1016/j.immuni.2016.04.023. [DOI] [PubMed] [Google Scholar]
- 46.Johnson D.B., Peng C., Sosman J.A. Nivolumab in melanoma: latest evidence and clinical potential. Ther. Adv. Med. Oncol. 2015;7:97–106. doi: 10.1177/1758834014567469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Puzanov I., Milhem M.M., Minor D., Hamid O., Li A., Chen L., Chastain M., Gorski K.S., Anderson A., Chou J. Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. J. Clin. Oncol. 2016;34:2619–2626. doi: 10.1200/JCO.2016.67.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chaurasiya S., Chen N.G., Fong Y. Oncolytic viruses and immunity. Curr. Opin. Immunol. 2018;51:83–90. doi: 10.1016/j.coi.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonteneau J.-F., Achard C., Zaupa C., Foloppe J., Erbs P. Oncolytic immunotherapy: The new clinical outbreak. OncoImmunology. 2015;5:e1066961. doi: 10.1080/2162402X.2015.1066961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grinde B. Herpesviruses: latency and reactivation - viral strategies and host response. J. Oral Microbiol. 2013;5:22766. doi: 10.3402/jom.v5i0.22766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosenfeld M.R., Meneses P., Dalmau J., Drobnjak M., Cordon-Cardo C., Kaplitt M.G. Gene transfer of wild-type p53 results in restoration of tumor-suppressor function in a medulloblastoma cell line. Neurology. 1995;45:1533–1539. doi: 10.1212/wnl.45.8.1533. [DOI] [PubMed] [Google Scholar]
- 52.Hoshi M., Harada A., Kawase T., Uyemura K., Yazaki T. Antitumoral effects of defective herpes simplex virus-mediated transfer of tissue inhibitor of metalloproteinases-2 gene in malignant glioma U87 in vitro: consequences for anti-cancer gene therapy. Cancer Gene Ther. 2000;7:799–805. doi: 10.1038/sj.cgt.7700177. [DOI] [PubMed] [Google Scholar]
- 53.Kim S.H., Carew J.F., Kooby D.A., Shields J., Entwisle C., Patel S., Shah J.P., Fong Y. Combination gene therapy using multiple immunomodulatory genes transferred by a defective infectious single-cycle herpes virus in squamous cell cancer. Cancer Gene Ther. 2000;7:1279–1285. doi: 10.1038/sj.cgt.7700231. [DOI] [PubMed] [Google Scholar]
- 54.Tung C., Federoff H.J., Brownlee M., Karpoff H., Weigel T., Brennan M.F., Fong Y. Rapid production of interleukin-2-secreting tumor cells by herpes simplex virus-mediated gene transfer: implications for autologous vaccine production. Hum. Gene Ther. 1996;7:2217–2224. doi: 10.1089/hum.1996.7.18-2217. [DOI] [PubMed] [Google Scholar]
- 55.Toda M., Martuza R.L., Kojima H., Rabkin S.D. In situ cancer vaccination: an IL-12 defective vector/replication-competent herpes simplex virus combination induces local and systemic antitumor activity. J. Immunol. 1998;160:4457–4464. [PubMed] [Google Scholar]
- 56.Kanno H., Hattori S., Sato H., Murata H., Huang F.H., Hayashi A., Suzuki N., Yamamoto I., Kawamoto S., Minami M. Experimental gene therapy against subcutaneously implanted glioma with a herpes simplex virus-defective vector expressing interferon-gamma. Cancer Gene Ther. 1999;6:147–154. doi: 10.1038/sj.cgt.7700008. [DOI] [PubMed] [Google Scholar]
- 57.Toda M., Martuza R.L., Rabkin S.D. Tumor growth inhibition by intratumoral inoculation of defective herpes simplex virus vectors expressing granulocyte-macrophage colony-stimulating factor. Mol. Ther. 2000;2:324–329. doi: 10.1006/mthe.2000.0130. [DOI] [PubMed] [Google Scholar]
- 58.Andtbacka R.H., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 59.Liu B.L., Robinson M., Han Z.Q., Branston R.H., English C., Reay P., McGrath Y., Thomas S.K., Thornton M., Bullock P. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 2003;10:292–303. doi: 10.1038/sj.gt.3301885. [DOI] [PubMed] [Google Scholar]
- 60.Kohlhapp F.J., Kaufman H.L. Molecular Pathways: Mechanism of Action for Talimogene Laherparepvec, a New Oncolytic Virus Immunotherapy. Clin. Cancer Res. 2016;22:1048–1054. doi: 10.1158/1078-0432.CCR-15-2667. [DOI] [PubMed] [Google Scholar]
- 61.Kaufman H.L., Kim D.W., DeRaffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 62.Rehman H., Silk A.W., Kane M.P., Kaufman H.L. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J. Immunother. Cancer. 2016;4:53. doi: 10.1186/s40425-016-0158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chesney J., Puzanov I., Collichio F., Singh P., Milhem M.M., Glaspy J., Hamid O., Ross M., Friedlander P., Garbe C. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination With Ipilimumab Versus Ipilimumab Alone in Patients With Advanced, Unresectable Melanoma. J. Clin. Oncol. 2018;36:1658–1667. doi: 10.1200/JCO.2017.73.7379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ribas A., Dummer R., Puzanov I., VanderWalde A., Andtbacka R.H.I., Michielin O., Olszanski A.J., Malvehy J., Cebon J., Fernandez E. Oncolytic Virotherapy Promotes Intratumoral T Cell Infiltration and Improves Anti-PD-1 Immunotherapy. Cell. 2017;170:1109–1119.e10. doi: 10.1016/j.cell.2017.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haddad D. Genetically Engineered Vaccinia Viruses As Agents for Cancer Treatment, Imaging, and Transgene Delivery. Front. Oncol. 2017;7:96. doi: 10.3389/fonc.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thorne S.H. Immunotherapeutic potential of oncolytic vaccinia virus. Front. Oncol. 2014;4:155. doi: 10.3389/fonc.2014.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kleinpeter P., Fend L., Thioudellet C., Geist M., Sfrontato N., Koerper V., Fahrner C., Schmitt D., Gantzer M., Remy-Ziller C. Vectorization in an oncolytic vaccinia virus of an antibody, a Fab and a scFv against programmed cell death -1 (PD-1) allows their intratumoral delivery and an improved tumor-growth inhibition. OncoImmunology. 2016;5:e1220467. doi: 10.1080/2162402X.2016.1220467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Z., Ravindranathan R., Kalinski P., Guo Z.S., Bartlett D.L. Rational combination of oncolytic vaccinia virus and PD-L1 blockade works synergistically to enhance therapeutic efficacy. Nat. Commun. 2017;8:14754. doi: 10.1038/ncomms14754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rojas J.J., Sampath P., Hou W., Thorne S.H. Defining Effective Combinations of Immune Checkpoint Blockade and Oncolytic Virotherapy. Clin. Cancer Res. 2015;21:5543–5551. doi: 10.1158/1078-0432.CCR-14-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fend L., Yamazaki T., Remy C., Fahrner C., Gantzer M., Nourtier V., Préville X., Quéméneur E., Kepp O., Adam J. Immune Checkpoint Blockade, Immunogenic Chemotherapy or IFN-α Blockade Boost the Local and Abscopal Effects of Oncolytic Virotherapy. Cancer Res. 2017;77:4146–4157. doi: 10.1158/0008-5472.CAN-16-2165. [DOI] [PubMed] [Google Scholar]
- 71.Heo J., Reid T., Ruo L., Breitbach C.J., Rose S., Bloomston M., Cho M., Lim H.Y., Chung H.C., Kim C.W. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nat. Med. 2013;19:329–336. doi: 10.1038/nm.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khanal S., Ghimire P., Dhamoon A.S. The Repertoire of Adenovirus in Human Disease: The Innocuous to the Deadly. Biomedicines. 2018;6:E30. doi: 10.3390/biomedicines6010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Buijs P.R., Verhagen J.H., van Eijck C.H., van den Hoogen B.G. Oncolytic viruses: From bench to bedside with a focus on safety. Hum. Vaccin. Immunother. 2015;11:1573–1584. doi: 10.1080/21645515.2015.1037058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cervera-Carrascon V., Siurala M., Santos J.M., Havunen R., Tähtinen S., Karell P., Sorsa S., Kanerva A., Hemminki A. TNFa and IL-2 armed adenoviruses enable complete responses by anti-PD-1 checkpoint blockade. OncoImmunology. 2018;7:e1412902. doi: 10.1080/2162402X.2017.1412902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fueyo J., Gomez-Manzano C., Alemany R., Lee P.S., McDonnell T.J., Mitlianga P., Shi Y.X., Levin V.A., Yung W.K., Kyritsis A.P. A mutant oncolytic adenovirus targeting the Rb pathway produces anti-glioma effect in vivo. Oncogene. 2000;19:2–12. doi: 10.1038/sj.onc.1203251. [DOI] [PubMed] [Google Scholar]
- 76.Lang F.F., Conrad C., Gomez-Manzano C., Yung W.K.A., Sawaya R., Weinberg J.S., Prabhu S.S., Rao G., Fuller G.N., Aldape K.D. Phase I Study of DNX-2401 (Delta-24-RGD) Oncolytic Adenovirus: Replication and Immunotherapeutic Effects in Recurrent Malignant Glioma. J. Clin. Oncol. 2018;36:1419–1427. doi: 10.1200/JCO.2017.75.8219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Koski A., Kangasniemi L., Escutenaire S., Pesonen S., Cerullo V., Diaconu I., Nokisalmi P., Raki M., Rajecki M., Guse K. Treatment of cancer patients with a serotype 5/3 chimeric oncolytic adenovirus expressing GMCSF. Mol. Ther. 2010;18:1874–1884. doi: 10.1038/mt.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ranki T., Pesonen S., Hemminki A., Partanen K., Kairemo K., Alanko T., Lundin J., Linder N., Turkki R., Ristimäki A. Phase I study with ONCOS-102 for the treatment of solid tumors - an evaluation of clinical response and exploratory analyses of immune markers. J. Immunother. Cancer. 2016;4:17. doi: 10.1186/s40425-016-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Joklik W.K. The structure and function of the reovirus genome. Ann. N Y Acad. Sci. 1980;354:107–124. doi: 10.1111/j.1749-6632.1980.tb27961.x. [DOI] [PubMed] [Google Scholar]
- 80.Strong J.E., Coffey M.C., Tang D., Sabinin P., Lee P.W. The molecular basis of viral oncolysis: usurpation of the Ras signaling pathway by reovirus. EMBO J. 1998;17:3351–3362. doi: 10.1093/emboj/17.12.3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chakrabarty R., Tran H., Selvaggi G., Hagerman A., Thompson B., Coffey M. The oncolytic virus, pelareorep, as a novel anticancer agent: a review. Invest. New Drugs. 2015;33:761–774. doi: 10.1007/s10637-015-0216-8. [DOI] [PubMed] [Google Scholar]
- 82.Errington F., Steele L., Prestwich R., Harrington K.J., Pandha H.S., Vidal L., de Bono J., Selby P., Coffey M., Vile R., Melcher A. Reovirus activates human dendritic cells to promote innate antitumor immunity. J. Immunol. 2008;180:6018–6026. doi: 10.4049/jimmunol.180.9.6018. [DOI] [PubMed] [Google Scholar]
- 83.Rajani K., Parrish C., Kottke T., Thompson J., Zaidi S., Ilett L., Shim K.G., Diaz R.M., Pandha H., Harrington K. Combination Therapy With Reovirus and Anti-PD-1 Blockade Controls Tumor Growth Through Innate and Adaptive Immune Responses. Mol. Ther. 2016;24:166–174. doi: 10.1038/mt.2015.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mostafa A.A., Meyers D.E., Thirukkumaran C.M., Liu P.J., Gratton K., Spurrell J., Shi Q., Thakur S., Morris D.G. Oncolytic Reovirus and Immune Checkpoint Inhibition as a Novel Immunotherapeutic Strategy for Breast Cancer. Cancers (Basel) 2018;10:E205. doi: 10.3390/cancers10060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ilett E., Kottke T., Thompson J., Rajani K., Zaidi S., Evgin L., Coffey M., Ralph C., Diaz R., Pandha H. Prime-boost using separate oncolytic viruses in combination with checkpoint blockade improves anti-tumour therapy. Gene Ther. 2017;24:21–30. doi: 10.1038/gt.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Samson A., Scott K.J., Taggart D., West E.J., Wilson E., Nuovo G.J., Thomson S., Corns R., Mathew R.K., Fuller M.J. Intravenous delivery of oncolytic reovirus to brain tumor patients immunologically primes for subsequent checkpoint blockade. Sci. Transl. Med. 2018;10:eaam7577. doi: 10.1126/scitranslmed.aam7577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mahalingam D., Goel S., Aparo S., Patel Arora S., Noronha N., Tran H., Chakrabarty R., Selvaggi G., Gutierrez A., Coffey M. A Phase II Study of Pelareorep (REOLYSIN®) in Combination with Gemcitabine for Patients with Advanced Pancreatic Adenocarcinoma. Cancers (Basel) 2018;10:E160. doi: 10.3390/cancers10060160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lundstrom K. New frontiers in oncolytic viruses: optimizing and selecting for virus strains with improved efficacy. Biologics. 2018;12:43–60. doi: 10.2147/BTT.S140114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chong P., Guo M.S., Lin F.H., Hsiao K.N., Weng S.Y., Chou A.H., Wang J.R., Hsieh S.Y., Su I.J., Liu C.C. Immunological and biochemical characterization of coxsackie virus A16 viral particles. PLoS ONE. 2012;7:e49973. doi: 10.1371/journal.pone.0049973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bradley S., Jakes A.D., Harrington K., Pandha H., Melcher A., Errington-Mais F. Applications of coxsackievirus A21 in oncology. Oncolytic Virother. 2014;3:47–55. doi: 10.2147/OV.S56322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pol J.G., Atherton M.J., Bridle B.W., Stephenson K.B., Le Boeuf F., Hummel J.L., Martin C.G., Pomoransky J., Breitbach C.J., Diallo J.S. Development and applications of oncolytic Maraba virus vaccines. Oncolytic Virother. 2018;7:117–128. doi: 10.2147/OV.S154494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Bourgeois-Daigneault M.C., Roy D.G., Aitken A.S., El Sayes N., Martin N.T., Varette O., Falls T., St-Germain L.E., Pelin A., Lichty B.D. Neoadjuvant oncolytic virotherapy before surgery sensitizes triple-negative breast cancer to immune checkpoint therapy. Sci. Transl. Med. 2018;10:eaao1641. doi: 10.1126/scitranslmed.aao1641. [DOI] [PubMed] [Google Scholar]
- 93.Pol J.G., Acuna S.A., Yadollahi B., Tang N., Stephenson K.B., Atherton M.J., Hanwell D., El-Warrak A., Goldstein A., Moloo B. Preclinical evaluation of a MAGE-A3 vaccination utilizing the oncolytic Maraba virus currently in first-in-human trials. Oncoimmunology. 2018;8:e1512329. doi: 10.1080/2162402X.2018.1512329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Colonno R.J., Banerjee A.K. Complete nucleotide sequence of the leader RNA synthesized in vitro by vesicular stomatitis virus. Cell. 1978;15:93–101. doi: 10.1016/0092-8674(78)90085-5. [DOI] [PubMed] [Google Scholar]
- 95.Stojdl D.F., Lichty B., Knowles S., Marius R., Atkins H., Sonenberg N., Bell J.C. Exploiting tumor-specific defects in the interferon pathway with a previously unknown oncolytic virus. Nat. Med. 2000;6:821–825. doi: 10.1038/77558. [DOI] [PubMed] [Google Scholar]
- 96.Ebert O., Shinozaki K., Huang T.G., Savontaus M.J., García-Sastre A., Woo S.L. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 2003;63:3605–3611. [PubMed] [Google Scholar]
- 97.Wongthida P., Diaz R.M., Pulido C., Rommelfanger D., Galivo F., Kaluza K., Kottke T., Thompson J., Melcher A., Vile R. Activating systemic T-cell immunity against self tumor antigens to support oncolytic virotherapy with vesicular stomatitis virus. Hum. Gene Ther. 2011;22:1343–1353. doi: 10.1089/hum.2010.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Diaz R.M., Galivo F., Kottke T., Wongthida P., Qiao J., Thompson J., Valdes M., Barber G., Vile R.G. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 2007;67:2840–2848. doi: 10.1158/0008-5472.CAN-06-3974. [DOI] [PubMed] [Google Scholar]
- 99.Altomonte J., Wu L., Meseck M., Chen L., Ebert O., Garcia-Sastre A., Fallon J., Mandeli J., Woo S.L. Enhanced oncolytic potency of vesicular stomatitis virus through vector-mediated inhibition of NK and NKT cells. Cancer Gene Ther. 2009;16:266–278. doi: 10.1038/cgt.2008.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Altomonte J., Wu L., Chen L., Meseck M., Ebert O., García-Sastre A., Fallon J., Woo S.L. Exponential enhancement of oncolytic vesicular stomatitis virus potency by vector-mediated suppression of inflammatory responses in vivo. Mol. Ther. 2008;16:146–153. doi: 10.1038/sj.mt.6300343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Marozin S., Altomonte J., Muñoz-Álvarez K.A., Rizzani A., De Toni E.N., Thasler W.E., Schmid R.M., Ebert O. STAT3 inhibition reduces toxicity of oncolytic VSV and provides a potentially synergistic combination therapy for hepatocellular carcinoma. Cancer Gene Ther. 2015;22:317–325. doi: 10.1038/cgt.2015.23. [DOI] [PubMed] [Google Scholar]
- 102.Huang T.-G., Ebert O., Shinozaki K., García-Sastre A., Woo S.L. Oncolysis of hepatic metastasis of colorectal cancer by recombinant vesicular stomatitis virus in immune-competent mice. Mol. Ther. 2003;8:434–440. doi: 10.1016/s1525-0016(03)00204-1. [DOI] [PubMed] [Google Scholar]
- 103.Conrad D.P., Tsang J., Maclean M., Diallo J.S., Le Boeuf F., Lemay C.G., Falls T.J., Parato K.A., Bell J.C., Atkins H.L. Leukemia cell-rhabdovirus vaccine: personalized immunotherapy for acute lymphoblastic leukemia. Clin. Cancer Res. 2013;19:3832–3843. doi: 10.1158/1078-0432.CCR-12-3199. [DOI] [PubMed] [Google Scholar]
- 104.Hanauer J.D.S., Rengstl B., Kleinlützum D., Reul J., Pfeiffer A., Friedel T., Schneider I.C., Newrzela S., Hansmann M.L., Buchholz C.J., Muik A. CD30-targeted oncolytic viruses as novel therapeutic approach against classical Hodgkin lymphoma. Oncotarget. 2018;9:12971–12981. doi: 10.18632/oncotarget.24191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simovic B., Walsh S.R., Wan Y. Mechanistic insights into the oncolytic activity of vesicular stomatitis virus in cancer immunotherapy. Oncolytic Virother. 2015;4:157–167. doi: 10.2147/OV.S66079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang L., Bridle B.W., Chen L., Pol J., Spaner D., Boudreau J.E., Rosen A., Bassett J.D., Lichty B.D., Bramson J.L., Wan Y. Delivery of viral-vectored vaccines by B cells represents a novel strategy to accelerate CD8(+) T-cell recall responses. Blood. 2013;121:2432–2439. doi: 10.1182/blood-2012-06-438481. [DOI] [PubMed] [Google Scholar]
- 107.Johnson J.E., Nasar F., Coleman J.W., Price R.E., Javadian A., Draper K., Lee M., Reilly P.A., Clarke D.K., Hendry R.M., Udem S.A. Neurovirulence properties of recombinant vesicular stomatitis virus vectors in non-human primates. Virology. 2007;360:36–49. doi: 10.1016/j.virol.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Shen W., Patnaik M.M., Ruiz A., Russell S.J., Peng K.W. Immunovirotherapy with vesicular stomatitis virus and PD-L1 blockade enhances therapeutic outcome in murine acute myeloid leukemia. Blood. 2016;127:1449–1458. doi: 10.1182/blood-2015-06-652503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alonso-Camino V., Rajani K., Kottke T., Rommelfanger-Konkol D., Zaidi S., Thompson J., Pulido J., Ilett E., Donnelly O., Selby P. The profile of tumor antigens which can be targeted by immunotherapy depends upon the tumor’s anatomical site. Mol. Ther. 2014;22:1936–1948. doi: 10.1038/mt.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cockle J.V., Rajani K., Zaidi S., Kottke T., Thompson J., Diaz R.M., Shim K., Peterson T., Parney I.F., Short S. Combination viroimmunotherapy with checkpoint inhibition to treat glioma, based on location-specific tumor profiling. Neuro-oncol. 2016;18:518–527. doi: 10.1093/neuonc/nov173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cameron C., Hota-Mitchell S., Chen L., Barrett J., Cao J.X., Macaulay C., Willer D., Evans D., McFadden G. The complete DNA sequence of myxoma virus. Virology. 1999;264:298–318. doi: 10.1006/viro.1999.0001. [DOI] [PubMed] [Google Scholar]
- 112.Lun X., Yang W., Alain T., Shi Z.Q., Muzik H., Barrett J.W., McFadden G., Bell J., Hamilton M.G., Senger D.L., Forsyth P.A. Myxoma virus is a novel oncolytic virus with significant antitumor activity against experimental human gliomas. Cancer Res. 2005;65:9982–9990. doi: 10.1158/0008-5472.CAN-05-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bartee M.Y., Dunlap K.M., Bartee E. Tumor-Localized Secretion of Soluble PD1 Enhances Oncolytic Virotherapy. Cancer Res. 2017;77:2952–2963. doi: 10.1158/0008-5472.CAN-16-1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schirrmacher V. Immunobiology of Newcastle Disease Virus and Its Use for Prophylactic Vaccination in Poultry and as Adjuvant for Therapeutic Vaccination in Cancer Patients. Int. J. Mol. Sci. 2017;18:1103. doi: 10.3390/ijms18051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kallio K., Hellström K., Balistreri G., Spuul P., Jokitalo E., Ahola T. Template RNA length determines the size of replication complex spherules for Semliki Forest virus. J. Virol. 2013;87:9125–9134. doi: 10.1128/JVI.00660-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lundstrom K. Oncolytic Alphaviruses in Cancer Immunotherapy. Vaccines (Basel) 2017;5:E9. doi: 10.3390/vaccines5020009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Quetglas J.I., Labiano S., Aznar M.Á., Bolaños E., Azpilikueta A., Rodriguez I., Casales E., Sánchez-Paulete A.R., Segura V., Smerdou C., Melero I. Virotherapy with a Semliki Forest Virus-Based Vector Encoding IL12 Synergizes with PD-1/PD-L1 Blockade. Cancer Immunol. Res. 2015;3:449–454. doi: 10.1158/2326-6066.CIR-14-0216. [DOI] [PubMed] [Google Scholar]
- 118.Engeland C.E., Grossardt C., Veinalde R., Bossow S., Lutz D., Kaufmann J.K., Shevchenko I., Umansky V., Nettelbeck D.M., Weichert W. CTLA-4 and PD-L1 checkpoint blockade enhances oncolytic measles virus therapy. Mol. Ther. 2014;22:1949–1959. doi: 10.1038/mt.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saha D., Martuza R.L., Rabkin S.D. Oncolytic herpes simplex virus immunovirotherapy in combination with immune checkpoint blockade to treat glioblastoma. Immunotherapy. 2018;10:779–786. doi: 10.2217/imt-2018-0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Saha D., Martuza R.L., Rabkin S.D. Macrophage Polarization Contributes to Glioblastoma Eradication by Combination Immunovirotherapy and Immune Checkpoint Blockade. Cancer Cell. 2017;32:253–267.e5. doi: 10.1016/j.ccell.2017.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]