Summary
Additive engineering has become increasingly important for making high-quality perovskite solar cells (PSCs), with a recent example involving acid during fabrication of cesium-based perovskites. Lately, it has been suggested that this process would introduce dimethylammonium ((CH3)2NH2+, DMA+) through hydrolysis of the organic solvent. However, material composition of the hydrolyzed product and its effect on the device performance remain to be understood. Here, we present an in-depth investigation of the hydrolysis-derived material (i.e., DMAPbI3) and detailed analysis of its role in producing high-quality PSCs. By varying the ratio of CsI/DMAPbI3 in the precursor, we achieve high-quality CsxDMA1-xPbI3 perovskite films with uniform morphology, low density of trap states, and good stability, leading to optimized power conversion efficiency up to 14.3%, with over 85% of the initial efficiency retained after ∼20 days in air without encapsulation. Our findings offer new insights into producing high-quality Cs-based perovskite materials.
Subject Areas: Energy Sustainability, Materials Characterization, Energy Materials
Graphical Abstract
Highlights
-
•
Dissolving PbI2 and HI in DMF is confirmed not to produce the “mythical” HPbI3
-
•
Detailed composition analyses show that DMAPbI3 is the hydrolysis product instead
-
•
Performance of devices can be optimized by tuning the CsI:DMAPbI3 ratio
-
•
The CsxDMA1-xPbI3 films remain stable in air for more than 20 days
Energy Sustainability; Materials Characterization; Energy Materials
Introduction
As an emerging alternative light-harvesting material in solar cells, cesium (Cs)-based halide perovskites have recently attracted booming attention due to their excellent charge transport properties and thermal stability (Wang et al., 2018a, Wang et al., 2018b, Wang et al., 2018d, Li et al., 2018b). It has been demonstrated that α-CsPbI3 (cubic phase) exhibits a band gap of ∼1.73 eV, highly desirable for building top cells in tandem perovskite solar cells (PSCs). However, α-CsPbI3 perovskite exhibits a tolerance factor of ∼0.85, making the materials unstable at room temperature due to the easily spontaneous transformation to the photo-inactive δ phase (Hu et al., 2017, Lau et al., 2018), severely hampering its application toward high-performance PSCs. Among various approaches (Wang et al., 2017, Wang et al., 2018a, Wang et al., 2018b, Wang et al., 2018c, Wang et al., 2018d, Hu et al., 2017, Lau et al., 2018, Jena et al., 2018, Swarnkar et al., 2016, Sanehira et al., 2017, Zhang et al., 2017, Liao et al., 2017, Luo et al., 2016, Fu et al., 2017, Li et al., 2018a, Li et al., 2018b), additive engineering, which involves careful selection and addition of components to the precursor solution, has been commonly employed for the perovskite deposition to achieve a better control of the crystallization process and the ensuing film quality (Sutherland, 2017). Optimization of the additive engineering has been demonstrated as an effective and facile route toward Cs-based photovoltaic devices with improved efficiency and stability and has become an emerging focus of PSC research.
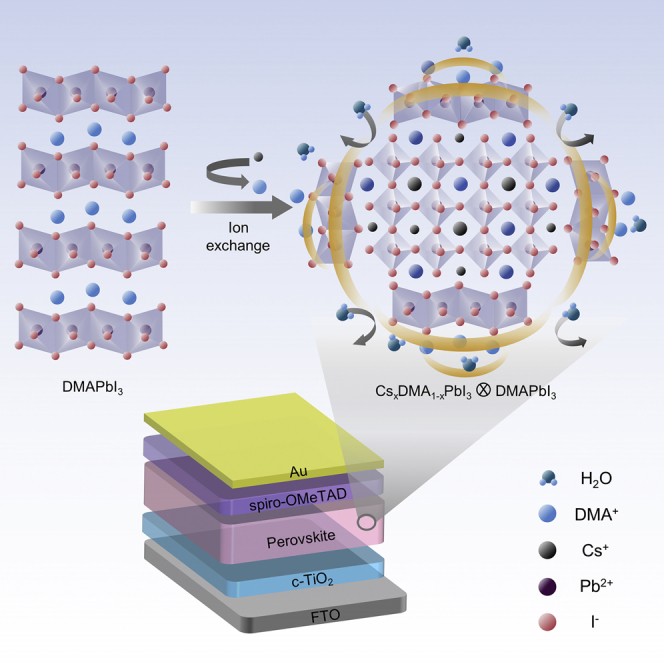
As an exemplary case, adding hydriodic acid (HI) to the N,N-dimethylformamide (DMF) solution of lead iodide (PbI2) and cesium iodide (CsI) has been demonstrated as a popular way to enhance the phase stability of the resultant Cs-based perovskite films (Eperon et al., 2015, Hu et al., 2017, Luo et al., 2016). However, the understanding of the detailed reaction mechanism and actual role of the additives has often remained elusive, which hinders further optimization of the fabrication process. For example, it has recently been suggested that the “mythical” hydrogen lead trihalide (HPbI3, also known as PbI2∙xHI), the often-assumed reaction product of HI and PbI2, does not actually exist (Ke et al., 2018). Instead, adding acid to DMF is known to generate a weak base dimethylamine (DMA) through hydrolysis (Noel et al., 2017, Sutherland, 2017, Daub and Hillebrecht, 2018), and with the presence of PbI2 the actual final product is believed to be a compound of DMAPbI3 (DMA+ = dimethylammonium, (CH3)2NH2+). Despite the broad adoption of such reaction route in fabricating Cs-based perovskite materials, systematic investigation of DMAPbI3 as the starting material and its effect on the performance of the resultant PSCs has been largely missing, breeding continued debates and confusion.
Here, we present a detailed characterization of the hydrolysis-derived DMAPbI3 and its role in producing high-quality Cs-based perovskite films. We employed an extensive set of techniques to accomplish in-depth analysis of the composition and properties of DMAPbI3 as the starting material, including in situ thermogravimetry Fourier transform infrared spectroscopy (TG-FTIR) coupled analysis, nuclear magnetic resonance (NMR), and FTIR. We systematically tuned the CsI/DMAPbI3 molar ratio in the precursor and investigated its effect on the film property of the perovskite material, including film morphology, defect states density, carrier lifetime, and material stability, among other PSC performance metrics. We achieve a power conversion efficiency (PCE) up to 14.3%, with over 85% of the initial efficiency retained after ∼20 days in ambient condition without encapsulation. Our findings offer new insights into this important fabrication process, which can lead to better implementation and further optimization in producing high-quality Cs-based perovskite materials for solar cells with improved efficiency and stability.
Results and Discussion
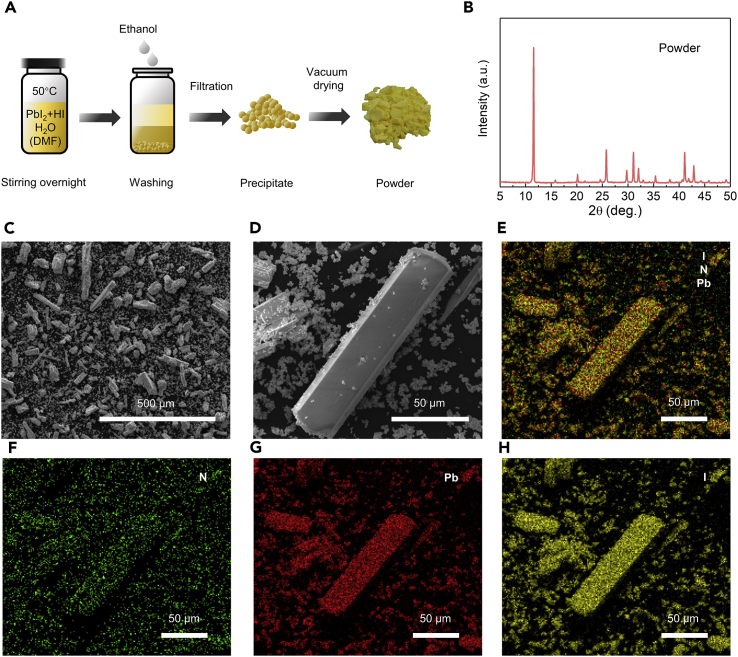
We synthesized the DMAPbI3 powder following the commonly used method by reacting PbI2 and HI in DMF (details in Methods section), which is used as the starting material for making the Cs-based perovskite films (Figure 1A). This method was once widely believed to produce the “mythical” HPbI3 (Wang et al., 2015, Pang et al., 2016, Long et al., 2016), whereas a recent study challenged such claim and proposed that the product would be DMAPbI3 (Ke et al., 2018, Daub and Hillebrecht, 2018), which is now confirmed, finally, by our detailed analysis as described below.
Figure 1.
Synthesis and Characterization of the DMAPbI3 Powder
(A) Schematic diagram for synthesizing DMAPbI3 powder.
(B) XRD pattern of the DMAPbI3 powder.
(C and D) SEM images of the DMAPbI3 powder with different resolutions, See also Figure S1.
(E–H) Energy-dispersive spectrometric (EDS) mapping for N, Pb, and I elements in the DMAPbI3 powder: EDS mapping of (E) N, Pb, and I elements overlay, (F) N element, (G) Pb element, and (H) I element distribution of the DMAPbI3 powder.
X-ray diffraction (XRD) patterns of the synthesized powder (Figure 1B) show peaks at 11.6°, 20.2°, 25.8°, 31.1°, 32.1°, 35.4°, 41.1°, and 42.8°, which can be well ascribed to DMAPbI3 (Mancini et al., 2016, Ke et al., 2018). In addition, the scanning electron microscopic (SEM) images (Figures 1C and 1D) show that the powder particles have the shape of hexagonal rods (Figures 1D and S1), consistent with DMAPbI3 crystals (Mancini et al., 2016, Ju et al., 2017). Furthermore, energy-dispersive spectrometric (EDS) mapping (Figures 1E–1H) measurements identify element N uniformly distributed in the entire sample (Figure 1F), together with elements Pb and I (Figures 1G and 1H). The above evidences strongly support that such reaction route in fact produces DMAPbI3; in particular, the universal existence of element N invalidates the misbelief of the delusional “HPbI3” product from this reaction.
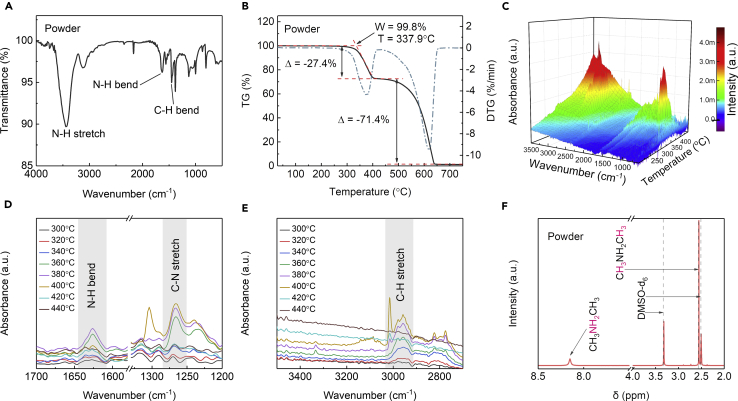
We further confirmed the material composition of the DMAPbI3 powder by performing FTIR spectrum, in situ TG-FTIR coupled analysis, and 1H NMR measurements. The FTIR spectrum confirms the presence of N-H and C-H bonds (Figure 2A), showing clear signatures of the N-H stretching mode (3,200–3,450 cm−1) and C-H bending mode (1,490–1,350 cm−1) (Jeon et al., 2014). The TG-differential TG data, together with in situ TG-FTIR, show two clear weight-losing stages during heat decomposition of the DMAPbI3 powder (Figure 2B). The first stage starts at 337.9°C with 24.7 wt % weight loss, corresponding to the volatilization of organic components, confirmed by the N-H, C-N, and C-H characteristic peaks in the released gases (Figures 2C–2E). During the second decomposition stage (∼400°C–640°C), the N-H, C-N, and C-H peaks disappear in the FTIR (Figures 2D and 2E) and the weight loss is consistent with the melting of lead iodide (PbI2) (bulk melting point at 402°C) (Schieber et al., 2008). In addition, the 1H NMR spectrum (Figure 2F) of the powder (dissolved in dimethyl sulfoxide-d6, DMSO-d6) shows clear signals at δ = 8.15 ppm and δ = 2.55 ppm, corresponding to protons in –NH2+– and –CH3, respectively (Ke et al., 2018). The integrated ratio from these two signals (corresponding to the molar ratio) is ∼1:3, also consistent with the chemical formula DMA+ ((CH3)2NH2+). All the results again strongly confirm DMAPbI3 as the product from the above reaction, which we will use as the Pb source for fabricating Cs-based perovskites.
Figure 2.
Compositional Analysis of DMAPbI3 Powder
(A) FTIR spectra.
(B) TG-differential TG curve.
(C) Three-dimensional in situ TG-FTIR spectra (temperature from 200°C to 440°C).
(D and E) In situ TG-FTIR spectra of volatilized products at various temperatures during the thermal degradation. The wavenumber ranges containing (D) N-H bend, C-N stretch and (E) C-H stretch were selected.
(F) 1H NMR spectra; the powder for NMR measurement was dissolved in DMSO-d6.
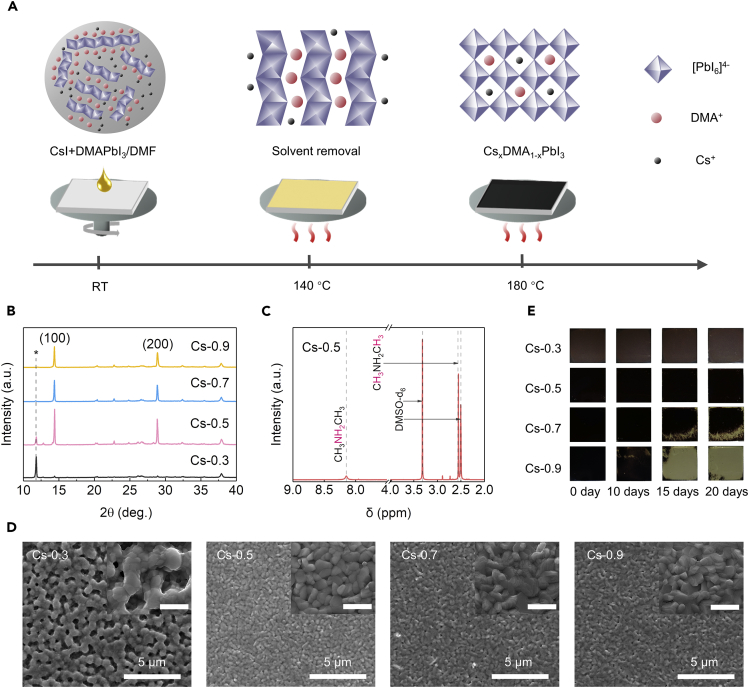
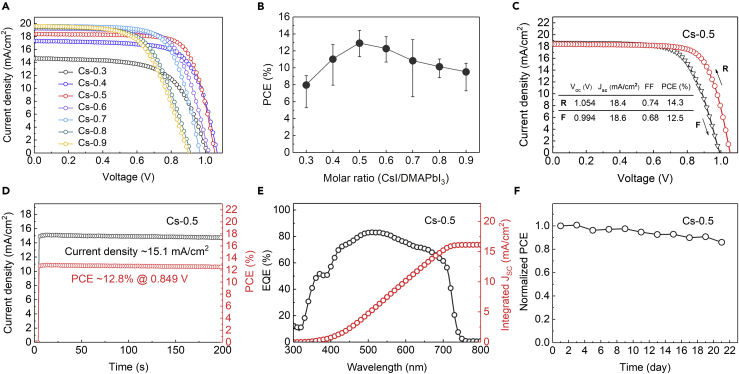
We prepared the perovskite precursor solution by dissolving DMAPbI3 powder and CsI in DMF, with a series of CsI/DMAPbI3 molar ratios (CsI from 0.3 to 0.9 M, DMAPbI3 fixed at 1 M, referred to as Cs-0.3, …, Cs-0.9) in the precursor (details in Methods section). A two-step annealing process (140°C + 180°C) was employed to fully crystallize the spin-coated films (Figure 3A), facilitating the formation of CsxDMA1-xPbI3 perovskites. The absorption property with different CsI/DMAPbI3 molar ratios was first investigated, showing similar optical features on the spectra (Figure S2). The XRD patterns (Figures 3B and S3) of the obtained films show clear peaks corresponding to CsxDMA1-xPbI3 (14.4°, 28.9°) and DMAPbI3 (11.8°), respectively (Ke et al., 2018). Remarkably, in contrast to perovskites fabricated from other conventional routes (such as reacting CsI and PbI2 with DMAI, Ke et al., 2018, or dissolving CsI and PbI2 in DMF with HI additive, Zhao et al., 2018), our perovskite films show more intense diffraction peaks of (100) (14.4°) and (200) (28.9°) without any splitting, along with other spurious peaks strongly suppressed. This result suggests that the introduction of DMA+ (also confirmed by 1H NMR in Figure 3C and FTIR in Figure S4) effectively optimizes tolerance factor, resulting in the high crystallinity and preferable orientation in the perovskite films (Shi et al., 2017).
Figure 3.
Fabrication and Characterization of CsxDMA1-xPbI3 Perovskite Films
(A) Schematic diagram of preparing CsxDMA1-xPbI3 perovskite films.
(B) XRD patterns of CsxDMA1-xPbI3 films with different molar ratios of CsI/DMAPbI3 in the precursor (Cs-0.9, 0.7, 0.5, and 0.3); peak for DMAPbI3 marked with *; see also Figure S3.
(C) 1H NMR spectra of the powder obtained from scratching the CsxDMA1-xPbI3 films when the molar ratio of CsI/DMAPbI3 in the precursor is 0.5/1.
(D) Top-view SEM images of the CsxDMA1-xPbI3 films with different concentration of Cs+; scale bar, 1 μm in insets; see also Figure S5.
(E) Optical photographs of CsxDMA1-xPbI3 films with different molar ratio of CsI/DMAPbI3 in ambient condition (RH 20% ± 5%) for 20 days.
We noticed that the molar ratio of CsI/DMAPbI3 has clear effects on the resulting CsxDMA1-xPbI3 films property. As the Cs+ ratio increases, the DMAPbI3 XRD peak (2θ = 11.8°) decreases and disappears when the molar ratio is over Cs-0.8. This indicates the existence of excess DMAPbI3 in the perovskite film when CsI molar ratio in the precursor falls below 0.8. SEM images (Figures 3D and S5) show that the Cs-0.5 precursor leads to the optimal film morphology with smooth and dense grains, whereas with insufficient Cs+ (Cs-0.3) the perovskite film displays many cavities and Cs-0.7, Cs-0.9 films also show emergence of pinholes. We further evaluated the stability of the films processed with different CsI/DMAPbI3 molar ratios. As shown in Figure 3E, the films with low concentrations of Cs+ (Cs-0.3, Cs-0.5) show negligible degradation after exposing in air for 20 days (relative humidity [RH] 20% ± 5%), whereas those with higher Cs+ ratio (Cs-0.7, Cs-0.9) quickly degrade from black (α) phase into yellow (δ) phase within a few days (see XRD data in Figure S6). The result suggests that an appropriate ratio of CsI/DMAPbI3 is required for achieving a phase-stable film, which could be attributed to the excess DMAPbI3 in the films acting as a moisture barrier, thus improving the stability of the perovskites.
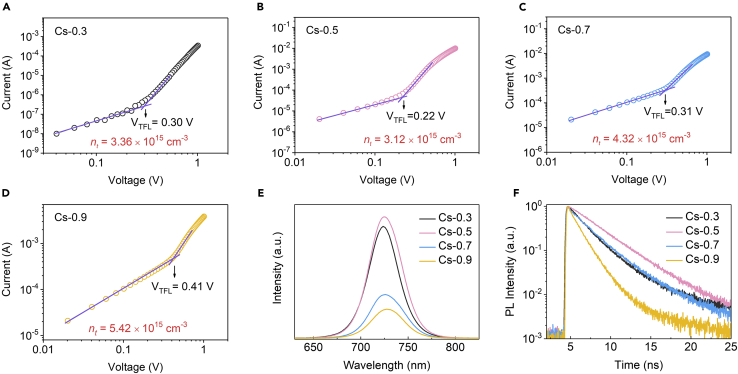
To understand the effect of the varied CsI/DMAPbI3 molar ratio on the defect property of the CsxDMA1-xPbI3 films, we employed the space-charge-limited current method to analyze the electron trap-state density (Figures 4A–4D). The current increases linearly as a function of voltage (indicating Ohmic conduction) up to kink, beyond which the current shows a rapid rise due to the filling of trap states by injected carriers. The voltage at the kink is known as trap-filled limit voltage (VTFL), often used to determine the density of trap states through the equation:
where e is the elementary charge, nt is the density of trap states, L is the film thickness between the two contacts, ε0 is the vacuum permittivity, and ε is the relative permittivity of CsPbI3 (ε = 6.32ε0) (Tong et al., 2016). VTFL and nt for different Cs+ concentrations are summarized in Table S1. We noticed that the Cs-0.5 film exhibits lowest density of trap states, which is further evidenced with the strongest luminescence intensity and the longest carrier lifetime from the photoluminescence (PL) and time-resolved PL spectra results, respectively (Figures 4E and 4F). Our results clearly demonstrate the positive effect of engineering the CsI/DMAPbI3 molar ratio on reducing the defect states in the resulting CsxDMA1-xPbI3 films.
Figure 4.
Evaluation of Defect States of CsxDMA1-xPbI3 Perovskite films
(A–D) Logarithmic plotted dark I-V curves of electron-only devices (FTO/TiO2/CsxDMA1-xPbI3 films/[6,6]-phenyl-C61-butyric acid methyl ester (PCBM)/Au)) based on (A) Cs-0.3, (B) Cs-0.5, (C) Cs-0.7, and (D) Cs-0.9 films; see also Table S1.
(E) PL spectra for CsxDMA1-xPbI3 perovskite films.
(F) Time-resolved PL decay trace of CsxDMA1-xPbI3 perovskite films; see also Table S2.
Having demonstrated the improved stability for films with optimized CsI/DMAPbI3 molar ratio, we further evaluated device efficiency and the stability of resulting complete devices (Figure S7). The PCEs with different ratios are shown in Figures 5A and 5B, and device parameters are summarized in Table S3. The optimal composition (i.e., Cs-0.5) yields a champion PCE of 14.3% with a Voc of 1.05 V, a short-circuit current density (Jsc) of 18.4 mA/cm2, and a filling factor of 0.74 (Figure 5C) based on the active layer processed at Cs-0.5, in good agreement with our findings when evaluating the perovskite film quality. We further found that the high performance for Cs-0.5 PSCs was consistent with their high crystal quality and good energy-level alignment at the electron transport interface (Figures S8 and S9). The steady power output for this champion device sustains at 12.8%, measuring at the fixed voltage of 0.849 V close to the maximum power point (Figure 5D), and the external quantum efficiency (EQE) shows an integrated Jsc value at 16.1 mA/cm2 (Figure 5E). We noted that the Cs-0.5 PSCs also exhibit good reproducibility (Figure S10) and good stability, with the best device maintaining 85% of its initial efficiency after 20 days of air exposure (RH 20% ± 5%) without encapsulation (Figure 5F).
Figure 5.
Photovoltaic Performance of CsxDMA1-xPbI3 Perovskite Solar Cells
(A) J-V curves of the solar cells based on CsxDMA1-xPbI3 perovskite films with different compositions (Cs from 0.3 to 0.9); see also Table S3.
(B) PCE of devices with varied CsxDMA1-xPbI3 films as a function of the ratio of CsI/DMAPbI3 in the precursor (Cs from 0.3 to 0.9).
(C) J-V curves of the champion device (Cs-0.5) in reverse and forward scan directions.
(D) Steady-state PCE and current density of the champion device (Cs-0.5) measured at maximum-power point of 0.849 V.
(E) External quantum efficiency spectra of the best solar cell based on the Cs-0.5 perovskite film.
(F) Evaluation of long-term stability of the device (Cs-0.5) stored in air (RH 20% ± 5%).
Conclusion
In summary, we performed comprehensive characterization of hydrolyzed DMAPbI3 material and systematic evaluation of its effect on fabricating high-quality Cs-based PSCs. Through a series of detailed analysis of the composition and properties of DMAPbI3, including in situ TG-FTIR and NMR, we unambiguously determine the existence of DMAPbI3 synthesized from the popular fabrication process—dissolving PbI2 in DMF with addition of HI—due to the hydrolysis of DMF. By carefully controlling the CsI and DMAPbI3 molar ratio in the precursor, we achieve an optimal composition essential for obtaining high-quality CsxDMA1-xPbI3 perovskite films with uniform morphology and low density of trap states, which further leads to optimized CsxDMA1-xPbI3 PSCs with highly reproducible PCEs up to 14.3%. Our findings provide an in-depth understanding on the product from hydrolyzed DMF and offer insightful guidelines for achieving high-efficiency stable Cs-based perovskite devices.
Limitation of the Study
In this work, we dissolve PbI2 and HI in DMF to synthesize DMAPbI3 and confirm that the “mythical” HPbI3 does not exist (which actually should be DMAPbI3) by providing a comprehensive analysis on the elementary information of the reaction products at every stage. However, the CsxDMA1-xPbI3 perovskite film is hard to be stabilized when x is approaching 1 by reacting such DMAPbI3 with CsI. It would be more interesting if the phase transition of CsxDMA1-xPbI3 perovskite films was studied through in situ measurement, to further investigate the effects of Cs+/DMA+ ratio on the phase transition of CsxDMA1-xPbI3 perovskites.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the National Key R&D Program of China (2017YFA0207400), the National Natural Science Foundation of China (61604032), and the Fundamental Research Funds for the Central Universities in China (ZYGX2016J206).
Author Contributions
M.L. conceived the idea of the work. Y.P. and S.B. proposed the experimental design. Y.L. synthesized and characterized the DMAPbI3 powder. Y.P. and F.L. performed the device fabrication and corresponding characterization. X.J. performed in situ TG-FTIR. All authors contributed to the manuscript.
Declaration of Interests
The authors declare no competing interests.
Published: May 31, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.04.024.
Supplemental Information
References
- Daub M., Hillebrecht H. On the demystification of “HPbI3” and the peculiarities of the non-innocent solvents H2O and DMF. Z. Anorg. Allg. Chem. 2018;644:1393–1400. [Google Scholar]
- Eperon G.E., Paterno G.M., Sutton R.J., Zampetti A., Haghighirad A.A., Cacialli F., Snaith H.J. Inorganic caesium lead iodide perovskite solar cells. J. Mater. Chem. A. 2015;3:19688–19695. [Google Scholar]
- Fu Y., Rea M.T., Chen J., Morrow D.J., Hautzinger M.P., Zhao Y., Pan D., Manger L.H., Wright J.C., Goldsmith R.H. Selective stabilization and photophysical properties of metastable perovskite polymorphs of CsPbI3 in thin films. Chem. Mater. 2017;29:8385–8394. [Google Scholar]
- Hu Y., Bai F., Liu X., Ji Q., Miao X., Qiu T., Zhang S. Bismuth incorporation stabilized α-CsPbI3 for fully inorganic perovskite solar cells. ACS Energy Lett. 2017;2:2219–2227. [Google Scholar]
- Jena A.K., Kulkarni A., Sanehira Y., Ikegami M., Miyasaka T. Stabilization of α-CsPbI3 in ambient room temperature conditions by incorporating Eu into CsPbI3. Chem. Mater. 2018;30:6668–6674. [Google Scholar]
- Jeon N.J., Noh J.H., Kim Y.C., Yang W.S., Ryu S., Seok S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014;13:897–903. doi: 10.1038/nmat4014. [DOI] [PubMed] [Google Scholar]
- Ju D., Zhao T., Yangyang D., Zhang G., Hu X., Cui D., Tao X. Gas induced conversion of hybrid perovskite single crystal to single crystal for great enhancement of their photoelectric properties. J. Mater. Chem. A. 2017;5:21919–21925. [Google Scholar]
- Ke W., Spanopoulos I., Stoumpos C.C., Kanatzidis M.G. Myths and reality of HPbI3 in halide perovskite solar cells. Nat. Commun. 2018;9:4785. doi: 10.1038/s41467-018-07204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau C.F.J., Deng X., Zheng J., Kim J., Zhang Z., Zhang M., Bing J., Wilkinson B., Hu L., Patterson R. Enhanced performance via partial lead replacement with calcium for a CsPbI3 perovskite solar cell exceeding 13% power conversion efficiency. J. Mater. Chem. A. 2018;6:5580–5586. [Google Scholar]
- Li B., Zhang Y., Fu L., Yu T., Zhou S., Zhang L., Yin L. Surface passivation engineering strategy to fully-inorganic cubic CsPbI3 perovskites for high-performance solar cells. Nat. Commun. 2018;9:1076. doi: 10.1038/s41467-018-03169-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Pei Y., Xiao F., Zeng T., Yang Z., Xu J., Sun J., Peng B., Liu M. Tailored dimensionality to regulate the phase stability of inorganic cesium lead iodide perovskites. Nanoscale. 2018;10:6318–6322. doi: 10.1039/c8nr00758f. [DOI] [PubMed] [Google Scholar]
- Liao J., Rao H., Chen B., Kuang D., Su C. Dimension engineering on cesium lead iodide for efficient and stable perovskite solar cells. J. Mater. Chem. A. 2017;5:2066–2072. [Google Scholar]
- Long M., Zhang T., Chai Y., Ng C.-F., Mak T.C.W., Xu J., Yan K. Nonstoichiometric acid–base reaction as reliable synthetic route to highly stable CH3NH3PbI3 perovskite film. Nat. Commun. 2016;7:13503. doi: 10.1038/ncomms13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P., Xia W., Zhou S., Sun L., Cheng J., Xu C., Lu Y. Solvent engineering for ambient-air-processed, phase-stable CsPbI3 in perovskite solar cells. J. Phys. Chem. Lett. 2016;7:3603–3608. doi: 10.1021/acs.jpclett.6b01576. [DOI] [PubMed] [Google Scholar]
- Mancini A., Quadrelli P., Amoroso G., Milanese C., Boiocchi M., Sironi A., Patrini M., Guizzetti G., Malavasi L. Synthesis, structural and optical characterization of APbX3 (A=methylammonium, dimethylammonium, trimethylammonium, X=I, Br, Cl) hybrid organic-inorganic materials. J. Solid State Chem. 2016;240:55–60. [Google Scholar]
- Noel N.K., Congiu M., Ramadan A.J., Fearn S., McMeekin D.P., Patel J.B., Johnston M.B., Wenger B., Snaith H.J. Unveiling the influence of pH on the crystallization of hybrid perovskites, delivering low voltage loss photovoltaics. Joule. 2017;1:328–343. [Google Scholar]
- Pang S., Zhou Y., Wang Z., Yang M., Krause A.R., Zhou Z., Zhu K., Padture N.P., Cui G. Transformative evolution of organolead triiodide perovskite thin films from strong room-temperature solid-gas interaction between HPbI3-CH3NH2 precursor pair. J. Am. Chem. Soc. 2016;138:750–753. doi: 10.1021/jacs.5b11824. [DOI] [PubMed] [Google Scholar]
- Sanehira E.M., Marshall A.R., Christians J.A., Harvey S.P., Ciesielski P.N., Wheeler L.M., Schulz P., Lin L.Y., Beard M.C., Luther J.M. Enhanced mobility CsPbI3 quantum dot arrays for record-efficiency, high-voltage photovoltaic cells. Sci. Adv. 2017;3:eaao4204. doi: 10.1126/sciadv.aao4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber M., Zamoshchik N., Khakhan O., Zuck A. Structural changes during vapor-phase deposition of polycrystalline-PbI2 films. J. Cryst. Growth. 2008;310:3168–3173. [Google Scholar]
- Shi Z., Zhang Y., Cui C., Li B., Zhou W., Ning Z., Mi Q. Symmetrization of the crystal lattice of MAPbI3 boosts the performance and stability of metal–perovskite photodiodes. Adv. Mater. 2017;29:1701656. doi: 10.1002/adma.201701656. [DOI] [PubMed] [Google Scholar]
- Sutherland B.R. Perovskite precursors get a pH tune-up. Joule. 2017;1:221–223. [Google Scholar]
- Swarnkar A., Marshall A.R., Sanehira E.M., Chernomordik B.D., Moore D.T., Christians J.A., Chakrabarti T., Luther J.M. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science. 2016;354:92–95. doi: 10.1126/science.aag2700. [DOI] [PubMed] [Google Scholar]
- Tong Y., Bladt E., Ayguler M.F., Manzi A., Milowska K.Z., Hintermayr V.A., Docampo P., Bals S., Urban A.S., Polavarapu L. Highly luminescent cesium lead halide perovskite nanocrystals with tunable composition and thickness by ultrasonication. Angew. Chem. Int. Ed. 2016;55:13887–13892. doi: 10.1002/anie.201605909. [DOI] [PubMed] [Google Scholar]
- Wang F., Yu H., Xu H., Zhao N. HPbI3: a new precursor compound for highly efficient solution-processed perovskite solar cells. Adv. Funct. Mater. 2015;25:1120–1126. [Google Scholar]
- Wang Q., Zheng X., Deng Y., Zhao J., Chen Z., Huang J. Stabilizing the α-phase of CsPbI3 perovskite by sulfobetaine zwitterions in one-step spin-coating films. Joule. 2017;1:371–382. [Google Scholar]
- Wang K., Jin Z., Liang L., Bian H., Bai D., Wang H., Zhang J., Wang Q., Liu S. All-inorganic cesium lead iodide perovskite solar cells with stabilized efficiency beyond 15% Nat. Commun. 2018;9:4544. doi: 10.1038/s41467-018-06915-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P., Zhang X., Zhou Y., Jiang Q., Ye Q., Chu Z., Li X., Yang X., Yin Z., You J. Solvent-controlled growth of inorganic perovskite films in dry environment for efficient and stable solar cells. Nat. Commun. 2018;9:2225. doi: 10.1038/s41467-018-04636-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhang T., Kan M., Li Y., Wang T., Zhao Y. Efficient α-CsPbI3 photovoltaics with surface terminated organic cations. Joule. 2018;2:2065–2075. [Google Scholar]
- Wang Y., Zhang T., Kan M., Zhao Y. Bifunctional Stabilization of All-inorganic α-CsPbI3 perovskite for 17% efficiency photovoltaics. J. Am. Chem. Soc. 2018;140:12345–12348. doi: 10.1021/jacs.8b07927. [DOI] [PubMed] [Google Scholar]
- Zhang T., Dar M.I., Li G., Xu F., Guo N., Gratzel M., Zhao Y. Bication lead iodide 2D perovskite component to stabilize inorganic α-CsPbI3 perovskite phase for high-efficiency solar cells. Sci. Adv. 2017;3:e1700841. doi: 10.1126/sciadv.1700841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Jin S., Huang S., Liu N., Ma J., Xue D., Han Q., Ding J., Ge Q., Feng Y. Thermodynamically stable orthorhombic γ-CsPbI3 thin films for high-performance photovoltaics. J. Am. Chem. Soc. 2018;140:11716–11725. doi: 10.1021/jacs.8b06050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.