Abstract
Background
Keratoconus is a slowly progressive ectatic deformity of the cornea with a prevalence of 200 to 400 cases per 100 000 persons. The cornea is thinner than normal and irregularly warped; irregular astigmatism and myopia result. Riboflavin-UVA crosslinking (collagen cross-linking) makes corneal tissue more rigid through a photochemical effect and can stop the progression of the disease.
Methods
This review is based on relevant publications retrieved by a selective search in Medline, as well as on meta-analyses, Cochrane Reviews, and reports of national and international health care institutions.
Results
Pertinent randomized controlled trials (RCTs) have shown that cross-linking prevents the progression of keratoconus to a statistically significant extent, as determined by measurement of topographic parameters. In the largest RCT to date (follow-up of 100 eyes for three years), the maximal corneal refractive power increased by 1.75 ± 0.38 diopters in the control group and decreased by -1.03 ± 0.19 diopters in the cross-linking group (p <0.001). This was also the only trial in which data were reported on the patient-relevant endpoint of uncorrected visual acuity, which mildly improved in the cross-linking group (-0.15 ± 0.06 logMAR, p = 0.009). Serious complications of cross-linking are known to date only from a few reports of individual cases. Cohort studies with follow-up times of up to ten years have shown that the condition can continue to progress after cross-linking, especially in younger patients.
Conclusion
Cross-linking is the first available treatment for keratoconus that can improve the natural course of the disease.
Keratoconus is a corneal disorder involving progressive deformation and thinning of the cornea due to hitherto unknown causes. The disorder often begins in the second decade of life and always affects both eyes, albeit sometimes to highly varying degrees. The disease incidence is approximately 13 cases/100 000 inhabitants per year, with the prevalence lying between 200 and 400 affected individuals/100 000 inhabitants (1). Children and adolescents with, e.g., atopic dermatitis, a positive family history, or trisomy 21 are at greater risk. Rahi et al. found atopy in 35% of individuals affected by keratoconus, whereas this was the case in only 12% of control subjects (2). However, frequent vigorous eye rubbing among atopic individuals might explain the correlation with keratoconus, since after a multivariate risk factor analysis only eye rubbing but not atopy remained a significant predictor of keratoconus (3). Therefore, early indications of keratoconus can also be identified by the dermatologist or pediatrician; patients who develop visual difficulties should be referred to an ophthalmologist.
Since the cornea with its refractive power plays an important role in the optical system of the eye, progressive corneal deformation results in an increase in refractive power and subsequent myopic shift, as well as in an (increasingly irregular) curvature of the cornea (astigmatism). Affected individuals first notice a non-specific deterioration in vision, which prompts a visit to the ophthalmologist, who often initially diagnoses moderate short-sightedness—and possibly astigmatism. The cone-shaped protrusion is typically displaced in a downwards direction, explaining the progressive irregularity of corneal refractive power. Ultimately, this leads to a situation in which visual function can no longer be adequately corrected with glasses, resulting in the suspected diagnosis of keratoconus. The older the affected individual, the slower the disease progresses (4). Whether a complete halt in disease progression can occur has not been conclusively established as yet, since there are no studies involving lifelong follow-up.
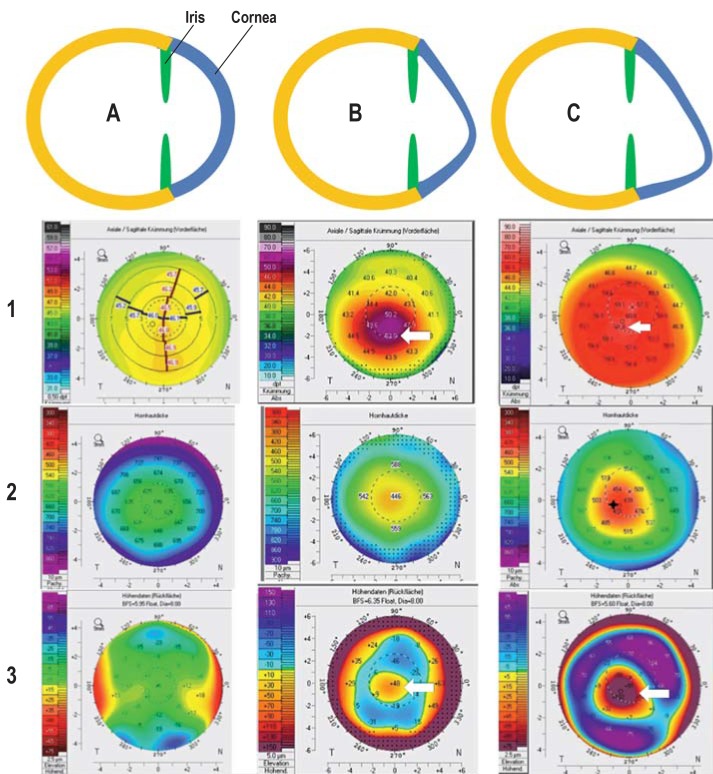
At early stages, the disease is generally not diagnosed in the context of routine ophthalmological examinations, since there are virtually no morphological changes. Computer-assisted measurement of the cornea (corneal topography or corneal tomography) can help to confirm or exclude the suspected diagnosis of keratoconus. Typical topo-/tomographic findings, such as increased paracentral corneal refractive power, protrusion of the anterior and/or posterior corneal surfaces, as well as paracentral corneal thinning, are seen in the case of keratoconus. In the case of progression, all these findings increase at varying degrees and speeds over the disease course and can be quantified by means of repeated topo-/tomographic examinations (figure 1). When interpreting examination results, one must bear in mind that examinations performed on different systems cannot be compared with one another (5) and that each method has system-specific measurement fluctuations (6, 7). Morphological findings that are visible to the ophthalmologist generally do not occur until later stages of the disease.
Figure 1.
Progressive disease course in keratoconus as a schematic representation with examples of tomographic findings
A: Normal corneal shape; unremarkable tomography with evenly distributed anterior corneal refractive power (1), corneal thickness (2), and curvature of the posterior corneal surface (3)
B: Early-stage keratoconus with marked deformation in a downward direction (increase in refractive power on the anterior corneal surface up to 52 diopters [1, arrow] and “island-shaped” protrusion of the posterior corneal surface [3, arrow]) and corneal thinning to 446 µm (2)
C: Late-stage keratoconus with marked deformation in a downward direction (increase in refractive power on the anterior corneal surface up to 61.8 diopters [1, arrow] and “island-shaped” protrusion of the posterior corneal surface [3, arrow]) and corneal thinning to 429 µm (2)
Figure: Archives of the Eye Center at the University of Freiburg Medical Center, Germany
The aim of this article is to critically present and discuss—in an evidence-based manner and using the currently available literature—the efficacy of cross-linking to halt the progression of keratoconus.
Disease course and treatment options to date
Early on in the disease, when affected individuals experience the first symptoms, changes in corneal refractive power can generally be corrected with glasses. As astigmatism becomes increasingly irregular, special dimensionally rigid contact lenses mostly need to be used. If, eventually, contact lenses can no longer be fitted, corneal transplantation may become necessary for the purposes of visual rehabilitation.
An observational study on 2363 patients found that approximately 22% required keratoplasty for visual rehabilitation (8). The prognosis for the majority of keratoconus patients following keratoplasty is excellent (9, 10), although one must anticipate that, in the often young patients, a second transplant may be necessary in the further course. Atopic patients have a somewhat poorer prognosis (11) since, due to chronic blepharokeratoconjunctivitis, they frequently experience inflammatory flare ups on the ocular surface and associated corneal vascularization, which increase the risk of transplant rejection following corneal transplantation.
It would be beneficial to all those affected if disease progression could be stopped or slowed down, thereby precluding the need for corneal transplantation. Early indirect evidence in the literature (12, 13) suggests that in recent years, since the introduction of cross-linking, ever fewer keratoconus patients require keratoplasty.
Progression of keratoconus
As a rule, progression of the disease differs considerably from individual to individual. The younger the affected individuals are, the higher their risk for (rapid and pronounced) progression (4). Progression may be stimulated by vigorous and frequent eye rubbing (14). There are currently no standardized guidelines for the definition of disease progression. Numerous clinical studies have used different parameters to this end. The most important parameters include (15):
An increase in maximum corneal refractive power (Kmax) by more that 1 dpt within 1 year
An increase in (corneal) myopia by more than 3 dpt or astigmatism by more than 1.5 dpt within 12 months
An increase in mean corneal refractive power by more than 1.5 dpt within 12 months
A reduction in minimal corneal thickness of more that 5% within 12 months.
A decline in visual acuity appears to be a less suitable parameter to determine progression, since keratoconus patients often report variable vision (16) and objective findings do not always correspond to subjective perception (17). In addition, eyeglass lenses can hamper the determination of refraction, or contact lens correction can compensate for altered values of corneal refractive power, meaning that no deterioration in vision can be determined despite altered corneal refractive power.
Regular topo-/tomographic examinations are required to identify disease progression. The measurement fluctuations for the respective parameters need to be taken into consideration in the diagnosis. Therefore, suspected progression should always be repeatedly confirmed in the further disease course. With regard to examination intervals, the individual risk profiles of affected individuals need to be taken into consideration (risk factors: eye rubbing, young patient age, steep corneal curvature gradient, high astigmatism, marked loss of vision, confirmed progression in the fellow eye, ocular allergies, atopic dermatitis, or trisomy 21). Therefore, it is recommended that young patients under the age of 25 years be monitored more frequently (e.g., every 3–6 months) and older patients less frequently (e.g., every 6–12 months).
Cross-linking in keratoconus
Much like the stiffening of heterologous heart valve transplants (18), the principle of riboflavin-UVA cross-linking is based on a photochemical effect that was first presented by Spoerl et al. in 1998 (19). Cross-linking was first used in patients at the end of the 1990s, while the first clinical results were published in 2003 (20).
Cross-linking procedure
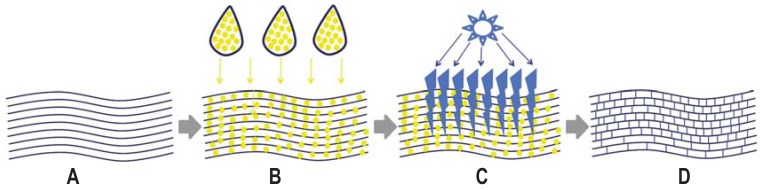
Following removal of the corneal epithelium, the riboflavin applied via eye drops penetrates to the deep corneal layers. There it absorbs the UVA light (370 nm wavelength, 3 mW/cm² irradiation) with which the cornea is irradiated for 30 min. This produces free oxygen radicals that lead to the creation of covalent bonds between the collagen fibrils in the corneal stroma (figure 2). Riboflavin also has a protective effect, since only when the stroma is saturated with riboflavin, will the high-energy UVA light be sufficiently absorbed in the cornea, thus preventing damage to intraocular structures.
Figure 2.
Mode of action of corneal cross-linking with riboflavin and UVA A: Parallel arrangement of fibrils in the corneal stroma following removal of the epithelium B: Application of riboflavin eye drops until saturation of the corneal stroma is achieved C: Corneal irradiation with UVA light (370 nm, 3 mW/cm²); for safety reasons, corneal thickness should not be less than 400 µm. D: Cross-linked collagen fibrils Figure: Archives of the Eye Center at the University of Freiburg Medical Center, Germany
Corneal thickness also plays a crucial role here: this should not become thinner than 400 µm during irradiation, since intraocular structures, such as the corneal endothelium, would otherwise be at risk (21). It is important to bear in mind in relation to corneal thickness that thinning can occur during treatment. This can be compensated in the short term by the use of hypotonic riboflavin eye drops, which in turn, can reduce the effectiveness of the treatment (22).
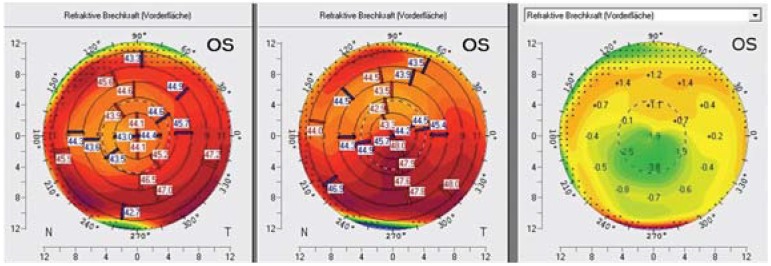
The treatment method described above has now become established as the “Dresdner protocol.” The aim of cross-linking is to stabilize the corneal tissue in order to halt or at least slow down disease progression; however, a cure as such is not possible. Thus, no further changes in topo-/tomographic parameters consistent with progression are seen following cross-linking. In some cases, a reduction in corneal refractive power and regularization of the corneal surface is seen, which can be associated with an improvement in visual acuity (figure 3). The investigation conducted by Wittig-Silva et al. found a reduction in maximum corneal refractive power by more than 2 dpt in 13% of participants in the cross-linking group (23).
Figure 3.
Course following cross-linking. Tomography at the time of cross-linking (center) and 3 months following cross-linking (left). The anterior corneal surface exhibits virtually normal refractive power (left) with a decrease of up to 3.8 dpt in the difference map of the two measurements (right, central green area).
Figure: Archives of the Eye Center at the University of Freiburg Medical Center, Germany
Also, by halting disease progression, it was possible to prevent keratoconus from advancing to a point where corneal transplantation becomes necessary.
Details on possible side effects and complications, as well as on current variants such as transepithelial or accelerated cross-linking can be found in the eMethods section.
Target group and indication for cross-linking
Cross-linking should be performed at a stage of disease in which affected individuals still have adequately good visual acuity. Furthermore, primarily those patients in whom progression has previously been identified should be treated.
Since disease progression is more pronounced in young individuals than it is in older patients, cross-linking is of considerable relevance to young patients in particular. For example, study results suggest that the treatment effect in young people could be more pronounced than in older patients, whereby a mean reduction in maximum corneal refractive power of 1.27 dpt was observed within 2 years following cross-linking in patients under 18 years of age (24).
In addition, complications also appear to occur less frequently in younger patients. For instance, a complication rate of 1% was observed in under 35-year-olds compared to a complication rate of 3% when all age groups were considered (25). However, a decline in treatment effect and renewed progression appear to occur more frequently in young patients. Mazzotta et al. reported that keratoconus progressed within a follow-up period of 10 years in 24% of young patients aged 15 years or younger (26). Therefore, regular check-ups (depending on the risk profile, e.g., patient age) should be performed even after treatment, initially every 6 months and later annually or, in the case of subjective symptoms, in the interim.
Methods
In addition to countless case series and cohort studies, a number of randomized controlled trials have now also been conducted. Therefore, we performed a literature search in Medline using the terms “keratoconus (cross-link* or crosslink*) trial,” which yielded six relevant studies out of 131 hits (inclusion criteria: randomized, controlled, at least 12 months follow-up; see Table). Meta-analyses, Cochrane reviews, and reports compiled by national and international healthcare institutions were also taken into account.
Table. Randomized controlled trials on cross-linking (CXL, Dresdner protocol) in keratoconus.
| Study | Patient number | Follow-up (months) | Age (mean)(years) | Intervention | Outcome | Primary endpoint | Limitations | Complications | Evidence level |
| O’Brart et al., 2011 (27) |
24 | 18 | 29.6 | Same patients with one eye as control, one eye CXL | Statistically significant effect of cross-linking on the change in maximum topographic K-reading | Mean change in Ksim at 18 months CXL: –0.66 D Control: +0.14 D |
Interdependence of eyes if both patient eyes included, no sham treatment | One Patient with temporary recurrent erosion | Ib |
| Hersh et al., 2011 (28) |
59 | 12 | n.s. | 28 patients sham-treated, 31 patients CXL, crossover pos‧sible after 3 months | Best-corrected visual acuity and Kmax were significantly better than baseline findings and control group findings | Mean change in Kmax after 12 months CXL: –2.0 ± 4.4 D Control: Not specified due to crossover |
Crossover design, only small number of patients in the control group for the analysis | Not specified | Ib |
| Wittig-Silva et al., 2014 (23) |
100 Eyes | 36 | 25.7 | 50 control eyes, 50 CLX eyes, in some cases both patient eyes randomized | Statistically significant difference in maximum corneal refractive power and uncorrected visual acuity | Mean change in Kmax after 36 months CXL: –1.03 ± 0.19 D Control: +1.75 ± 0.38 D |
Interdependence of eyes if both patient eyes included, no sham treatment | Two sterile temporary infiltrates with no permanent damage | Ib |
| Lang et al., 2015 (29) |
29 | 36 | 29.5 | 14 sham-treatedpatients, 15 CXL patients | Statistically significant effect of cross-linking on the change in maximum topographic K-reading | Mean change in Kmax after 36 months CXL: –0.35 ± 0.58 D Control: +0.11 ± 0.61 D |
Low patient number (weak power) | Temporary corneal opacities (haze) and complete remission | Ib |
| Seyedian et al., 2015 (30) |
26 | 12 | 25.6 | Same patients with one eye as control, one eye CXL | Statistically significant effect of cross-linking on the change in maximum topographic K-reading | Mean change in Kmax after 12 months CXL: –0.22 ± 0.6 D Control: +0.41 ± 0.74 D |
Interdependence of eyes if both patient eyes included, no sham treatment | Temporary corneal opacities (haze) and complete remission | Ib |
| Hersh et al., 2017 (31) |
205 | 12 | 33.0 | 102 CXL; 103 sham-treated patients | Statistically significant effect of cross-linking on the change in maximum topographic K-reading and corrected visual acuity | Kmax after 12 months CXL: –1.6 ± 4.2 D Control: +1.0 ± 5.1 D |
High drop-out rate, summary of two independent studies (published in part in Hersh et al. 2011 [28], see above), crossover ‧design, only a small number of patients in the control group (n = 2) for the analysis | Short term: one patient with ulcerative keratitis; long term: persistent corneal opacities (haze) in two eyes, ‧endothelial folds in one eye; corneal scarring in one eye, irregular epithelium in one eye | Ib |
CXL = cross-linking; n. s.= not specified; Kmax = maximum keratometry reading; Control = control group; Ksim = simulated ketatometry
Results
Randomized controlled trials (evidence level Ib)
All studies identified and included on the basis of the literature search showed a statistically significantly positive effect for cross-linking on the change in maximum corneal refractive power (Kmax). Furthermore, some studies also found a positive effect on uncorrected or corrected visual acuity. Wittig-Silva et al. (23), who included and followed up 100 eyes with progressive keratoconus for 3 years, found an increase in maximum corneal refractive power of 1.75 ± 0.38 dpt in the control group, whereas a flattening of -1.03 ± 0.19 dpt was seen in the cross-linking group (p <0.001). Moreover, a deterioration in uncorrected visual acuity of + 0.1 ± 0.04 logMAR (logarithm of the minimum angle of resolution) was observed in the control group, while a mild improvement in uncorrected visual acuity of -0.15 ± 0.06 logMAR was seen in the cross-linking group (p = 0.009). However, all studies published to date (23, 27– 31) have methodological weaknesses that need to be taken into account when interpreting their results. Overall, only very few complications and adverse effects were reported in the studies discussed here. Detailed information on the studies’ methods, effects, complications, and methodological deficiencies can be found in the Table.
Meta-analyses (evidence level Ia)
Despite the methodological weaknesses described in the Table and differences in the randomized controlled trials published to date, a number of working groups have attempted to bring these studies together in meta-analyses. However, the results of these systematic reviews should be interpreted with caution, since it is difficult to statistically combine the respective studies due to their considerable heterogeneity. Kobashi et al. (who included five studies with altogether 289 eyes, [32]) reached the conclusion in their systematic review that cross-linking can effectively halt the progression of keratoconus, although the evidence for this is limited due to the heterogeneity and methodological weaknesses of the individual studies. Therefore, it was not possible to meta-analytically summarize the results on maximum corneal refractive power due to the high heterogeneity (I² = 81%). Li et al. (who included six studies with 261 eyes in total, [33]) also confirmed the efficacy of cross-linking to halt the progression of keratoconus (the weighted mean difference for maximum corneal refractive power was -2.05; 95% confidence interval: [-3.10;–1.00]; p <0.00001). However, it was not possible at the time of the study to estimate medium- and long-term effects, since most studies had short follow-up periods.
Cochrane Review and other reports
In a 2015 Cochrane Review, Sykakis et al. (34) concluded that there is still insufficient evidence to demonstrate the efficacy of cross-linking, despite almost 700 published studies. The National Institute for Health and Care Excellence (NICE) in Great Britain came to the conclusion that there is sufficient qualitative as well as quantitative evidence for the efficacy of cross-linking, on the basis of which approval was recommended (35). The procedure was also approved by the FDA in the US due to a lack of alternatives, despite the fact that the evidence is classified as weak (31, 36). In its report, the German Institute for Quality and Efficiency in Health Care (Deutsche Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen, IQWiG) came to the conclusion that there is an indication pointing to the efficacy of cross-linking with regard to uncorrected visual acuity (1). It must be borne in mind here that this report only took into consideration patient-relevant endpoints (visual acuity) in the randomized controlled trials, meaning that, once the raw data had been statistically processed, the results of only one single study (23) lead to this conclusion.
As already discussed in the section “Progression of keratoconus,” it seems reasonable from an ophthalmologist’s point of view, on the other hand, to consider not only visual acuity but also the change in corneal shape or refractive power when assessing disease course in keratoconus patients, since these changes generally precede a deterioration in vision. The German Federal Joint Committee (Gemeinsamer Bundesausschuss, G-BA) has now decided, on the basis of the current evidence, to include cross-linking in the catalog of procedures covered by statutory health insurance in Germany.
Summary and outlook
Randomized controlled trials have demonstrated in recent years that riboflavin-UVA cross-linking is successfully able to halt disease progression in keratoconus patients. What is of particular importance here is that keratoconus progression is reliably identified, before the indication for treatment is made. This standardized treatment procedure with a low side-effects profile has now become firmly established in Germany. There is also an increasing number of reports on further developments such as transepithelial or accelerated cross-linking, both of which promise benefits for patients, but whose efficacy compared to standard cross-linking has not yet been demonstrated.
Supplementary Material
eMethods
Side effects and complications
Overall, cross-linking is a procedure with a low side-effects profile. However, the literature reports a complication risk of 1%–10% (e1), although the complications frequently seen are transient effects such as impaired epithelial healing. Only isolated cases of severe complications (such as corneal melting or corneal perforation) have been reported. Since cross-linking according to the Dresdner protocol involves initial removal of the cornea, severe pain occurs during the first 1–3 days following the procedure; this generally needs to be managed with pain medication. There is also a risk of infection during this period, as a result of which preventive treatment with antibiotic eye drops or eye ointments is necessary. If corneal infiltration develops nevertheless, a distinction needs to be made between sterile infiltrates (0%–8% of cases, [24]) and infectious infiltrates (individual case reports [e2]). Both scenarios can lead to the formation of corneal scarring, which may cause visual impairment in the long term (0%–3%, [24]). Irrespective of this, persistent scarring following cross-linking is reported in 3%–9% of cases (e3). The side effect most frequently observed is so-called haze, involving a fine haziness in the anterior corneal stroma, which generally has no effect on vision and resolves completely within a number of months (24). Ultimately, cross-linking is not able to stabilize disease course in all patients. For example, renewed progression was reported in up to 24% of cases (25) within 10 years following cross-linking in children.
Modified treatment procedure
Since cross-linking according to the Dresdner protocol has already been in use for almost 20 years, various modified treatment procedures have now been developed and investigated in studies and will be briefly discussed below.
Transepithelial cross-linking
The aim of transepithelial cross-linking it to dispense with removal of the corneal epithelium at the start of treatment. This reduces pain and the risk of infection (e4). However, since riboflavin cannot diffuse through the intact corneal epithelium due to tight junctions (e5), there are a variety of approaches to achieve penetration of riboflavin into the stroma (e.g. addition of benzalkonium chloride [e6], iontophoresis [e7]). Since there are already numerous studies comparing transepithelial cross-linking with standard cross-linking, some of which report conflicting results, an attempt was made to summarize the in part highly heterogeneous studies in review articles and meta-analyses (evidence level Ia). A recent meta-analysis (e8) based on a review of randomized controlled trials came to the conclusion that transepithelial cross-linking is inferior to standard cross-linking according to the Dresdner protocol in terms of preventing further progression. Therefore, transepithelial cross-linking protocols should currently only be used in studies after patients have received all relevant information on the procedure.
Accelerated cross-linking
The aim of accelerated cross-linking is to shorten the irradiation time by intensifying UVA irradiation, meaning that the 70-min procedure according to the Dresdner protocol can be shortened, thereby reducing the burden on the patient and saving resources. The various protocols always comply with the same total energy density (5.4 J/cm²) set out in the Dresdner protocol. Thus, depending on the power of the UVA lamp used, the irradiation time can be varied (e.g., 10 min irradiation at 9 mW/cm² power). Early clinical studies at shortened irradiation times showed similar effects to those with the Dresdner protocol (e9), although the typically observed reduction in maximum corneal refractive power appears to be less marked with shorter irradiation (e10). However, it has not been conclusively elucidated as yet whether the same amount of covalent bonds can be achieved in less time. The availability of oxygen in the corneal stroma could be a limiting factor here. A recent meta-analysis revealed the Dresdner protocol to be superior in terms of halting progression compared to accelerated cross-linking (e11). Therefore, further study results also need to be awaited for accelerated cross-linking before it can be routinely used in patients. Finally, there are already novel approaches that attempt to increase the availability of oxygen in tissue by means of irradiation pauses during treatment (pulsed corneal cross-linking), which could result in more oxygen radicals and, in turn, enhance the cross-linking effect (e12). Further controlled studies need to investigate whether this approach is able to achieve equivalent efficacy compared to standard cross-linking.
Key Messages.
The diagnosis of keratoconus can be confirmed using corneal topography or tomography.
Regular topo-/tomographic check-ups are able to identify disease progression.
By means of a photochemical effect, riboflavin-UVA cross-linking results in a stabilization of corneal tissue, which, for the first time, offers the possibility to prevent disease progression in affected individuals.
A number of randomized controlled trials have demonstrated that cross-linking can halt or slow down disease progression.
Since the duration of efficacy for cross-linking is not yet sufficiently known, regular topo- or tomographic follow-up examinations are still required even after treatment.
Acknowledgments
Translated from the original German by Christine Schaefer-Tsorpatzidis.
Footnotes
Conflict of interest statement
The authors state that there are no conflicts of interest.
References
- 1.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen. Abschlussbericht N15-05 Hornhautvernetzung bei Keratokonus Oct 2016 p. 198. Report No.: 436. www.iqwig.de/de/projekte-ergebnisse/projekte/nichtmedikamentoese-verfahren/n15-05-uv-vernetzung-mit-riboflavin-bei-keratokonus.6714.html (last accessed on 14 February 2019) [Google Scholar]
- 2.Rahi A, Davies P, Ruben M, Lobascher D, Menon J. Keratoconus and coexisting atopic disease. Br J Ophthalmol. 1977;61:761–764. doi: 10.1136/bjo.61.12.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bawazeer AM, Hodge WG, Lorimer B. Atopy and keratoconus: a multivariate analysis. Br J Ophthalmol. 2000;84:834–836. doi: 10.1136/bjo.84.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McMahon TT, Edrington TB, Szczotka-Flynn L, Olafsson HE, Davis LJ, Schechtman KB. Longitudinal changes in corneal curvature in keratoconus. Cornea. 2006;25:296–305. doi: 10.1097/01.ico.0000178728.57435.df. [DOI] [PubMed] [Google Scholar]
- 5.Meyer JJ, Gokul A, Vellara HR, Prime Z, McGhee CNJ. Repeatability and agreement of Orbscan II, Pentacam HR, and Galilei tomography systems in corneas with keratoconus. Am J Ophthalmol. 2017;175:122–128. doi: 10.1016/j.ajo.2016.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Wonneberger W, Sterner B, MacLean U, Claesson M, Zetterberg M. Repeated same-day versus single tomography measurements of keratoconic eyes for analysis of disease progression. Cornea. 2018;37:474–479. doi: 10.1097/ICO.0000000000001513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomes JAP, Tan D, Rapuano CJ, et al. Global consensus on keratoconus and ectatic diseases. Cornea. 2015;34:359–369. doi: 10.1097/ICO.0000000000000408. [DOI] [PubMed] [Google Scholar]
- 8.Tuft SJ, Moodaley LC, Gregory WM, Davison CR, Buckley RJ. Prognostic factors for the progression of keratoconus. Ophthalmology. 1994;101:439–447. doi: 10.1016/s0161-6420(94)31313-3. [DOI] [PubMed] [Google Scholar]
- 9.Böhringer D, Schindler A, Reinhard T. [Satisfaction with penetrating keratoplasty Results of a questionnaire census] Ophthalmologe. 2006;103:677–681. doi: 10.1007/s00347-006-1373-0. [DOI] [PubMed] [Google Scholar]
- 10.Böhringer D, Böhringer S, Poxleitner K, et al. Long-term graft survival in penetrating keratoplasty: the biexponential model of chronic endothelial cell loss revisited. Cornea. 2010;29:1113–1117. doi: 10.1097/ICO.0b013e3181d21d07. [DOI] [PubMed] [Google Scholar]
- 11.Reinhard T, Möller M, Sundmacher R. Penetrating keratoplasty in patients with atopic dermatitis with and without systemic cyclosporin A. Cornea. 1999;18:645–651. doi: 10.1097/00003226-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 12.Lang SJ, Bischoff M, Bohringer D, Seitz B, Reinhard T. Analysis of the changes in keratoplasty indications and preferred techniques. PLoS One. 2014;9 doi: 10.1371/journal.pone.0112696. e112696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Godefrooij DA, Gans R, Imhof SM, Wisse RPL. Nationwide reduction in the number of corneal transplantations for keratoconus following the implementation of cross-linking. Acta Ophthalmol. 2016;94:675–678. doi: 10.1111/aos.13095. [DOI] [PubMed] [Google Scholar]
- 14.Sugar J, Macsai MS. What causes keratoconus? Cornea. 2012;31:716–719. doi: 10.1097/ICO.0b013e31823f8c72. [DOI] [PubMed] [Google Scholar]
- 15.Maier P, Reinhard T. [Riboflavin UVA crosslinking in progressive keratoconus] Ophthalmologe. 2017;114:571–586. doi: 10.1007/s00347-017-0500-4. [DOI] [PubMed] [Google Scholar]
- 16.Gordon MO, Schechtman KB, Davis LJ, McMahon TT, Schornack J, Zadnik K. Visual acuity repeatability in keratoconus: impact on sample size Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Optom Vis Sci. 1998;75:249–257. doi: 10.1097/00006324-199804000-00021. [DOI] [PubMed] [Google Scholar]
- 17.Davis LJ, Schechtman KB, Begley CG, Shin JA, Zadnik K. Repeatability of refraction and corrected visual acuity in keratoconus The CLEK Study Group. Collaborative Longitudinal Evaluation of Keratoconus. Optom Vis Sci. 1998;75:887–896. doi: 10.1097/00006324-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Sung HW, Chang Y, Chiu CT, Chen CN, Liang HC. Mechanical properties of a porcine aortic valve fixed with a naturally occurring crosslinking agent. Biomaterials. 1999;20:1759–1772. doi: 10.1016/s0142-9612(99)00069-1. [DOI] [PubMed] [Google Scholar]
- 19.Spoerl E, Huhle M, Seiler T. Induction of cross-links in corneal tissue. Exp Eye Res. 1998;66:97–103. doi: 10.1006/exer.1997.0410. [DOI] [PubMed] [Google Scholar]
- 20.Wollensak G, Spörl E, Seiler T. [Treatment of keratoconus by collagen cross linking] Ophthalmologe. 2003;100:44–49. doi: 10.1007/s00347-002-0700-3. [DOI] [PubMed] [Google Scholar]
- 21.Lange C, Böhringer D, Reinhard T. Corneal endothelial loss after crosslinking with riboflavin and ultraviolet-A. Graefes Arch Clin Exp Ophthalmol. 2012;250:1689–1691. doi: 10.1007/s00417-012-2101-x. [DOI] [PubMed] [Google Scholar]
- 22.Kaya V, Utine CA, Yilmaz ÖF. Intraoperative corneal thickness measurements during corneal collagen cross-linking with hypoosmolar riboflavin solution in thin corneas. Cornea. 2012;31:486–490. doi: 10.1097/ICO.0b013e31821e4286. [DOI] [PubMed] [Google Scholar]
- 23.Wittig-Silva C, Chan E, Islam FMA, Wu T, Whiting M, Snibson GR. A randomized, controlled trial of corneal collagen cross-linking in progressive keratoconus: three-year results. Ophthalmology. 2014;12:812–821. doi: 10.1016/j.ophtha.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 24.Vinciguerra P, Albé E, Frueh BE, Trazza S, Epstein D. Two-year corneal cross-linking results in patients younger than 18 years with documented progressive keratoconus. Am J Ophthalmol. 2012;154:520–526. doi: 10.1016/j.ajo.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Koller T, Mrochen M, Seiler T. Complication and failure rates after corneal crosslinking. J Cataract Refract Surg. 2009;35:1358–1362. doi: 10.1016/j.jcrs.2009.03.035. [DOI] [PubMed] [Google Scholar]
- 26.Mazzotta C, Traversi C, Baiocchi S, et al. Corneal collagen cross-linking with riboflavin and ultraviolet a light for pediatric keratoconus: ten-year results. Cornea. 2018;37:560–566. doi: 10.1097/ICO.0000000000001505. [DOI] [PubMed] [Google Scholar]
- 27.O’Brart DPS, Chan E, Samaras K, Patel P, Shah SP. A randomised, prospective study to investigate the efficacy of riboflavin/ultraviolet A (370 nm) corneal collagen cross-linkage to halt the progression of keratoconus. Br J Ophthalmol. 2011;95:1519–1524. doi: 10.1136/bjo.2010.196493. [DOI] [PubMed] [Google Scholar]
- 28.Hersh PS, Greenstein SA, Fry KL. Corneal collagen crosslinking for keratoconus and corneal ectasia: one-year results. J Cataract Refract Surg. 2011;37:149–160. doi: 10.1016/j.jcrs.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Lang SJ, Messmer EM, Geerling G, et al. Prospective, randomized, double-blind trial to investigate the efficacy and safety of corneal cross-linking to halt the progression of keratoconus. BMC Ophthalmol. 2015;15 doi: 10.1186/s12886-015-0070-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Seyedian MA, Aliakbari S, Miraftab M, Hashemi H, Asgari S, Khabazkhoob M. Corneal collagen cross-linking in the treatment of progressive keratoconus: a randomized controlled contralateral eye study. Middle East Afr J Ophthalmol. 2015;22:340–345. doi: 10.4103/0974-9233.159755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hersh PS, Stulting RD, Muller D, Durrie DS, Rajpal RK. United States multicenter clinical trial of corneal collagen crosslinking for keratoconus treatment. Ophthalmology. 2017;124:1259–1270. doi: 10.1016/j.ophtha.2017.03.052. [DOI] [PubMed] [Google Scholar]
- 32.Kobashi H, Rong SS. Corneal collagen cross-linking for keratoconus: systematic review. Biomed Res Int. 2017;2017 doi: 10.1155/2017/8145651. 8145651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li J, Ji P, Lin X. Efficacy of corneal collagen cross-linking for treatment of keratoconus: a meta-analysis of randomized controlled trials. PLoS One. 2015;10 doi: 10.1371/journal.pone.0127079. e0127079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sykakis E, Karim R, Evans JR, et al. Corneal collagen cross-linking for treating keratoconus. Cochrane Database Syst Rev. 2015 doi: 10.1002/14651858.CD010621.pub2. CD010621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.National Institute for Health and Care Excellence (NICE) Interventional procedures guidance. www.nice.org.uk/guidance/ipg466 (last accessed on 9 February 2019) [Google Scholar]
- 36.Jeng BH, Farid M, Patel SV, Schwab IR. Corneal cross-linking for keratoconus: a look at the data, the food and drug administration, and the future. Ophthalmology. 2016;123:2270–2272. doi: 10.1016/j.ophtha.2016.08.006. [DOI] [PubMed] [Google Scholar]
- E1.Kohlhaas M. [Complications and postoperative therapeutic strategies in cross-linking] Ophthalmol Z Dtsch Ophthalmol Ges. 2017;114:693–696. doi: 10.1007/s00347-017-0511-1. [DOI] [PubMed] [Google Scholar]
- E2.Eberwein P, Auw-Hädrich C, Birnbaum F, Maier PC, Reinhard T. [Corneal melting after cross-linking and deep lamellar keratoplasty in a keratoconus patient] Klin Monatsblätter Für Augenheilkd. 2008;225:96–98. doi: 10.1055/s-2008-1027128. [DOI] [PubMed] [Google Scholar]
- E3.Raiskup F, Hoyer A, Spoerl E. Permanent corneal haze after riboflavin-UVA-induced cross-linking in keratoconus. J Refract Surg. 2009;25:S824–S828. doi: 10.3928/1081597X-20090813-12. [DOI] [PubMed] [Google Scholar]
- E4.Ashwin PT, McDonnell PJ. Collagen cross-linkage: a comprehensive review and directions for future research. Br J Ophthalmol. 2010;94:965–970. doi: 10.1136/bjo.2009.164228. [DOI] [PubMed] [Google Scholar]
- E5.Baiocchi S, Mazzotta C, Cerretani D, Caporossi T, Caporossi A. Corneal crosslinking: riboflavin concentration in corneal stroma exposed with and without epithelium. J Cataract Refract Surg. 2009;35:893–899. doi: 10.1016/j.jcrs.2009.01.009. [DOI] [PubMed] [Google Scholar]
- E6.Koppen C, Wouters K, Mathysen D, Rozema J, Tassignon MJ. Refractive and topographic results of benzalkonium chloride-assisted transepithelial crosslinking. J Cataract Refract Surg. 2012;38:1000–1005. doi: 10.1016/j.jcrs.2012.01.024. [DOI] [PubMed] [Google Scholar]
- E7.Vinciguerra P, Randleman JB, Romano V, et al. Transepithelial iontophoresis corneal collagen cross-linking for progressive keratoconus: initial clinical outcomes. J Refract Surg. 2014;30:746–753. doi: 10.3928/1081597X-20141021-06. [DOI] [PubMed] [Google Scholar]
- E8.Li W, Wang B. Efficacy and safety of transepithelial corneal collagen crosslinking surgery versus standard corneal collagen crosslinking surgery for keratoconus: a meta-analysis of randomized controlled trials. BMC Ophthalmol. 2017;17 doi: 10.1186/s12886-017-0657-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Hashemi H, Miraftab M, Seyedian MA, et al. Long-term results of an accelerated corneal cross-linking protocol (18 mW/cm2) for the treatment of progressive keratoconus. Am J Ophthalmol. 2015;160:1164–1170e1. doi: 10.1016/j.ajo.2015.08.027. [DOI] [PubMed] [Google Scholar]
- E10.Shetty R, Pahuja NK, Nuijts RMMA. Current protocols of corneal collagen cross-linking: visual, refractive, and tomographic outcomes. Am J Ophthalmol. 2015;160:243–249. doi: 10.1016/j.ajo.2015.05.019. [DOI] [PubMed] [Google Scholar]
- E11.Liu Y, Liu Y, Zhang YN, et al. Systematic review and meta-analysis comparing modified cross-linking and standard cross-linking for progressive keratoconus. Int J Ophthalmol. 2017;10:1419–1429. doi: 10.18240/ijo.2017.09.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Mazzotta C, Traversi C, Paradiso AL, Latronico ME, Rechichi M. Pulsed light accelerated crosslinking versus continuous light accelerated crosslinking: one-year results. J Ophthalmol. 2014;2014 doi: 10.1155/2014/604731. 604731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Side effects and complications
Overall, cross-linking is a procedure with a low side-effects profile. However, the literature reports a complication risk of 1%–10% (e1), although the complications frequently seen are transient effects such as impaired epithelial healing. Only isolated cases of severe complications (such as corneal melting or corneal perforation) have been reported. Since cross-linking according to the Dresdner protocol involves initial removal of the cornea, severe pain occurs during the first 1–3 days following the procedure; this generally needs to be managed with pain medication. There is also a risk of infection during this period, as a result of which preventive treatment with antibiotic eye drops or eye ointments is necessary. If corneal infiltration develops nevertheless, a distinction needs to be made between sterile infiltrates (0%–8% of cases, [24]) and infectious infiltrates (individual case reports [e2]). Both scenarios can lead to the formation of corneal scarring, which may cause visual impairment in the long term (0%–3%, [24]). Irrespective of this, persistent scarring following cross-linking is reported in 3%–9% of cases (e3). The side effect most frequently observed is so-called haze, involving a fine haziness in the anterior corneal stroma, which generally has no effect on vision and resolves completely within a number of months (24). Ultimately, cross-linking is not able to stabilize disease course in all patients. For example, renewed progression was reported in up to 24% of cases (25) within 10 years following cross-linking in children.
Modified treatment procedure
Since cross-linking according to the Dresdner protocol has already been in use for almost 20 years, various modified treatment procedures have now been developed and investigated in studies and will be briefly discussed below.
Transepithelial cross-linking
The aim of transepithelial cross-linking it to dispense with removal of the corneal epithelium at the start of treatment. This reduces pain and the risk of infection (e4). However, since riboflavin cannot diffuse through the intact corneal epithelium due to tight junctions (e5), there are a variety of approaches to achieve penetration of riboflavin into the stroma (e.g. addition of benzalkonium chloride [e6], iontophoresis [e7]). Since there are already numerous studies comparing transepithelial cross-linking with standard cross-linking, some of which report conflicting results, an attempt was made to summarize the in part highly heterogeneous studies in review articles and meta-analyses (evidence level Ia). A recent meta-analysis (e8) based on a review of randomized controlled trials came to the conclusion that transepithelial cross-linking is inferior to standard cross-linking according to the Dresdner protocol in terms of preventing further progression. Therefore, transepithelial cross-linking protocols should currently only be used in studies after patients have received all relevant information on the procedure.
Accelerated cross-linking
The aim of accelerated cross-linking is to shorten the irradiation time by intensifying UVA irradiation, meaning that the 70-min procedure according to the Dresdner protocol can be shortened, thereby reducing the burden on the patient and saving resources. The various protocols always comply with the same total energy density (5.4 J/cm²) set out in the Dresdner protocol. Thus, depending on the power of the UVA lamp used, the irradiation time can be varied (e.g., 10 min irradiation at 9 mW/cm² power). Early clinical studies at shortened irradiation times showed similar effects to those with the Dresdner protocol (e9), although the typically observed reduction in maximum corneal refractive power appears to be less marked with shorter irradiation (e10). However, it has not been conclusively elucidated as yet whether the same amount of covalent bonds can be achieved in less time. The availability of oxygen in the corneal stroma could be a limiting factor here. A recent meta-analysis revealed the Dresdner protocol to be superior in terms of halting progression compared to accelerated cross-linking (e11). Therefore, further study results also need to be awaited for accelerated cross-linking before it can be routinely used in patients. Finally, there are already novel approaches that attempt to increase the availability of oxygen in tissue by means of irradiation pauses during treatment (pulsed corneal cross-linking), which could result in more oxygen radicals and, in turn, enhance the cross-linking effect (e12). Further controlled studies need to investigate whether this approach is able to achieve equivalent efficacy compared to standard cross-linking.